Abstract
Thrombosed aneurysms are difficult to visualize with digital subtraction angiography. We report a case of subarachnoid hemorrhage from a thrombosed ruptured aneurysm which was undetected on digital subtraction angiography but was visualized with cone beam volume CT. To our knowledge, this is the first report highlighting the utility of cone beam volume CT in identifying such aneurysms.
Keywords: Aneurysm, Subarachnoid, CT
Background
Ten to twenty per cent of patients with spontaneous subarachnoid hemorrhage (SAH) have culprit aneurysm(s) that are not visualized by conventional cerebral angiogram.1 This apparent non-visualization has been related to aneurysmal geometry, vasospasm, cerebral hypoperfusion, or thrombosis of the aneurysms.2 3 Cone beam volume CT is a promising new technique for these cases. Siemens has developed flat panel detector technology that can be used to produce cone beam volume CT inside the angiography suite with C arm motion (DynaCT; Siemens Medical Solutions, Erlagen, Germany), thus combining the utility of a CT scan simultaneously with that of an angiogram. We present a case that shows the ability of DynaCT in identifying a thrombosed aneurysm that was undetected with digital subtraction angiography (DSA).
Case presentation
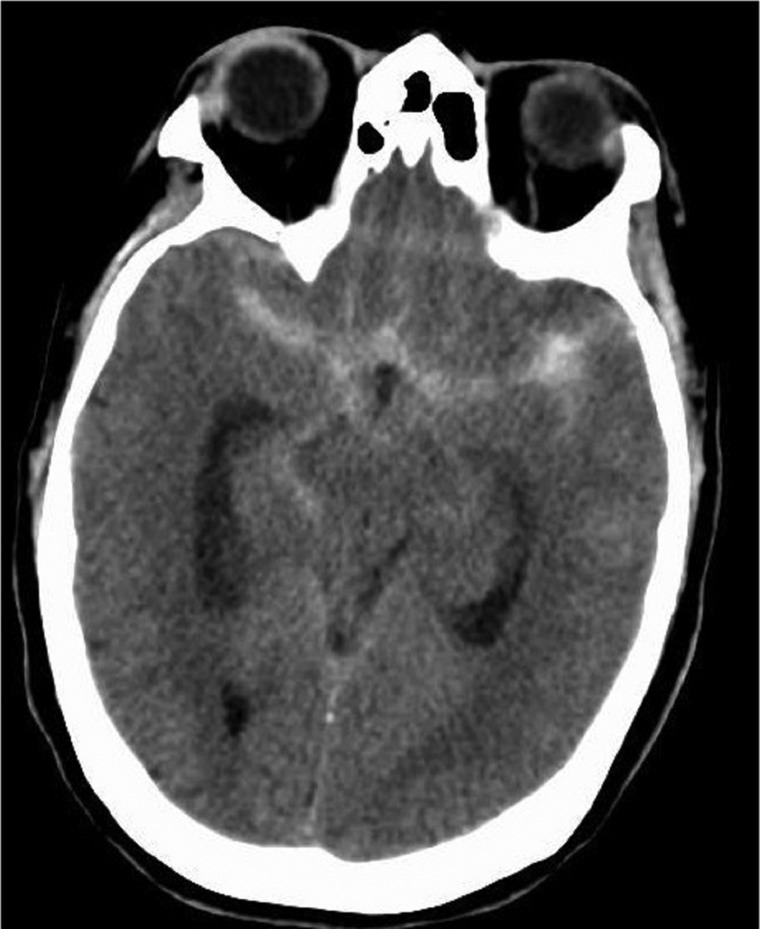
A 60-year-old woman presented with a sudden onset, worst headache of her life, nuchal rigidity, weakness, and lethargy. CT scan of the brain showed SAH in the suprasellar cistern, extending into the anterior interhemispheric fissure, bilateral perisylvian, prepontine, and right perimesencephalic cisterns, and also extending into the third and fourth ventricles (figure 1). The patient's Hunt and Hess grade was 2. After placement of an external ventricular drain, an emergent biplane cerebral angiogram was performed, which demonstrated two 1 mm saccular aneurysms at the right middle cerebral artery bifurcation and an unremarkable vasculature otherwise (figure 2A). Repeat imaging using DynaCT showed the presence of a culprit 3 mm ruptured and thrombosed aneurysm at the right anterior cerebral artery bifurcation of the pericallosal and callosal marginal branches (figure 2B). Following the identification of thrombosed aneurysm, the patient was immediately moved to the operating room and the aneurysm was clipped surgically. The patient was monitored in the neurological intensive care unit afterwards. She received nimodipine prophylaxis and daily transcranial Doppler monitoring. Her hospital course was complicated by symptomatic vasospasm of the left middle cerebral artery and right anterior cerebral artery on hospital day 11 which was treated with balloon angioplasty and intra-arterial nicardipine therapy. She was discharged to home on day 14 without residual neurological deficits. At the 30 day follow-up, she was ambulatory and independently taking care of her activities of daily living.
Figure 1.
Axial CT scan showing moderate volume subarachnoid hemorrhage involving the bilateral perisylvian and perimesencephalic cisterns.
Figure 2.
Lateral view from digital subtraction angiography with selective right common carotid injection. No aneurysm is visible on this image (A) but reconstructed images obtained with DynaCT show the thrombosed right anterior cerebral arteryaneurysm (B).
Discussion
DSA is considered to be a gold standard investigation in identifying an aneurysm following SAH. Visualization of an aneurysm on DSA requires filling of a contrast agent across the region of interest. A lateral aneurysm with a narrow neck or obliteration of the aneurysm by hemorrhage or thrombosis can obscure the visualization of the culprit aneurysm on DSA.3 4 Autopsy series have reported an incidence of thrombosis of intracranial aneurysm of 9–13%.5 Thrombosis is a well known complication of giant intracranial aneurysm.6 Thrombosis of the aneurysm is often associated with thrombosis of the parent artery.7 While the exact mechanism for aneurysm thrombosis remains to be elucidated, various factors such as cerebral vasospasm, local damage to the arterial wall due to aneurysmal rupture, aneurysm geometry, and atherosclerotic disease causing reduced blood flow are thought to be related.7 8 Routine cerebral angiogram often shows normal vasculature in the presence of a thrombosed aneurysm.9 While contrast enhanced MR angiography and CT angiography may be able to visualize such an aneurysm,10 as our case highlights, DynaCT can provide a way to diagnose a thrombosed aneurysm within the angiography suite where therapeutic decisions can be made on the table, avoiding delay in treatment.
DynaCT combines cone beam CT mounted on a C arm with a flat panel detector within the angiographic suite. It provides a multiplanar, cross sectional imaging method with a high spatial and contrast resolution, allowing imaging of the intracranial brain parenchyma, subarachnoid space, and ventricular system. Three-dimensional reconstructions with DynaCT allow a better understanding of the complex vascular pathologies and their anatomical relationship with surrounding structures. With the availability of DynaCT it has become feasible to perform CT scan-like images within the confinement of the angiographic suit, eliminating the necessity of moving the patient out of the interventional suite. DynaCT is especially helpful for early detection of complications encountered during interventional procedures, such as rupture of the aneurysm during embolization and tailoring of further therapeutic measures.11 It is also useful in visualizing stent struts and their relationship to arterial walls.12 Although the quality of images produced by DynaCT is inferior to conventional CT, it achieves results with less radiation and intravenous contrast doses and is generally sufficient to make a diagnosis.11 13
Our case highlights another important advantage offered by DynaCT in identifying culprit aneurysms in angiogram negative cases.
Learning points.
Routine cerebral angiogram is often unrevealing in patients with subarachnoid hemorrhage.
Thrombosis, hemorrhage, and narrow neck are factors that can obscure an aneurysm on the angiogram.
DynaCT is a useful modality to identify such aneurysms.
Footnotes
Contributors: SS: designing, drafting and revising the manuscript. SBM: designing the manuscript. YH: clinical examination of the patient. CPVR: interpretation of the imaging study and clinical examination, and revising the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Juul R, Fredriksen TA, Ringkjob R. Prognosis in subarachnoid hemorrhage of unknown etiology. J Neurosurg 1986;64:359–62 [DOI] [PubMed] [Google Scholar]
- 2.Rinkel GJ, van Gijn J, Wijdicks EF. Subarachnoid hemorrhage without detectable aneurysm. A review of the causes. Stroke 1993;24:1403–9 [DOI] [PubMed] [Google Scholar]
- 3.Jou LD, Mohamed A, Lee DH, et al. 3D rotational digital subtraction angiography may underestimate intracranial aneurysms: findings from two basilar aneurysms. AJNR Am J Neuroradiol 2007;28:1690–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ernemann UU, Gronewaller E, Duffner FB, et al. Influence of geometric and hemodynamic parameters on aneurysm visualization during three-dimensional rotational angiography: an in vitro study. AJNR Am J Neuroradiol 2003;24:597–603 [PMC free article] [PubMed] [Google Scholar]
- 5.Housepian EM, Pool JL. A systematic analysis of intracranial aneurysms from the autopsy file of the Presbyterian Hospital, 1914 to 1956. J Neuropathol Exp Neurol 1958;17:409–23 [DOI] [PubMed] [Google Scholar]
- 6.Sugita M, Sasaki H, Kakizawa T, et al. Giant middle cerebral artery aneurysm with parent artery occlusion—case report. Neurol Med Chir (Tokyo) 1991;31:37–40 [DOI] [PubMed] [Google Scholar]
- 7.Fodstad H, Liliequist B. Spontaneous thrombosis of ruptured intracranial aneurysms during treatment with tranexamic acid (AMCA). Report of three cases. Acta Neurochir (Wien) 1979;49:129–44 [DOI] [PubMed] [Google Scholar]
- 8.Black SP, German WJ. Observations on the relationship between the volume and the size of the orifice of experimental aneurysms. J Neurosurg 1960;17:984–90 [DOI] [PubMed] [Google Scholar]
- 9.Wakui K, Kamijo Y, Seguchi K, et al. Thrombosed aneurysm of the middle cerebral artery with occlusion of the distal parent artery—case report. Neurol Med Chir (Tokyo) 1992;32:842–5 [DOI] [PubMed] [Google Scholar]
- 10.Renowden SA, Molyneux AJ, Anslow P, et al. The value of MRI in angiogram-negative intracranial haemorrhage. Neuroradiology 1994;36:422–5 [DOI] [PubMed] [Google Scholar]
- 11.Heran NS, Song JK, Namba K, et al. The utility of DynaCT in neuroendovascular procedures. AJNR Am J Neuroradiol 2006;27:330–2 [PMC free article] [PubMed] [Google Scholar]
- 12.Benndorf G, Strother CM, Claus B, et al. Angiographic CT in cerebrovascular stenting. AJNR Am J Neuroradiol 2005;26:1813–18 [PMC free article] [PubMed] [Google Scholar]
- 13.Akpek S, Brunner T, Benndorf G, et al. Three-dimensional imaging and cone beam volume CT in C-arm angiography with flat panel detector. Diagn Interv Radiol 2005;11:10–13 [PubMed] [Google Scholar]