Abstract
Background
The balance between endothelial injury and repair in childhood is poorly understood. We examined this relationship in healthy children, in adults and in children with familial hypercholesterolaemia (FH).
Methodology
Circulating endothelial cells (CECs) were measured as a marker of vascular injury, with vascular repair assessed by counting colony forming units (CFUs), also known as endothelial progenitor cells.
Results
CEC number increased with age. Children with FH had elevated CECs compared to healthy children , with similar levels numerically to those found in healthy adults. CFU numbers were higher in healthy children than either healthy adults or children with FH. Endothelium dependent vascular function, measured by flow-mediated dilatation (FMD), was positively associated with CFU number, even following adjustment for confounding risk variables.
Conclusion
Levels of CECs increase and CFUs decrease with age. In childhood, before the onset of clinically detectable cardiovascular dysfunction, children with a major risk factor for atherosclerotic disease have levels of these indices of vascular injury and repair approaching those seen in adults.
Introduction
Although atherosclerosis has long been regarded as a degenerative disorder associated with ageing(1) considerable evidence now demonstrates that the underlying pathological process is highly modifiable and amenable to prevention. The vascular damage begins in childhood and develops silently for decades before clinical events such as myocardial infarction or stroke occur(2).Although clinical events rarely occur during childhood, sub-clinical atherosclerosis and the health of the vascular wall can be reliably detected at this early stage by non-invasive measures such as; flow mediated vasodilatation (FMD), levels of circulating mature endothelial cells or endothelial microparticles found in the blood (3-5). This early progression however, exists as a delicate balance between vascular endothelial injury and the endogenous ability to repair this damage.
Circulating cholesterol level, particularly low-density lipoprotein cholesterol, is one factor that maintains a strong , continuous association with cardiovascular risk (6) from childhood onwards, even at low concentrations. Only modest increases are typically seen in early life in relation to dietary habits, sedentary lifestyle and obesity. More extreme elevation in cholesterol level during childhood is usually the consequence of genetic abnormalities(7).
A population of immature cells with the potential to recognize and repair sites of endothelial injury, identified in blood, may act as endogenous vascular repair mechanisms offsetting the damaging effect of risk factors (RFs) on the endothelium, potentially attenuating atherogenesis. These cells have been labelled as endothelial progenitor cells (EPCs) (8) and although the literature variably describes heterogeneous populations of cells the exposure to RFs consistently appears to have an adverse impact on them. ‘EPC’ number is not only associated with endothelium-dependent vasodilatation but also predictive of cardiovascular prognosis independently of the conventional RF profile in higher risk patients (9-11).
Indeed, elevated levels of circulating LDL cholesterol in animals and adults with dyslipidaemia are associated with decreased ‘EPC’ number and function (12-14) that can increase following statin therapy and cholesterol reduction by non-pharmacological measures(15) .
Currently, neither the role of these putative ‘EPCs’ in childhood is known, nor is it known how exposure to RFs during childhood influences their biology. We hypothesised that risk factors for atherosclerosis, specifically ageing and hypercholesterolemia, adversely affect the balance between vascular injury and repair.
In this study we report on changes in biomarkers of endothelial injury and repair, in relation to age, hypercholesterolemia and vascular function.
Results
Relationship between age, cellular and vascular parameters in healthy subjects
We studied 26 healthy children aged between 10 and 18 years and 82 healthy adults aged between 18 and 67 years. The baseline characteristics are summarised in Table 1. Systolic and diastolic BP; total LDL and HDL cholesterol and triglycerides increased modestly with age (Table 1).
Table 1.
Baseline characteristics of healthy volunteers.
| Group: (Years) | Child: (10-18) |
Adult: (19-67) |
p value |
|---|---|---|---|
| Age median [Interquartile range] |
14[12-16] | 42[31-51] | |
| Number of subjects | 26 | 82 | |
| Gender (%male) | 34 | 37 | |
| WHR | 0.8 ± 0.03 | 0.83 ± 0.02 | 0.33 |
| Glucose, mmol/L | 4.7± 0.06 | 4.9 ± 0.06 | 0.133 |
| Total Cholesterol, mmol/L |
4.1 ± 0.13 | 4.9 ± 0.1 | <0.001* |
| LDL Cholesterol, mmol/L |
2.41 ± 0.11 | 2.94 ± 0.09 | 0.001* |
| HDL Cholesterol, mmol/L |
1.34 ± 0.06 | 1.53 ± 0.05 | 0.012* |
| Triglycerides, mmol/L |
0.72[ 0.65-0.92] | 0.85 [0.72-1.21] | 0.001* |
| Systolic BP, mmHg | 104 ± 1.87 | 117.8 ± 1.6 | <0.001* |
| Diastolic BP, mmHg | 60.5 ± 1.5 | 71.0 ± 1.04 | <0.001* |
Values represent mean ± SEM, age values represent median ±interquartile range.
HDL: High density Lipoprotein; LDL: Low density Lipoprotein, WHR: Waist Hip ratio. Values compared between 10-18, 19-67 year old age groups. Unpaired t-test between between child age group and adult age group.
Represent significant differences between child and adult age groups , p<0.05.
Relationship between colony-forming unit number and age
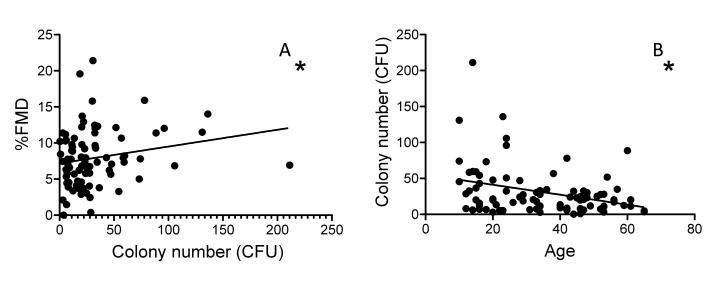
CFUs were measured in 20 children and in 66 adults, figure 1a. There was a significant correlation between CFU number and age (r=−0.29, p=0.038) in the whole population, figure 2a. Children had higher CFU numbers than adults (39[13-59]; 20[9-31], p=0.012, median ± interquartile range; children and adults respectively).
Figure 1.
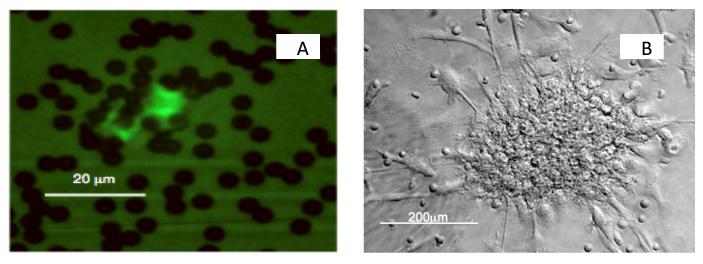
Enumeration of cell types. (a) Immunomagnetic beads coated with CD146 were incubated with whole blood, then adherent cells stained with FITC-conjugated ULEX. ULEX bright cells with more than 5 beads attached and greater than 10μm in size were considered CECs. (b) Phase contrast image of a CFU. Nonadherent cells were selected from PBMCS cultured for 2 days. They were grown in culture medium supplemented with cytokines and growth factors at 37°C, 5%CO2 for 7days, and CFUs then counted.
Figure 2.
Alterations in CFU number with age and with %FMD in healthy subjects. (a) CFUs decreased with age starting from 10 years of age to 67 (r=−0.287, *p=0.007). Furthermore, the increase in CFU number was associated with an augmented ability to vasodilatate, (%FMD )(r=0.238, *p=0.038) (b).
Weight, waist size, glucose, cholesterol, HDL and LDL levels, and systolic and diastolic BP did not correlate with CFU. However triglyceride levels (r=−0.28, p=0.011) and LDL (r=−0.31, p=0.005) correlated with CFU. Moreover, the inverse relationship between CFU number and age remained after multivariable adjustment (β=−0.32, p=0.03).
Relationship between circulating endothelial cells and age
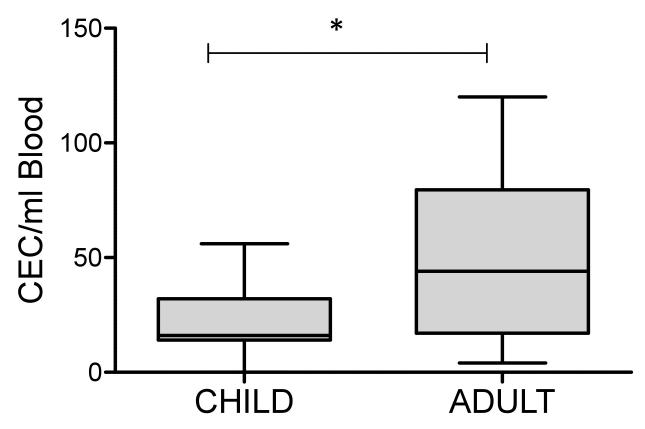
CEC number increased with age in the entire cohort (r=0.44, p<0.005). CEC numbers were also greater in adults than in children (44[17-80] vs 16[14-32] median ± interquartile range; children vs adults respectively, p<0.001), figure 3. The relationship between age and CEC number in healthy subjects remained after multivariable adjustment for vascular risk factors, (β=0.703, p<0.001).
Figure 3.
Alterations in CEC number with age in healthy subjects. CEC number, which is known to represent endothelial damage and apoptosis, were greater in adults (19–67 years) than in children (10–18 years) (44[17-80] vs 16[14-32] median ± interquartile range; children vs adults respectively, *p<0.001 represents significant difference between adults and children).
Relationship Among age, vascular measures, CFU, and CECs
To explore these relationships across the whole cohort, multiple regression analyses were conducted. A strong inverse relationship between FMD and age existed in the entire cohort, including children, after adjusting for baseline arterial diameter as well as gender and modifiable risk factors in the model (β=0.453, p<0.001).
A significant relationship was observed between CFU and FMD in the whole cohort (r=0.238, p=0.038), figure 2b, which remained after multivariable adjustment for risk factors and baseline brachial artery diameter (β=0.245, p=0.019). However, no relationship was observed between CEC levels and FMD
The influence of familial hypercholesterolemia on vascular and cellular parameters in childhood
We studied 26 control children, eight of whom were unaffected siblings of FH patients, and 29 children with FH. Baseline characteristics are summarised in Table 2. Total and LDL cholesterol levels were greatly elevated and systolic BP was slightly higher in FH children.
Table 2.
Baseline characteristics of child cohorts.
| Group: (Years) | Child: (10-18) |
FH : (10-18) |
p value |
|---|---|---|---|
| Age median [Interquartile range] | 14[12-16] | 12[11-13] | |
| Number of subjects | 26 | 29 | |
| Gender (%male) | 34 | 49 | |
| WHR | 0.8 ± 0.03 | 0.85 ± 0.18 | 0.22 |
| Glucose, mmol/L | 4.7± 0.06 | 4.76 ± 0.07 | 0.76 |
| Total Cholesterol, mmol/L | 4.1 ± 0.13 | 7.06 ± 0.33 | <0.001* |
| LDL Cholesterol, mmol/L | 2.41 ± 0.11 | 5.29 ± 0.31 | <0.001* |
| HDL Cholesterol, mmol/L | 1.34 ± 0.06 | 1.45± 0.05 | 0.19 |
| Triglycerides, mmol/L | 0.72 [0.65-0.92] | 0.79[0.6- 1.1] |
0.49 |
| Systolic BP, mmHg | 104 ± 1.87 | 110.6 ± 2.50 |
0.02* |
| Diastolic BP, mmHg | 60.5 ± 1.5 | 62.7 ± 1.9 | 0.366 |
Values represent mean ± SEM, age values represent median ± interquartile range.
HDL: High density Lipoprotein; LDL: Low density Lipoprotein, WHR: Waist Hip ratio. Values compared between 10-18 year old healthy and FH children. Unpaired t-test between child age group and FH group.
Represent significant values , p<0.05.
Colony-Forming Unit number and Circulating Endothelial Cells in Childhood FH
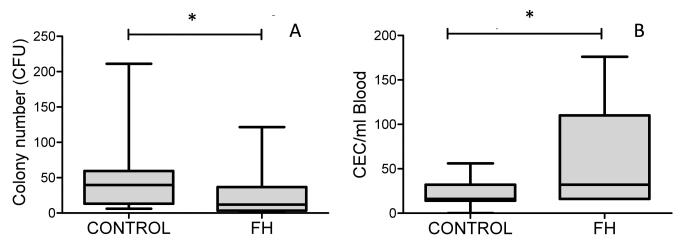
CFU numbers were greatly reduced in the FH group compared with healthy children (12[4-36] vs 39[13-59], median ± interquartile range respectively, p=0.028), figure 4a. Moreover, multivariable analyses demonstrated a significant association between CFU and FH after adjusting for age, gender and BP in the model (β=0.357, p=0.045).
Figure 4.
Alterations in CFUs and CECs in children with FH. (a) Numbers of CFUs were reduced in FH children compared with healthy children (12[4-36] vs 39[13-59], p=0.028). Conversely, the numbers of CECs in children with FH were greater than those found in age matched controls (36[16-161]; 16[14-32], p=0.031) (b). Data presented as median ± interquartile range, *p<0.05 represents significant difference between healthy children and children with FH.
Children with FH also had elevated CECs compared to age matched controls (36[16-161] vs 16[14-32], median ± interquartile range respectively, p=0.031), figure 4b.
Relationship Among Vascular Measures, CFU and CECs in Childhood Familial Hypercholesterolemia
FMD was similar in healthy children and those with FH (6.9[4.3-8.4] vs 7.0 [3.7-9.67, p=0.79, median ± interquartile range respectively. No relationship between CFU or CECs and the vascular parameters was seen in children with FH.
Discussion
Our study is the first to examine the relationships between CFUs, CECs and vascular dysfunction with healthy ageing from childhood to adulthood and in children with FH. We have shown that the number of EPCs declines with age with a concomitant increase in CEC number, a recognised marker of endothelial injury. Consistent with previous data in adults with a range of risk factors, we also find an independent relationship between CFUs and endothelial function in a healthy population, including children. This is in agreement with that the concept that maintaining the balance between vascular injury and repair becomes more challenging with advancing age.
We have also seen that children with FH have values for CFUs and CECs similar to those found in adults, implying that exposure to this vascular risk factor has an adverse impact on endothelial homeostasis even at this early stage of life. Although our sample number is relatively small, the only RF that differed between healthy and FH children was the (LDL) cholesterol level, suggesting that this alone is sufficient to account for the raised CEC counts in FH children at levels similar to adult participants who are at greater CVD risk than control children.
There is still very limited data on these markers of endothelial damage and repair in healthy children. Our data is consistent with, and extends the implications of previous studies that have demonstrated a decrease in CFU number with age (16) and show increased concentrations of circulating angiogenic cell populations in children (17). Our data also demonstrate that with hypercholesterolaemia, CFU numbers are lower in children with FH than in healthy age matched children and of a similar level to those seen in adults.
There is considerable controversy surrounding the true nature of EPCs and the significance of their numbers in humans. Since they were first discovered (18) , a number of distinct populations of EPCs have been identified and defined. The phenotpye of our colonies was found be composed of hematopoietic cells enriched with T cells and monocytes-macrophages (data not shown) and are therefore an assay to measure cell-cell interactions rather than a postnatal primary vasculogenic cell population (8, 19).
In humans it has been difficult to definitively demonstrate that EPC’s of any phenotype directly replace endothelial cells in vivo, although cells of recipient origin have been identified within the coronary arterial endothelium in biopsy samples from heart transplant patients (20). Moreover, the absence of any bone-marrow derived cells in the endothelium of atherosclerotic mouse plaques in a bone-marrow transplant model has recently questioned how EPCs contribute to endothelial maintenance in-vivo (21) despite evidence that cell transplantation accelerates endothelial healing in vascular injury models (22) .
Nonetheless, the morphological identification of the colony forming units (CFU or CFU-endothelial cells (CFU-EC) (23) ) used here have been consistently identified as a predictive biomarker for disease (9, 10).
Indeed, our observations that CFU numbers fall from childhood into adulthood and that children with FH have fewer CFUs are consistent with the opinion that an increased vascular risk with ageing and early exposure to lipid abnormalities may in part be explained by reduced CFU numbers (3, 12) . It has also been suggested that the phenotype of these cells may be altered following chronic exposure to LDL, supported by data from in-vitro studies demonstrating augmented senescence and a decreased capacity to form tubules in the presence of oxLDL (13, 14).
Studies in adults have consistently demonstrated a strong association between classical RFs and the numbers of both CFUs and CECs, suggesting a relevant biological link between risk factors, these cells and progression of vascular injury (9, 10, 12, 24-28) although the causal relationship and extent of residual confounding by other influences remains to be determined.
Numbers of CECs increased with healthy ageing from childhood and with childhood FH in our study, extending previous observations in healthy adults (29) .There are a number of different ways of measuring CECs and comparison of absolute numbers of CECs between different studies can be problematic because of the inherent variability in the techniques used. We used a previously validated method which provides a robust characterisation of these cells and also fragments of senescent endothelial cells that may be lost using flow cytometric methods (3).Circulating markers of endothelial cell damage/apoptosis, such as CECs, are measureable in healthy adults and children ‘at risk’ for cardiovascular disease(30), for example levels of CECs are elevated in very young children with irreversible pulmonary hypertension(31) and in children with vasculitis(3). Consequently, increased CEC levels are consistent with an increased endothelial cell turnover as a result of greater demand on the systems that maintain integrity of the vascular endothelium. Considered together with the lower CFU numbers, these CEC data are consistent with an adverse shift in the balance of endothelial injury and repair promoting a more rapid progression of disease observed with aging and FH (32, 33).
The relationship between endothelium-dependent vasomotor function and age was unclear and made difficult by the continuous altering arterial diameter from childhood to adulthood. Furthermore in contrast to previous reports (34) vascular function in children with FH was no different from healthy children. It is likely that the children recruited with FH were “healthier” than subjects recruited to previous studies. Although not receiving statins, all children were managed in a specialist clinic where multiple healthy lifestyle measures are promoted. The preserved endothelial vasomotor function observed in our FH group, suggests that in childhood vascular injury induced by one or more risk factors may still be compensated for but is unlikely to be sufficient in the presence advancing age and increasing risk factor burden.
Nevertheless, the association between CFU numbers and FMD, even following adjustment for the global risk factor profile, remained consistent with previous reports and supports the hypothesis that these cells are influenced by risk factors and implicated in the maintenance of a healthy endothelium (9).
Efforts to modulate this balance of injury and repair may prove to be an important means of preventing or reversing vascular injury as exercise can elevate EPCs (CD133+/KDR + and CD34+/KDR+ cells) in children (35). This simple and effective means of enhancing vascular repair mechanisms may supplement the established benefits of exercise on weight, metabolic parameters and general fitness and is likely to be of greatest incremental value in children with known risk factors.
We conclude, using ageing and childhood FH as models of increased cardiovascular risk, that sub-clinical endothelial pathophysiology is detectable at an early stage in life through the measurement of CFUs and CECs, even before functional vascular changes have become apparent, although a relationship between CFU numbers and FMD remains. Other disease states in children that are also associated with accelerated vascular injury from early life such as obesity (36), chronic inflammatory disease or infection (37) and renal failure (38), would be amenable to similar analyses. Early detection of endothelial and vascular injury in these diseases could provide the information necessary to inform implementation of global and specific interventions that include; exercise, dietary or novel pharmacological approaches to improve long-term cardiovascular outcomes.
Methods
Study Population
We conducted 2 studies. The first examined the relationship between age, indices of vascular injury and repair in healthy individuals from childhood onwards. The second was a case-control study exploring the impact of FH on these parameters during childhood. The study was approved by the Great Ormond Street Hospital for Children NHS Trust, the Institute of Child Health Research Ethics Committee and informed consent was obtained from all the subjects. All the procedures were undertaken in accordance with national and institutional guidelines.
Participants underwent assessment of blood pressure (BP) and anthropometric parameters (height, weight, body mass). Colony forming unit assays were performed as previously described (9, 39). Briefly, PBMCs at 5×106 cells/well were cultured in 6-well fibronectin-coated plates containing RPMI (Gibco, Invitrogen, CA) supplemented with 20% fetal calf serum (FCS; Imclone, New York, NY) and antibiotics. After 2 days, the nonadherent cell population present in the supernatant was selected and these cells cultured at a concentration of 1×106/well in fibronectin-coated 24-well plates containing the following culture media (MCDB 131 medium; Invitrogen, Carlsbad, CA; supplemented with: L-glutamine; 50ng/ml endothelial cell growth serum (Sigma-Aldrich, St. Louis, MO); 20ng/ml Vascular Endothelial Growth Factor (VEGF; R&D Systems, Boston, MA); 5ng/ml basic human fibroblast growth factor; 20% fetal bovine serum (Imclone); heparin 5U/ml; penicillin 100,000 U/ml; and streptomycin 100,000 mg/ml (39). All cells were cultured at 37°C, 5% CO2 and the media changed after 4 days.
After a further 7 days of culture, the cells were washed gently with warmed media and the number of colonies in each well counted using a phase contrast microscope at x100 magnification (Leica axiovert; Leica Microsytems, Wetzlar, Germany). A colony forming unit (CFU) was defined as a central core of round cells with more elongated cells at the periphery (9, 39). Colony counting and preparation was carried out with an interobserver correlation of 0.95 for the final colony count.
Circulating Endothelial Cells
Circulating endothelial cells (CECs) were extracted and enumerated in nested sub-groups of healthy subjects and children with FH using a protocol previously described by our group (3). Briefly, 1ml of blood collected in EDTA tubes was mixed with 1ml buffer (0.1%Na Azide, 0.6%Na Citrate, 0.1% BSA in PBS) and 20μl FcR blocking agent (Miltenyi Biotec, Cologne, Germany). To this 50μl of a preparation of Dynal beads (Dynal Biotech, Bromborough, Wirral, UK) linked to CD146 (Biocytex, Marseille, France) was incubated for 30 minutes at 4°C. The cells bound to CD146 immunomagnetic beads were separated using a magnet (MPC-l, Dynal Biotech) and washed 3 times in a buffer before resuspension in 100μl of buffer containing 2mg/ml FITC labelled Ulex (Ulex Europus Lectin, Sigma-Aldrich). These cells were incubated in the dark at room temperature for one hour before washing 3 times in buffer and finally resuspended in 200 μl buffer. Cells were counted using a Nageotte chamber and a fluorescent microscope (Leica Microsystems). Brightly fluorescent cells greater than 10 μM in diameter had more than 5 CD146 beads attached were considered CECs (3, 29). CEC counting was carried out with an inter-observer correlation of 0.95 for the final CEC count.
Noninvasive Vascular Studies
All studies were performed in a temperature-controlled vascular laboratory by a trained operator. Studies commenced after an acclimatization period of at least 15 minutes.
Brachial Artery Vasomotor Function
High-resolution ultrasound imaging with an Acuson 5- to 10-MHz linear probe (Acuson, Mountain View, CA) was used to assess flow mediated dilatation as previously reported (40). Brachial artery diameter was measured offline by an automatic edge detection system (Brachial Tools, Medical Imaging Applications, Coralville, IA) and expressed as a percentage change from baseline diameter. Doppler-derived flow measurements (using a pulsed-wave Doppler signal at a 70° angle) were also obtained continuously. The increase in blood flow after the release of the cuff was expressed as a percentage change from the baseline flow.
Statistics (Data analysis)
Data are expressed as mean ± standard error of the mean (SEM) or median [interquartile range] for non-parametric data, unless otherwise stated. In descriptive analyses, parametric summary statistics and significance tests were used when the data were normally distributed. Nonparametric tests were used for analysis of gender, triglycerides, CFU number, and CEC data. For z-scores we used the European reference curves.
Univariate comparisons between groups were analysed by t-test for normally distributed data and Mann-Whitney-U test or Kruskal Wallis test for non-parametric data. The Chi-squared test was used to assess differences in gender.
Bivariate correlations were determined using Pearson or Spearman correlation coefficients as appropriate. Multivariable logistic regression analyses were performed to explore relationships between age and CFU and/or vascular measures with adjustments for potential confounders (gender, waist circumference, systolic BP and fasting glucose and lipid levels). All statistical analyses were performed with SPSS, version 16 (SPSS INC).
Acknowledgments
We would like to thank the Vasculopathy Consortium at the Institute of Child Health, which helped with the endothelial assays and with patient recruitment.
Statement of financial support: The work was supported by the British Heart Foundation (grant PG/04/081/17384).
References
- 1.Grundy SM. Age as a risk factor: you are as old as your arteries. Am J Cardiol. 1999;83:1455–7. doi: 10.1016/s0002-9149(99)00125-3. [DOI] [PubMed] [Google Scholar]
- 2.Dawber TR. The Framingham Study: the epidemiology of atherosclerotic disease. Harvard University Press; Cambridge, MA: 1980. [Google Scholar]
- 3.Clarke LA, Shah V, Arrigoni F, et al. Quantitative detection of circulating endothelial cells in vasculitis: comparison of flow cytometry and immunomagnetic bead extraction. J Thromb Haemost. 2008;6:1025–32. doi: 10.1111/j.1538-7836.2008.02953.x. [DOI] [PubMed] [Google Scholar]
- 4.Deanfield J, Donald A, Ferri C, et al. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:7–17. doi: 10.1097/00004872-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Jarvisalo MJ, Jartti L, Nanto-Salonen K, et al. Increased aortic intima-media thickness: a marker of preclinical atherosclerosis in high-risk children. Circulation. 2001;104(24):2943–7. doi: 10.1161/hc4901.100522. [DOI] [PubMed] [Google Scholar]
- 6.Murray C, Lopez A. The World Health Report 2002: Reducing risks, Promoting Healthy life. World Health Organization; Geneva, Switzerland: 2002. p. 230. [Google Scholar]
- 7.Virkola K, Pesonen E, Akerblom HK, Siimes MA. Cholesterol and carotid artery wall in children and adolescents with familial hypercholesterolemia: a controlled study by ultrasound. Acta Pediatr. 1997;86:1203–7. doi: 10.1111/j.1651-2227.1997.tb14847.x. [DOI] [PubMed] [Google Scholar]
- 8.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–9. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 10.Werner N, Kosiol S, T. S, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt-Lucke C, Rossig L, Fichtlscherer S, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–7. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 12.Vasa M, Fichtlscherer S, Adler K, et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–90. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 13.Imanishi T, Hano T, Matsuo Y, Nishio I. Oxidised Low-density lipoprotein inhibits vascular endothelial growth factor-induced endothelial progenitor cell differentiation. Clin Exp Pharmacol Physiol. 2003;30:665–670. doi: 10.1046/j.1440-1681.2003.03894.x. [DOI] [PubMed] [Google Scholar]
- 14.Imanishi T, Hano T, Sawamura T, Nishio I. Oxidised Low density lipoprotein induceds endothelial progenitor cell senescence, leading to cellular dysfunction. Clin Exp Pharmacol Physiol. 2004;31:407–13. doi: 10.1111/j.1440-1681.2004.04022.x. [DOI] [PubMed] [Google Scholar]
- 15.Croce G, Passacquale G, Necozione S, Ferri C, Desideri G. Nonpharmacological treatment of hypercholesterolemia increases circulating endothelial progenitor cell population in adults. Arterioscl Throm Vasc Biol. 2006;26:e38–9. doi: 10.1161/01.ATV.0000218504.71680.b5. [DOI] [PubMed] [Google Scholar]
- 16.Hoetzer GL, MacEneaney OJ, Irmiger HM, et al. Gender differences in circulating endothelial progenitor cell colony-forming capacity and migratory activity in middle-aged adults. Am J Cardiol. 2007;99:46–8. doi: 10.1016/j.amjcard.2006.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jie KE, Goossens MH, van Oostrom O, Lilien MR, Verhaar MC. Circulating endothelial progenitor cell levels are higher during childhood than in adult life. Atherosclerosis. 2009;202:345–7. doi: 10.1016/j.atherosclerosis.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 19.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–95. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minami E, Laflamme MA, Saffitz JE, Murry CE. Extracardiac progenitor cells repopulate most major cell types in the transplanted human heart. Circulation. 2005;8:19. doi: 10.1161/CIRCULATIONAHA.105.576017. [DOI] [PubMed] [Google Scholar]
- 21.Hagensen MK, Shim J, Thim T, Falk E, Bentzon JF. Circulating endothelial progenitor cells do not contribute to plaque endothelium in murine atherosclerosis. Circulation. 2010;121:898–905. doi: 10.1161/CIRCULATIONAHA.109.885459. [DOI] [PubMed] [Google Scholar]
- 22.Gulati R, Jevremovic D, Witt TA, et al. Modulation of the vascular response to injury by autologous blood-derived outgrowth endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H512–7. doi: 10.1152/ajpheart.00063.2004. [DOI] [PubMed] [Google Scholar]
- 23.Gehling UM, Ergun S, Fielder W. CFU-EC: How they were originally defined. Blood. 2007;110:1073. doi: 10.1182/blood-2007-03-081638. [DOI] [PubMed] [Google Scholar]
- 24.Werner N, Wassmann S, Ahlers P, et al. Endothelial progenitor cells correlate with endothelial function in patients with coronary artery disease. Basic Res Cardiol. 2007;102:565–71. doi: 10.1007/s00395-007-0680-1. [DOI] [PubMed] [Google Scholar]
- 25.Fadini GP, Coracina A, Baesso I, et al. Peripheral blood CD34+KDR+ endothelial progenitor cells are determinants of subclinical atherosclerosis in a middle-aged general population. Stroke. 2006;37:2277–82. doi: 10.1161/01.STR.0000236064.19293.79. [DOI] [PubMed] [Google Scholar]
- 26.Westerweel PE, Visseren FL, Hajer GR, et al. Endothelial progenitor cell levels in obese men with the metabolic syndrome and the effect of simvastatin monotherapy vs. simvastatin/ezetimibe combination therapy. Eur Heart J. 2008;29:2808–17. doi: 10.1093/eurheartj/ehn431. [DOI] [PubMed] [Google Scholar]
- 27.Tepper OM, Galiano RD, Capla JM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–6. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 28.Fadini G, de Kreutzenberg S, Agostini C, et al. Low CD34+ cell count and metabolic syndrome synergistically increase the risk of adverse outcomes. Atherosclerosis. 2009;207:213–9. doi: 10.1016/j.atherosclerosis.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 29.Woywodt A, Blann AD, Kirsch T, et al. Isolation and enumeration of circulating endothelial cells by immunomagnetic isolation: proposal of a definition and a consensus protocol. J Thromb Haemost. 2006;4:671–7. doi: 10.1111/j.1538-7836.2006.01794.x. [DOI] [PubMed] [Google Scholar]
- 30.Bulut D, Tüns H, Mügge A. CD31+/Annexin V+ microparticles in healthy offsprings of patients with coronary artery disease. Eur J Clin Invest. 2009;39:17–22. doi: 10.1111/j.1365-2362.2008.02058.x. [DOI] [PubMed] [Google Scholar]
- 31.Smadja DM, Gaussem P, Mauge L, et al. Circulating endothelial cells: a new candidate biomarker of irreversible pulmonary hypertension secondary to congenital heart disease. Circulation. 2009;119:374–81. doi: 10.1161/CIRCULATIONAHA.108.808246. [DOI] [PubMed] [Google Scholar]
- 32.Wiegman A, de Groot E, Hutten BA, et al. Arterial intima-media thickness in children heterozygous for familial hypercholesterolaemia. Lancet. 2004;363:369–70. doi: 10.1016/S0140-6736(04)15467-6. [DOI] [PubMed] [Google Scholar]
- 33.Smilde TJ, van WS, Wollersheim H, Kastelein JJ, Stalenhoef AF. Genetic and metabolic factors predicting risk of cardiovascular disease in familial hypercholesterolemia. Neth J Med. 2001;59:184–95. doi: 10.1016/s0300-2977(01)00155-3. [DOI] [PubMed] [Google Scholar]
- 34.Sorensen KE, Celermajer DS, Georgakopoulos D, Hatcher GD, Betteridge DJ, Deanfield JE. Impairment of endothelium-dependent dilation is an early event in children with familial hypercholesterolemia and is related to the lipoprotein(a) level. J Clin Invest. 1994;93:50–5. doi: 10.1172/JCI116983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walther C, Adams V, Bothur I, et al. Increasing physical education in high school students: effects on concentration of circulating endothelial progenitor cells. Eur J Cardiovasc Prev Rehabil. 2008;15:416–22. doi: 10.1097/HJR.0b013e3282fb2df1. [DOI] [PubMed] [Google Scholar]
- 36.Reilly JJ, Methven E, McDowell ZC, et al. Health consequences of obesity. Arch Dis Child. 2003;88:748–52. doi: 10.1136/adc.88.9.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charakida M, Donald AE, Green H, et al. Early structural and functional changes of the vasculature in HIV-infected children: impact of disease and antiretroviral therapy. Circulation. 2005;112:103–9. doi: 10.1161/CIRCULATIONAHA.104.517144. [DOI] [PubMed] [Google Scholar]
- 38.Kari JA, Donald AE, Vallance DT, et al. Physiology and biochemistry of endothelial function in children with chronic renal failure. Kidney Int. 1997;52:468–72. doi: 10.1038/ki.1997.354. [DOI] [PubMed] [Google Scholar]
- 39.Arrigoni FI, Matarin M, Thompson P, et al. Extended extraocular phenotype of PROM1 mutation in kindreds with known autosomal dominant macular dystrophy. Eur J Hum Genet. 2011;19:131–7. doi: 10.1038/ejhg.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donald AE, Halcox JP, Charakida M, et al. Methodological approaches to optimize reproducibility and power in clinical studies of flow-mediated dilation. J Am Coll Cardiol. 2008;51:1959–64. doi: 10.1016/j.jacc.2008.02.044. [DOI] [PubMed] [Google Scholar]