Abstract
Obesity and excessive lipolysis are implicated in preeclampsia. Intrauterine growth restriction is associated with low maternal body mass index and decreased lipolysis. Our aim was to assess how maternal and offspring fatty acid metabolism is altered in mothers in the third trimester of pregnancy with preeclampsia (n=62) or intrauterine growth restriction (n=23) compared to healthy pregnancies (n=164). Markers of lipid metabolism and erythrocyte fatty acid concentrations were measured. Maternal adipose tissue fatty acid composition and mRNA expression of adipose tissue fatty acid metabolizing enzymes and placental fatty acid transporters were compared. Mothers with preeclampsia had higher plasma triglyceride (21%, p<0.001) and non-esterified fatty acid (50%, p<0.001) concentrations than Controls. Concentrations of major n-6 and n-3 long chain polyunsaturated fatty acids in erythrocytes were 23-60% lower (all p<0.005) in preeclampsia and intrauterine growth restriction mothers and offspring compared to Controls. Subcutaneous adipose tissue Δ-5 and Δ-6 desaturase and very long chain fatty acid elongase mRNA expression was lower in preeclampsia than Controls [Control 3.38(2.96) vs preeclampsia 1.83(1.91), p=0.030; 3.33(2.25) vs 1.03(0.96), p<0.001; 0.40 (0.81) vs 0.00 (0.00), p=0.038 (square root) expression relative to control gene respectively]. Low maternal and fetal long chain polyunsaturated fatty acid concentrations in preeclampsia may be the result of decreased maternal synthesis.
Keywords: fatty acid, pregnancy, pre-eclampsia, intrauterine growth restriction
Introduction
Pre-eclampsia (PE), a multi-system disorder particular to pregnancy, is a leading cause of maternal and neonatal morbidity and mortality. Preeclampsia is characterized by widespread endothelial dysfunction, resulting in hypertension due to vasoconstriction, proteinuria attributable to glomerular damage and oedema secondary to increased vascular permeability. Maternal obesity, increased insulin resistance and aberrant fatty acid metabolism are involved in its pathogenesis1. Excessive non-esterified fatty acid (NEFA) flux in PE, similar to that seen in non-alcoholic fatty liver disease, may instigate ectopic lipid accumulation in the liver and other tissues2 and interfere with long chain polyunsaturated fatty acid (LC PUFA) synthesis3. Intrauterine growth restriction (IUGR) can occur independently or simultaneously with PE. Isolated IUGR pregnancies are characterized by low maternal BMI, low plasma lipid levels and reduced lipolysis4, 5. Few data exist on the impact of PE or IUGR on maternal fatty acid mobilisation during pregnancy and that available has focussed on percentage of total fatty acids in plasma6-9 rather than absolute amounts or erythrocyte composition. These latter measures allow primary independent effects on individual fatty acids to be determined and better reflect tissue fatty acid composition and long term nutritional status rather than recent dietary intake10.
Long chain polyunsaturated fatty acids of the n-3 and n-6 series such as docosahexaenoic acid (22:6n-3, DHA) and arachidonic acid (20:4n-6) are required for fetal growth11 and brain development12. Thus a potential long term consequence of disturbed LC PUFA synthesis in PE is sub-optimal neurodevelopment of the infants13. Maternal LC PUFA are mobilized by week 13 of gestation14 but their source(s) are not established. The n-6 and n-3 LC PUFA are synthesized from essential shorter chain precursors (18:2n-6 and 18:3n-3 respectively) via Δ5-desaturase, Δ6-desaturase and elongase enzymes (Figure S1). Pregnant women accumulate adipose tissue in the early anabolic stage of pregnancy but in later gestations, due to insulin resistance, adipose tissue fatty acid mobilization increases15. Placental transfer of LC PUFA to the fetus is obligatory12 and both circulating NEFA and fatty acids released from lipolysis of lipoprotein triglycerides are transported across the placenta by a series of fatty acid binding and transfer proteins16. Amongst these, pFABPpm a membrane transporter, and FABP7, an intracellular transporter, have high affinities for 22:6n-3 and are expressed by trophoblasts17-19.
We hypothesized that, in an analogous way to non-alcoholic fatty liver disease, excessive NEFA flux in PE provokes ectopic lipid accumulation in the liver and inhibits LC PUFA synthesis. Our aim was to assess whether maternal fatty acid metabolism is altered in mothers with PE or IUGR compared to healthy pregnancy and impacts on offspring LC PUFA status. We carried out erythrocyte fatty acid compositional analysis of paired maternal and fetal samples from healthy and complicated pregnancies. Markers of lipid metabolism were also assessed to describe the gross differences in fuel metabolism. To assess whether LC PUFA status was related to the composition of stored fatty acids we analysed both visceral and upper body subcutaneous adipose tissue fatty acid composition in a subset of the pregnancies. The mRNA expression of desaturase and elongase enzymes was quantitated in adipose tissue as a marker of LC PUFA synthesis in this and other tissues. To test whether offspring LC PUFA status was related to placental transfer of fatty acids, mRNA expression of key enzymes and transporters in LC PUFA metabolism was quantitated in placental biopsies.
Methods
Subjects
Subjects with PE (n=62), with IUGR (n=23) and Controls with uncomplicated pregnancies (n=164) in the third trimester of a singleton pregnancy were recruited. The study was approved by the Glasgow Royal Infirmary Local Research Ethics Committee and women gave written informed consent. For further details on recruitment and biopsy sampling see the Online Supplement.
Biochemical analyses
Total cholesterol, triglyceride and HDL cholesterol20, glucose and high sensitivity CRP assays21 were performed by Clinical Biochemistry, Glasgow Royal Infirmary. Other analytes were assayed using commercially available kits (Online Supplement). Fatty acids were extracted from erythrocyte membranes14 and adipose tissue and identified by gas chromatography14, 22 (details in Online Supplement).
Messenger RNA expression
Total RNA was isolated from placenta (Control n=57, PE n=17 and IUGR n=11), subcutaneous adipose tissue (Control n=50, PE n=12 and IUGR n=13) and visceral adipose tissue (Control n=25, PE n=12 and IUGR n=5) and cDNA synthesized. Target gene expression was quantitated relative to a control gene by Taqman real time PCR using commercial primer probe sets (Applied Biosystems) (details in Online Supplement).
Statistical analysis
Details of statistical analysis are provided in the Online Supplement. Unsaturation index (UI) is the average number of double bonds per fatty acid residue multiplied by 100; average chain length (CL) is the sum of mol% times chain length for each reported fatty acid divided by 100; C20-22 is the total percentage of LC PUFA with ≥20 carbon units; DHA deficiency index is 22:5n-6/22:4n-6 and EFA deficiency index is (n-3+n-6)/(n-7+n-9). Due to multiple testing significance levels were set at P<0.005 for plasma metabolic markers and fatty acid analysis.
Results
Maternal metabolic and inflammatory profile
Maternal antenatal booking characteristics (Table S1) and third trimester plasma profiles (Table S2) are shown. Mothers with PE had higher triglyceride, NEFA, leptin, adiponectin and IL-6 than Controls, and these differences were maintained after adjustment for maternal BMI, parity, smoking status and gestational age at sampling.
The impact of PE and IUGR on maternal LC PUFA status
Maternal third trimester erythrocyte fatty acid concentrations are shown in Table 1. There were no differences in concentrations of saturated fatty acids (SAFA) between groups apart from a lower concentration of the minor fatty acid 22:0 in IUGR. For the monounsaturated fatty acids (MUFA) there was a 19% lower concentration of 24:1n-9 in IUGR. Concentrations of all n-6 PUFA, apart from the minor fatty acids 18:3n-6 and 22:2n-6, were 23% to 60% lower in both PE and IUGR mothers. Of the n-3 PUFA, 22:5n-3 and 22:6n-3 were lower in PE and IUGR mothers to a similar extent as for the n-6 PUFA. Interestingly, 20:5n-3 was not different between groups.
Table 1.
Maternal erythrocyte fatty acid concentrations (nmol/mL blood) from third trimester Control, preeclampsia (PE) and intrauterine growth restriction (IUGR) mothers
| Fatty Acid | Control (n=164) |
PE (n=62) |
IUGR (n=23) |
P* |
†Adjusted P |
|---|---|---|---|---|---|
| SAFA | |||||
| 12:0 | 0.3 (2) | 1.4 (5) | 0 (0) | 0.026 | 0.099 |
| 14:0 | 15 (9) | 17 (8) | 16 (8) | 0.26 | 0.48 |
| 16:0 | 516 (116) | 531 (107) | 447 (128) | 0.010 | 0.016 |
| 17:0 | 6 (5) | 5 (5) | 7 (4) | 0.21 | 0.19 |
| 18:0 | 318 (74) | 294 (70) | 294 (83) | 0.055 | 0.021 |
| 20:0 | 11 (4) | 10 (4) | 11 (4) | 0.65 | 0.11 |
| 22:0 | 22 (14)a | 17 (16)a | 9 (12)b | <0.001 | <0.001 |
| 24:0 | 60 (12) | 61 (17) | 61 (13) | 0.80 | 0.85 |
| MUFA | |||||
| 14:1n-7 | 0 (0) | 0 (0) | 0 (0) | - | - |
| 16:1n-7 | 17 (10) | 20 (9) | 17 (10) | 0.20 | 0.079 |
| 17:1n-7 | 17 (28) | 15 (27) | 15 (27) | 0.81 | 0.69 |
| 18:1n-7 | 5 (10) | 5 (11) | 3 (9) | 0.71 | 0.96 |
| 18:1n-9 | 288 (80) | 283 (65) | 238 (71) | 0.012 | 0.007 |
| 20:1n-9 | 8 (5) | 6 (5) | 6 (4) | 0.025 | 0.007 |
| 22:1n-9 | 1 (3) | 1 (3) | 0 (0) | 0.21 | 0.11 |
| 24:1n-9 | 86 (22)a | 80 (20)a,b | 70 (21)b | 0.002 | 0.004 |
| PUFA n-6 | |||||
| 18:2n-6 | 171 (54)a | 130 (51)b | 115 (49)b | <0.001 | <0.001 |
| 18:3n-6 | 1.8 (3) | 1.3 (3) | 2.1 (3) | 0.43 | 0.38 |
| 20:2n-6 | 4 (5)a | 2 (3)b | 2 (3)b | <0.001 | <0.001 |
| 20:3n-6 | 32 (15)a | 23 (14)b | 20 (11)b | <0.001 | <0.001 |
| 20:4n-6 | 225 (89)a | 142 (97)b | 128 (82)b | <0.001 | <0.001 |
| 22:2n-6 | 0.8 (1.8) | 0.7 (1.5) | 0.8 (1.6) | 0.97 | 0.64 |
| 22:4n-6 | 43 (20)a | 26 (20)b | 25 (17)b | <0.001 | <0.001 |
| 22:5n-6 | 10 (6)a | 5 (6)b | 4 (5)b | <0.001 | <0.001 |
| PUFA n-3 | |||||
| 18:3n-3 | 5 (4) | 3 (3) | 4 (3) | 0.028 | <0.001 |
| 20:3n-3 | 1.1 (2.8) | 0.1 (0.6) | 0 (0) | 0.006 | 0.002 |
| 20:5n-3 | 16 (10) | 16 (10) | 17 (12) | 0.91 | 0.86 |
| 22:3n-3 | 3.6 (5.1) | 2.6 (4.0) | 1.5 (2.3) | 0.080 | 0.012 |
| 22:5n-3 | 32 (14)a | 19 (14)b | 17 (14)b | <0.001 | <0.001 |
| 22:6n-3 | 65 (30)a | 40 (35)b | 39 (31)b | <0.001 | <0.001 |
| Summary indices | |||||
| % SAFA | 49 (7)a | 54 (9)b | 56 (10)b | <0.001 | <0.001 |
| % MUFA | 21 (2)a | 23 (2)b | 22 (4)a | <0.001 | <0.001 |
| % PUFA | 30 (7)a | 22 (9)b | 22 (9)b | <0.001 | <0.001 |
| % UNSAT | 51 (7)a | 46 (9)b | 44 (10)b | <0.001 | <0.001 |
| UI | 131 (29)a | 102 (35)b | 101 (36)b | <0.001 | <0.001 |
| Av CL | 18.5 (0.3)a | 18.3 (0.3)b | 18.3 (0.3)b | <0.001 | <0.001 |
| C 20-22 | 26 (6)a | 20 (7)b | 21 (6)b | <0.001 | <0.001 |
| n-6/n-3 ratio | 4.2 (1.2) | 4.5 (2.0) | 4.5 (2.1) | 0.21 | 0.21 |
| DHA deficiency index |
0.20 (0.11)a | 0.13 (0.16)b | 0.11 (0.11)b | <0.001 | <0.001 |
| EFA deficiency index |
1.44 (0.39)a | 0.98 (0.42)b | 1.02 (0.41)b | <0.001 | <0.001 |
Values are mean and standard deviation (SD).
ANOVA was used to test for differences among groups. Different superscript letters indicate differences between individual groups using post hoc Tukey test. Significance level P<0.005.
adjusted for maternal body mass index (BMI), parity, smoking status and gestational age at sampling.
Summary indices of maternal fatty acid status are shown in Table 1. The percentage SAFA is higher and the percentage unsaturated fatty acids and PUFA significantly lower in PE and IUGR. The main driver for the change in proportions is the lower PUFA as concentrations of SAFA are similar between groups. The lower PUFA concentrations account for the lower unsaturation index and average chain length observed in PE and IUGR. Since concentrations of both n-6 and n-3 PUFA are lower, the n-6/n-3 ratio is similar across groups. PE and IUGR mothers are deficient in EFA and 22:6n-3. All observed differences were maintained after adjustment for potential confounders. Women with severe PE had significantly lower concentrations than those with mild PE for the majority of fatty acid parameters measured (Table S3). There was no relationship with gestational age at PE onset (data not shown).
Offspring metabolic and inflammatory profile
Cord blood total cholesterol, but not HDL, concentration was significantly higher in PE offspring (Table S2). Conversely both total and HDL cholesterol concentrations were lower in IUGR compared to Control offspring. Cord blood triglyceride concentrations were higher in PE and IUGR offspring compared to Controls. There were no differences in cord blood NEFA, glucose, insulin, HOMA or inflammatory marker levels between groups. Cord blood leptin (46-68%) and adiponectin (38-60%) levels were significantly lower in PE and IUGR offspring, associations which were lost after adjusting for maternal BMI and gestation at delivery for leptin and maternal smoking for adiponectin as described by others23-25.
The impact of PE and IUGR on offspring LC-PUFA status
Cord blood erythrocyte SAFA concentrations were similar between groups apart from higher levels of the minor fatty acids 14:0 and 17:0 in PE and IUGR (Table 2). There were no differences in MUFA concentrations between groups. There were significantly lower 20:3n-6, 22:4n-6 and 22:5n-6 concentrations in PE and IUGR and a trend towards reduced 20:4n-6. Similar to their mothers, there were significantly lower concentrations of 22:5n-3 and 22:6n-3 in PE and IUGR offspring. The lower 22:5n-3 concentration was lost on adjustment for confounders, particularly parity. Interestingly, there was a trend towards higher 18:3n-3 concentration in PE and IUGR.
Table 2.
Cord erythrocyte fatty acid concentrations (nmol/mL blood) from Control, preeclampsia (PE) and intrauterine growth restriction (IUGR) offspring
| Fatty Acid | Control (n=85) |
PE (n=21) |
IUGR (n=13) |
P* |
†Adjusted P |
|---|---|---|---|---|---|
| SAFA | |||||
| 12:0 | 0 (0) | 0 (0) | 0 (0) | - | - |
| 14:0 | 8 (4)a | 11 (5)b | 12 (5)b | 0.001 | 0.044 |
| 16:0 | 429 (99) | 446 (78) | 451 (90) | 0.60 | 0.81 |
| 17:0 | 3 (2)a | 4 (2)a,b | 5 (1)b | <0.001 | 0.045 |
| 18:0 | 275 (59) | 265 (43) | 272 (35) | 0.74 | 0.86 |
| 20:0 | 8 (3) | 8 (3) | 7 (2) | 0.20 | 0.18 |
| 22:0 | 21 (7) | 19 (4) | 17 (5) | 0.08 | 0.044 |
| 24:0 | 62 (14) | 59 (9) | 57 (9) | 0.31 | 0.078 |
| MUFA | |||||
| 14:1n-7 | 1.0 (3.8) | 1.0 (2.2) | 1.5 (3.5) | 0.87 | 0.82 |
| 16:1n-7 | 10 (6) | 10 (5) | 8 (2) | 0.34 | 0.53 |
| 17:1n-7 | 29 (24) | 38 (21) | 45 (16) | 0.023 | 0.14 |
| 18:1n-7 | 14 (16) | 9 (14) | 5 (13) | 0.13 | 0.47 |
| 18:1n-9 | 171 (54) | 168 (38) | 163 (33) | 0.88 | 0.82 |
| 20:1n-9 | 1.5 (1.6) | 2.4 (2.6) | 2.3 (1.2) | 0.087 | 0.85 |
| 22:1n-9 | 0.2 (1.1) | 0 (0) | 0 (0) | 0.71 | 0.38 |
| 24:1n-9 | 57 (16) | 57 (15) | 56 (11) | 0.97 | 0.73 |
| PUFA n-6 | |||||
| 18:2n-6 | 57 (18) | 55(16) | 57 (17) | 0.91 | 0.67 |
| 18:3n-6 | 0.4 (1.1) | 0.6 (0.8) | 0.9 (1.2) | 0.22 | 0.26 |
| 20:2n-6 | 5 (6) | 4 (6) | 5 (4) | 0.76 | 0.094 |
| 20:3n-6 | 41 (16)a | 30 (15)b | 27 (11)b | 0.002 | 0.016 |
| 20:4n-6 | 239 (90) | 176 (90) | 193 (66) | 0.007 | 0.008 |
| 22:2n-6 | 1.9 (3.6) | 0.4 (1.9) | 0 (0) | 0.037 | 0.055 |
| 22:4n-6 | 48 (19)a | 33 (18)b | 37 (13)a,b | 0.002 | 0.016 |
| 22:5n-6 | 18 (8)a | 10 (7)b | 12 (5)b | <0.001 | 0.006 |
| PUFA n-3 | |||||
| 18:3n-3 | 0.08 (0.3) | 0.39 (0.8) | 0.37 (0.6) | 0.011 | 0.087 |
| 20:5n-3 | 6.1 (7) | 5.0 (8) | 2.6 (4) | 0.22 | 0.52 |
| 22:3n-3 | 0.1 (0.4) | 0 (0) | 0 (0) | 0.67 | 0.16 |
| 22:5n-3 | 11 (5)a | 7 (6)b | 8 (5)a,b | 0.004 | 0.11 |
| 22:6n-3 | 73 (33)a | 49 (31)b | 53 (29)a,b | 0.003 | 0.03 |
| Summary Indices | |||||
| % SAFA | 51 (7) | 56 (10) | 55 (9) | 0.007 | 0.007 |
| % MUFA | 18 (2)a | 19 (4)b | 19 (2)a,b | 0.002 | 0.094 |
| % PUFA | 31 (7)a | 24 (9)b | 26 (9)a,b | <0.001 | 0.002 |
| % UNSAT | 49 (7) | 44 (10) | 45 (9) | 0.007 | 0.007 |
| UI | 141 (29)a | 114 (36)b | 122 (35)a,b | <0.001 | 0.003 |
| Av CL | 18.7 (0.3)a | 18.4 (0.3)b | 18.4 (0.3)b | <0.001 | <0.001 |
| C 20-22 | 33 (6.2)a | 27 (6.4)b | 28 (6.9)b | <0.001 | <0.001 |
| n-6/n-3 ratio | 4.8 (1.2) | 5.1 (2.1) | 5.1 (2.0) | 0.63 | 0.98 |
| DHA deficiency index |
0.36 (0.10) | 0.30 (0.12) | 0.32 (0.08) | 0.019 | 0.13 |
| EFA deficiency index |
1.84 (0.53)a | 1.33 (0.55)b | 1.48 (0.39)a,b | <0.001 | 0.001 |
Values are mean and standard deviation (SD).
ANOVA was used to test for differences among groups. Different superscript letters indicate differences between individual groups using post hoc Tukey test. Significance level P<0.005.
adjusted for maternal body mass index (BMI), parity, smoking status and gestational age at delivery.
Summary measures for cord blood erythrocytes are shown in Table 2. The pattern in cord blood is the same as that observed in their mothers i.e. a higher proportion of SAFA and a reduced proportion of unsaturated fatty acids and PUFA, driven by the lower levels of PUFA. PE offspring had lower 22:6n-3 and were classed as being EFA deficient, whereas in IUGR offspring there was only a trend towards a deficiency in 22:6n-3 and EFA. The study was underpowered to examine the impact of severity of preeclampsia on offspring fatty acid composition.
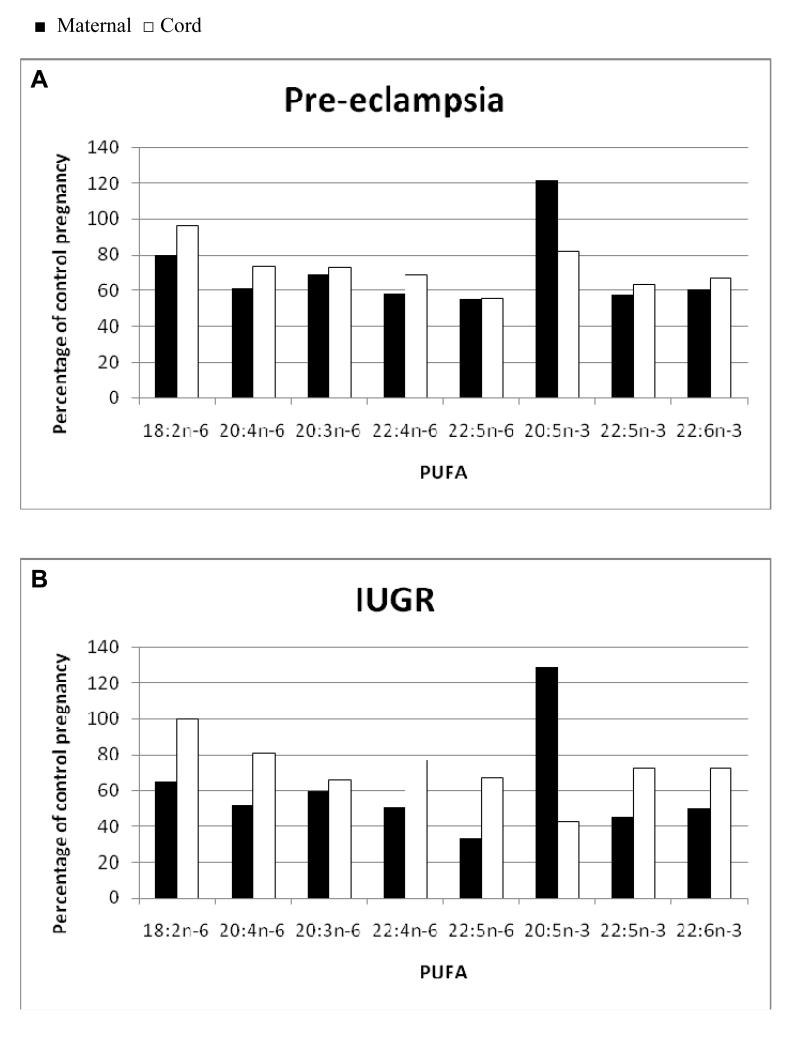
Figure 1 shows percent maternal and cord blood PUFA concentrations relative to the Control concentration for PE (Fig 1A) and IUGR (Fig 1B). Values below 100% indicate a relative fatty acid deficiency compared to Controls. IUGR and PE maternal fatty acids levels are all deficient apart from 20:5n-3. Cord blood levels of PUFA were deficient to a lesser degree than mothers. For the majority of PUFA the relative deficiency in the IUGR offspring is less than that in PE but this did not reach statistical significance due to high inter-individual variability.
Figure 1.
Maternal (■) and cord (□) plasma polyunsaturated fatty acid (PUFA) concentration expressed as a percentage of Control maternal and cord fatty acid concentration respectively in pregnancies complicated by A. Preeclampsia (PE). B. Intrauterine growth restriction (IUGR).
Maternal adipose tissue as a potential source of LC PUFA
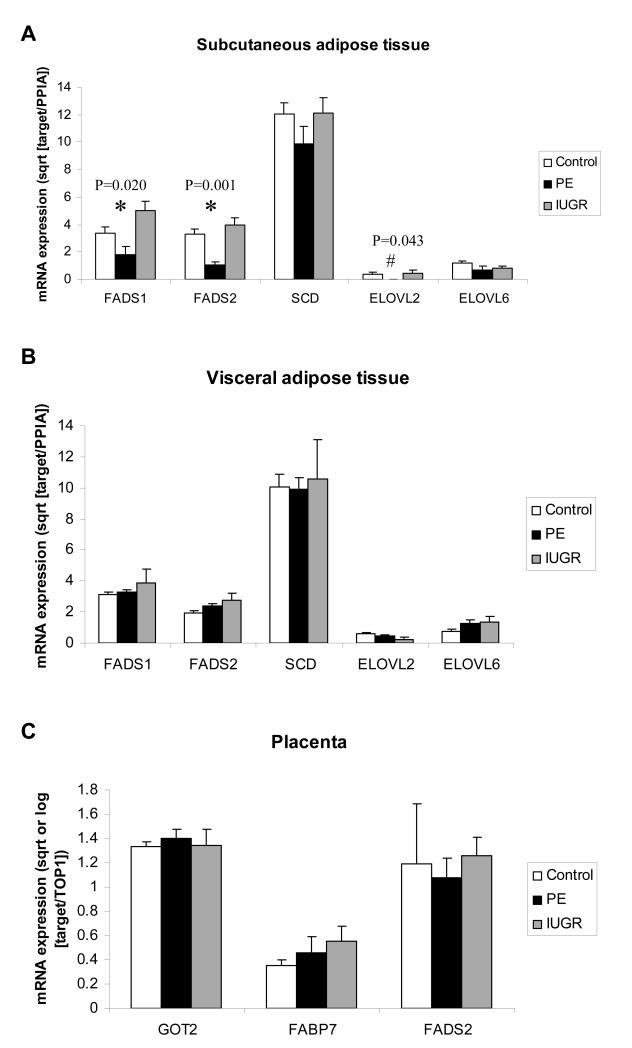
There were no differences in subcutaneous or visceral adipose tissue fatty acid composition between Control, PE and IUGR mothers (Tables S4 and S5). The predominant fatty acids in both tissues were 16:0, 18:1n-9 and 18:2n-6. Long chain PUFA was a minor component of adipose tissue. There were significant differences in subcutaneous adipose tissue Δ5-desaturase (FADS1) and Δ6-desaturase (FADS2) mRNA expression between groups (Fig 2A). Subcutaneous adipose tissue FADS1 and FADS2 expression in PE was lower than in Controls [mean (SD) Control 3.38 (2.96) vs PE 1.83 (1.91) square root [sqrt] (FADS1 expression relative to PPIA), P=0.030] and [Control 3.33 (2.25) vs PE 1.03 (0.96) sqrt (FADS2 expression relative to PPIA), P<0.001]. All PE subcutaneous adipose tissue samples showed no detectable expression of subcutaneous very long chain fatty acid elongase (ELOVL2). There was a significantly higher proportion of detectable subcutaneous adipose tissue ELOVL2 expression in Control compared to PE (P=0.038). There were no significant differences in FADS1, FADS2 or ELOVL2 mRNA expression in visceral adipose tissue (Fig 2B). Stearoyl CoA desaturase (SCD) and long chain fatty acid elongase (ELOVL6) mRNA expression did not differ among groups in either subcutaneous or visceral adipose tissue.
Figure 2.
A. Maternal subcutaneous adipose tissue gene expression in control (n=50), preeclampsia (PE) (n=12) and intrauterine growth restriction (IUGR) (n=13), B. maternal visceral adipose tissue gene expression in control (n=25), PE (n=12) and IUGR (n=5), C. placental gene expression in control (n=57), PE (n=17) and IUGR (n=11) pregnancy relative to control gene (PPIA for adipose tissue and TOP1 for placenta). Means (standard error), of transformed (log [GOT2 and FADS2] or square root) target gene expression relative to control gene expression values, are presented. * significant difference among groups on ANOVA; # significant difference among groups on Kruskal-Wallis test.
Placental fatty acid metabolism and transport markers
To confirm whether low cord levels of 20:3n-6 in the presence of normal levels of 18:2n-6 was indicative of low Δ6-desaturase activity in PE and IUGR offspring, Δ6-desaturase (FADS2) mRNA expression in a fetal tissue (placenta) was quantitated and found not to be different among groups (Figure 2C). In order to test whether lower offspring LC PUFA levels might be due to lower placental expression of fatty acid transfer proteins, mRNA expression of placental fatty acid transporters (pFABPpm [GOT2] and FABP7) was assessed and found not to differ among groups (Figure 2C). The lack of difference in placental gene expression was independent of mode of delivery.
Discussion
Absolute amounts of maternal erythrocyte n-6 and n-3 PUFA concentrations were approximately 60% lower in PE and IUGR compared to Controls and cord blood LC PUFA deficiency is also common to PE and IUGR. Low amounts of maternal LC PUFA could result from inhibition of synthesis, decreased release from maternal stores and/or reduced acquisition from diet.
In PE the metabolic pattern is of high BMI and high plasma triglyceride and NEFA concentrations. Although we could not assess maternal insulin resistance due to the random nature of maternal blood samples, second trimester insulin resistance has previously been shown to be associated with PE26. The complete biochemical profile of PE women observed here is indicative of metabolic syndrome, a biochemical manifestation of insulin resistance which is typical of PE27. The observed reduced adipose tissue Δ5- and Δ6-desaturase and ELOVL2 expression (Figure S2) suggests that low LC PUFA in PE could be due to decreased synthesis. We have previously hypothesized that increased NEFA flux in pregnancy can lead to mitochondrial dysfunction2 thus impairing LC PUFA synthesis, analogous to the situation in non-alcoholic fatty liver disease which is associated with obesity, insulin resistance and ectopic lipid accumulation in the liver. In non-alcoholic fatty liver disease liver LC PUFA are depleted possibly via reduced Δ5- and Δ6-desaturase activities3. It was notable that the magnitude of reduction in LC PUFA was greater in women with severe PE.
Mothers with IUGR are reported to have low lipolysis rates4 and low BMI28. In IUGR we observed trends towards lower maternal erythrocyte concentrations of the major fatty acids stored in adipose tissue, 18:1n-9 and 16:029, which is consistent with lower adipocyte lipolysis. The metabolic pattern in IUGR mothers thus may indicate a primary defect in fat storage and mobilization from adipose tissue (Figure S2). IUGR cord leptin levels are extremely low reflecting reduced adipose tissue depots, however cord triglyceride is high and NEFA concentrations normal suggesting that although maternal fatty acid supply may be abnormal, there is still fetal capacity for triglyceride synthesis.
The ratio of the n-6 to the n-3 series remains similar among groups suggesting that both pathways are affected equally. It is notable that PE and IUGR mothers are not deficient in 20:5n-3 which suggests it can be synthesized from 18:3n-3. Elongation or desaturation pathways downstream of this point may be impaired (Figure S1 and S2). We observed that subcutaneous adipose tissue mRNA expression of Δ5- and Δ6-desaturase and very long chain fatty acid elongase (ELOVL2 which acts on C20 and C22 fatty acids) was significantly lower in PE mothers suggesting compromized synthesis downstream of C20. Adipose tissue is not a major site of LC PUFA synthesis but expression here may reflect expression of synthetic enzymes in inaccessible tissue e.g. the liver.
It is not known from which maternal stores LC PUFA are mobilized in pregnancy. Potential sites are adipose tissue, liver and membranes (including the brain). As in the non-pregnant state29, maternal adipose tissue contains reasonable quantities of 18:2n-6, but very little 18:3n-3 and minor amounts of LC PUFA. There were also no differences in adipose tissue fatty acid composition between groups that could explain the large difference in maternal erythrocyte LC PUFA concentrations. Together these data suggest that maternal adipose tissue does not act as a short-term store or site of synthesis of PUFA. It is possible that maternal membrane (brain) and liver LC PUFA stores are affected in PE and IUGR especially if synthetic pathways are impeded.
Differing diet between PE and IUGR mothers and Controls is another potential explanation for reduced PUFA. The extent of deficiency (up to 60%) would suggest that diets would have to be substantially different to have that magnitude of effect on erythrocyte PUFA concentrations. Vegan mothers are not specifically susceptible to PE or IUGR30, 31 nor do they demonstrate a similar degree of EFA deficiency32. In our study, the ratio of 22:5n-6/22:6n-3, a measure of dietary omega-3 or 22:6n-3 deficiency33, was not significantly different between PE cases and Controls, further suggesting that diet alone is not the cause of the fatty acid changes. There is also no evidence that dietary supplementation with LC PUFA impacts on incidence of PE or IUGR34, 35.
The fetus is dependent on the mother for its EFA supply therefore it is not surprising that we observed reduced LC PUFA in PE and IUGR offspring. The degree to which the babies are deficient is less than expected from the lack of mobilization in the mothers (Fig 1). DHA is an extremely important fatty acid for fetal neural development and PE cord blood erythrocytes have a lesser degree of DHA deficiency than EFA deficiency (Table 2). This suggests that there are mechanisms to compensate such as up-regulation of placental transport or fetal synthesis. There was no difference in placental expression of pFABPpm (GOT2) and FABP7 between groups. However there are a wide array of placental fatty acid transport proteins16 and a more systematic analysis is required to eliminate the possibility of up-regulation of placental fatty acid transport. There was a trend towards increased levels of 18:3n-3, the precursor of n-3 PUFA in cord erythrocytes for PE and IUGR. However, placental Δ6-desaturase mRNA expression did not differ. Thus there is no evidence for up-regulation of placental or fetal fatty acid synthesis pathways. Both PE and IUGR offspring had significantly lower cord blood adiponectin levels than controls (Table S2) which may result from reduced n-3 LC PUFA-induced adiponectin release from adipocytes36, 37.
The strength of our data is the use of absolute fatty acid concentrations which allows us to understand which specific fatty acids are driving the differences in composition between healthy and complicated pregnancies. There were limitations to our study. Blood samples were random and women were not all fasted which will have an impact on some maternal variables (triglyceride and NEFA) but not erythrocyte fatty acid composition. No samples from pre-pregnancy or early gestation were available for these women. There were few IUGR subjects, the PE group were a mixture of primiparous and parous women with potentially different underlying risk factors, and there were no dietary intake data.
Perspectives
At birth, the offspring of PE and IUGR pregnancies are deficient in essential LC PUFA which may have long term consequences for their development. The mechanisms by which these deficiencies arise differ between PE and IUGR and suggest different interventions. In IUGR LC PUFA supplementation may overcome the lack of maternal fatty acid mobilization. In PE insulin-sensitizing treatments such as metformin may reduce ectopic fat accumulation and upregulate LC PUFA synthetic pathways.
Supplementary Material
Novelty and significance.
1) What is new?
This study shows that levels of a class of fats that are particularly important for fetal brain development (long chain polyunsaturated fatty acids) are lower in maternal and cord blood in pregnancies complicated by preeclampsia and intrauterine growth restriction. We provide evidence that in preeclampsia this might be due to a decreased ability of the mother to make these fats from dietary precursors.
2) What is relevant?
The findings are important as they indicate that offspring of pregnancies complicated by preeclampsia or intrauterine growth restriction may be at risk of impaired neural development and suggest that the approaches to reducing this risk would differ. In preeclampsia, drug interventions that may improve the mother’s metabolism and ability to make long chain polyunsaturated fatty acids are indicated, whereas in intrauterine growth restriction supplementation of the diet with long chain polyunsaturated fatty acids would be indicated.
3) Summary
Low maternal and offspring levels of fats important for offspring neural development in preeclampsia may be due to an impaired ability of the mother to synthesize them.
Acknowledgements
We appreciate the technical support of Liz Grigonis-Deanne and Dr Alice Owen.
Sources of funding SCPMDE Clinical Research Fellowship (Vanessa MacKay [nee Rodie]), Wellcome Trust Student Elective Prize and Carnegie Trust Undergraduate Vacation Scholarship (Louise McKenna), Chief Scientist’s Office CZG/1/74, GRI Endowments 09REFGEN03, British Heart Foundation (PG/03/147/16351 and PG/02/167/14801), Carnegie Trust, Lister Bellahouston Travelling Fellowship, Heart UK Sue McCarthy Travelling Fellowship, Royal Society Study Visit to Australia Travel Award, SHERT Medical Research Foreign Travel Grant and University of Wollongong Australia study leave assistance grant.
Footnotes
Conflicts of Interest None.
References
- 1.Sattar N, Gaw A, Packard CJ, Greer IA. Potential pathogenic roles of aberrant lipoprotein and fatty acid metabolism in pre-eclampsia. Br J Obstet Gynaecol. 1996;103:614–620. doi: 10.1111/j.1471-0528.1996.tb09827.x. [DOI] [PubMed] [Google Scholar]
- 2.Jarvie E, Hauguel-de-Mouzon S, Nelson SM, Sattar N, Catalano PM, Freeman DJ. Lipotoxicity in obese pregnancy and its potential role in adverse pregnancy outcome and obesity in the offspring. Clin Sci (Lond) 2010;119:123–129. doi: 10.1042/CS20090640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Videla LA, Rodrigo R, Araya J, Poniachik J. Oxidative stress and depletion of hepatic long-chain polyunsaturated fatty acids may contribute to nonalcoholic fatty liver disease. Free Radic Biol Med. 2004;37:1499–1507. doi: 10.1016/j.freeradbiomed.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 4.Diderholm B, Stridsberg M, Norden-Lindeberg S, Gustafsson J. Decreased maternal lipolysis in intrauterine growth restriction in the third trimester. BJOG. 2006;113:159–164. doi: 10.1111/j.1471-0528.2005.00825.x. [DOI] [PubMed] [Google Scholar]
- 5.Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Divergent metabolic and vascular phenotypes in pre-eclampsia and intrauterine growth restriction: relevance of adiposity. J Hypertens. 2004;22:2177–2183. doi: 10.1097/00004872-200411000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Cetin I, Giovannini N, Alvino G, Agostoni C, Riva E, Giovannini M, Pardi G. Intrauterine growth restriction is associated with changes in polyunsaturated fatty acid fetal-maternal relationships. Pediatr Res. 2002;52:750–755. doi: 10.1203/00006450-200211000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Mahomed K, Williams MA, King IB, Mudzamiri S, Woelk GB. Erythrocyte omega-3, omega-6 and trans fatty acids in relation to risk of preeclampsia among women delivering at Harare Maternity Hospital, Zimbabwe. Physiol Res. 2007;56:37–50. doi: 10.33549/physiolres.930859. [DOI] [PubMed] [Google Scholar]
- 8.Ogburn PL, Jr., Williams PP, Johnson SB, Holman RT. Serum arachidonic acid levels in normal and preeclamptic pregnancies. Am J Obstet Gynecol. 1984;148:5–9. doi: 10.1016/s0002-9378(84)80023-x. [DOI] [PubMed] [Google Scholar]
- 9.Ortega-Senovilla H, Alvino G, Taricco E, Cetin I, Herrera E. Enhanced circulating retinol and non-esterified fatty acids in pregnancies complicated with intrauterine growth restriction. Clin Sci (Lond) 2010;118:351–358. doi: 10.1042/CS20090292. [DOI] [PubMed] [Google Scholar]
- 10.Arab L. Biomarkers of fat and fatty acid intake. J Nutr. 2003;133(Suppl 3):925S–932S. doi: 10.1093/jn/133.3.925S. [DOI] [PubMed] [Google Scholar]
- 11.Makrides M, Gibson RA. Long-chain polyunsaturated fatty acid requirements during pregnancy and lactation. Am J Clin Nutr. 2000;71:307S–311S. doi: 10.1093/ajcn/71.1.307S. [DOI] [PubMed] [Google Scholar]
- 12.Al MD, van Houwelingen AC, Kester AD, Hasaart TH, de Jong AE, Hornstra G. Maternal essential fatty acid patterns during normal pregnancy and their relationship to the neonatal essential fatty acid status. Br J Nutr. 1995;74:55–68. doi: 10.1079/bjn19950106. [DOI] [PubMed] [Google Scholar]
- 13.Cetin I, Koletzko B. Long-chain omega-3 fatty acid supply in pregnancy and lactation. Curr Opin Clin Nutr Metab Care. 2008;11:297–302. doi: 10.1097/MCO.0b013e3282f795e6. [DOI] [PubMed] [Google Scholar]
- 14.Stewart F, Rodie VA, Ramsay JE, Greer IA, Freeman DJ, Meyer BJ. Longitudinal assessment of erythrocyte fatty acid composition throughout pregnancy and post partum. Lipids. 2007;42:335–344. doi: 10.1007/s11745-006-3005-5. [DOI] [PubMed] [Google Scholar]
- 15.Huda SSSN, Freeman DJ. Lipoprotein metabolism and vascular complications in pregnancy. Clinical Lipidology. 2009;4:91–102. [Google Scholar]
- 16.Cunningham P, McDermott L. Long chain PUFA transport in human term placenta. J Nutr. 2009;139:636–639. doi: 10.3945/jn.108.098608. [DOI] [PubMed] [Google Scholar]
- 17.Campbell FM, Clohessy AM, Gordon MJ, Page KR, Dutta-Roy AK. Uptake of long chain fatty acids by human placental choriocarcinoma (BeWo) cells: role of plasma membrane fatty acid-binding protein. J Lipid Res. 1997;38:2558–2568. [PubMed] [Google Scholar]
- 18.Larque E, Krauss-Etschmann S, Campoy C, Hartl D, Linde J, Klingler M, Demmelmair H, Cano A, Gil A, Bondy B, Koletzko B. Docosahexaenoic acid supply in pregnancy affects placental expression of fatty acid transport proteins. Am J Clin Nutr. 2006;84:853–861. doi: 10.1093/ajcn/84.4.853. [DOI] [PubMed] [Google Scholar]
- 19.Xu LZ, Sanchez R, Sali A, Heintz N. Ligand specificity of brain lipid-binding protein. J Biol Chem. 1996;271:24711–24719. doi: 10.1074/jbc.271.40.24711. [DOI] [PubMed] [Google Scholar]
- 20.Lipid Research Clinics Program . Lipid and lipoprotein analysis. Manual of Laboratory Operations Vol. 1. Dept.of Health, Education and Welfare, National Institutes of Health; Bethesda, MD: 1974. Publication No. 75-628. [Google Scholar]
- 21.Packard CJ, O’Reilly DS, Caslake MJ, McMahon AD, Ford I, Cooney J, Macphee CH, Suckling KE, Krishna M, Wilkinson FE, Rumley A, Lowe GD. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention Study Group. N Engl J Med. 2000;343:1148–1155. doi: 10.1056/NEJM200010193431603. [DOI] [PubMed] [Google Scholar]
- 22.Evans K, Burdge GC, Wootton SA, Clark ML, Frayn KN. Regulation of dietary fatty acid entrapment in subcutaneous adipose tissue and skeletal muscle. Diabetes. 2002;51:2684–2690. doi: 10.2337/diabetes.51.9.2684. [DOI] [PubMed] [Google Scholar]
- 23.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32:1076–1080. doi: 10.2337/dc08-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pardo IM, Geloneze B, Tambascia MA, Barros AA. Inverse relationship between cord blood adiponectin concentrations and the number of cigarettes smoked during pregnancy. Diabetes Obes Metab. 2005;7:144–147. doi: 10.1111/j.1463-1326.2004.00379.x. [DOI] [PubMed] [Google Scholar]
- 25.Stoll-Becker S, Kreuder J, Reiss I, Etspuler J, Blum WF, Gortner L. Influence of gestational age and intrauterine growth on leptin concentrations in venous cord blood of human newborns. Klin Padiatr. 2003;215:3–8. doi: 10.1055/s-2003-36892. [DOI] [PubMed] [Google Scholar]
- 26.Hauth JC, Clifton RG, Roberts JM, Myatt L, Spong CY, Leveno KJ, Varner MW, Wapner RJ, Thorp JM, Jr., Mercer BM, Peaceman AM, Ramin SM, Carpenter MW, Samuels P, Sciscione A, Tolosa JE, Saade G, Sorokin Y, Anderson GD. Maternal insulin resistance and preeclampsia. Am J Obstet Gynecol. 2011;204:327, e321–326. doi: 10.1016/j.ajog.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodie VA, Freeman DJ, Sattar N, Greer IA. Pre-eclampsia and cardiovascular disease: metabolic syndrome of pregnancy? Atherosclerosis. 2004;175:189–202. doi: 10.1016/j.atherosclerosis.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 28.Ehrenberg HM, Dierker L, Milluzzi C, Mercer BM. Low maternal weight, failure to thrive in pregnancy, and adverse pregnancy outcomes. Am J Obstet Gynecol. 2003;189:1726–1730. doi: 10.1016/s0002-9378(03)00860-3. [DOI] [PubMed] [Google Scholar]
- 29.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res. 2008;47:348–380. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Carter JP, Furman T, Hutcheson HR. Preeclampsia and reproductive performance in a community of vegans. South Med J. 1987;80:692–697. doi: 10.1097/00007611-198706000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Fikree FF, Berendes HW, Midhet F, D’Souza RM, Hussain R. Risk factors for intrauterine growth retardation: results of a community-based study from Karachi. J Pak Med Assoc. 1994;44:30–34. [PubMed] [Google Scholar]
- 32.Lakin V, Haggarty P, Abramovich DR, Ashton J, Moffat CF, McNeill G, Danielian PJ, Grubb D. Dietary intake and tissue concentration of fatty acids in omnivore, vegetarian and diabetic pregnancy. Prostaglandins Leukot Essent Fatty Acids. 1998;59:209–220. doi: 10.1016/s0952-3278(98)90065-5. [DOI] [PubMed] [Google Scholar]
- 33.Fokkema MR, Smit EN, Martini IA, Woltil HA, Boersma ER, Muskiet FA. Assessment of essential fatty acid and omega3-fatty acid status by measurement of erythrocyte 20:3omega9 (Mead acid), 22:5omega6/20:4omega6 and 22:5omega6/22:6omega3. Prostaglandins Leukot Essent Fatty Acids. 2002;67:345–356. doi: 10.1054/plef.2002.0440. [DOI] [PubMed] [Google Scholar]
- 34.Oken E, Kleinman KP, Olsen SF, Rich-Edwards JW, Gillman MW. Associations of seafood and elongated n-3 fatty acid intake with fetal growth and length of gestation: results from a US pregnancy cohort. Am J Epidemiol. 2004;160:774–783. doi: 10.1093/aje/kwh282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szajewska H, Horvath A, Koletzko B. Effect of n-3 long-chain polyunsaturated fatty acid supplementation of women with low-risk pregnancies on pregnancy outcomes and growth measures at birth: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2006;83:1337–1344. doi: 10.1093/ajcn/83.6.1337. [DOI] [PubMed] [Google Scholar]
- 36.Flachs P, Mohamed-Ali V, Horakova O, Rossmeisl M, Hosseinzadeh-Attar MJ, Hensler M, Ruzickova J, Kopecky J. Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed a high-fat diet. Diabetologia. 2006;49:394–397. doi: 10.1007/s00125-005-0053-y. [DOI] [PubMed] [Google Scholar]
- 37.Krebs JD, Browning LM, McLean NK, Rothwell JL, Mishra GD, Moore CS, Jebb SA. Additive benefits of long-chain n-3 polyunsaturated fatty acids and weight-loss in the management of cardiovascular disease risk in overweight hyperinsulinaemic women. Int J Obes (Lond) 2006;30:1535–1544. doi: 10.1038/sj.ijo.0803309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.