Experts debate the clinical evidence standards needed to approve major manufacturing changes for biologics, biosimilars, and interchangeable biosimilars. As sponsors consider their development plans, the resource investment required to develop an analytically highly similar candidate must be balanced by regulatory relief for the clinical studies required to achieve the necessary indications for the marketed product. This article discusses biosimilarity and comparability as related scientific and regulatory concepts and the usefulness of clinical data for both.
Biological Variation
The product attributes of biologics vary over their manufacturing lifetime due to the intrinsic variability of different batches of product (that must fit predetermined release specifications) as well as the larger variation that occurs after manufacturing changes (approved by regulatory authorities).
When a sponsor uses a single facility to supply the global market and the manufacturing changes are minimal, modern analytics are often accepted as being sufficiently sensitive to demonstrate that the postmanufacturing product is “the same” as the prechange product. When more complex manufacturing changes occur, the regulatory agency determines whether additional data, including preclinical and clinical data, are necessary too. The peer-reviewed literature includes examples of acceptable differences found between pre- and postmanufacturing changes with a number of leading recombinant biopharmaceuticals, such as Aranesp, Enbrel, and Rituxan.1 In Europe, the use of comparability is sometimes disclosed in the European public assessment report,2 but in the United States, equivalent information is never made public.
The basis for the regulatory oversight of these products throughout their life cycle relies on the established regulatory principles of comparability. Clinical outcomes between batches have been indistinguishable as a practical matter for most patients, and, although comparability is still largely based only on analytical studies, instances of comparability failures are very rare. Switching patients between batches of product, or between pre- and postmanufacturing-change products, during their course of treatment has not led to problems in clinical practice. Because the label does not change, providers and their patients are generally unaware of any change to their biologic or of comparability having been applied.
The regulatory science on which comparability is based has subsequently been applied to the review and approval of biosimilars.3
One Standard for all Biologics
Established in highly regulated markets in support of manufacturing changes, the “highly similar” analytical standard became part of US law for biosimilars in the Biologics Price Competition and Innovation Action (Title VII of the Patient Protection and Affordable Care Act) enacted in March 2010. The Food and Drug Administration (FDA) has extensive practical experience applying the standard to a wide variety of products, simple and complex, both recombinant and naturally sourced, and as the basis for both extrapolation between indications and interchangeability for all indications, given that the pre- and postmanufacturing-change products always have a identical labels.
The standard is formally represented by the International Conference on Harmonisation Q5E Guideline: Comparability of Biotechnological/Biological Products Subject to Changes in Their Manufacturing Process, CPMP/ICH/5721/03; the definition of “comparable” is as follows:
“Comparable: A conclusion that products have highly similar quality attributes before and after manufacturing process changes and that no adverse impact on the safety or efficacy, including immunogenicity, of the drug product occurred. This conclusion can be based on an analysis of product quality attributes. In some cases, nonclinical or clinical data might contribute to the conclusion.”
In the United States, the FDA prefers to use the term “comparability” for changes made by a single manufacturer and “similarity” when applying this same process to biosimilars. By contrast, in Europe, the European Medicines Agency uses the term “comparability” when evaluating both inter- as well as intramanufacturing changes and as the explicit basis for Similar Biologics Medicinal Products (“biosimilars”), on the market in the European Union since early 2006. More recently, the European Medicines Agency has used the expression “biosimilar comparability” to clarify the context but not to change the concept as a scientific matter.
Europe has approved 14 biosimilars for somatropin, epoetin, and filgrastim3 and is currently evaluating monoclonal antibodies. The extent of data required for approval of these biosimilars is remarkably close to that required, e.g., for a major manufacturing change to Aranesp, according to the European public assessment report.2 A major issue that must be addressed is the presence of any new and/or different proportions of glycosylation patterns because these can create “residual uncertainty” as to the clinical performance of the new product, including the potential for immunogenicity. Regulators accept that, if the product attributes, including posttranslational modifications, are “highly similar” to the prior product, there is limited residual uncertainty, and clinical data would not generally be required in the case of a manufacturing change.
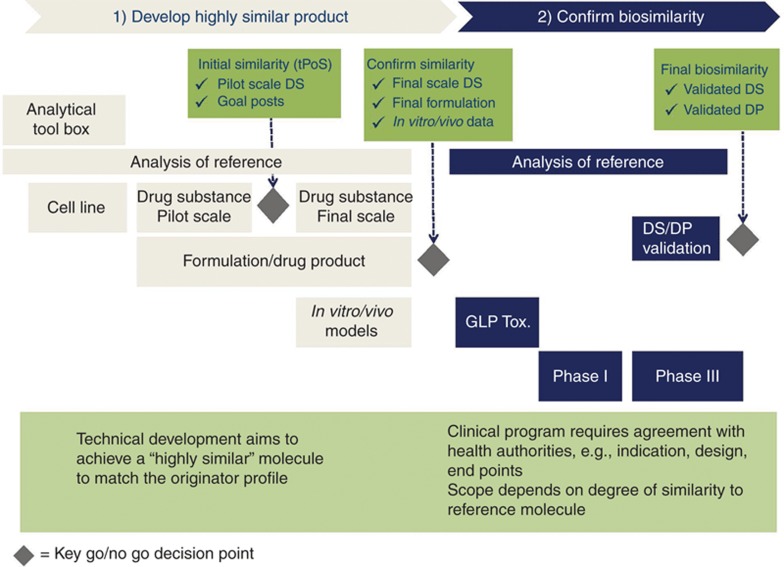
For biosimilars, the concept of “goal posts,” whereby the attributes of the biosimilar fall within the demonstrated variation of the originator reference over its lifetime,4 is becoming a widely accepted approach for creation of a “highly similar” product. This reflects a fundamentally different philosophy for the development of biosimilars as compared with originator products (which additionally themselves extend their own “goal posts” by using comparability during their own lifetime). Specifically, the biosimilar sponsor can rely on the prior finding of quality, safety, and efficacy (European Union) or safety, purity, and potency (United States) of the originator reference product on the basis of analytical similarity (Figure 1). Clinical studies, if conducted, target any “residual uncertainty” and are not considered in isolation as the basis for a conclusion of “comparability” (manufacturing changes and European Union biosimilars) or “similarity” (US biosimilars). Such studies can, however, be designed to identify any unusual or unexpected immunogenicity issues and have value for informing the development of both originator and biosimilar products, as well as products after manufacturing changes.
Figure 1.
Biosimilar development requires a paradigm shift—the focus is on similarity and not on de novo efficacy. DP, drug product; DS, drug substance; GLP Tox., good laboratory practice toxicology studies in animals; tPoS, technical proof of similarity.
Because of the specificity of analytical and biological characterization technologies now available, originator biologics and biosimilars can be fully characterized so that functional integrity and performance can be assured before clinical trials. If critical product attribute differences are detected, the impact on receptor binding and other functional mechanisms such as antibody-dependent cell cytotoxicity can be assessed, but it is not currently possible to evaluate the impact of these differences on immunogenicity. Therefore, clinical trials evaluating residual uncertainty are usually focused on safety and/or immunogenicity and to a lesser extent on sensitive efficacy end points. Because clinical end points are far less sensitive than analytics at picking up even moderate differences between products (pre- and postmajor manufacturing changes or reference product compared with biosimilars) and are conducted subsequent to those analytics, such studies may require unique designs to probe differences between molecules. It is arguably irresponsible to require clinical studies for which no meaningful scientific conclusions can be drawn because the hypothesis of “sameness” cannot be refuted.5 However, in cases in which analytical differences are found, it can be important to confirm that they have no obvious clinical impact.
The Value of the Scientific Principles of Comparability
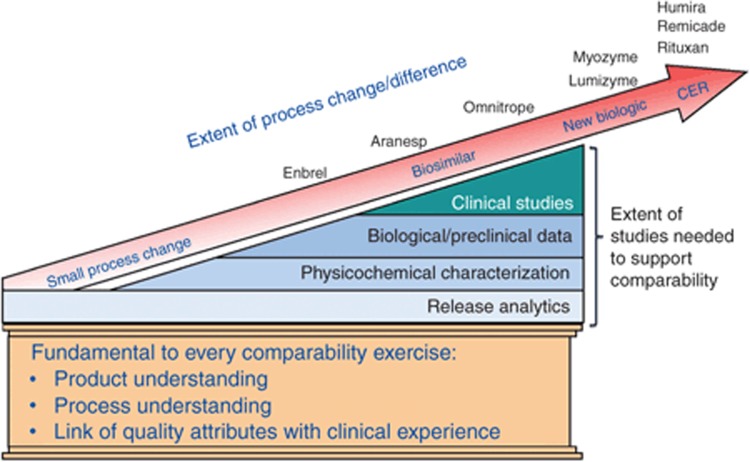
Fortunately, we can rely on the progress made in regulatory science over 20 years to come to a consistent, fair, and science-based conclusion as to what is “showable” clinically and therefore “knowable” as the basis for regulatory decision making (Figure 2). The higher fidelity of analytics provides the basic measure of quality and manufacturing control for all biologics irrespective of whether those analytics are identifying differences that are clinically relevant.
Figure 2.
The continuum of the science of comparability. CER, comparative effectiveness research.
When analytics demonstrate that the active moieties in two products are “highly similar,” then the only “unknown” is the potential for immunogenicity because the clinical outcomes will otherwise be the same. Thus, in the case of a biologic for which a manufacturing change has been made or for a biosimilar (whether interchangeability is sought for the biosimilar or not), the focus of any clinical study can be ruling out major immunogenicity for the product. Subsequent postmarket surveillance provides important confirmation and is equally important for all medicines.
Conclusion
Analytical high similarity is the most robust scientific basis for comparing independently sourced biologics. For two decades, comparability has reduced the need for clinical studies after manufacturing changes and has been used as the basis for complete extrapolation between all indications as well as full interchangeability for each indication, irrespective of whether the mechanism of action is known or whether any clinical studies at all have been conducted on the postchange product. Therefore, comparability is the de facto interchangeability standard in use today for biologics.
That analytics provide more sensitive information than clinical studies is not disputed. Nonetheless, the comparability approach can address what is “knowable” and avoid what is merely “doable” to minimize clinical studies that will not benefit patients. For manufacturing changes, as well as biosimilarity, clinical trials can focus on exploring residual uncertainty.5 This requires a paradigm shift in thinking for regulators and product sponsors to create and appropriately use unique and innovative end points.
M.M. is head of Global Pharmaceutical Development at Sandoz International. Sandoz is the leading sponsor of approved similar biological medicinal products in Europe, with three products: somatropin, epoetin alfa, and filgrastim. G.W. is vice president at Avalere Health and works with multiple stakeholders in health care including, but not limited to, biopharma companies (originator and generic).
References
- Schiestl M., Stangler T., Torella C., Cepeljnik T., Toll H., Grau R. Acceptable changes in quality attributes of glycosylated biopharmaceuticals. Nat. Biotechnol. 2011;29:310–312. doi: 10.1038/nbt.1839. [DOI] [PubMed] [Google Scholar]
- Assessment Report for Aranesp®, July 2008 EPAR, Dod. Ref No: EMEA/478499/2008 ; < http://www.ema.europa.eu/docs/en_GB/document_library/ EPAR_-_Assessment_Report_-_Variation/human/000332/WC500026148.pdf>. Accessed 30 November 2012.
- Weise M., et al. Biosimilars: what clinicians should know. Blood. 2012;120:5111–5117. doi: 10.1182/blood-2012-04-425744. [DOI] [PubMed] [Google Scholar]
- McCamish M., Woollett G.Worldwide experience with biosimilar development. mAbs 3209–217.2011. <http://www.landesbioscience.com/journals/mabs/article/15005/>. Accessed 20 November 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple R. A regulators view of CER. Clinical Trials. 2011;0:1–10. [Google Scholar]