Abstract
INTRODUCTION
Giant inguinoscrotal bladder hernias are very rare and require surgical intervention. They usually do not cause any specific symptoms and thus, they are often misdiagnosed. If left untreated though, they might lead to severe medical conditions, such as renal failure.
PRESENTATION OF CASE
We present the case of a 71-year-old male patient suffering from a giant inguinoscrotal mass, accompanied by symptoms of the lower urinary track (LUTS) and chronic renal failure.
DISCUSSION
In our case, the patient presented with bladder hernia causing non specific symptoms of renal failure. In contrast to acute renal failure, a chronic renal impairment most often comes with no specific symptoms and thus, it can be present for many years before the diagnosis is made. It is evident that such serious conditions should be suspected and treated.
CONCLUSION
Inguinoscrotal bladder hernias may be associated with severe medical conditions, such as renal deterioration, and should be considered in the differential diagnosis of renal failure, when accompanied by any inguinal, scrotal, or low abdominal wall hernia.
Keywords: Chronic renal failure, Bladder outlet obstruction (BOO), Lower urinary track symptoms (LUTS), Giant inguinoscrotal bladder hernia
1. Introduction
Urinary bladder herniation through the inguinal canal is an uncommon disorder that requires surgical intervention. Resulting from the combination of insufficient abdominal wall tissue and increased intra-abdominal pressure, the bladder prolapse into the scrotum is very rare and often misdiagnosed. Elderly males most usually are affected and present with scrotal swelling and obstructive urinary tract symptoms, typically symptoms of bladder outlet obstruction (BOO) and/or urinary infection.1
Yet, even most patients suffer from mild to moderate symptoms, a massive inguinoscrotal bladder hernia, if left untreated, might lead to severe medical conditions, such as renal failure.2 We report on a case of a 71-year-old male patient presenting with chronic renal failure, urinary dysuria complaints and clinically palpable large inguinoscrotal mass.
2. Presentation of case
A 71-year-old man referred to the urology outpatients complaining of a large, long standing, left scrotal mass, weak urinary stream, nocturia and mild dysuria. He reported that the mass was there for the past ten years, without causing him any symptoms. For the last three years, he had some difficulties in the micturition, which made him to address a urologist. He particularly mentioned that he had to compress his left hemiscrotum in order to initiate the urination and that he was going to the toilet every 2 h at daytime and 3–4 times at night. He was feeling fatigued all the time and looked pale, which was attributed to his bad sleep habits. From his medical history, he had no previous operations in the left inguinal area and suffered from mild hypertension, treated with antidiuretics.
Physical examination revealed a large left hemiscrotum, with inability to palpate the left testicle, the spermatic cord and the external inguinal ring. An ultrasound was arranged, which showed dilatation of the left kidney and ureter, with thinning of the renal parenchyma and a sliding urinary bladder into the left hemiscrotum (Fig. 1). An abdominal CT scan confirmed our initial findings (Fig. 2), while the laboratory tests showed signs of chronic renal failure (blood urea nitrogen was 49 mg/dl and creatinine was 4.3 mg/dl, with mild anemia).
Fig. 1.
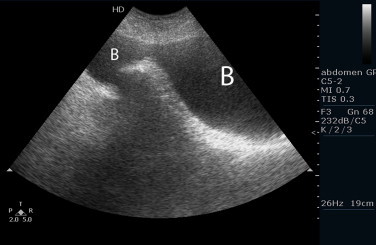
Ultrasound of the sliding bladder into the scrotum (B).
Fig. 2.
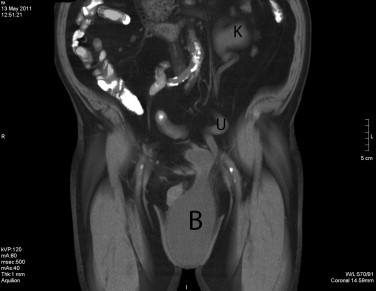
Abdominal and scrotal CT: clear visualization of the giant inguinoscrotal bladder hernia (B), with the dilated left ureter (U) and left renal kidney (K).
A decision was then made to operate on the patient. A ureteral stent was placed preoperatively in the left ureter, aiming at relieving the obstruction on one hand, but mainly, at guiding us during the groin exploration on the other. The stent was placed retrogradely, finding no particular difficulty during the procedure. The patient was operated under spinal anesthesia. After incising the skin, subcutaneous tissue and external oblique aponeurosis, the spermatic cord was elevated from the posterior wall of the inguinal canal. An indirect hernia was discovered. The bladder was revealed and dissected off the spermatic cord (Fig. 3). It was then replaced into its native position. A Lichtenstein open tension free mesh hernioplasty was employed for the surgical reconstruction of the inguinal hernia.3 A polypropylene plug was inserted into the inguinal ring and a polypropylene mesh (3 × 5 in.) was trimmed to fit the floor of the inguinal canal.
Fig. 3.
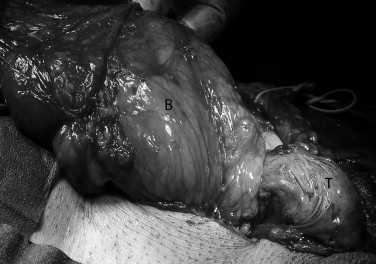
Surgical exploration of the left groin: clear visualization of the bladder. (B): Bladder, (T): left testicle.
There were no complications in the early post-operative period. The patient was mobilized early and the Foley catheter was left in place for 7 days. The ureteral stent remained for 3 months, during which the patient gradually improved his renal function (blood urea nitrogen was 23 mg/dl and creatinine was 1.9 mg/dl, six months after the operation). After hernia restoration, the patient improved significantly his LUTS. He stopped complaining for urgency and frequency, while on the other hand, he restored his micturition; he had improved his stream and had no significant strain in order to begin his urination. He had been checked periodically with uroflowmetry and ultrasound, which revealed an average Q max: 18 ml/s and PVR not more than 20–30 cc. Follow up cystography was done after one and six months, without any evidence of bladder inguinal hernia.
Finally, the patient gave his written consent and our paper has been approved by the Ethics Committee of the University Hospital of Larissa, Greece.
3. Discussion
The incidence of bladder involvement in inguinal hernias is less than 4% and may reach 10% in the elderly patients. Predisposing factors may be obesity, weakened lower abdominal musculature and BOO.3 They are more often direct and can be limited to the inguinal canal or can reach the scrotum. Most cases are asymptomatic and are usually found incidentally at the time of herniorrhaphy.2 If BOO is present though, patients are not asymptomatic and they mostly complain of their lower urinary track symptoms (LUTS), rather than the hernia itself. It is these patients that most often are misdiagnosed of having benign prostate hyperplasia or some form of prostatitis, and in the cases of large scrotal masses, this could lead to some major complications, such as renal failure, bladder necrosis and scrotal abscess.4
It is evident that such serious conditions should be suspected and treated. Both ultrasonography and CT of the lower abdomen and scrotum may aid in the diagnosis of a bladder hernia, and should be immediately performed when a suspicious comorbidity exists.5,6 Still, compression of the scrotum in order to initiate the urination can be often diagnostic of this condition.
In our case, the patient presented with LUTS and non specific symptoms of renal failure. In contrast to acute renal failure, a chronic renal impairment most often comes with no specific symptoms and thus, it can be present for many years before the diagnosis is made. It is crucial that a high risk patient be monitored regularly; otherwise the diagnosis may delay until the kidneys have already been damaged. Furthermore, some of the symptoms – such as fatigue – may have been present for some time, but can develop so gradually, that they are not noticed or attributed to renal failure.7
Our patient suffered from hypertension, which had been treated with antidiuretics, and fatique, which was falsely initially attributed to his LUTS. He had an obstructed left ureter, due to the bladder hernia, which added to the known risk factor of hypertension in the developing of renal damage, even if the other kidney seemed at the time of diagnosis not severely impaired. Indeed, our evidence showed an improvement of the total renal function six months after the surgical repair of the inguinal bladder hernia.In the case of renal deterioration due to obstruction from a large inguinoscrotal bladder hernia, stenting the ureter is of little or temporary benefit for the kidney. The ureter is usually strangled into the inguinal canal, which makes the relief from the obstruction less feasible, while the risk of damaging the ureter remains high. Thus, surgical repair is recommended, aiming at the replacement of the bladder into the Retzius space. In the past, a partially resection of the herniated portion of the bladder was routinely performed, but nowadays our approach has changed in that the bladder should be reduced and resected only in the presence of a tumor in the herniated bladder, bladder necrosis, or herniated bladder diverticulum. In our patient the bladder replacement was followed by a standard Mesh procedure, as recommended.8–10
4. Conclusion
In rare cases, inguinoscrotal bladder hernias may be associated with severe medical conditions, such as renal deterioration, and should be considered in the differential diagnosis of renal failure, when accompanied by any inguinal, scrotal, or low abdominal wall hernia. The bladder should be completely replaced back into the retroperitoneal space whenever possible. A tension-free herniorraphy with or without mesh should be performed for the correction of the anatomical defect.
Conflict of interest statement
The authors report no declarations of interest.
Funding
None.
Ethical approval
The patient gave his written consent and our paper has been approved by the Ethics Committee of the University Hospital of Larissa, Greece. Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request
Author contributions
A. Karatzas: case-study design, data collection, OR team, writing; G. Christodoulidis: OR team; M. Spyridakis: OR team; C. Stavaras: data collection; E. Aravantinos: writing; M. Melekos: supervisior.
References
- 1.Bjurlin M.A., Delaurentis D.A., Jordan M.D., Richter H.M., 3rd Clinical and radiographic findings of a sliding inguinoscrotal hernia containing the urinary bladder. Hernia. 2010;14(December (6)):635–638. doi: 10.1007/s10029-009-0597-8. [DOI] [PubMed] [Google Scholar]
- 2.Wagner A.A., Arcand P., Bamberger M.H. Acute renal failure resulting from huge inguinal bladder hernia. Urology. 2004;64(July (1)):156–157. doi: 10.1016/j.urology.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 3.Vindlacheruvu R.R., Zayyan K., Burgess N.A., Wharton S.B., Dunn D.C. Extensive bladder infarction in a strangulated inguinal hernia. British Journal of Urology. 1996;77(June (6)):926–927. doi: 10.1046/j.1464-410x.1996.07035.x. [DOI] [PubMed] [Google Scholar]
- 4.Oruc M.T., Akbulut Z., Ozozan O., Coskun F. Urological findings in inguinal hernias: a case report and review of the literature. Hernia. 2004;8(February (1)):76–79. doi: 10.1007/s10029-003-0157-6. [DOI] [PubMed] [Google Scholar]
- 5.Verbeeck N., Larrousse C., Lamy S. Diagnosis of inguinal bladder hernias: the current role of sonography. JBR-BTR. 2005;88(September–October (5)):233–236. [PubMed] [Google Scholar]
- 6.Andac N., Baltacioglu F., Tuney D., Cimsit N.C., Ekinci G., Biren T. Inguinoscrotal bladder herniation: is CT a useful tool in diagnosis? Clinical Imaging. 2002;26(September–October (5)):347–348. doi: 10.1016/s0899-7071(02)00447-3. [DOI] [PubMed] [Google Scholar]
- 7.Jafar T.H., Stark P.C., Schmid C.H., Landa M., Maschio G., de Jong P.E. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Annals of Internal Medicine. 2003;139(August (4)):244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- 8.Thompson J.E., Jr., Taylor J.B., Nazarian N., Bennion R.S. Massive inguinal scrotal bladder hernias: a review of the literature with 2 new cases. Journal of Urology. 1986;136(December (6)):1299–1301. doi: 10.1016/s0022-5347(17)45321-3. [DOI] [PubMed] [Google Scholar]
- 9.Bisharat M., O’Donnell M.E., Thompson T., MacKenzie N., Kirkpatrick D., Spence R.A. Complications of inguinoscrotal bladder hernias: a case series. Hernia. 2009;13(February (1)):81–84. doi: 10.1007/s10029-008-0389-6. [DOI] [PubMed] [Google Scholar]
- 10.Zhao G., Gao P., Ma B., Tian J., Yang K. Open mesh techniques for inguinal hernia repair: a meta-analysis of randomized controlled trials. Annals of Surgery. 2009;250(July (1)):35–42. doi: 10.1097/SLA.0b013e3181ad63cc. [DOI] [PubMed] [Google Scholar]


