Abstract
Background/Aims
Individuals being treated with tumor necrosis factor (TNF)-α inhibitors are at increased risk of developing tuberculosis (TB). We determined the clinical characteristics and treatment response of patients who developed TB after using TNF-α inhibitors.
Methods
Patients with TB detected within 12 months of the initiation of TNF-α inhibitor treatment were included, if seen from January 1, 2000 to August 31, 2011. We retrospectively reviewed the clinical records, results of bacteriological examinations, and radiographs of the included patients and the response to anti-TB treatment.
Results
We indentified seven cases of TB in 457 patients treated with TNF-α inhibitors during the study period. TB developed a median of 123 days (range, 48 to 331) after the first dose of TNF-α inhibitor. Pulmonary TB, including TB pleuritis, was diagnosed in three patients and extrapulmonary TB in four. Favorable treatment outcomes were achieved in six of seven patients.
Conclusions
Among the TNF-α inhibitor users who contracted TB, extrapulmonary sites were common and the treatment response was satisfactory.
Keywords: Tumor necrosis factor-alpha, Tuberculosis, Mycobacterium
INTRODUCTION
Tuberculosis (TB) is a leading cause of mortality and morbidity worldwide. In 2010, there were 8.8 million incident cases of TB, 1.1 million deaths from TB among human immunodeficiency virus (HIV)-negative people, and an additional 0.35 million deaths from HIV-associated TB [1]. The majority of infected persons will develop a latent TB infection, and the infection will eventually reactivate in about 10% of these subjects, leading to active TB [2].
Tumor necrosis factor (TNF) and TNF receptors are important regulators of immune cell activation, proliferation, differentiation, survival, and apoptosis [3-5]. TNF-α is a key cytokine in the immune response to infection with Mycobacterium tuberculosis [6], and is critical for the formation and maintenance of the granuloma [7]. TNF-α, together with interferon (IFN)-γ, increases the phagocytic capacity of macrophages and enhances the killing of M. tuberculosis via the generation of reactive nitrogen and oxygen intermediates [8]. TNF-α, deficient mice are unable to control M. tuberculosis infection, and granulomas do not form properly in their lungs [9,10].
Several TNF-α inhibitors are used widely in the treatment of chronic inflammatory diseases, such as rheumatoid arthritis, inflammatory bowel disease, and several other conditions [11-15]. Unfortunately, individuals treated with TNF-α inhibitors are reportedly at an increased risk of developing TB [11,14,16,17]. However, the characteristics and treatment results of subsequent TB cases have not yet been reported. In this study, we investigated the clinical characteristics and treatment responses of TB that developed after TNF-α inhibitor treatment.
METHODS
Study setting and patients
Patients with TB that was detected within 12 months of the initiation of TNF-α inhibitor treatment between January 1, 2000 and August 31, 2011 at Seoul National University Hospital, a tertiary referral hospital in South Korea, were included in the study. We excluded patients with any other risk factors for TB reactivation, such as HIV infection, silicosis, or other immunosuppressive treatment, including anticancer chemotherapy. Patients who used TNF-α inhibitors for less than 4 weeks were also excluded. TB was diagnosed using all clinical, radiological, microbiological, and pathological information collected during the diagnostic process and follow-up period. The study protocol was approved by the Ethics Review Committee of Seoul National University Hospital.
Data collection
We retrospectively reviewed the clinical records, results of bacteriological examinations, patient radiographs, and responses to anti-TB treatment. Patient clinical variables were analyzed using descriptive statistics. The results are expressed as means and standard deviations or median values with ranges.
RESULTS
Demographic and clinical characteristics of patients
During the study period, 457 patients were treated with TNF-α inhibitors in our hospital. Of these, 11 (2.4%) patients were diagnosed with TB. Four TB patients diagnosed more than 12 months after initiating TNF-α inhibitor treatment were excluded. In total, seven patients who were diagnosed with TB within 12 months of TNF-α inhibitor initiation were included in the analysis. The median patient age was 62 years (range, 32 to 67). Four of the patients were female and one had diabetes. Of the seven patients with TB, one completed a 9-month course of isoniazid prophylaxis before developing active TB.
Use of TNF-α inhibitors
Rheumatoid arthritis was the most common indication for TNF-α inhibitor use (three patients). TNF-α inhibitors were used in one patient each with Crohn's disease, ulcerative colitis, ankylosing spondylitis, and reactive arthritis. Infliximab was the most commonly prescribed (three patients). The median duration of TNF-α inhibitor use was 167 days (range, 42 to 1,704) (Table 1).
Table 1.
Demographic and clinical characteristics of seven patients with tuberculosis (TB) that developed following tumor necrosis factor (TNF)-α inhibitor use
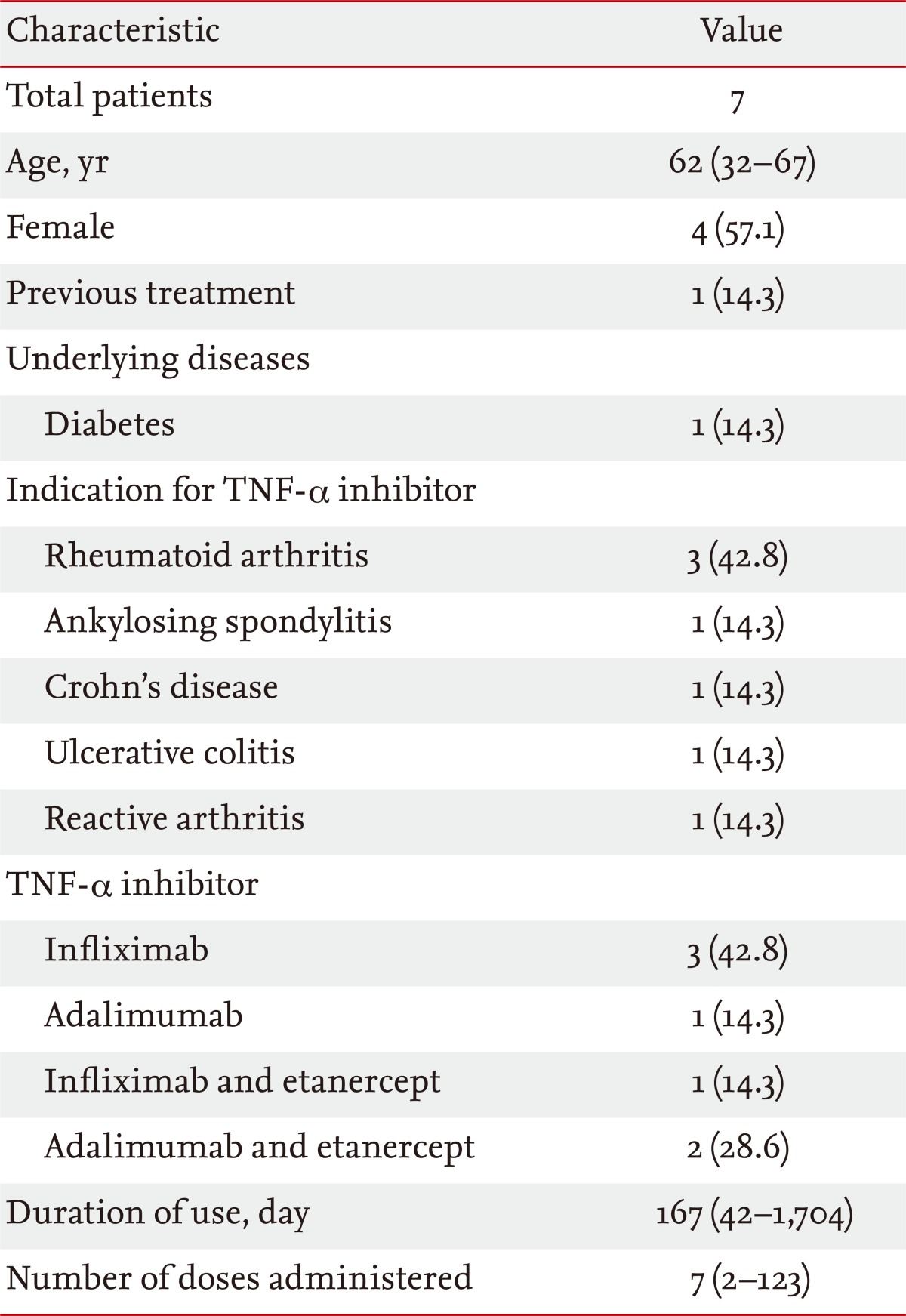
Values are presented as median (range) or number (%).
Results of tuberculin skin tests and IFN-γ release assays
Tests for latent TB infection were performed in five of the seven patients. The tuberculin skin test was negative in one patient. In addition, IFN-γ release assays performed in four patients were negative.
TB developed after using TNF-α inhibitors
TB developed a median of 123 days (range, 48 to 331) after the first dose of TNF-α inhibitor. The median number of TNF-α inhibitor doses before developing TB was 16 doses (range, 2 to 123). TB was diagnosed a median of 25 days (range, 3 to 80) after the last dose of TNF-α inhibitor. TB was diagnosed in three patients based on sputum M. tuberculosis culture, in one patient with TB-polymerase chain reaction of a sputum specimen, and in three other patients based on symptoms, compatible chest radiograph findings, and clinical responses to anti-TB medication. Pulmonary TB, including TB pleuritis, was diagnosed in three patients and extrapulmonary TB, including disseminated TB, was diagnosed in four. The extrapulmonary sites were the pericardium, intestine, and bone (Table 2).
Table 2.
Characteristics of tuberculosis (TB) that developed following tumor necrosis factor (TNF)-α inhibitor use
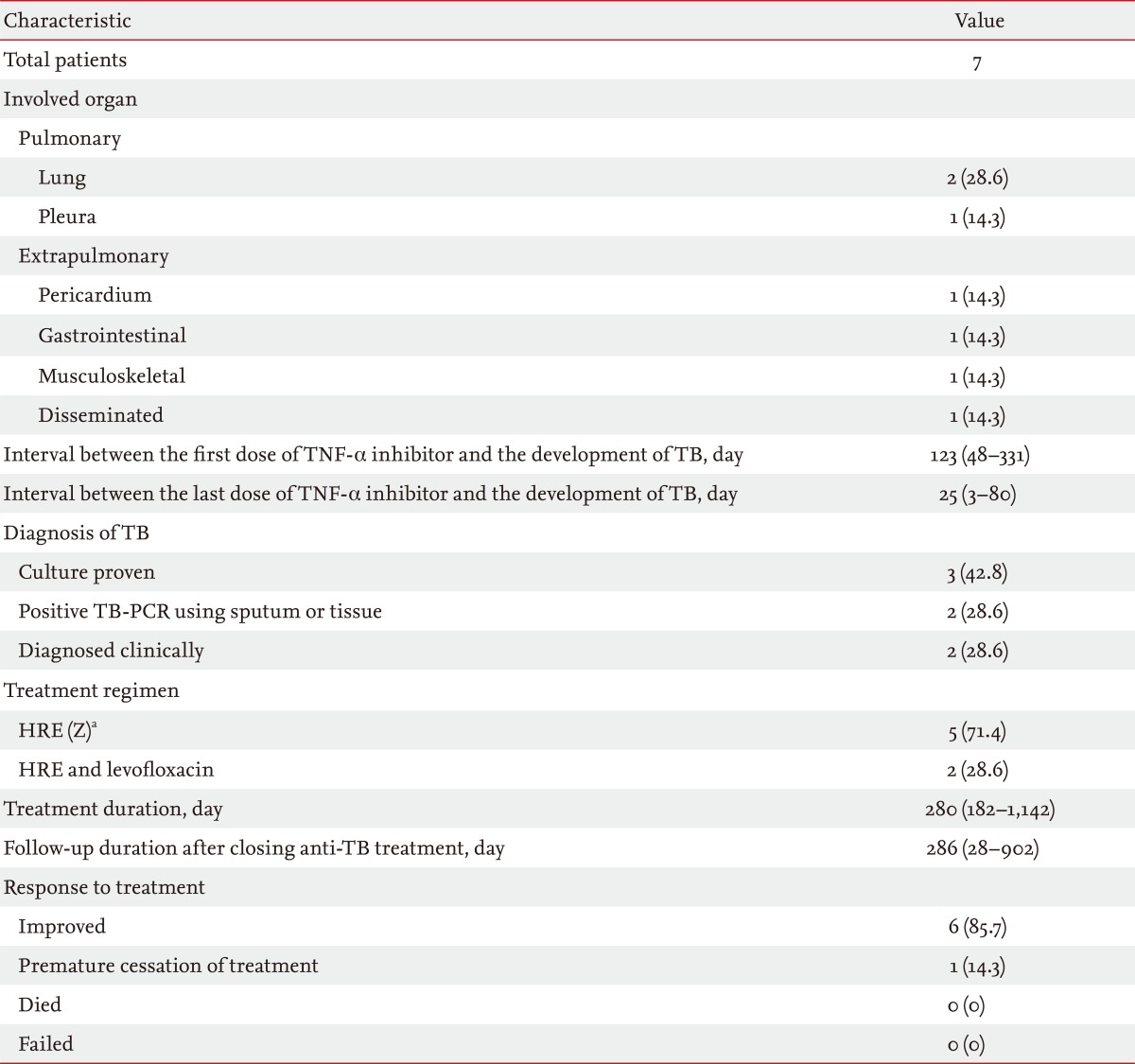
Values are presented as median (range) or number (%).
PCR, polymerase chain reaction.
aIsoniazid (H), rifampicin (R), ethambutol (E), pyrazinamide (Z).
Anti-TB treatment and responses
All patients were treated with combinations of first-line anti-TB medications. The median treatment duration was 280 days (range, 182 to 1,142). In two patients, levofloxacin was used instead of pyrazinamide due to abnormal liver function.
Of the three patients with pulmonary TB, two were cured following treatment, and confirmed by negative sputum conversion in one patient. In the other patient, treatment was completed without evidence of aggravation or recurrence. Three of four patients with extrapulmonary TB improved clinically and radiographically with treatment. In one patient, however, the anti-TB treatment was stopped prematurely after 130 days of medication, because of worsening of ulcerative colitis (Table 2).
DISCUSSION
In this study, we found that active TB developed within 12 months of TNF-α inhibitor initiation in 7 of 457 patients (1.5%). Pulmonary TB, including TB pleuritis, was diagnosed in three patients and extrapulmonary TB was diagnosed in the other four. Since extrapulmonary TB constitutes 18.9% of all TB in South Korea [18], the proportion of extrapulmonary TB among TNF-α inhibitor users was somewhat higher than expected. In fact, an earlier study that showed a higher risk of TB developing in TNF-α inhibitor users also showed that extrapulmonary TB constituted 56% of the TB cases [19]. It is possible that the high proportion of extrapulmonary TB among TNF-α inhibitor users is associated with inadequate compartmentalization of viable mycobacterial bacilli by granulomas due to TNF-α antagonism [10].
The treatment responses of TB in the TNF-α inhibitor users were satisfactory. Favorable outcomes were achieved in all but one patient, who could not take anti-TB medication because of worsening ulcerative colitis. This concurs with the favorable treatment responses of other immunocompromised TB patients, such as those with HIV infection [20], organ transplant [21,22], and long-term steroid users [23].
Although the use of TNF-α inhibitors is regarded as one of the indications of treatment for latent TB infection [24-26], only one of the seven patients in our series started a 9-month course of isoniazid prophylaxis before initiating TNF-α inhibitor treatment. A possible explanation is that the official Korean guidelines recommending the treatment of latent TB in TNF-α inhibitor users were published in 2011 [27]. Most of the patients in our study started TNF-α inhibitors before the guideline was publish.
In summary, we showed that among TNF-α inhibitor users who contracted TB, extrapulmonary sites were common and the treatment responses were satisfactory.
KEY MESSAGE
1. We found seven cases of tuberculosis (TB) from 457 patients treated with tumor necrosis factor (TNF)-α inhibitors during study period of 12 years.
2. Among TNF-α inhibitor users who contracted TB, extrapulmonary site were commonly involved.
Footnotes
No potential conflict of interest relevant to this article is reported.
References
- 1.WHO global tuberculosis control report 2010: summary. Cent Eur J Public Health. 2010;18:237. [PubMed] [Google Scholar]
- 2.Lin PL, Plessner HL, Voitenok NN, Flynn JL. Tumor necrosis factor and tuberculosis. J Investig Dermatol Symp Proc. 2007;12:22–25. doi: 10.1038/sj.jidsymp.5650027. [DOI] [PubMed] [Google Scholar]
- 3.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 4.Hehlgans T, Pfeffer K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: players, rules and the games. Immunology. 2005;115:1–20. doi: 10.1111/j.1365-2567.2005.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 6.Serbina NV, Flynn JL. Early emergence of CD8(+) T cells primed for production of type 1 cytokines in the lungs of Mycobacterium tuberculosis-infected mice. Infect Immun. 1999;67:3980–3988. doi: 10.1128/iai.67.8.3980-3988.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 8.Bekker LG, Freeman S, Murray PJ, Ryffel B, Kaplan G. TNF-alpha controls intracellular mycobacterial growth by both inducible nitric oxide synthase-dependent and inducible nitric oxide synthase-independent pathways. J Immunol. 2001;166:6728–6734. doi: 10.4049/jimmunol.166.11.6728. [DOI] [PubMed] [Google Scholar]
- 9.Bean AG, Roach DR, Briscoe H, et al. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol. 1999;162:3504–3511. [PubMed] [Google Scholar]
- 10.Flynn JL, Goldstein MM, Chan J, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 11.Askling J, Fored CM, Brandt L, et al. Risk and case characteristics of tuberculosis in rheumatoid arthritis associated with tumor necrosis factor antagonists in Sweden. Arthritis Rheum. 2005;52:1986–1992. doi: 10.1002/art.21137. [DOI] [PubMed] [Google Scholar]
- 12.Dixon WG, Watson K, Lunt M, Hyrich KL, Silman AJ, Symmons DP. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2006;54:2368–2376. doi: 10.1002/art.21978. [DOI] [PubMed] [Google Scholar]
- 13.Ellerin T, Rubin RH, Weinblatt ME. Infections and anti-tumor necrosis factor alpha therapy. Arthritis Rheum. 2003;48:3013–3022. doi: 10.1002/art.11301. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD BIOBADASER Group. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum. 2003;48:2122–2127. doi: 10.1002/art.11137. [DOI] [PubMed] [Google Scholar]
- 15.Singh JA, Wells GA, Christensen R, et al. Adverse ef fects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;(2):CD008794. doi: 10.1002/14651858.CD008794.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallis RS, Broder M, Wong J, Beenhouwer D. Granulomatous infections due to tumor necrosis factor blockade: correction. Clin Infect Dis. 2004;39:1254–1255. doi: 10.1086/424455. [DOI] [PubMed] [Google Scholar]
- 17.Wolfe F, Michaud K, Anderson J, Urbansky K. Tuberculosis infection in patients with rheumatoid arthritis and the effect of infliximab therapy. Arthritis Rheum. 2004;50:372–379. doi: 10.1002/art.20009. [DOI] [PubMed] [Google Scholar]
- 18.Korean National Tuberculosis Association. Trend of case notification rate per 100,000 by year, 2004-2011 [Internet] Seoul (KR): Korean National Tuberculosis Association; c2011. [cited 2013 Feb 1]. Available from: http://www.knta.or.kr. [Google Scholar]
- 19.Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 20.Khan FA, Minion J, Pai M, et al. Treatment of active tuberculosis in HIV-coinfected patients: a systematic review and meta-analysis. Clin Infect Dis. 2010;50:1288–1299. doi: 10.1086/651686. [DOI] [PubMed] [Google Scholar]
- 21.Morris MI, Daly JS, Blumberg E, et al. Diagnosis and management of tuberculosis in transplant donors: a donor-derived infections consensus conference report. Am J Transplant. 2012;12:2288–2300. doi: 10.1111/j.1600-6143.2012.04205.x. [DOI] [PubMed] [Google Scholar]
- 22.Ersan S, Celik A, Atila K, et al. Tuberculosis in renal transplant recipients. Ren Fail. 2011;33:753–757. doi: 10.3109/0886022X.2011.599095. [DOI] [PubMed] [Google Scholar]
- 23.Gaitonde S, Pathan E, Sule A, Mittal G, Joshi VR. Efficacy of isoniazid prophylaxis in patients with systemic lupus erythematosus receiving long term steroid treatment. Ann Rheum Dis. 2002;61:251–253. doi: 10.1136/ard.61.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solovic I, Sester M, Gomez-Reino JJ, et al. The risk of tuberculosis related to tumour necrosis factor antagonist therapies: a TBNET consensus statement. Eur Respir J. 2010;36:1185–1206. doi: 10.1183/09031936.00028510. [DOI] [PubMed] [Google Scholar]
- 25.Salgado E, Gomez-Reino JJ. The risk of tuberculosis in patients treated with TNF antagonists. Expert Rev Clin Immunol. 2011;7:329–340. doi: 10.1586/eci.11.6. [DOI] [PubMed] [Google Scholar]
- 26.Migliori GB, Zellweger JP, Abubakar I, et al. European union standards for tuberculosis care. Eur Respir J. 2012;39:807–819. doi: 10.1183/09031936.00203811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joint Committee for the Development of Korean Guidelines for Tuberculosis; Korea Centers for Disease Control and Prevention. Korean Guidelines for Tuberculosis. 1st ed. Cheongwon: Korea Centers for Disease Control and Prevention; 2011. [Google Scholar]


