Abstract
Hypertension and heart failure (HF) are common diseases that, despite advances in medical therapy, continue to be associated with high morbidity and mortality. Therefore, innovative therapeutic strategies are needed. Inhibition of the neutral endopeptidase (NEPinh) had been investigated as a potential novel therapeutic approach because of its ability to increase the plasma concentrations of the natriuretic peptides (NPs). Indeed, the NPs have potent natriuretic and vasodilator properties, inhibit the activity of the renin–angiotensin–aldosterone system, lower sympathetic drive, and have antiproliferative and antihypertrophic effects. Such potentially beneficial effects can be theoretically achieved by the use of NEPinh. However, studies have shown that NEPinh alone does not result in clinically meaningful blood pressure-lowering actions. More recently, NEPinh has been used in combination with other cardiovascular agents, such as angiotensin-converting enzyme inhibitors, and antagonists of the angiotensin receptor. Another future possible combination would be the use of NEPinh with NPs or their newly developed chimeric peptides. This review summarizes the current knowledge of the use and effects of NEPinh alone or in combination with other therapeutic agents for the treatment of human cardiovascular disease such as HF and hypertension.
Keywords: Neutral endopeptidase inhibition, Natriuretic peptide system, Evolving strategy in cardiovascular therapeutics
Introduction
The burden of cardiovascular disease (CVD) continues to increase worldwide. The final common pathway in CVD is heart failure (HF), which often is mediated by progressive uncontrolled hypertension. Indeed, the important link between hypertension and HF is underscored by the recent report from the landmark ALLHAT Study that demonstrated that the development of HF in hypertensive patients was a powerful predictor for increased mortality1 A recent report further suggested the importance of the control of hypertension for the reduction in HF.2
Today, there continues to be a high priority for the development of innovative therapeutic agents that better control blood pressure (BP) and also have a therapeutic potential in the setting of HF. Such agents should enhance current therapies for CVD and, importantly, prevent target-organ damage. Such therapeutics could be especially useful for high-risk populations such as the elderly, diabetics, African-Americans, and other populations in whom adequate BP control is of great importance. Currently, there is a widespread use of modulators of the renin–angiotensin–aldosterone system (RAAS) which may inhibit release of renin, antagonize angiotensin II (Ang II) at its receptor level, or block the actions of aldosterone. Such strategies underscore the deleterious properties of over-activated RAAS, which is a hallmark of CVD.
In this review, we will focus on an endogenous peptide system, the natriuretic peptide (NP) system, and on novel strategies aimed to enhance the biological activities of the NPs via inhibition of their degradation.
As will be discussed below, manipulation of this system so as to achieve target-organ protection, BP control, optimal volume homeostasis, and the inhibition or reversal of myocardial and renal remodeling represents a new therapeutic opportunity. Our focus will be upon inhibition of a key enzyme which degrades the NPs, specifically, neprilysin (neutral endopeptidase or EC 3.4.24.11 or NEP), and on the combination of NEP inhibition and RAAS antagonism by novel molecules.
Natriuretic peptide system
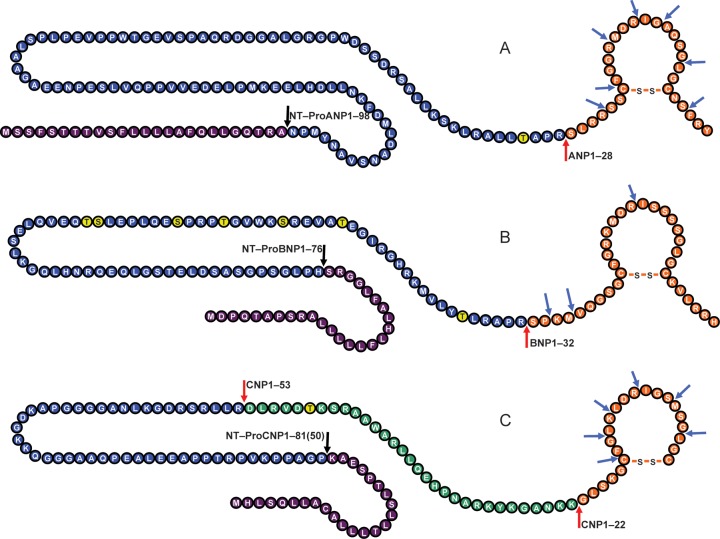
The NP system consists primarily of three well-characterized peptides, with each being a distinct gene product with structural similarity (Figure 1): atrial natriuretic peptide (ANP)3 and B-type natriuretic peptide (BNP)4 are mainly from cardiomyocytes, and C-type natriuretic peptide (CNP) is mostly from endothelial and renal cells.5–8 These three peptides all have cardiorenal protective properties. Of note, BNP is also produced by cardiofibroblasts where it elicits its anti-fibrotic actions in the heart.9 While ANP is secreted from the myocytes as the active hormone ANP1–28, BNP is produced and released from the myocytes as a 108 amino acid prohormone, proBNP1–108, in a glycosylated form.10 In the circulation, glycosylated proBNP1–108 is gradually deglycosylated and further processed by corin into the biologically active BNP1–32 and into the inactive N-terminal (NT)-proBNP1–76 linear fragment.11 Like ANP and BNP, CNP is formed by cleavage of a precursor protein, proCNP1–103, by proteolytic enzymes to produce the active forms CNP1–22 and CNP1–53.12
Figure 1.
The three major human endogenous natriuretic peptides, their processing and degradation. (A) ProANP1–126 and signal peptide, cleaved to NT-ProANP1–98 and the active hormone ANP1–28. (B) ProBNP1–108 and signal peptide, cleaved to NT-ProBNP1–76 and the active hormone BNP1–32. (C) ProCNP1–103 and signal peptide, cleaved to NT-ProCNP1–80(50) and the active hormones CNP1–22 and CNP1–53. Red arrows indicate processing site by corin or furin. Blue arrows indicate neutral endopeptidase cleavage sites. Yellow-labelled amino acids indicate glycosylation sites that may prevent processing. Purple-labelled amino acid sequence indicates the signal peptide.
While ANP and BNP are released from the myocytes in response to cardiac stretch, CNP is released from endothelial cells in response to cytokines and endothelium-dependent agonists, such as acetylcholine. Like ANP and BNP, CNP has potent systemic cardiovascular actions, which include reductions in cardiac filling pressures and output, secondary to vasorelaxation and decreases in venous return, but has minimal renal actions.13 CNP is the most anti-fibrotic of the three native endogenous NPs.
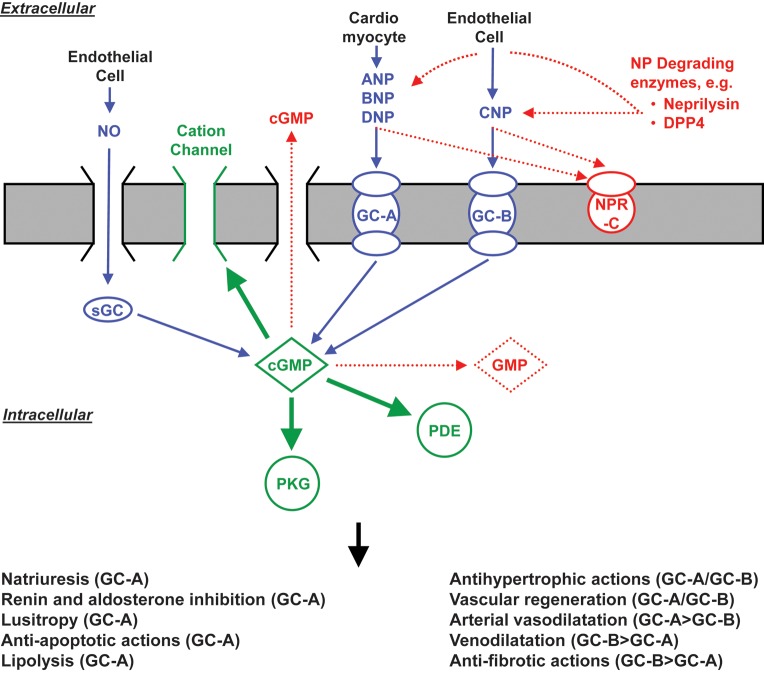
As illustrated in Figure 2, all NPs function via the second messenger cGMP. Atrial natriuretic peptide and BNP bind to GC-A while CNP binds to the GC-B14 (Figure 2). All three peptides are cleared by the clearance receptor, NPR-C, which is a receptor that is not linked to a GC.15 New evidence, however, indicates that the NPR-C is also involved in the anti-fibrotic actions of the NP by mechanisms that are independent of cGMP activation.16 The NPs are also cleared from the circulation via enzymatic degradation by NEP.14 A comprehensive view of the biology of these peptides has emerged following studies in cell systems, murine models of altered NP production or receptor function, and integrative physiological studies in disease models and in humans. The biological properties of these peptides, which include natriuresis, vasodilatation, inhibition of the RAAS, positive lusitropism, and inhibition of fibrosis, have led to the unique concept of cardiorenal protection by activation of cGMP.15,17–21 Such a view has been strengthened most recently by successful chronic therapeutic strategies in experimental CVD states. Specifically, oral delivery of BNP with novel conjugation technologies has been reported in dogs.22 Most exciting is the recent success of direct cardiac BNP overexpression achieved with a novel gene delivery system in rats via an adeno-associated virus (AAV9) vector. Indeed, this approach allows long-term cardiac delivery of BNP for the treatment of hypertensive heart disease and induces improvement of cardiac function and structure.23
Figure 2.
Known actions of the natriuretic peptides through GC-A and GC-B activation. ANP, atrial natriuretic peptide; BNP, B-type natriuretic peptide; CNP, C-type natriuretic peptide; DNP, dendroaspis natriuretic peptide; cGMP, cyclic guanosine monophospate; sGC, soluble guanylyl cyclase; GC-A, guanylyl cyclase-A; GC-B, guanylyl cyclase-B; NO, nitric oxide; NPR-C, natriuretic peptide receptor-C; PDE, phosphodiesterase; PKG, protein kinase G; DPP4, dipeptidyl peptidase IV.
Most recently, there has been a flurry of new data demonstrating the actions of the NPs, particularly the GC-A agonists ANP and BNP, on metabolic regulation. Among the many potentially beneficial findings are that GC-A activation increases lipid oxidation in transgenic rodents, inhibits adipocyte growth, increases oxygen consumption, increases mitochondrial biogenesis in the skeletal muscle in rodents, delays gastric emptying, activates adiponectin, converts white adipocytes to brown adipocytes, lowers insulin levels, and improves glucose tolerance.24–32 Furthermore, a specific human ANP genetic variant rs5068, which increases circulating ANP, protects against both hypertension and metabolic syndrome.33,34 Of note, hypertension and obesity are both associated with reduced ANP and BNP levels, suggesting the existence of a deficiency of the NP system in these conditions thus supporting a need for NP therapy.35–38
Although initially studied for their diagnostic and predictive value in human HF, the NPs are now also viewed as potential, innovative therapeutic agents for cardiorenal and, perhaps, even in metabolic disease syndromes. Indeed, a seminal work published back in 1986 in Science, marked the beginning of the use of the NPs as markers of human CVD.39 Since then, the NPs and, in particular, the BNP1–32 and NT-proBNP 1–76 molecular forms have been used for the diagnosis and prognosis of HF. However, due to the inherent biological properties of the NPs, these hormones are now considered as useful treatment of HF and other CV morbidities such as hypertension.40–43 To date, ANP and BNP are currently approved for the treatment of acute HF in Japan and in USA, respectively. While the ASCEND-HF trial could not demonstrate reduced rate of death or rehospitalization or symptom relief with Nesiritide (recombinant BNP), it confirmed the safety of the drug regarding renal function.44 The higher occurrence of hypotension after Nesiritide in this trial may suggest that this type of treatment could be dose adjusted so as to avoid excessive hypotension and could be targeted to benefit certain subgroups within the acute HF population.44 Most recently, Cataliotti et al.45 have demonstrate the efficacy of small dose (10 μg/kg twice a day) of subcutaneously delivered BNP in normalizing BP in a patient with uncontrolled hypertension even when the subject's usual anti-hypertensive therapy was not given. Indeed, this is the first evidence of the efficacy of these peptides in human hypertension and may represent a new concept in the treatment of resistant hypertension.
Most recently, a designer NP, Cenderitide or CD-NP, has entered clinical trials for HF.46,47 Cenderitide, is a newly designed chimeric peptide that simultaneously co-activates GC-A and the GC-B by fusing together the amino acid sequence of CNP, a pure GC-B agonist, with the elongated C-terminal tail sequence of a GC-A agonist found in snake venom, dendroaspis NP.48 Neutel et al.49 recently reported the results of a Phase I clinical trial evaluating the pharmacokinetics, pharmacodynamics, safety, and tolerability of subcutaneous bolus and subcutaneous infusion of Cenderitide in patients with chronic HF. Cenderitide was well tolerated and, compared with native NPs, possesses greater resistance to degradation by NEP.50 Given the encouraging results obtained with the use of the native NPs and their chimerics, like Cenderitide, in the treatment of human CVD and yet the difficulties associated with the chronic delivery of proteins such as the NPs, the prevention of their enzymatic degradation by NEP, so as to enhance the NP system, is a target worth pursuing. Thus, the effects of NEP inhibition (NEPinh) have been explored both in animal models and in humans either alone or in combination with the inhibition of other systems involved in the progression of CVD.
Dysregulation of the natriuretic peptide system in hypertension and heart failure
There is a growing body of evidence that dysregulation of the NP system exists in human CVD. As stated above, human hypertension, HF, and obesity may all be an NP deficiency state.36–38 Initially, it was thought that during the evolution of HF, the heart increases its production and release of ANP and BNP, in order to compensate for the increased water retention and cardiac stress that characterizes left ventricular (LV) systolic dysfunction.39 Indeed, elevated circulating levels of such hormones, as detected by commercially available immunoreactive assays, have been associated with worsening of HF and with poor prognosis.51 Recent studies using more sophisticated technologies (sensitive mass spectrometric techniques) have reported that in patients with congestive HF and high plasma BNP levels assessed by a conventional diagnostic assay (Biosite), there is actually a lack of mature BNP1–32.52 Thus, this suggested that altered processing of proBNP1–108 and/or of BNP1–32 might occur in HF and hence a relative deficiency of this protective hormone. Studies have now confirmed our previous report and support the concept that altered processing of BNP does indeed occur in HF. Niederkofler et al.,53 using an extremely sensitive mass spectrometry-based method, have recently reported very low levels of circulating BNP1–32 in subjects with advanced HF despite markedly elevated levels of immunoreactive BNP by the Biosite assay. Furthermore, they also demonstrated the presence of shorter, less biologically active, proteolytically processed forms of BNP1–32. These results were further confirmed in a later study by Miller et al.54 Here, the authors also used a quantitative mass spectrometry approach to determine the circulating molecular forms of BNP in patients with HF. They demonstrated that less biologically active precursors and multiple degraded forms of BNP are present and are included in the measurements that come from the current clinical assays. The authors suggested that most of the ‘BNP’ that is detected by clinical BNP assays is actually different degradation products of BNP and the less biologically active prohormone, proBNP1–108. Taken together, these data help explain the blunting of the expected physiological responses to apparently high levels of BNP. Furthermore, it suggests that patients with advanced HF may actually be in a state of NP deficiency and may benefit from exogenous administration of recombinant NP or by the inhibition of NP proteolytic degradation.
Similar to what was observed in HF, patients with essential hypertension may also have an NP deficiency state. Although initially thought that this hormone was activated in hypertension, Belluardo et al.37 reported in 2006 the presence of a relative deficiency of the BNP system in early stages of essential hypertension. In particular, BNP1–32 was not activated in hypertensive patients, while NT-proBNP was lower in mildly hypertensive patients when compared with normotensive subjects. This suggested that a deficiency of bioactive BNP may be present in the early stages of hypertension thus favoring the progression of this condition. Importantly, we have now extended this original observation to a large well-characterized adult general population from Olmsted County. Here, we also defined the ANP system to investigate whether the deficiency of the BNP system in subjects with mild hypertension is counterbalanced by the activation of the other cardiac hormone. However, we not only confirmed that the BNP system including BNP1–32, NT-proBNP, and the precursor proBNP1–108 was not activated, but also demonstrated that the ANP system, by ANP1–28 and NT-proANP1–98, was likewise lower in pre-hypertensives than in normotensive subjects.38
Dries et al.55 have also reported that the presence of two missense mutations in the corin gene (the enzyme that is known to process proBNP1–108 to its active form BNP1–32 as well as non-active proANP1–126 to mature ANP1–28) is associated with high BP and hypertension in African-Americans. This and similar findings reported in a mouse model, in which the deletion of corin induced hypertension, support the hypothesis that an altered processing pathway of proBNP1–108 to mature BNP1–32 also occurs in hypertension.56 Perhaps, this impaired processing of the NP results in reduced BP-lowering effects of these vasoactive hormones and so leads to an increased risk of developing hypertension and ultimately to more severe CVD such as overt HF. Complementing the elegant work of Dries are the studies of Newton-Cheh et al. Here, these investigators employing large populations have identified single-nucleotide polymorphisms of the ANP and BNP gene as well as the NP clearance receptor, which are linked to a protection from hypertension when associated with elevated circulating NP levels.33,57 Taken all together, these studies support the existence of a deficiency state of biologically active cardiac NPs in HF and hypertension. The use of these hormones or of strategies aimed at preventing their excessive degradation for the treatment of CVD is therefore a logical pursuit.
Neutral endopeptidase
Neutral endopeptidase is a type II integral membrane metallopeptidase. Specifically, it is a zinc-dependent, membrane bound endopeptidase that hydrolyses peptides on the amino side of hydrophobic residues.58–61 Neutral endopeptidase has a short NT cytoplasmic domain, a single transmembrane helix, and a C-terminal extracellular domain with a zinc atom at the active site.62,63 In mammals, NEP is widely expressed, e.g. kidney, lung, endothelial cells, vascular smooth muscle cells, cardiac myocytes, fibroblasts, neutrophils, adipocytes, testes, and brain, with the highest concentrations being present in the renal proximal tubule. In lymphocytes, NEP expression is developmentally regulated.61,64–67 Neutral endopeptidase is critical for the processing and catabolism of vasoactive peptides and peptides involved in diuresis and natriuresis, e.g. the NPs, angiotensin I (Ang I), bradykinin (BK), and endothelin-1 (ET-1).68–70 Many other substrates for NEP exist, including opioid peptides, Substance P, peptides involved in the regulation of inflammation, amyloid β-protein, and gastrin. Neutral endopeptidase's ability to degrade multiple substrates also means that the sole inhibition of NEP yields broader effects than anticipated and explains why NEPinh is best combined with the inhibition of other vasoactive compounds.
Selective neutral endopeptidase inhibition
Medical therapy for hypertension and HF is usually aimed at decreasing cardiac load by haemodynamic modulation. Typical ways of accomplishing this are by arterial dilatation, venous dilatation, and increased sodium and water excretion. Indeed, angiotensin-converting enzyme (ACE)-inhibitors, which block the conversion of Ang I to Ang II, suppress the RAAS, and increase BK levels, are known to decrease cardiac afterload and reduce HF morbidity and mortality.71,72
Since many substrates for NEP are peptides with vasoactive and diuretic/natriuretic actions; hence, NEPinh has been examined as a potential therapeutic modality. The key role that NEP plays in the degradation of the NPs initially provided the rationale for NEPinh. Atrial natriuretic peptide is cleaved by NEP at seven different sites, but the initial cleavage occurs between Cys7 and Phe8. Cleavage here destroys ANP's ring structure and so inactivates it.68 Likewise, CNP is cleaved between Cys7 and Phe8 and between other hydrophobic amino acids.73,74 B-type natriuretic peptide, although hydrolysed by NEP, is slightly more resistant to NEP than ANP or CNP. Neutral endopeptidase cleaves BNP first between Met4 and Val5 and then at other sites.75,76
If NEP solely acted on NPs, NEPinh alone might be expected to augment the vasodilating, natriuretic, and diuretic actions seen with NPs. However, NEP hydrolyses other vasoactive peptides with opposing physiological actions.77 Thus, NEPinh alone results in both desirable and undesirable effects. For example, NEP hydrolyses Ang I to angiotensin 1–7, and since Ang 1–7 counters the action of the vasoconstrictor Ang II, the hydrolysis of Ang I to Ang 1–7 by NEP produces a potentially beneficial BP-lowering effect. Also desirable, NEP catabolizes the potent vasoconstrictor ET-1. However, NEP also hydrolyses BK to the inactivated BK 1–7, which results in the undesirable effect of BP increasing. In short, NEPinh would help increase the circulating levels of the NPs and BK, but it would also increase the vasoconstrictors Ang II and ET-1. Some studies have even reported greater vasoconstrictor than vasodilator effects from NEPinh alone.77
One of the first NEP inhibitor developed for clinical use was candoxatril, a potent, orally available NEP inhibitor that is, however, no longer clinically studied (Candoxatrilat was a related, intravenously administered compound). In humans candoxatril caused a dose-dependent increase in plasma ANP, natriuresis, and the NP second messenger, cGMP.78,79 However, NEPinh with candoxatril was also found to increase Ang II levels. This Ang II increase was not accompanied by elevated aldosterone levels, most likely secondary to the concomitant increase in NPs, which are known to suppress aldosterone.80–83 Ultimately, candoxatril's effects on BP in hypertensive patients were not consistently impressive. Higher doses (200 mg) were more natriuretic than lower doses.84 When candoxatril 200 mg, twice daily for 28 days, was compared with placebo in essential hypertension patients, no relevant BP decrease occurred despite significantly increased ANP levels.85 Taken together, NEPinh in hypertensive subjects produces the competing effects of increased pressors and increased vasodilators, with an insignificant BP-lowering result.
Candoxatril was also studied in the setting of chronic LV dysfunction, and this has been extensively reviewed elsewhere.79 In LV dysfunction, vasoconstrictor and vasodilator balance is paramount. In a canine model of mild HF (characterized by elevations of NPs but not RAAS), candoxatril improved renal haemodynamics and increased urinary ANP and cGMP excretion. These beneficial actions were not found when a severe HF model (characterized by both NP elevation and RAAS activation) was similarly treated with candoxatrilat.86 In human HF, candoxatril or candoxatrilat treatment increased ANP and BNP levels, produced diuresis and natriuresis, and decreased clearance of ANP administered exogenously. However, systemic and pulmonary vascular resistances were not affected.79 In small studies of chronic HF patients, candoxatril increased levels of the vasoconstrictor ET-1 as well as ANP levels87 and also caused a dose-dependent increase in systemic vascular resistance and decrease in cardiac index.88
Dual inhibition of neutral endopeptidase and angiotensin-converting enzyme
Given that NEPinh alone leads to an increase in circulating levels of both vasodilators as well as vasoconstrictors, drugs that inhibited both NEP and ACE were developed and are referred to as vasopeptidase inhibitors (VPIs).89 The appeal of this combination is that NEPinh increases endogenous NP levels and that ACE inhibition attenuates the Ang II increases seen with NEPinh alone. Thus, the combination should decrease systemic and renal vascular resistance, suppress aldosterone, and increase natriuresis and diuresis.90 Currently, however, none of these compounds are in clinical development. In preclinical studies, these compounds showed promise for hypertension, HF, and renal disease treatment; however, their side effect profile in clinical trials stunted development. Of particular concern was the high incidence of angioedema, which is typically manifested as swelling of the skin and mucous membranes of the face, lips, tongue, oropharynx, upper respiratory tract, and occasionally intestines.91
Omapatrilat was the VPI in the most advanced stage of development. It was compared with lisinopril in HF patients in the IMPRESS trial. It performed well in the composite of death, admission, or study treatment discontinuation for worsening HF, and it improved NYHA class more than lisinopril.92 After these promising results, the OVERTURE trial compared omapatrilat with enalapril in chronic HF patients. Unfortunately, here the investigators did not find superiority of omapatrilat over enalapril with respect to the primary endpoint, the combined risk of death or hospitalization for HF requiring intravenous treatment.93 Eventually, a Food and Drug Administration (FDA) review of an omapatrilat safety database found a relatively high occurrence (compared with ACE inhibition alone) of severe angioedema, especially in African-Americans and smokers, and did not approve the drug.94 The OCTAVE trial was designed as a large (n = 25 302), randomized, active-controlled, multicentred trial that compared 6 months of treatment with omapatrilat or enalapril in hypertensive patients.95 Patients were started at a low dose of omapatrilat and titrated up in the hope of decreasing the incidence of angioedema. By Week 8, omapatrilat reduced systolic BP 3.6 mmHg more than enalapril. By Week 24, omapatrilat-treated subjects required less adjunctive antihypertensive therapy than enalapril-treated patients. Omapatrilat-treated subjects were more likely to reach goal BP, but again they had a higher incidence of angioedema (2.17% for omapatrilat vs. 0.68% for enalapril), which occurred early in the course of therapy.95 Ultimately, omapatrilat was not FDA approved.
Angioedema development with VPIs remains a persistent concern. It had been simply thought that increased levels of BK secondary to dual ACE and NEP inhibition were the cause of the angioedema. We now know that inhibition of aminopeptidase P (APP), which was also potently inhibited by omapatrilat, is an important consideration.96 Aminopeptidase P has a role both in BK degradation when ACE is inhibited and in the inactivation of the pro-inflammatory BK metabolite, des-Arg9-BK. Indeed, subjects with lower APP levels are predisposed to develop angioedema with ACE-inhibitor therapy.97 Similarly, individuals with XPNPEP2 genetic variations that result in lower APP levels are also more apt to have ACE-inhibitor-associated angioedema.98,99 In this age of pharmacogenomics and personalized therapy, the incidence of angioedema may be decreased by not giving genetically predisposed subjects such a medication.
Angiotensin receptor blockade with neutral endopeptidase inhibition
An alternative dual NEP and ACE inhibition strategy can be achieved by the combination of NEPinh with an angiotensin receptor blocker (ARB). Angiotensin receptor blockers do not disrupt BK metabolism as much as ACE-inhibitors, and it has been reported that some patients with ACE-inhibitor-associated angioedema can be switched over to an ARB without the occurrence of angioedema.97 This has led to the development of a novel class of drugs that combines the actions of NEPinh and ARB, known as angiotensin receptor blockade with neutral endopeptidase inhibition (ARNi).
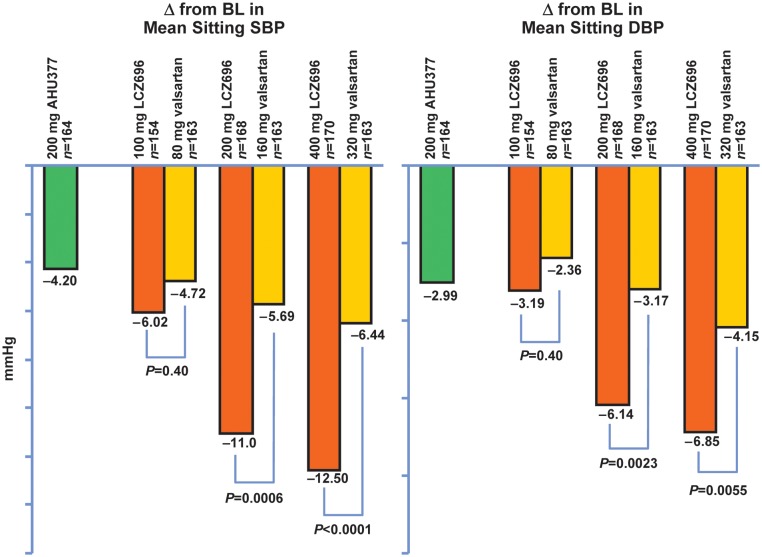
LCZ696 is the first compound of this category and the one in the most advanced stage of development. This ARNi is orally available and provides in a 1:1 ratio blockade of the angiotensin type 1 receptor (AT1R) with a valsartan moiety and NEPinh via an AHU377 prodrug moiety, which is metabolized within an hour to the active moiety LBQ657.100 In an 8-week, randomized, double-blind Phase II trial in hypertensive patients, various doses of LCZ696 were compared with those of comparable doses of valsartan. ARNi-treated subjects had greater reductions in sitting systolic and diastolic BP than valsartan-treated subjects. This was significant for the group treated with 200 mg LCZ696, (‘mid range’ dose) vs. 160 mg valsartan and for 400 mg LCZ696 (highest dose) vs. 320 mg valsartan. The ARNi-treated subjects also had greater systolic and diastolic BP reductions than found in a separate group treated with AHU377 alone (Figure 3). Angioedema did not occur, with ∼8% of the ARNi-treated subjects being black.101
Figure 3.
Angiotensin receptor blockade with neutral endopeptidase inhibition effects: decreased blood pressure as a result of the direct antihypertrophic, anti-fibrotic, endothelial, and renal-enhancing actions mediated by increased biological activities of the natriuretic peptides and by increased angiotensin type 2 receptor binding (adapted from Ruilope et al.,101 Copyright 2010, with permission from Elsevier).
There are currently ongoing trials examining LCZ696 both in chronic HF and in chronic HF with preserved ejection fraction (EF), and studies evaluating sodium excretion in healthy, hypertensive, and HF patients treated with LCZ696. Of great interest, the outcome of the PARADIGM-HF trial is eagerly awaited. In this Phase III study in symptomatic HF, the ability of LCZ696 to delay the first occurrence of HF hospitalization or CV mortality will be compared with enalapril.102 Paramount HF will test the hypothesis that in patients with HF and preserved EF, LCZ696 will have favorable actions on neurohormones and on echocardiographic findings with chronic therapy compared with ACE inhibition.103
To complement LCZ696, other innovative ARNi's are being developed. Specifically, a highly potent, oral, dual inhibitor of the AT1R and neprilysin for the treatment of hypertension and/or chronic HF is under development. This newest ARNi, still in preclinical development, is a single molecule exhibiting pharmacological properties of both NEPinh and AT1R blockade.104
Summary
Using NEPinh to increase the circulating plasma concentrations of NPs in disease states such as HF and hypertension, which are marked by relative deficiencies of bioactive NPs, set the way for VPIs and ARNi's. Unfortunately, these early VPIs, in addition to favourable therapeutic actions, were also associated with an increased risk of angioedema. This side effect caused the FDA not to approve the most clinically advanced VPI, omapatrilat.
ARNi's represent the latest advance combining an ARB with NEPinh, which are now being evaluated in preclinical and clinical studies. Indeed, the combination of an NEP inhibitor and ARB is very appealing. Blockade of the AT1R by ARB facilitates the binding of Ang II to the angiotensin type 2 receptor that elicits several favorable actions (Table 1). LCZ696 stands out as one of the first drugs of this ARNi class that combines inhibition of NEP and blockade of the AT1R. Importantly, this novel class of drugs seems to be safe and not to share with their VPIs precursors an increased risk for angioedema neither in more at-risk subjects such as African-Americans. The dual inhibition of the Ang II receptor and NEP could provide clinical benefits in a wide range of CVDs, particularly hypertension and HF. We eagerly await the result of clinical trials examining ARNi both in chronic HF and in chronic HF with preserved EF.
Table 1.
Main actions of angiotensin type 1 and 2 receptor activation
| Receptors | Local actions |
Clinical effects |
||||||
|---|---|---|---|---|---|---|---|---|
| AT1R | Vasoconstriction | Hypertrophy | Proliferation | Pro-fibrotic | Pro-oxidation | Hypertension | Cardiac and vascular remodelling | Atherosclerosis |
| AT2R | Vasodilatation | Antihypertrophy | Antiproliferation | Anti-fibrotic | Anti-oxidation | Hypotension | Anti-cardiac and -vascular remodelling | Anti-atherosclerosis |
Funding
Supported by grants from the National Institutes of Health: HL R01 36634, HL P01 76611, HL R01 098502-01A1, and the Mayo Foundation, Rochester, MN, USA.
Conflict of interest: Mayo Clinic has licensed Cenderitide (CD-NP) to Nile Therapeutics and J.C.B. is Chair of the Scientific Advisory Board of Nile Therapeutics.
References
- 1.Piller LB, Baraniuk S, Simpson LM, Cushman WC, Massie BM, Einhorn PT, Oparil S, Ford CE, Graumlich JF, Dart RA, Parish DC, Retta TM, Cuyjet AB, Jafri SZ, Furberg CD, Saklayen MG, Thadani U, Probstfield JL, Davis BR. Long-term follow-up of participants with heart failure in the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) Circulation. 124:1811–1818. doi: 10.1161/CIRCULATIONAHA.110.012575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodeheffer RJ. Hypertension and heart failure: the ALLHAT imperative. Circulation. 2011;124:1803–1805. doi: 10.1161/CIRCULATIONAHA.111.059303. [DOI] [PubMed] [Google Scholar]
- 3.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 4.Sudoh T, Kangawa K, Minamino N, Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988;332:78–81. doi: 10.1038/332078a0. [DOI] [PubMed] [Google Scholar]
- 5.Heublein DM, Clavell AL, Stingo AJ, Lerman A, Wold L, Burnett JC., Jr C-type natriuretic peptide immunoreactivity in human breast vascular endothelial cells. Peptides. 1992;13:1017–1019. doi: 10.1016/0196-9781(92)90065-b. [DOI] [PubMed] [Google Scholar]
- 6.Cataliotti A, Giordano M, De Pascale E, Giordano G, Castellino P, Jougasaki M, Costello LC, Boerrigter G, Tsuruda T, Belluardo P, Lee SC, Huntley B, Sandberg S, Malatino LS, Burnett JC., Jr CNP production in the kidney and effects of protein intake restriction in nephrotic syndrome. Am J Physiol Renal Physiol. 2002;283:F464–F472. doi: 10.1152/ajprenal.00372.2001. [DOI] [PubMed] [Google Scholar]
- 7.Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y, Shirakami G, Jougasaki M, Obata K, Yasue H. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest. 1991;87:1402–1412. doi: 10.1172/JCI115146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen HH, Cataliotti A, Schirger JA, Martin FL, Burnett JC., Jr Equimolar doses of atrial and brain natriuretic peptides and urodilatin have differential renal actions in overt experimental heart failure. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1093–R1097. doi: 10.1152/ajpregu.00682.2004. [DOI] [PubMed] [Google Scholar]
- 9.Tsuruda T, Boerrigter G, Huntley BK, Noser JA, Cataliotti A, Costello-Boerrigter LC, Chen HH, Burnett JC., Jr Brain natriuretic peptide is produced in cardiac fibroblasts and induces matrix metalloproteinases. Circ Res. 2002;91:1127–1134. doi: 10.1161/01.res.0000046234.73401.70. [DOI] [PubMed] [Google Scholar]
- 10.Tonne JM, Campbell JM, Cataliotti A, Ohmine S, Thatava T, Sakuma T, Macheret F, Huntley BK, Burnett JC, Jr, Ikeda Y. Secretion of glycosylated pro-B-type natriuretic peptide from normal cardiomyocytes. Clin Chem. 57:864–873. doi: 10.1373/clinchem.2010.157438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ichiki T, Huntley BK, Heublein DM, Sandberg SM, McKie PM, Martin FL, Jougasaki M, Burnett JC., Jr Corin is present in the normal human heart, kidney, and blood, with pro-B-type natriuretic peptide processing in the circulation. Clin Chem. 2011;57:40–47. doi: 10.1373/clinchem.2010.153908. [DOI] [PubMed] [Google Scholar]
- 12.Chauhan SD, Hobbs AJ, Ahluwalia A. C-type natriuretic peptide: new candidate for endothelium-derived hyperpolarising factor. Int J Biochem Cell Biol. 2004;36:1878–1881. doi: 10.1016/j.biocel.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Chen HH, Burnett JC., Jr C-type natriuretic peptide: the endothelial component of the natriuretic peptide system. J Cardiovasc Pharmacol. 1998;32(Suppl. 3):S22–S28. [PubMed] [Google Scholar]
- 14.Charles CJ, Espiner EA, Nicholls MG, Richards AM, Yandle TG, Protter A, Kosoglou T. Clearance receptors and endopeptidase 24.11: equal role in natriuretic peptide metabolism in conscious sheep. Am J Physiol. 1996;271(2 Pt 2):R373–R380. doi: 10.1152/ajpregu.1996.271.2.R373. [DOI] [PubMed] [Google Scholar]
- 15.Kuhn M. Molecular physiology of natriuretic peptide signalling. Basic Res Cardiol. 2004;99:76–82. doi: 10.1007/s00395-004-0460-0. [DOI] [PubMed] [Google Scholar]
- 16.Anand-Srivastava MB. Natriuretic peptide receptor-C signaling and regulation. Peptides. 2005;26:1044–1059. doi: 10.1016/j.peptides.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Burnett JC, Jr, Granger JP, Opgenorth TJ. Effects of synthetic atrial natriuretic factor on renal function and renin release. Am J Physiol. 1984;247(5 Pt 2):F863–F866. doi: 10.1152/ajprenal.1984.247.5.F863. [DOI] [PubMed] [Google Scholar]
- 18.Wada A, Tsutamoto T, Matsuda Y, Kinoshita M. Cardiorenal and neurohumoral effects of endogenous atrial natriuretic peptide in dogs with severe congestive heart failure using a specific antagonist for guanylate cyclase-coupled receptors. Circulation. 1994;89:2232–2240. doi: 10.1161/01.cir.89.5.2232. [DOI] [PubMed] [Google Scholar]
- 19.Lee SC, Stevens TL, Sandberg SM, Heublein DM, Nelson SM, Jougasaki M, Redfield MM, Burnett JC., Jr The potential of brain natriuretic peptide as a biomarker for New York Heart Association class during the outpatient treatment of heart failure. J Card Fail. 2002;8:149–154. doi: 10.1054/jcaf.2002.125368. [DOI] [PubMed] [Google Scholar]
- 20.Cataliotti A, Boerrigter G, Costello-Boerrigter LC, Schirger JA, Tsuruda T, Heublein DM, Chen HH, Malatino LS, Burnett JC., Jr Brain natriuretic peptide enhances renal actions of furosemide and suppresses furosemide-induced aldosterone activation in experimental heart failure. Circulation. 2004;109:1680–1685. doi: 10.1161/01.CIR.0000124064.00494.21. [DOI] [PubMed] [Google Scholar]
- 21.Cataliotti A, Schirger JA, Martin FL, Chen HH, McKie PM, Boerrigter G, Costello-Boerrigter LC, Harty G, Heublein DM, Sandberg SM, James KD, Miller MA, Malkar NB, Polowy K, Burnett JC., Jr Oral human brain natriuretic peptide activates cyclic guanosine 3′,5′-monophosphate and decreases mean arterial pressure. Circulation. 2005;112:836–840. doi: 10.1161/CIRCULATIONAHA.105.538520. [DOI] [PubMed] [Google Scholar]
- 22.Cataliotti A, Chen HH, Schirger JA, Martin FL, Boerrigter G, Costello-Boerrigter LC, James KD, Polowy K, Miller MA, Malkar NB, Bailey KR, Burnett JC., Jr Chronic actions of a novel oral B-type natriuretic peptide conjugate in normal dogs and acute actions in angiotensin II-mediated hypertension. Circulation. 2008;118:1729–1736. doi: 10.1161/CIRCULATIONAHA.107.759241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cataliotti A, Tonne JM, Bellavia D, Martin FL, Oehler EA, Harders GE, Campbell JM, Peng KW, Russell SJ, Malatino LS, Burnett JC, Jr, Ikeda Y. Long-term cardiac pro-B-type natriuretic peptide gene delivery prevents the development of hypertensive heart disease in spontaneously hypertensive rats. Circulation. 2011;123:1297–1305. doi: 10.1161/CIRCULATIONAHA.110.981720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitsuishi M, Miyashita K, Itoh H. cGMP rescues mitochondrial dysfunction induced by glucose and insulin in myocytes. Biochem Biophys Res Commun. 2008;367:840–845. doi: 10.1016/j.bbrc.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Miyashita K, Itoh H, Tsujimoto H, Tamura N, Fukunaga Y, Sone M, Yamahara K, Taura D, Inuzuka M, Sonoyama T, Nakao K. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 2009;58:2880–2892. doi: 10.2337/db09-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarzani R, Marcucci P, Salvi F, Bordicchia M, Espinosa E, Mucci L, Lorenzetti B, Minardi D, Muzzonigro G, Dessi-Fulgheri P, Rappelli A. Angiotensin II stimulates and atrial natriuretic peptide inhibits human visceral adipocyte growth. Int J Obes (Lond) 2008;32:259–267. doi: 10.1038/sj.ijo.0803724. [DOI] [PubMed] [Google Scholar]
- 27.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Addisu A, Gower WR, Jr, Landon CS, Dietz JR. B-type natriuretic peptide decreases gastric emptying and absorption. Exp Biol Med (Maywood) 2008;233:475–482. doi: 10.3181/0708-RM-216. [DOI] [PubMed] [Google Scholar]
- 29.Meirhaeghe A, Sandhu MS, McCarthy MI, de Groote P, Cottel D, Arveiler D, Ferrieres J, Groves CJ, Hattersley AT, Hitman GA, Walker M, Wareham NJ, Amouyel P. Association between the T-381C polymorphism of the brain natriuretic peptide gene and risk of type 2 diabetes in human populations. Hum Mol Genet. 2007;16:1343–1350. doi: 10.1093/hmg/ddm084. [DOI] [PubMed] [Google Scholar]
- 30.Choquet H, Cavalcanti-Proenca C, Lecoeur C, Dina C, Cauchi S, Vaxillaire M, Hadjadj S, Horber F, Potoczna N, Charpentier G, Ruiz J, Hercberg S, Maimaitiming S, Roussel R, Boenhnke M, Jackson AU, Patsch W, Krempler F, Voight BF, Altshuler D, Groop L, Thorleifsson G, Steinthorsdottir V, Stefansson K, Balkau B, Froguel P, Meyre D. The T-381C SNP in BNP gene may be modestly associated with type 2 diabetes: an updated meta-analysis in 49 279 subjects. Hum Mol Genet. 2009;18:2495–2501. doi: 10.1093/hmg/ddp169. [DOI] [PubMed] [Google Scholar]
- 31.Tsukamoto O, Fujita M, Kato M, Yamazaki S, Asano Y, Ogai A, Okazaki H, Asai M, Nagamachi Y, Maeda N, Shintani Y, Minamino T, Asakura M, Kishimoto I, Funahashi T, Tomoike H, Kitakaze M. Natriuretic peptides enhance the production of adiponectin in human adipocytes and in patients with chronic heart failure. J Am Coll Cardiol. 2009;53:2070–2077. doi: 10.1016/j.jacc.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 32.Birkenfeld AL, Budziarek P, Boschmann M, Moro C, Adams F, Franke G, Berlan M, Marques MA, Sweep FC, Luft FC, Lafontan M, Jordan J. Atrial natriuretic peptide induces postprandial lipid oxidation in humans. Diabetes. 2008;57:3199–3204. doi: 10.2337/db08-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newton-Cheh C, Larson MG, Vasan RS, Levy D, Bloch KD, Surti A, Guiducci C, Kathiresan S, Benjamin EJ, Struck J, Morgenthaler NG, Bergmann A, Blankenberg S, Kee F, Nilsson P, Yin X, Peltonen L, Vartiainen E, Salomaa V, Hirschhorn JN, Melander O, Wang TJ. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet. 2009;41:348–353. doi: 10.1038/ng.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cannone V, Boerrigter G, Cataliotti A, Costello-Boerrigter LC, Olson TM, McKie PM, Heublein DM, Lahr BD, Bailey KR, Averna M, Redfield MM, Rodeheffer RJ, Burnett JC., Jr A genetic variant of the atrial natriuretic peptide gene is associated with cardiometabolic protection in the general community. J Am Coll Cardiol. 58:629–636. doi: 10.1016/j.jacc.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magnusson M, Jujic A, Hedblad B, Engstrom G, Persson M, Struck J, Morgenthaler NG, Nilsson P, Newton-Cheh C, Wang TJ, Melander O. Low plasma level of atrial natriuretic peptide predicts development of diabetes: the prospective Malmo Diet and Cancer study. J Clin Endocrinol Metab. 97:638–645. doi: 10.1210/jc.2011-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, Vasan RS. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 37.Belluardo P, Cataliotti A, Bonaiuto L, Giuffre E, Maugeri E, Noto P, Orlando G, Raspa G, Piazza B, Babuin L, Chen HH, Martin FL, McKie PM, Heublein DM, Burnett JC, Jr, Malatino LS. Lack of activation of molecular forms of the BNP system in human grade 1 hypertension and relationship to cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2006;291:H1529–H1535. doi: 10.1152/ajpheart.00107.2006. [DOI] [PubMed] [Google Scholar]
- 38.Macheret F, Heublein D, Costello-Boerrigter LC, Boerrigter G, McKie P, Bellavia D, Mangiafico S, Ikeda Y, Bailey K, Scott CG, Sandberg S, Chen HH, Malatino LS, Redfield MM, Rodeheffer R, Burnett JC, Jr, Cataliotti A. Human hypertension is characterized by a lack of activation of the anti-hypertensive cardiac hormones ANP and BNP. J Am Coll Cardiol. 2011;57:1386–1395. doi: 10.1016/j.jacc.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burnett JC, Jr, Kao PC, Hu DC, Heser DW, Heublein D, Granger JP, Opgenorth TJ, Reeder GS. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science. 1986;231:1145–1147. doi: 10.1126/science.2935937. [DOI] [PubMed] [Google Scholar]
- 40.Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF. Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. J Am Med Assoc. 2002;287:1531–1540. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 41.Colucci WS, Elkayam U, Horton DP, Abraham WT, Bourge RC, Johnson AD, Wagoner LE, Givertz MM, Liang CS, Neibaur M, Haught WH, LeJemtel TH. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N Engl J Med. 2000;343:246–253. doi: 10.1056/NEJM200007273430403. [DOI] [PubMed] [Google Scholar]
- 42.Hobbs RE, Mills RM, Young JB. An update on nesiritide for treatment of decompensated heart failure. Expert Opin Investig Drugs. 2001;10:935–942. doi: 10.1517/13543784.10.5.935. [DOI] [PubMed] [Google Scholar]
- 43.Boerrigter G, Burnett JC., Jr Recent advances in natriuretic peptides in congestive heart failure. Expert Opin Investig Drugs. 2004;13:643–652. doi: 10.1517/13543784.13.6.643. [DOI] [PubMed] [Google Scholar]
- 44.O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF, Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 45.Cataliotti A, Costello-Boerrigter LC, Chen HH, Textor SC, Burnett JC., Jr Sustained blood pressure-lowering actions of subcutaneous B-type natriuretic peptide (nesiritide) in a patient with uncontrolled hypertension. Mayo Clin Proc. 2012;87:413–415. doi: 10.1016/j.mayocp.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee CY, Chen HH, Lisy O, Swan S, Cannon C, Lieu HD, Burnett JC., Jr Pharmacodynamics of a novel designer natriuretic peptide, CD-NP, in a first-in-human clinical trial in healthy subjects. J Clin Pharmacol. 2009;49:668–673. doi: 10.1177/0091270009336233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.A study assessing the pharmacokinetics, pharmacodynamics, safety and tolerability of subcutaneous bolus and subcutaneous infusion of cenderitide in patients with chronic heart failure with volume overload (HF) http://clinicaltrials.gov/ct2/show/NCT01316432 .
- 48.Lisy O, Huntley BK, McCormick DJ, Kurlansky PA, Burnett JC., Jr Design, synthesis, and actions of a novel chimeric natriuretic peptide: CD-NP. J Am Coll Cardiol. 2008;52:60–68. doi: 10.1016/j.jacc.2008.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neutel JM, Rolston W, Maddock S, Goldsmith S, Koren M, Antwerp B, Burnett JC, Lieu HD. Initial experience with subcutaneous infusion of cenderitide in chronic heart failure patients. J Am Coll Cardiol. 2012;59:E1037. [Google Scholar]
- 50.Dickey DM, Potter LR. Dendroaspis natriuretic peptide and the designer natriuretic peptide, CD-NP, are resistant to proteolytic inactivation. J Mol Cell Cardiol. 2011;51:67–71. doi: 10.1016/j.yjmcc.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wright GA, Struthers AD. Natriuretic peptides as a prognostic marker and therapeutic target in heart failure. Heart. 2006;92:149–151. doi: 10.1136/hrt.2003.018325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hawkridge AM, Heublein DM, Bergen HR, 3rd, Cataliotti A, Burnett JC, Jr, Muddiman DC. Quantitative mass spectral evidence for the absence of circulating brain natriuretic peptide (BNP-32) in severe human heart failure. Proc Natl Acad Sci USA. 2005;102:17442–7. doi: 10.1073/pnas.0508782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niederkofler EE, Kiernan UA, O'Rear J, Menon S, Saghir S, Protter AA, Nelson RW, Schellenberger U. Detection of endogenous B-type natriuretic peptide at very low concentrations in patients with heart failure. Circ Heart Fail. 2008;1:258–264. doi: 10.1161/CIRCHEARTFAILURE.108.790774. [DOI] [PubMed] [Google Scholar]
- 54.Miller WL, Phelps MA, Wood CM, Schellenberger U, Van Le A, Perichon R, Jaffe AS. Comparison of mass spectrometry and clinical assay measurements of circulating fragments of B-type natriuretic peptide in patients with chronic heart failure. Circ Heart Fail. 2011;4:355–360. doi: 10.1161/CIRCHEARTFAILURE.110.960260. [DOI] [PubMed] [Google Scholar]
- 55.Dries DL, Victor RG, Rame JE, Cooper RS, Wu X, Zhu X, Leonard D, Ho SI, Wu Q, Post W, Drazner MH. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation. 2005;112:2403–2410. doi: 10.1161/CIRCULATIONAHA.105.568881. [DOI] [PubMed] [Google Scholar]
- 56.Chan JC, Knudson O, Wu F, Morser J, Dole WP, Wu Q. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc Natl Acad Sci USA. 2005;102:785–790. doi: 10.1073/pnas.0407234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, Smith AV, Tobin MD, Verwoert GC, Hwang SJ, Pihur V, Vollenweider P, O'Reilly PF, Amin N, Bragg-Gresham JL, Teumer A, Glazer NL, Launer L, Zhao JH, Aulchenko Y, Heath S, Sober S, Parsa A, Luan J, Arora P, Dehghan A, Zhang F, Lucas G, Hicks AA, Jackson AU, Peden JF, Tanaka T, Wild SH, Rudan I, Igl W, Milaneschi Y, Parker AN, Fava C, Chambers JC, Fox ER, Kumari M, Go MJ, van der Harst P, Kao WH, Sjogren M, Vinay DG, Alexander M, Tabara Y, Shaw-Hawkins S, Whincup PH, Liu Y, Shi G, Kuusisto J, Tayo B, Seielstad M, Sim X, Nguyen KD, Lehtimaki T, Matullo G, Wu Y, Gaunt TR, Onland-Moret NC, Cooper MN, Platou CG, Org E, Hardy R, Dahgam S, Palmen J, Vitart V, Braund PS, Kuznetsova T, Uiterwaal CS, Adeyemo A, Palmas W, Campbell H, Ludwig B, Tomaszewski M, Tzoulaki I, Palmer ND, Aspelund T, Garcia M, Chang YP, O'Connell JR, Steinle NI, Grobbee DE, Arking DE, Kardia SL, Morrison AC, Hernandez D, Najjar S, McArdle WL, Hadley D, Brown MJ, Connell JM, Hingorani AD, Day IN, Lawlor DA, Beilby JP, Lawrence RW, Clarke R, Hopewell JC, Ongen H, Dreisbach AW, Li Y, Young JH, Bis JC, Kahonen M, Viikari J, Adair LS, Lee NR, Chen MH, Olden M, Pattaro C, Bolton JA, Kottgen A, Bergmann S, Mooser V, Chaturvedi N, Frayling TM, Islam M, Jafar TH, Erdmann J, Kulkarni SR, Bornstein SR, Grassler J, Groop L, Voight BF, Kettunen J, Howard P, Taylor A, Guarrera S, Ricceri F, Emilsson V, Plump A, Barroso I, Khaw KT, Weder AB, Hunt SC, Sun YV, Bergman RN, Collins FS, Bonnycastle LL, Scott LJ, Stringham HM, Peltonen L, Perola M, Vartiainen E, Brand SM, Staessen JA, Wang TJ, Burton PR, Artigas MS, Dong Y, Snieder H, Wang X, Zhu H, Lohman KK, Rudock ME, Heckbert SR, Smith NL, Wiggins KL, Doumatey A, Shriner D, Veldre G, Viigimaa M, Kinra S, Prabhakaran D, Tripathy V, Langefeld CD, Rosengren A, Thelle DS, Corsi AM, Singleton A, Forrester T, Hilton G, McKenzie CA, Salako T, Iwai N, Kita Y, Ogihara T, Ohkubo T, Okamura T, Ueshima H, Umemura S, Eyheramendy S, Meitinger T, Wichmann HE, Cho YS, Kim HL, Lee JY, Scott J, Sehmi JS, Zhang W, Hedblad B, Nilsson P, Smith GD, Wong A, Narisu N, Stancakova A, Raffel LJ, Yao J, Kathiresan S, O'Donnell CJ, Schwartz SM, Ikram MA, Longstreth WT, Jr, Mosley TH, Seshadri S, Shrine NR, Wain LV, Morken MA, Swift AJ, Laitinen J, Prokopenko I, Zitting P, Cooper JA, Humphries SE, Danesh J, Rasheed A, Goel A, Hamsten A, Watkins H, Bakker SJ, van Gilst WH, Janipalli CS, Mani KR, Yajnik CS, Hofman A, Mattace-Raso FU, Oostra BA, Demirkan A, Isaacs A, Rivadeneira F, Lakatta EG, Orru M, Scuteri A, Ala-Korpela M, Kangas AJ, Lyytikainen LP, Soininen P, Tukiainen T, Wurtz P, Ong RT, Dorr M, Kroemer HK, Volker U, Volzke H, Galan P, Hercberg S, Lathrop M, Zelenika D, Deloukas P, Mangino M, Spector TD, Zhai G, Meschia JF, Nalls MA, Sharma P, Terzic J, Kumar MV, Denniff M, Zukowska-Szczechowska E, Wagenknecht LE, Fowkes FG, Charchar FJ, Schwarz PE, Hayward C, Guo X, Rotimi C, Bots ML, Brand E, Samani NJ, Polasek O, Talmud PJ, Nyberg F, Kuh D, Laan M, Hveem K, Palmer LJ, van der Schouw YT, Casas JP, Mohlke KL, Vineis P, Raitakari O, Ganesh SK, Wong TY, Tai ES, Cooper RS, Laakso M, Rao DC, Harris TB, Morris RW, Dominiczak AF, Kivimaki M, Marmot MG, Miki T, Saleheen D, Chandak GR, Coresh J, Navis G, Salomaa V, Han BG, Zhu X, Kooner JS, Melander O, Ridker PM, Bandinelli S, Gyllensten UB, Wright AF, Wilson JF, Ferrucci L, Farrall M, Tuomilehto J, Pramstaller PP, Elosua R, Soranzo N, Sijbrands EJ, Altshuler D, Loos RJ, Shuldiner AR, Gieger C, Meneton P, Uitterlinden AG, Wareham NJ, Gudnason V, Rotter JI, Rettig R, Uda M, Strachan DP, Witteman JC, Hartikainen AL, Beckmann JS, Boerwinkle E, Vasan RS, Boehnke M, Larson MG, Jarvelin MR, Psaty BM, Abecasis GR, Chakravarti A, Elliott P, van Duijn CM, Newton-Cheh C, Levy D, Caulfield MJ, Johnson T. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kerr MA, Kenny AJ. The purification and specificity of a neutral endopeptidase from rabbit kidney brush border. Biochem J. 1974;137:477–488. doi: 10.1042/bj1370477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kerr MA, Kenny AJ. The molecular weight and properties of a neutral metallo-endopeptidase from rabbit kidney brush border. Biochem J. 1974;137:489–495. doi: 10.1042/bj1370489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malfroy B, Swerts JP, Guyon A, Roques BP, Schwartz JC. High-affinity enkephalin-degrading peptidase in brain is increased after morphine. Nature. 1978;276:523–526. doi: 10.1038/276523a0. [DOI] [PubMed] [Google Scholar]
- 61.Turner AJ, Tanzawa K. Mammalian membrane metallopeptidases: NEP, ECE, KELL, and PEX. FASEB J. 1997;11:355–364. doi: 10.1096/fasebj.11.5.9141502. [DOI] [PubMed] [Google Scholar]
- 62.Emoto N, Yanagisawa M. Endothelin-converting enzyme-2 is a membrane-bound, phosphoramidon-sensitive metalloprotease with acidic pH optimum. J Biol Chem. 1995;270:15262–8. doi: 10.1074/jbc.270.25.15262. [DOI] [PubMed] [Google Scholar]
- 63.Spyroulias GS, Cordopatis P. Current inhibition concepts of zinc metallopeptidases involved in BP regulation. Curr Enzyme Inhib. 2005;1:29–42. [Google Scholar]
- 64.Shima M, Seino Y, Torikai S, Imai M. Intrarenal localization of degradation of atrial natriuretic peptide in isolated glomeruli and cortical nephron segments. Life Sci. 1988;43:357–363. doi: 10.1016/0024-3205(88)90113-0. [DOI] [PubMed] [Google Scholar]
- 65.Graf K, Koehne P, Grafe M, Zhang M, Auch-Schwelk W, Fleck E. Regulation and differential expression of neutral endopeptidase 24.11 in human endothelial cells. Hypertension. 1995;26:230–235. doi: 10.1161/01.hyp.26.2.230. [DOI] [PubMed] [Google Scholar]
- 66.Dussaule JC, Stefanski A, Bea ML, Ronco P, Ardaillou R. Characterization of neutral endopeptidase in vascular smooth muscle cells of rabbit renal cortex. Am J Physiol. 1993;264(1 Pt 2):F45–F52. doi: 10.1152/ajprenal.1993.264.1.F45. [DOI] [PubMed] [Google Scholar]
- 67.Standeven KF, Hess K, Carter AM, Rice GI, Cordell PA, Balmforth AJ, Lu B, Scott DJ, Turner AJ, Hooper NM, Grant PJ. Neprilysin, obesity and the metabolic syndrome. Int J Obes (Lond) 2011;35:1031–1040. doi: 10.1038/ijo.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stephenson SL, Kenny AJ. The hydrolysis of alpha-human atrial natriuretic peptide by pig kidney microvillar membranes is initiated by endopeptidase-24.11. Biochem J. 1987;243:183–187. doi: 10.1042/bj2430183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stephenson SL, Kenny AJ. Metabolism of neuropeptides. Hydrolysis of the angiotensins, bradykinin, substance P and oxytocin by pig kidney microvillar membranes. Biochem J. 1987;241:237–247. doi: 10.1042/bj2410237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vijayaraghavan J, Scicli AG, Carretero OA, Slaughter C, Moomaw C, Hersh LB. The hydrolysis of endothelins by neutral endopeptidase 24.11 (enkephalinase) J Biol Chem. 1990;265:14150–5. [PubMed] [Google Scholar]
- 71.Konstam MA, Rousseau MF, Kronenberg MW, Udelson JE, Melin J, Stewart D, Dolan N, Edens TR, Ahn S, Kinan D. Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dysfunction in patients with heart failure. SOLVD Investigators. Circulation. 1992;86:431–438. doi: 10.1161/01.cir.86.2.431. [DOI] [PubMed] [Google Scholar]
- 72.Konstam MA, Kronenberg MW, Rousseau MF, Udelson JE, Melin J, Stewart D, Dolan N, Edens TR, Ahn S, Kinan D. Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dilatation in patients with asymptomatic systolic dysfunction. SOLVD (Studies of Left Ventricular Dysfunction) Investigators. Circulation. 1993;88(5 Pt 1):2277–2283. doi: 10.1161/01.cir.88.5.2277. [DOI] [PubMed] [Google Scholar]
- 73.Potter LR. Natriuretic peptide metabolism, clearance and degradation. FEBS J. 2011;278:1808–1817. doi: 10.1111/j.1742-4658.2011.08082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Watanabe Y, Nakajima K, Shimamori Y, Fujimoto Y. Comparison of the hydrolysis of the three types of natriuretic peptides by human kidney neutral endopeptidase 24.11. Biochem Mol Med. 1997;61:47–51. doi: 10.1006/bmme.1997.2584. [DOI] [PubMed] [Google Scholar]
- 75.Kenny AJ, Bourne A, Ingram J. Hydrolysis of human and pig brain natriuretic peptides, urodilatin, C-type natriuretic peptide and some C-receptor ligands by endopeptidase-24.11. Biochem J. 1993;291(Pt 1):83–88. doi: 10.1042/bj2910083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dickey DM, Potter LR. Human B-type natriuretic peptide is not degraded by meprin A. Biochem Pharmacol. 2010;80:1007–1011. doi: 10.1016/j.bcp.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferro CJ, Spratt JC, Haynes WG, Webb DJ. Inhibition of neutral endopeptidase causes vasoconstriction of human resistance vessels in vivo. Circulation. 1998;97:2323–2330. doi: 10.1161/01.cir.97.23.2323. [DOI] [PubMed] [Google Scholar]
- 78.Ando S, Rahman MA, Butler GC, Senn BL, Floras JS. Comparison of candoxatril and atrial natriuretic factor in healthy men. Effects on hemodynamics, sympathetic activity, heart rate variability, and endothelin. Hypertension. 1995;26(6 Pt 2):1160–1166. doi: 10.1161/01.hyp.26.6.1160. [DOI] [PubMed] [Google Scholar]
- 79.McDowell G, Nicholls DP. The endopeptidase inhibitor, candoxatril, and its therapeutic potential in the treatment of chronic cardiac failure in man. Expert Opin Investig Drugs. 1999;8:79–84. doi: 10.1517/13543784.8.1.79. [DOI] [PubMed] [Google Scholar]
- 80.Richards AM, Wittert G, Espiner EA, Yandle TG, Frampton C, Ikram H. Prolonged inhibition of endopeptidase 24.11 in normal man: renal, endocrine and haemodynamic effects. J Hypertens. 1991;9:955–962. [PubMed] [Google Scholar]
- 81.Sagnella GA, Singer DR, Markandu ND, Buckley MG, MacGregor GA. Is atrial natriuretic peptide-guanosine 3′,5′ cyclic monophosphate coupling a determinant of urinary sodium excretion in essential hypertension? J Hypertens. 1992;10:349–354. doi: 10.1097/00004872-199204000-00005. [DOI] [PubMed] [Google Scholar]
- 82.O'Connell JE, Jardine AG, Davies DL, McQueen J, Connell JM. Renal and hormonal effects of chronic inhibition of neutral endopeptidase (EC 3.4.24.11) in normal man. Clin Sci (Lond) 1993;85:19–26. doi: 10.1042/cs0850019. [DOI] [PubMed] [Google Scholar]
- 83.Sagnella GA, Markandu ND, Buckley MG, Miller MA, Blackwood A, Singer DR, MacGregor GA. Hormonal and renal responses to neutral endopeptidase inhibition in normal humans on a low and on a high sodium intake. Eur J Clin Invest. 1995;25:165–170. doi: 10.1111/j.1365-2362.1995.tb01543.x. [DOI] [PubMed] [Google Scholar]
- 84.O'Connell JE, Jardine AG, Davidson G, Connell JM. Candoxatril, an orally active neutral endopeptidase inhibitor, raises plasma atrial natriuretic factor and is natriuretic in essential hypertension. J Hypertens. 1992;10:271–277. doi: 10.1097/00004872-199203000-00011. [DOI] [PubMed] [Google Scholar]
- 85.Bevan EG, Connell JM, Doyle J, Carmichael HA, Davies DL, Lorimer AR, McInnes GT. Candoxatril, a neutral endopeptidase inhibitor: efficacy and tolerability in essential hypertension. J Hypertens. 1992;10:607–613. [PubMed] [Google Scholar]
- 86.Chen HH, Schirger JA, Chau WL, Jougasaki M, Lisy O, Redfield MM, Barclay PT, Burnett JC., Jr Renal response to acute neutral endopeptidase inhibition in mild and severe experimental heart failure. Circulation. 1999;100:2443–2448. doi: 10.1161/01.cir.100.24.2443. [DOI] [PubMed] [Google Scholar]
- 87.McDowell G, Coutie W, Shaw C, Buchanan KD, Struthers AD, Nicholls DP. The effect of the neutral endopeptidase inhibitor drug, candoxatril, on circulating levels of two of the most potent vasoactive peptides. Br J Clin Pharmacol. 1997;43:329–332. doi: 10.1046/j.1365-2125.1997.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kentsch M, Otter W, Drummer C, Notges A, Gerzer R, Muller-Esch G. Neutral endopeptidase 24.11 inhibition may not exhibit beneficial haemodynamic effects in patients with congestive heart failure. Eur J Clin Pharmacol. 1996;51:269–272. doi: 10.1007/s002280050196. [DOI] [PubMed] [Google Scholar]
- 89.Burnett JC., Jr Vasopeptidase inhibition: a new concept in blood pressure management. J Hypertens Suppl. 1999;17:S37–S43. [PubMed] [Google Scholar]
- 90.Cataliotti A, Boerrigter G, Chen HH, Jougasaki M, Costello LC, Tsuruda T, Lee SC, Malatino LS, Burnett JC., Jr Differential actions of vasopeptidase inhibition versus angiotensin-converting enzyme inhibition on diuretic therapy in experimental congestive heart failure. Circulation. 2002;105:639–644. doi: 10.1161/hc0502.102962. [DOI] [PubMed] [Google Scholar]
- 91.Hoover T, Lippmann M, Grouzmann E, Marceau F, Herscu P. Angiotensin converting enzyme inhibitor induced angio-oedema: a review of the pathophysiology and risk factors. Clin Exp Allergy. 2010;40:50–61. doi: 10.1111/j.1365-2222.2009.03323.x. [DOI] [PubMed] [Google Scholar]
- 92.Rouleau JL, Pfeffer MA, Stewart DJ, Isaac D, Sestier F, Kerut EK, Porter CB, Proulx G, Qian C, Block AJ. Comparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure: IMPRESS randomised trial. Lancet. 2000;356:615–620. doi: 10.1016/s0140-6736(00)02602-7. [DOI] [PubMed] [Google Scholar]
- 93.Packer M, Califf RM, Konstam MA, Krum H, McMurray JJ, Rouleau JL, Swedberg K. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE) Circulation. 2002;106:920–926. doi: 10.1161/01.cir.0000029801.86489.50. [DOI] [PubMed] [Google Scholar]
- 94.Tabrizchi R. Omapatrilat. Bristol-Myers Squibb. Curr Opin Investig Drugs. 2001;2:1414–1422. [PubMed] [Google Scholar]
- 95.Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. 2004;17:103–111. doi: 10.1016/j.amjhyper.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 96.Fryer RM, Segreti J, Banfor PN, Widomski DL, Backes BJ, Lin CW, Ballaron SJ, Cox BF, Trevillyan JM, Reinhart GA, von Geldern TW. Effect of bradykinin metabolism inhibitors on evoked hypotension in rats: rank efficacy of enzymes associated with bradykinin-mediated angioedema. Br J Pharmacol. 2008;153:947–955. doi: 10.1038/sj.bjp.0707641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Adam A, Cugno M, Molinaro G, Perez M, Lepage Y, Agostoni A. Aminopeptidase P in individuals with a history of angio-oedema on ACE inhibitors. Lancet. 2002;359:2088–2089. doi: 10.1016/S0140-6736(02)08914-6. [DOI] [PubMed] [Google Scholar]
- 98.Duan QL, Nikpoor B, Dube MP, Molinaro G, Meijer IA, Dion P, Rochefort D, Saint-Onge J, Flury L, Brown NJ, Gainer JV, Rouleau JL, Agostoni A, Cugno M, Simon P, Clavel P, Potier J, Wehbe B, Benarbia S, Marc-Aurele J, Chanard J, Foroud T, Adam A, Rouleau GA. A variant in XPNPEP2 is associated with angioedema induced by angiotensin I-converting enzyme inhibitors. Am J Hum Genet. 2005;77:617–626. doi: 10.1086/496899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.La Corte AL, Carter AM, Rice GI, Duan QL, Rouleau GA, Adam A, Grant PJ, Hooper NM. A functional XPNPEP2 promoter haplotype leads to reduced plasma aminopeptidase P and increased risk of ACE inhibitor-induced angioedema. Hum Mutat. 2011;32:1326–1331. doi: 10.1002/humu.21579. [DOI] [PubMed] [Google Scholar]
- 100.Gu J, Noe A, Chandra P, Al-Fayoumi S, Ligueros-Saylan M, Sarangapani R, Maahs S, Ksander G, Rigel DF, Jeng AY, Lin TH, Zheng W, Dole WP. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi) J Clin Pharmacol. 2010;50:401–414. doi: 10.1177/0091270009343932. [DOI] [PubMed] [Google Scholar]
- 101.Ruilope LM, Dukat A, Bohm M, Lacourciere Y, Gong J, Lefkowitz MP. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet. 2010;375:1255–1266. doi: 10.1016/S0140-6736(09)61966-8. [DOI] [PubMed] [Google Scholar]
- 102.This Study Will Evaluate the Efficacy and Safety of LCZ696 Compared to Enalapril on Morbidity and Mortality of Patients With Chronic Heart Failure (PARADIGM-HF) NCT1035255 http://clinicaltrials.gov/ct2/show/NCT01035255?term=PARADIGM-HF&rank=1 .
- 103.LCZ696 Compared to Valsartan in Patients with Chronic Heart Failure and Preserved Left-ventricular Ejection Fraction. http://clinicaltrials.gov/ct2/show/NCT00887588?term=NCT00887588&rank=1 .
- 104.Kurtz TW, Klein U. Next generation multifunctional angiotensin receptor blockers. Hypertens Res. 2009;32:826–834. doi: 10.1038/hr.2009.135. [DOI] [PubMed] [Google Scholar]