Description
A 48 -year-old previously healthy woman presented with a 1-month history of increasing clumsiness, ataxia, bilateral swaying while walking with frequent falls and a 20-day history of slurred speech, difficulty swallowing and nasal regurgitation. There was no recent decline in memory, no seizures and no blurred vision. A CT scan of the brain carried out in a private hospital where the patient was admitted for 4 days revealed no abnormality. On presentation to our institute, the patient was afebrile with a regular pulse rate of 86/min and blood pressure of 126/80 mm Hg. She was conscious, oriented and could obey simple commands. Dysarthria was present and the gag reflex was absent. Titubation was present. Bilateral cerebellar signs were present (ie, impaired finger to nose test with past pointing and impaired knee to heel test) as was dysdiadokinesia. Power in all four limbs was grade 4. Reflexes bilaterally were sluggish and both plantars were flexor. The remainder of the physical examination was insignificant. A nasogastric feeding tube was inserted and the patient was started on nasogastric feeds.
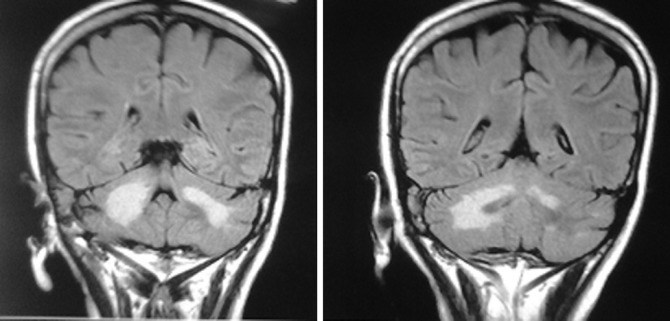
Routine laboratory investigations (ie, complete blood count, renal and liver function tests and blood sugars) were within normal limits. An MRI of the brain revealed an asymmetric T2 hyperintense signal in bilateral middle cerebellar peduncles and cerebellar white matter from right to left. Similar signal abnormality was also seen in the upper pons and medulla (figure 1). Signals in the rest of the brain parenchyma were within normal limits. These findings were suggestive of progressive multifocal leucoencephalopathy (PML) involving the posterior fossa. The patient's reports obtained later revealed that she had retroviral disease and was HIV-1 positive with a CD4 count of 65. Cerebrospinal fluid (CSF) analysis was normal and a cryptococcal antigen test was negative. John Cunningham (JC) virus polymerase chain reaction analysis of CSF was advised but could not be carried out due to financial constraints.
Figure 1.
An MRI of the brain showing asymmetric T2 hyperintense signals in bilateral middle cerebellar peduncles and cerebellar white matter, from right to left. A similar signal abnormality is also seen in the upper pons and medulla. The rest of the brain parenchyma is within normal limits.
A final diagnosis of PML limited to the posterior fossa in a patient with retroviral disease was made. The patient was started on antiretroviral therapy with stavudine, lamivudine and efavirenz and prophylaxis for opportunistic infections. The patient has been discharged and is currently able to take her drugs orally. Also, the cerebellar signs and symptoms have reduced.
Discussion
We here describe a case of HIV presenting with PML in a previously asymptomatic woman with the demyelination predominantly involving the cerebellar peduncles and cerebellar white matter, an uncommon site of involvement, without any involvement of the cortical white matter. Recognition of PML presenting with predominant cerebellar symptoms is important since treatment with highly active antiretroviral therapy (HAART) can slow progression and improve survival in these patients.
PML is a rare life threatening infection caused by the JC virus, seen in immunocompromised individuals with AIDS or leukaemia, on chemotherapy or being treated with immune suppressants such as monoclonal antibodies. The diagnostic suspicion is high in the setting of HIV.1
PML is a demyelinating disease caused by the polyoma JC virus, in which the myelin sheath covering the axons of nerve cells is gradually destroyed, impairing the transmission of nerve impulses. It affects the subcortical white matter, particularly that of the parietal and occipital lobes. PML destroys oligodendrocytes and produces intranuclear inclusions. Symptoms include weakness or paralysis, vision loss, impaired speech and cognitive deterioration. Its presentation confined to isolated brainstem and cerebellum lesions is unusual.
Diagnosis of PML may be difficult. Because 80% of the population are seropositive, the presence of antibodies to JC is not of diagnostic value. CSF identification of JC virus by polymerase chain reaction is useful, and studies suggest a sensitivity of 95% and specificity of 90%–99%.2 The MRI findings are characteristic in PML, with a high T2 signal and a low T1 signal, which does not enhance with gadolinium, showing multifocal non-enhancing lesions without mass effect with a common area of involvement being the cortical white matter, but the brainstem and cerebellum may also be involved.3 Brain biopsy is the only definitive way to make the diagnosis.4
There is no known cure for PML. In some cases, the disease slows or stops if the patient's immune system improves; some AIDS patients with PML have been able to survive for several years with the advent of HAART. There are recent reports of improved outcome following aggressive HAART in HIV and specific JC antiviral treatment with interferon α or cidofovir.5 6
Learning points.
Progressive multifocal leucoencephalopathy (PML) should be considered in all patients with an underlying disease process associated with altered cell-mediated immunity, where there is a progressive neurological picture that is possibly, but not definitely, of multifocal origin.
Devastating opportunistic John Cunningham viral infection should be considered in the differential diagnosis of isolated progressive posterior fossa white matter disease.
Although there is no known cure for PML, the disease slows or stops if the patient's immune system improves, and patients with PML have been able to survive for several years with the advent of HAART.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Astrom K-E, Mancall EL, Richardson EP. Progressive multi-focal leukoencephalopathy: a hitherto unrecognised complication of chronic lymphatic leukaemia and Hodgkin's disease. Brain 1958;81:93–111 [DOI] [PubMed] [Google Scholar]
- 2.Cinque P, Vago L, Dahl H, et al. Polymerase chain reaction on cerebrospinal fluid for diagnosis of virus-associated opportunistic diseases of the central nervous system in HIV-infected patients. AIDS 1996;10:951–8 [DOI] [PubMed] [Google Scholar]
- 3.Rosas MJ, Simoes-Ribeiro F, An SF, et al. Progressive multi-focal leukoencephalopathy: unusual MRI findings and prolonged survival in a pregnant woman. Neurology 1999;52:657–9 [DOI] [PubMed] [Google Scholar]
- 4.Jones HR, Jr, Hedley-Whyte ET, Freidberg SR, et al. Primary cerebellopontine progressive multifocal leukoencephalopathy diagnosed premortem by cerebellar biopsy. Ann Neurol 1982;11:199–202 [DOI] [PubMed] [Google Scholar]
- 5.Huang SS, Skolasky RL, Dal Pan GJ, et al. Survival prolongation in HIV-associated progressive multi-focal leukoencephalopathy treated with alpha-interferon: an observational study. J Neurovirol 1998;4:324–32 [DOI] [PubMed] [Google Scholar]
- 6.De Luca A, Fantoni M, Tartaglione T, et al. Response to cidofovir after failure of antiretroviral therapy alone in AIDS-associated progressive multifocal leukoencephalopathy. Neurology 1999;52:891–2 [DOI] [PubMed] [Google Scholar]