Abstract
Selective inhibitors of sodium-glucose cotransporter 2 (SGLT2)-mediated reabsorption of glucose in the proximal tubule of the kidney are being developed for the treatment of diabetes. SGLT2 shares high degree of homology with SGLT3; however, very little is known about the expression and functional role of SGLT3 in the human kidney. Indeed, the SGLT2 inhibitors that are currently in clinical trials might affect the expression and/or the activity of SGLT3. Therefore, the present study examined the expression of SGLT3 mRNA and protein in human kidney and in a human proximal tubule HK-2 cell line. The results indicated that human SGLT3 (hSGLT3) message and protein are expressed both in vivo and in vitro. We also studied the activity of hSGLT3 protein following its over-expression in mammalian kidney-derived COS-7 cells and in HK-2 cells treated with the imino sugar deoxynojirimycin (DNJ), a potent agonist of hSGLT3. Over-expression of hSGLT3 in COS-7 cells increased intracellular sodium concentration by 3-fold without affecting glucose transport. Activation of hSGLT3 with DNJ (50 μM) increased sodium uptake in HK-2 cells by 5.5 fold and this effect could be completely blocked with SGLT inhibitor phlorizin (50 μM). These results suggest that SGLT3 is expressed in human proximal tubular cells where it serves as a novel sodium transporter. Up-regulation of the expression of SGLT3 in the proximal tubule in diabetic patients may contribute to the elevated sodium transport in this segment of the nephron that has been postulated to promote hyperfiltration and renal injury.
Keywords: Human kidney, HK-2 cells, Proximal tubule, SGLT3, Sodium, Glucose
1. Introduction
A new class of drugs that inhibits glucose reabsorption in the kidney is being developed for the treatment of diabetes (DeFronzo et al., 2012). When blood is filtered at glomerulus of the kidney nephron, glucose leaves blood but then almost all of the filtered glucose is reabsorbed by the sodium-glucose cotransporters, SGLT1 and SGLT2, that are localized on the apical side of the proximal tubule cells (Wright et al., 2011). Genetic mutations in human (Santer and Calado, 2010) as well as gene knock out studies in mouse (Vallon et al., 2011) have revealed that SGLT2 is responsible for most of the glucose reabsorbed from the glomerular filtrate. Results from recent clinical trials have shown that treatment of diabetic patients with inhibitors of SGLT2 increases the urinary excretion of glucose and lowers the serum concentration of glucose and improves symptoms of diabetes (Abdul-Ghani et al., 2011; Chao and Henry, 2010). Although SGLT2 is highly homologous to SGLT3 (Wright et al., 2011), the effects of these drugs on activity or expression of SGLT3 in the kidney have not been studied previously.
Indeed, very little is known about the expression or activity of SGLT3 in the kidney. Human SGLT3 is encoded by SLC5A4 gene (Wright et al., 2011) and its transcript has previously been found in RNA obtained from a pool of adult human kidney tissues (Nishimura and Naito, 2005) and in cultured renal carcinoma cells (Veyhl et al., 1998). Mouse and rat both have two genes, SLC5A4a and SLC5A4b, (Pletcher et al., 2000) that encode SGLT3a and SGLT3b (Tabatabai et al., 2003). We have previously reported that both genes are expressed in the kidney of mouse (Tabatabai et al., 2003), and have found mRNA for SGLT3b in the rat kidney (GenBank DQ054787). In addition, we reported that SGLT3a and SGLT3b mRNAs are expressed in primary cultures of mouse kidney cells (Tabatabai et al., 2001, 2003). SGLT3 transcript has also been found in LLC-PK1, a proximal tubule cell line derived from pig (Clancey and Lever, 2000).
Human SGLT3 shares 70% amino acid sequence identity with SGLT1 (Diez-Sampedro et al., 2003) and has 55% homology with SGLT2. Despite sequence similarity, electrophysiological studies performed with human SGLT3 (hSGLT3) over-expressed in Xenopus oocytes suggested that this protein does not transport glucose but that it facilitated influx of Na+ in the presence of extracellular glucose (Diez-Sampedro et al., 2003). In these studies, SGLT3 was reported to have an apparent affinity (K0.5) for D-glucose of 19 mM, and the imino sugar deoxynojirimycin (DNJ) was identified as a potent activator of hSGLT3 with a K0.5 of 0.5 μM (Voss et al., 2007).
The expression of SGLT3 protein in the kidney has not been studied previously and very little is known about its role in kidney function. In the present study, we generated an antibody against hSGLT3 and determined whether SGLT3 protein is expressed in the human kidney and also examined its effects on intracellular sodium concentration in COS-7 cells expressing hSGLT3 and in human proximal tubule HK-2 cell line.
2. Materials and Methods
2.1. Chemicals
Deoxynojirimycin (DNJ), phlorizin, Hoechst 33258, and antibiotics were purchased from Sigma (St. Louis, MO). All other chemicals were molecular biology grade and were obtained from either Sigma or Thermo Fisher Scientific (Waltham, MA).
2.2. Human kidney tissue
Three previously collected tissues from kidney biopsies from different patients were obtained and maintained at −80 °C until use. These tissues were obtained without identifiers by following a protocol that had been approved by the Institutional Review Board Committee at the Medical College of Wisconsin.
2.3. Cell culture
Cell lines were obtained from American Type Culture Collection (Manassas, VA). HK-2 cells were cultured in Keratinocyte serum-free medium supplemented with bovine pituitary extract (0.05 mg/ml) and epidermal growth factor (5 ng/ml) (Kit Cat. No. 17005-04; Invitrogen, Carlsbad, CA). COS-7 cells were grown in Dulbecco’s Modified Eagle Medium containing 25 mM glucose (Cat. No. 11995-065; Invitrogen) that was supplemented with 25 mM Hepes and 10% fetal bovine serum (Atlanta Biologicals; Lawrenceville, GA). All media were supplemented with ampicillin (5,000 U/ml) and streptomycin (5 mg/ml). Cells were grown in 35 mm culture plates at 37 °C and 5% CO2 atmosphere in a humidified incubator.
2.4. Transient over-expressions of human SGLT1 and SGLT3
TrueORF cDNA clones SC119918 and SC304066 purchased from OriGene (Rockville, MD) were used to over-express hSGLT1 and hSGLT3 proteins in COS-7 cells. To propagate these constructs, vectors were transformed into E. coli JM109 competent cells (Promega; Madison, WI) and the transformed cells selected by resistance to ampicillin (100 μg/ml). Plasmid constructs were prepared using a kit from QIAGEN (Valencia, CA). COS-7 cells were transfected with each expression vector (1 μg per plate) using TurboFectin 8.0 transfection reagent and following the manufacturer’s instructions (OriGene). Cells treated with TurboFectin alone were used as a vehicle control and untreated cells were used as additional control. The cells were collected at different time points up to 48 hours post transfection and used in different experiments as described.
2.5. Gene expression
RNA was extracted from the human kidney samples by homogenization with a PowerGen 125 homogenizer (Fisher) in TRIzol® reagent and then total RNA was prepared using PureLink™ RNA Mini Kit (Invitrogen). Total RNA from HK-2 or COS-7 cells were prepared with RNeasy Mini kit from QIAGEN as described before (Tabatabai et al., 2001). All RNA preparations were subjected to on-the-column treatment with DNase I (QIAGEN). After quantification, RNA samples were used in reverse-transcription and polymerase chain reaction (RT-PCR) using protocols that we have previously described in detail (Tabatabai et al., 2001). Briefly, cDNA was prepared by incubating 1-3 μg total RNA with 0.5 μg Oligo(dT) primer and reverse transcribed with 50 units of Superscript II enzyme for 50 minutes at 42 °C in a total volume of 20 μl. As controls, duplicate reactions were set up without the RT enzyme. To amplify hSGLT3 cDNA, the sequences 5′-GTCGGAGAAAGAGCTCCTGA-3′ and 5′-CATCATCTGTTTCTTCCTGA-3′ (GenBank No. NM_014227) were selected as forward and reverse primers and synthesized by Integrated DNA Technologies (Coralville, IA). All PCRs contained 2 μl of the RT reaction and 0.2 μM of the forward and reverse primers in 50 μl volume. For regular PCR, Platinum Taq DNA polymerase (Invitrogen) was used and reactions were heated to 94 °C for 2 min followed by up to 45 cycles of 94 °C for 30 s, 61 or 56 °C for 30 s, 72 °C for 55 s, and then heated at 72 °C for additional 5 min in a GeneAmp 9700 thermocycler (Applied Biosystems; Foster City, CA). For real time PCR, Power SYBR® Green (Applied Biosystems) was used and reactions were heated to 95 °C for 10 min followed by 40 cycles of 95 °C for 30 s, 61 °C for 1 min, 72 °C for 55 s in Stratagene Mx3000P thermocycler (Santa Clara, CA).
The PCR products were separated by agarose gel electrophoresis, stained with ethidium bromide, and documented with Kodak EDAS 290 System. The sequence of the hSGLT3 cDNAs from human tissue and COS-7 samples were determined by Functional Biosciences, Inc. (Madison, WI) and that from HK-2 cells had previously been determined at a Core facility at the Medical College of Wisconsin. Sequence analysis was performed with software programs ClustalW2 (ebi.ac.uk/Tools/msa/clustalw2), MultAlin (multalin.toulouse.inra.fr), and BLAST (ncbi.nlm.nih.gov/Blast.cgi).
2.6. Protein expression
Total kidney proteins were prepared by homogenizing tissue with a glass Dounce homogenizer in RIPA buffer [50mM Tris HCl pH 8, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS] containing 1% (V/V) protease inhibitor cocktail (Cat. No. P8340; Sigma). After centrifugation, the supernatant was collected and protein concentration was determined by Bio-Rad DC Protein Assay (Hercules, CA). Total proteins from HK-2 or COS-7 were prepared by direct lysis of cells on culture plates in a buffer containing 0.063 M Tris-HCl (pH 6.8), 8.0 M urea, 2% SDS, 5% β mercaptoethanol, and 10% glycerol with bromophenol blue.
Human SGLT1 (hSGLT1) and hSGLT3 polyclonal antibodies were raised in rabbits against synthetic peptides C-Ahx-ENIQEGPKETIEIETQVP and C-Ahx-EKSQEETDDGVEEDYPEKSRG, respectively, and affinity purified (21st Century Biochemicals, Inc.; Marlboro, MA). These antibodies were used in Western blot experiments. Briefly, total proteins from human kidney tissues (100 μg) or cell lysates (4-15 μl) were separated on Novex 4-12% Bis-Tris polyacrylamide gels (Invitrogen) and then transferred onto Immobilion-FL (Millipore; Billerica, MA) or Immun-Blot (BioRad) PVDF membranes. Blots were then blocked in casein blocking buffer (LI-COR; Lincoln, Nebraska). Following overnight incubation at 4 °C with hSGLT1 or hSGLT3 primary antibodies diluted to 1-2 μg/ml in hybridization buffer (blocking buffer containing 0.1% Tween 20) the blots were incubated for 1 hour at room temperature with LI-COR IRDye® 800CW conjugated goat anti-rabbit IgG secondary antibody (Cat. No. 827-08365) diluted to 10,000 in hybridization buffer. Infrared (IR) signals were detected at 800 nm by LI-COR Odyssey IR Imaging System. To re-probe with actin, the blots were rehydrated by brief exposure to methanol, rinsed in PBS containing 0.1% Tween, and then incubated for 1 hour at room temperature with 1:5,000 dilution of actin sc-1616-R antibody (Santa Cruz Biotechnology; Santa Cruz, CA). Detection was carried out using 1:10,000 dilution of IRDye 680 conjugated goat anti-rabbit IgG (Cat. No. 926-32221, LI-COR); and the signal was detected at 700 nm.
2.7. 2-NBDG uptake
We have previously described in detail a method for fluorescence measurement of glucose uptake using 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG; Invitrogen) (Blodgett et al., 2011). 2-NBDG uptake experiments were performed on COS-7 cells transfected for 48 hours with vector for over-expression of hSGLT3 or hSGLT1. Cells treated with TurboFectin alone and untreated cells were used as additional controls. Three culture plates were used for each uptake experiment. Briefly, the media was removed and the cells were incubated for one hour at 37 °C with 100 μM 2-NBDG in Na+ buffer containing (in mM) [140 NaCl, 5 KCl, 2.5 CaCl2, 1 MgSO4, 1 KH2PO4, and 10 Hepes (pH 7.4)]. Uptake was also performed with 2-NBDG (100 μM) in Na+-free buffer containing equal molar choline chloride. Cell homogenates were prepared and fluorescence was measured respectively at excitation and emission wavelengths of 485 and 528 nm in BioTek Synergy HT microplate reader (Winooski, VT). Cellular DNA content was used for normalization. DNA was measured by addition of Hoechst stain (1 μg/ml) to the homogenates and fluorescence was measured using excitation and emission wavelengths of 360 and 460 nm, respectively. Cellular concentrations of 2-NBDG and DNA were quantified from standard curve graphs and the intracellular level of 2-NBDG, [2-NBDG]i, was expressed as μM 2-NBDG/μg DNA. 2-NBDG transported in presence of Na+ is considered to be total uptake, which is the sum of contributions from Na+-dependent (SGLT) and Na+-independent (GLUT) transport activities. The difference between the total and the Na+-independent uptakes was taken as the contribution from Na+-dependent transport.
2.8.1. Intracellular concentration of Na+ in hSGLT3-expressing COS-7 cells
Intracellular Na+ concentration, [Na+]i, was measured by using the fluorescent Na+ indicator dye CoroNa™ Green. Three culture plates were used per condition. COS-7 cells were either transfected with hSGLT3 expression vector, treated with transfection reagent alone, or left untreated as above. After 48 hours, the cells were rinsed in a Na+-free buffer containing (in mM) [140 choline chloride, 2 KCl, 1 MgCl2, 1 CaCl2, 10 Hepes-Tris (pH 7.4)]. The cells from three plates were collected in 600 μl of 20 mM Tris buffer (pH 7.4) and homogenized by sonication using Omni-Ruptor 250 Ultrasonic Homogenizer (OMNI International; Kennesaw, GA). The homogenates were centrifuged at 18,000g for 15 minutes at 4 °C and the supernatants were collected. CoroNa™ Green (Cat. No. C36675, Invitrogen) was added to supernatants at the final concentration of 16 μM and fluorescence was measured using excitation and emission wavelengths of 485 and 528 nm in microplate reader. The concentrations of Na+ in the homogenates were determined from standard curves generated over the range of 0.1 to 1 mM NaCl.
The Na+ concentration was normalized to the amount of DNA. The DNA concentration of the cell homogenates was determined by diluting aliquots of the sample in TNE buffer [10 mM Tris (pH 7.4), 200 mM NaCl, 1 mM EDTA]. Hoechst stain was then added (1 μg/ml) and fluorescence was measured as described above. Standard curves were generated using 1 to 5 μg/ml calf thymus DNA in TNE.
2.8.2. Measurement of Na+ uptake in HK-2 cells
HK-2 cultures were grown until they were about 80% confluent. The media was then removed and plates were rinsed in Na+-free buffer. Baseline level of Na+ uptake was measured by incubating cells for 20 minutes at 37 °C in Na+ containing buffer. Cells were also incubated in Na+ buffer containing 50 μM of hSGLT3 agonist, deoxynojirimycin (DNJ), in presence or absence of 50 μM phlorizin. After incubation, the plates were rinsed in Na+-free buffer and [Na+]i was determined as described above.
2.9. Statistical analysis
Mean values ± standard error are presented. ANOVA with Holm-Sidak post hoc test and all other data analyses were performed with SigmaPlot 11.2 (Systat Software, Inc.; San Jose, CA).
3. Results
3.1. Human SGLT3 expression controls
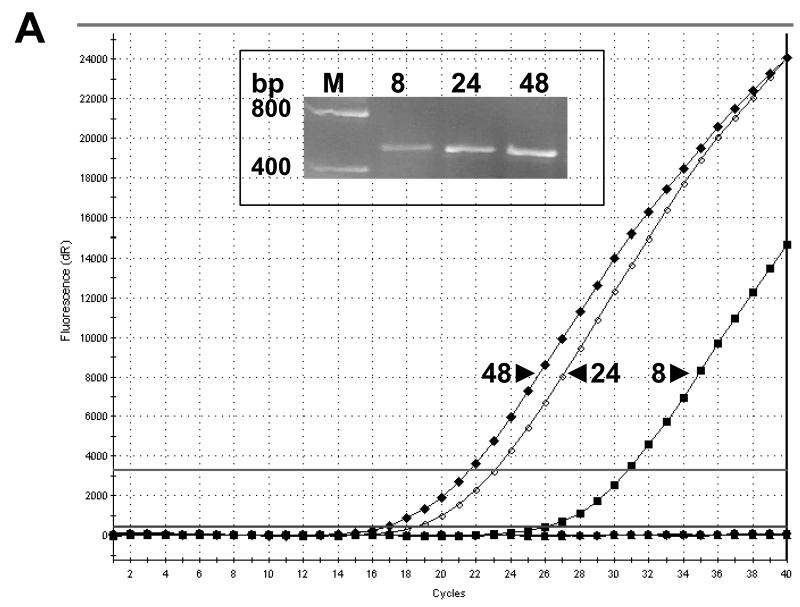
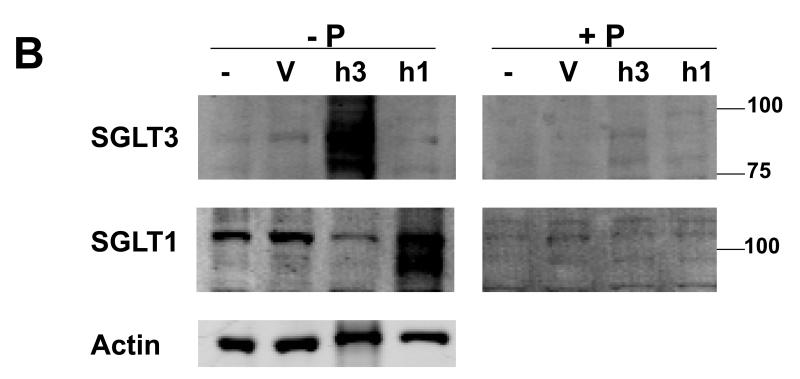
COS-7 cells that were transfected with a commercially available vector to express either hSGLT3 or hSGLT1 protein were used to validate the specificity of an hSGLT3 antibody that we generated. We first examined the expression of hSGLT3 mRNA at various time following transfection of the cells using real time PCR with hSGLT3 primers (Fig. 1A). The results indicate that the expression of hSGLT3 mRNA is relatively low in COS-7 cells 8 hours after transfection and that the levels reach a peak 48 hours post transfection. Gel analysis of these reactions showed a product with the expected size of ~0.5 kb (Fig. 1A inset), and DNA sequencing confirmed that the amplified product exhibited 100% homology with hSGLT3 (not shown). Similar experiments were performed using COS-7 cells transfected with hSGLT1 over-expression vector. The activity and specificity of the hSGLT1 and hSGLT3 antibodies were then examined in Western blot experiments with whole cell lysates extracted from COS-7 cells expressing hSGLT1 (h1) and hSGLT3 (h3). The results of these experiments are presented in Fig. 1B. The SGLT3 antibody produced a strong signal from hSGLT3 transfected cells but not from cells transfected with hSGLT1 or control cells treated with the vehicle or untreated controls. The SGLT1 antibody reacted strongly with hSGLT1 transfected cells but only background bands were observed in samples obtained from hSGLT3-expressing cells and controls. Incubation of each of the antibodies with its respective antigenic peptide abolished the signal.
Fig. 1. Human SGLT1 and SGLT3 specific antibodies.
COS-7 cells transiently transfected with vectors for over-expression of hSGLT1 (h1) or hSGLT3 (h3) were used to examine the reactivity and specificity of antibodies. (A) Total RNA samples were prepared 8, 24, and 48 hours post transfection and used in RT-real time PCR with hSGLT3-specific primers. Amplification profiles are shown in the graph and gel electrophoresis analysis of PCR products are shown in the inset. M, DNA size marker; bp, base pairs. (B) Forty eight hours post transfection with h1 or h3 vectors, total cell lysates were prepared from COS-7 cells and used in Western blot. Cell lysates from transfection vehicle (V) and untreated (−) cells were used as controls. Blots were probed with 1 μg/ml of hSGLT1 or hSGLT3 antibody and infrared (IR) signals were detected at 800 nm; -P, antibody alone. Above experiments were repeated with peptide-blocked antibodies; +P. Blots were re-probed with actin antibody and IR detection was carried out at 700 nm. Positions of MW size markers are shown.
3.2.1. SGLT3 expression in the human kidney
Human tissue samples were used to investigate the in vivo expression of SGLT3 in the kidney. Total RNA was prepared from the kidneys of individuals designated as K1, K2, and K3, and used in RT-PCR experiments. PCR using the RT reactions from K1, K2, and K3 tissue RNA produced a band with the expected size of ~0.5 kb in all samples. BLAST analysis on nucleotide sequences from these cDNA products confirmed that all three had the expected sequence of the SLC5A4 gene (not shown). A partial sequence from K1 encodes peptide sequence encompassing P442 to F486 (not shown) and that from K2 (GenBank No. JQ340171) and K3 (GenBank No. JQ519903) encode A425 to K578 of human SGLT3. Western blot analysis was performed on homogenates prepared from K1, K2, and K3 tissues to investigate the expression of SGLT3 protein. The SGLT3 antibody produced a band with MW size similar to the estimated 72 kDa size of hSGLT3 (Fig. 2A). Another band with MW size of ~80 kDa was also observed. Incubation of SGLT3 antibody with the antigen peptide abolished both the 72 and 80 kDa signals (Fig. 2A). Post-translational modification such as glycosylation has been shown to affect migration of SGLT1 in denaturing gels (Wright et al., 2011). The predicted glycosylation site in hSGLT1 is conserved in hSGLT3 (Wright et al., 2011); therefore, this modification may explain the appearance of a slow migrating hSGLT3.
Fig. 2. SGLT3 expression in human kidney tissue and in human proximal tubule HK-2 cell line.
Total proteins were prepared from human kidney tissues designated as K1, K2 and K3, and whole cell lysates were prepared from cultures of HK-2 cells. (A) To examine SGLT3 expression, Western blot was performed with 100 μg of human kidney total proteins and 15 μl of HK-2 whole cell lysates. Blot was probed with SGLT3 antibody (2 μg/ml) and IR signal was detected at 800 nm. (B) Western blot was repeated with SGLT1 antibody (1 μg/ml). P; antibodies pre-incubated with antigen peptides were used. Positions of MW size markers are shown.
3.2.2. SGLT1 in SGLT3-expressing human kidney tissues
Western blot analysis on tissue proteins was repeated to examine whether SGLT1 was also present. Probing the kidney protein blot with SGLT1 antibody resulted in a band with MW size of ~100 kDa (Fig. 2B). This signal could be blocked by pre-incubating the antibody with the antigenic peptide.
3.3.1. SGLT3 expression in HK-2 cells
We also examined whether SGLT3 is expressed in HK-2 cells as a model of human proximal tubule cells. Amplification of cDNA from RNA extracted from HK-2 cells produced a PCR product with the anticipated size of ~0.5 kb (not shown). The sequence of the PCR product was determined and submitted to the GenBank (No. GQ292716). BLAST analysis confirmed that this sequence is identical to that of the sequence encompassing nucleotides 1273 to 1737 of human SLC5A4 mRNA (GenBank No. NM_014227). Western blot analysis was performed on whole cell lysate isolated from HK-2 cells. Similar to the results with the human kidney, a band with MW size of ~72 kDa was observed when blot was probed with SGLT3 antibody (Fig. 2A). A ~80 kDa band was also present in HK-2 protein sample. Both the 72 and the 80 kDa bands were abolished when antibody was pre-incubated with the antigenic peptide.
3.3.2. SGLT1 expression in HK-2 cells
Expression of hSGLT1 has not been previously reported in HK-2 cells. When HK-2 whole cell lysate protein blot was probed with hSGLT1 antibody, a signal with the MW size of ~100 kDa was detected (Fig. 2B), and the signal was not present when the SGLT1 antibody was pre-incubated with the antigenic peptide.
3.4.1. Human SGLT3 expressed in COS-7 cells does not transport glucose
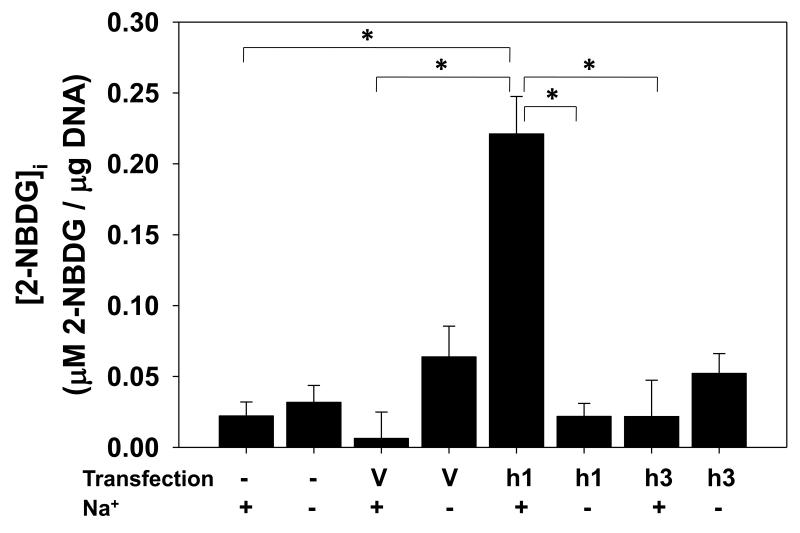
Activity of hSGLT3 has not previously been examined in a mammalian cell expression system. We over-expressed hSGLT3 in COS-7 cells (Fig. 1B) and examined its glucose transport activity by fluorescent 2-NBDG (Blodgett et al., 2011). Cells expressing hSGLT1 (Fig. 1B) served as controls for 2-NBDG uptake. The results of these experiments presented in Fig. 3 indicate that the intracellular concentration of 2-NBDG, [2-NBDG]i, was averaged 0.02 μM/μg DNA in control cells and was increased by 10-fold to 0.2 μM/μg DNA in cells expressing hSGLT1. In contrast, [2-NBDG]i did not increase in cells expressing hSGLT3 and remained 0.02 μM/μg DNA. In the absence of Na+, 2-NBDG uptake in hSGLT1 and hSGLT3 expressing COS-7 cells were both similar to controls.
Fig. 3. Human SGLT3 over-expressed in COS-7 cells does not transport 2-NBDG.
COS-7 cells transfected for 48 hours with vectors for over-expression of hSGLT1 (h1) or hSGLT3 (h3) were incubated at 37 °C with 2-NBDG (100 μM) in Na+ and Na+-free uptake buffers. Cells treated with transfection vehicle (V) and untreated cells (−) were used as controls. After 1 hour, cells were collected and intracellular concentration of 2-NBDG was measured by fluorescence spectroscopy and values were normalized to the amount of DNA (N = 3-5); Na+-dependent uptake, (+); Na+-independent uptake, (−). * represents P < 0.001.
3.4.2. Expression of human SGLT3 increases intracellular sodium in COS-7 cells
We also examined the effect of over-expression of hSGLT3 on intracellular Na+ concentration, [Na+]i, in COS-7 cells. The results indicate that the basal [Na+]i in COS-7 culture was 41±13 μM/μg DNA and that [Na+]i was 3-fold higher in cells expressing hSGLT3 (Fig. 4).
Fig. 4. Over-expression of hSGLT3 in COS-7 cells increases intracellular level of Na+.
COS-7 cells were either transfected with a hSGLT3 over-expression vector (h3), treated with the transfection reagent vehicle (V), or left untreated (−). After 48 hours, intracellular Na+ concentration was measured with CoroNa Green fluorescent dye and values were normalized to the amount of DNA (N = 6-7). *, P = 0.02 between hSGLT3-expressing cells and controls.
3.5. SGLT3 activity in HK-2 cells
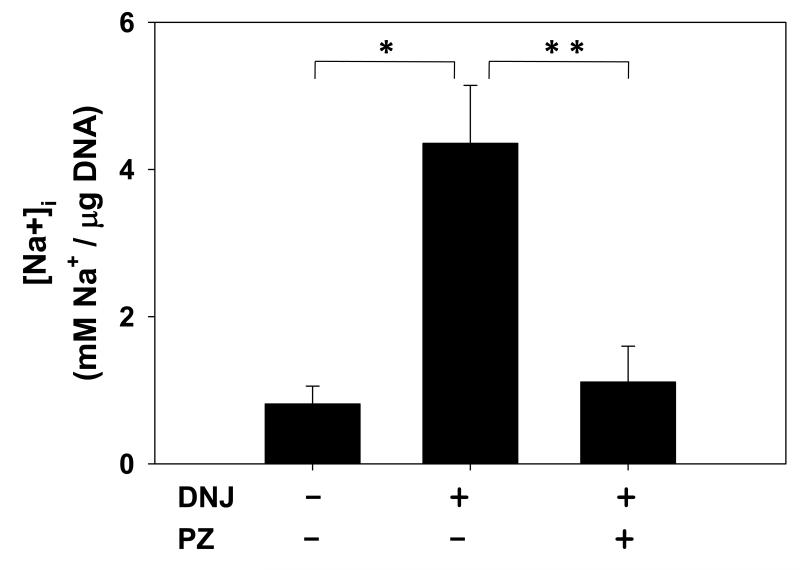
Deoxynojirimycin (DNJ) is a potent agonist of hSGLT3 (Voss et al., 2007). We used DNJ in sodium uptake experiments to investigate the role of this protein on intracellular Na+ levels in HK-2 cell line. Baseline [Na+]i in untreated cells averaged 0.8±0.2 mM/μg DNA (Fig. 5). Exposure of cells to DNJ strongly stimulated Na+ uptake in HK-2 cells and increased [Na+]i from 0.8 to 4.4±0.8 mM/μg DNA in 20 minutes. Co-exposure of cells to phlorizin (50 μM) completely blocked the effect of DNJ on Na+ uptake.
Fig. 5. DNJ stimulates Na+ uptake in HK-2 cells.
Cultures of HK-2 cells were incubated at 37 °C for 20 minutes in Na+ uptake buffer with or without 50 μM DNJ. Cells were also co-treated with DNJ (50 μM) and phlorizin (PZ, 50 μM) in Na+ buffer. Intracellular level of Na+ was measured and was normalized to the amount of DNA (N = 5-11). P < 0.001 and P = 0.006 are represented by * and **, respectively.
4. Discussion
Glucose reabsorption occurs in the proximal tubule and is mediated by Na+-dependent transporters SGLT1 and SGLT2 (Wright et al., 2011). Absorbed glucose then diffuses back into the blood from basolateral side of cells via GLUT transporters in a Na+-independent manner (Wright et al., 2011). Humans with mutations in SLC5A1 gene encoding SGLT1 suffer from an intestinal disease known as glucose-galactose malabsorption but have little or no sign of glucosuria (Soylu et al., 2008; Wright et al., 2011; Xin and Wang, 2011). However, people with mutations in SLC5A2 gene encoding SGLT2 develop severe glucosuria (Lee et al., 2012; Santer and Calado, 2010; Yu et al., 2011) with urinary excretions of glucose as high as 160 g per day (Bakris et al., 2009). Despite massive glucosuria, these patients appear not to suffer from other symptoms (Calado et al., 2011). Unique localization of SGLT2 in the early S1 and S2 segment of the proximal tubule and its major role in reabsorption of glucose were crucial factors in the development of a new class of drugs that specifically inhibit SGLT2, but not SGLT1, activity (Hardman and Dubrey, 2011). Based on sequence homology, human SLC5A4, which is commonly known as SGLT3, is highly similar to SGLT1 and SGLT2 (Wright et al., 2011). The effects of these drugs on SGLT3 expression or activity have not been considered in the development of the SGLT2 inhibitors for diabetes since very little is known about expression of this protein in the human kidney or its function.
Human SGLT3 is considered to have unique transport properties. Previous studies with human SGLT3 expressed in Xenopus oocytes suggested that this protein does not transport glucose (Diez-Sampedro et al., 2003). Inability of hSGLT3 to transport glucose is suggested to be related to single amino acid variation at position 457 of hSGLT3 as compared to SGLT1 or SGLT2 (Bianchi and Diez-Sampedro, 2010). Site directed mutagenesis of glutamate (E) to glutamine (Q) at the position of 457 restores glucose transport function (Bianchi and Diez-Sampedro, 2010). In our study, the SGLT3 mRNA sequences from three human kidney tissues and from human proximal tubule HK-2 cell line also show the conservation of E457 codon. Other electrophysiological studies in Xenopus oocytes suggested that hSGLT3 appears to be a glucose-stimulated Na+ channel (Diez-Sampedro et al., 2003), but no studies have been done to confirm this in a mammalian expression system or human kidney cells. We examined hSGLT3 activity by transiently over-expressing this protein in a mammalian kidney-derived cell line COS-7 cells, and then measured glucose uptake and the intracellular level of Na+. We found that expression of hSGLT3 protein in COS-7 cells (Fig. 1B) had no effect on Na+-dependent or – independent uptakes of 2-NBDG, a fluorescent derivative of glucose (Fig. 3). We had previously shown Na+-dependent transport of 2-NBDG by mouse SGLT1 and SGLT2 expressed in COS-7 cells (Blodgett et al., 2011) and in the present study we showed similar results with COS-7 cells that over-expressed human SGLT1 (Fig. 3). In addition, expression of hSGLT3 in COS-7 cells markedly increased intracellular sodium levels (Fig. 4). These results are consistent with the view that SGLT3 does not transport glucose in mammalian cells but serves as a glucose-stimulated Na+ transporter.
HK-2 is a proximal tubule cell line which was isolated from a human normal kidney (Ryan, 1994). Here, we showed that SGLT1 is expressed in these cells (Fig. 2B). This result provides additional support that HK-2 culture has properties similar to the proximal tubule cells in the kidney. Findings of SGLT3 mRNA and protein expression in this in vitro model of human proximal tubule cells, together with their in vivo expression along with SGLT1 in the human kidney (Fig. 2) provide further evidence for proximal tubule localization of hSGLT3.
Membrane depolarization in Xenopus oocytes over-expressing hSGLT3 was stimulated in response to μM amounts of imino sugar deoxynojirimycin (DNJ) while mM concentrations of DNJ had no effect on membrane depolarization in SGLT1-expressing cells (Voss et al., 2007). We used this potent agonist of hSGLT3 to assess SGLT3 activity in HK-2 cells. Our results indicate that 20 minute exposure of cells to DNJ (50 μM) stimulated Na+ uptake by 5.5 fold (Fig. 5). Phlorizin inhibits the activity of hSGLT3 over-expressed in Xenopus oocytes (Diez-Sampedro et al., 2003), and it also blocked the stimulatory effect of DNJ on the uptake of Na+ in HK-2 cells (Fig. 5). These results are consistent with Na+ transport activity of SGLT3 in HK-2 cells.
Enhanced proximal tubule sodium reabsorption has been observed in diabetic patients (Hannedouche et al., 1990; O’Hagan et al., 1991; Pruijm et al., 2010) and this may contribute to the increased incidence of salt-sensitive hypertension in diabetic patients. A role for SGLT in hyperglycemia-induced increased absorption of sodium in the early proximal tubule has been demonstrated in animal models of diabetes (Bank and Aynedjian, 1990; Kumar et al., 1988; Pollock et al., 1991). Consistently, the delivery of sodium to the late proximal and distal tubule of streptozotocin (STZ)-induced diabetic rats have been found to be lower than that seen in non-diabetic controls and administration of phlorizin restores normal delivery of sodium to the distal nephron (Pollock et al., 1991; Vallon et al., 1999). It has been proposed that the development of glomerular hyperfiltration in diabetic patients can be the result of decreased sodium delivery to macula densa and reduced activation of tubulo-glomerular feedback (Vervoort et al., 2005; Vallon and Thomson, 2012). Indeed, Thomson et al. (2012) recently reported that single nephron glomerular filtration rate (SNGFR) was elevated in STZ-treated diabetic animals and that administration of an SGLT2 inhibitor to inhibit Na+-glucose transport in the proximal tubule increased distal delivery of fluid and prevented the hyperfiltration in this model. The results from this study now indicate that the expression of SGLT3 is elevated in the proximal tubule in diabetic animals and are consistent with the hypothesis that SGLT3 may also play a role in enhancing early proximal tubule sodium absorption and modulating renal hemodynamics via changes in tubulo-glomerular feedback.
In summary, present study indicates that SGLT3 is expressed in the human kidney and HK-2 cells and that over-expression of hSGLT3 in COS-7 cells or treatment of HK-2 cells with an activator of hSGLT3 increases intracellular levels of Na+. These findings support the view that SGLT3 serves as a novel Na+ transporter in the proximal tubule. Up-regulation of the expression of SGLT3 in the proximal tubule in diabetic patients may contribute to the elevated sodium transport in this segment of the nephron that has been postulated to promote hyperfiltration and renal injury.
Given the high degree of homology of SGLT3 and SGLT2, the effects of the new anti- SGLT2 drugs on activity and expression of SGLT3 in the human kidney should be considered as a potential non-specific target that may contribute to the effects of this class of drugs on sodium transport.
Acknowledgements
This study was supported by funds from the National Institutes of Health grants R01DK085031 (to NMT) and HL36279 (to RJR). Additional funds were provided by a Pilot and Feasibility grant (to NMT), that was awarded by the parent grant P50DK079306. The current affiliation for A.B. Blodgett is U.S. Forest Service, Madison, WI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdul-Ghani MA, Norton L, DeFronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr.Rev. 2011;32:515–531. doi: 10.1210/er.2010-0029. [DOI] [PubMed] [Google Scholar]
- Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int. 2009;75:1272–1277. doi: 10.1038/ki.2009.87. [DOI] [PubMed] [Google Scholar]
- Bank N, Aynedjian HS. Progressive increases in luminal glucose stimulate proximal sodium absorption in normal and diabetic rats. J.Clin.Invest. 1990;86:309–316. doi: 10.1172/JCI114700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi L, Diez-Sampedro A. A single amino acid change converts the sugar sensor SGLT3 into a sugar transporter. PLoS One. 2010;5:e10241. doi: 10.1371/journal.pone.0010241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blodgett AB, Kothinti RK, Kamyshko I, Petering DH, Kumar S, Tabatabai NM. A fluorescence method for measurement of glucose transport in kidney cells. Diabetes Technol.Ther. 2011;13:743–751. doi: 10.1089/dia.2011.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calado J, Santer R, Rueff J. Effect of kidney disease on glucose handling (including genetic defects) Kidney Int.Suppl. 2011:S7–13. doi: 10.1038/ki.2010.510. [DOI] [PubMed] [Google Scholar]
- Chao EC, Henry RR. SGLT2 inhibition--a novel strategy for diabetes treatment. Nat.Rev.Drug Discov. 2010;9:551–559. doi: 10.1038/nrd3180. [DOI] [PubMed] [Google Scholar]
- Clancey CJ, Lever JE. Differential regulation of three glucose transporter genes in a renal epithelial cell line. J.Cell Physiol. 2000;185:244–252. doi: 10.1002/1097-4652(200011)185:2<244::AID-JCP9>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Davidson JA, Del PS. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes.Metab. 2012;14:5–14. doi: 10.1111/j.1463-1326.2011.01511.x. [DOI] [PubMed] [Google Scholar]
- Diez-Sampedro A, Hirayama BA, Osswald C, Gorboulev V, Baumgarten K, Volk C, Wright EM, Koepsell H. A glucose sensor hiding in a family of transporters. Proc.Natl.Acad.Sci.U.S.A. 2003;100:11753–11758. doi: 10.1073/pnas.1733027100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorboulev V, Schurmann A, Vallon V, Kipp H, Jaschke A, Klessen D, Friedrich A, Scherneck S, Rieg T, Cunard R, Veyhl-Wichmann M, Srinivasan A, Balen D, Breljak D, Rexhepaj R, Parker HE, Gribble FM, Reimann F, Lang F, Wiese S, Sabolic I, Sendtner M, Koepsell H. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes. 2012;61:187–196. doi: 10.2337/db11-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannedouche TP, Delgado AG, Gnionsahe DA, Boitard C, Lacour B, Grunfeld JP. Renal hemodynamics and segmental tubular reabsorption in early type 1 diabetes. Kidney Int. 1990;37:1126–1133. doi: 10.1038/ki.1990.95. [DOI] [PubMed] [Google Scholar]
- Hardman TC, Dubrey SW. Development and potential role of type-2 sodium-glucose transporter inhibitors for management of type 2 diabetes. Diabetes Ther. 2011;2:133–145. doi: 10.1007/s13300-011-0004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AM, Gupta RK, Spitzer A. Intracellular sodium in proximal tubules of diabetic rats. Role of glucoseKidney Int. 1988;33:792–797. doi: 10.1038/ki.1988.69. [DOI] [PubMed] [Google Scholar]
- Lee H, Han KH, Park HW, Shin JI, Kim CJ, Namgung MK, Kim KH, Koo JW, Chung WY, Lee DY, Kim SY, Cheong HI. Familial renal glucosuria: a clinicogenetic study of 23 additional cases. Pediatr.Nephrol. 2012 doi: 10.1007/s00467-012-2109-9. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug Metab Pharmacokinet. 2005;20:452–477. doi: 10.2133/dmpk.20.452. [DOI] [PubMed] [Google Scholar]
- O’Hagan M, Howey J, Greene SA. Increased proximal tubular reabsorption of sodium in childhood diabetes mellitus. Diabet.Med. 1991;8:44–48. doi: 10.1111/j.1464-5491.1991.tb01515.x. [DOI] [PubMed] [Google Scholar]
- Pletcher MT, Roe BA, Chen F, Do T, Do A, Malaj E, Reeves RH. Chromosome evolution: the junction of mammalian chromosomes in the formation of mouse chromosome 10. Genome Res. 2000;10:1463–1467. doi: 10.1101/gr.146600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock CA, Lawrence JR, Field MJ. Tubular sodium handling and tubuloglomerular feedback in experimental diabetes mellitus. Am.J.Physiol. 1991;260:F946–F952. doi: 10.1152/ajprenal.1991.260.6.F946. [DOI] [PubMed] [Google Scholar]
- Pruijm M, Wuerzner G, Maillard M, Bovet P, Renaud C, Bochud M, Burnier M. Glomerular hyperfiltration and increased proximal sodium reabsorption in subjects with type 2 diabetes or impaired fasting glucose in a population of the African region. Nephrol.Dial.Transplant. 2010;25:2225–2231. doi: 10.1093/ndt/gfq008. [DOI] [PubMed] [Google Scholar]
- Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994;45:48–57. doi: 10.1038/ki.1994.6. [DOI] [PubMed] [Google Scholar]
- Santer R, Calado J. Familial renal glucosuria and SGLT2: from a mendelian trait to a therapeutic target. Clin.J.Am.Soc.Nephrol. 2010;5:133–141. doi: 10.2215/CJN.04010609. [DOI] [PubMed] [Google Scholar]
- Soylu OB, Ecevit C, Altinoz S, Ozturk AA, Temizkan AK, Maeda M, Kasahara M. Nephrocalcinosis in glucose-galactose malabsorption: nephrocalcinosis and proximal tubular dysfunction in a young infant with a novel mutation of SGLT1. Eur.J.Pediatr. 2008;167:1395–1398. doi: 10.1007/s00431-008-0681-6. [DOI] [PubMed] [Google Scholar]
- Tabatabai NM, Blumenthal SS, Lewand DL, Petering DH. Differential regulation of mouse kidney sodium-dependent transporters mRNA by cadmium. Toxicol.Appl.Pharmacol. 2001;177:163–173. doi: 10.1006/taap.2001.9321. [DOI] [PubMed] [Google Scholar]
- Tabatabai NM, Blumenthal SS, Lewand DL, Petering DH. Mouse kidney expresses mRNA of four highly related sodium-glucose cotransporters: regulation by cadmium. Kidney Int. 2003;64:1320–1330. doi: 10.1046/j.1523-1755.2003.00201.x. [DOI] [PubMed] [Google Scholar]
- Thomson SC, Rieg T, Miracle C, Mansoury H, Whaley J, Vallon V, Singh P. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am.J.Physiol Regul.Integr.Comp Physiol. 2012;302:R75–R83. doi: 10.1152/ajpregu.00357.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V, Richter K, Blantz RC, Thomson S, Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J.Am.Soc.Nephrol. 1999;10:2569–2576. doi: 10.1681/ASN.V10122569. [DOI] [PubMed] [Google Scholar]
- Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, Koepsell H, Rieg T. SGLT2 mediates glucose reabsorption in the early proximal tubule. J.Am.Soc.Nephrol. 2011;22:104–112. doi: 10.1681/ASN.2010030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V, Thomson SC. Renal function in diabetic disease models: the tubular system in the pathophysiology of the diabetic kidney. Annu.Rev.Physiol. 2012;74:351–375. doi: 10.1146/annurev-physiol-020911-153333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervoort G, Veldman B, Berden JH, Smits P, Wetzels JF. Glomerular hyperfiltration in type 1 diabetes mellitus results from primary changes in proximal tubular sodium handling without changes in volume expansion. Eur.J.Clin.Invest. 2005;35:330–336. doi: 10.1111/j.1365-2362.2005.01497.x. [DOI] [PubMed] [Google Scholar]
- Veyhl M, Wagner K, Volk C, Gorboulev V, Baumgarten K, Weber WM, Schaper M, Bertram B, Wiessler M, Koepsell H. Transport of the new chemotherapeutic agent beta- D-glucosylisophosphoramide mustard (D-19575) into tumor cells is mediated by the Na+-D- glucose cotransporter SAAT1. Proc.Natl.Acad.Sci.U.S.A. 1998;95:2914–2919. doi: 10.1073/pnas.95.6.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss AA, Diez-Sampedro A, Hirayama BA, Loo DD, Wright EM. Imino sugars are potent agonists of the human glucose sensor SGLT3. Mol.Pharmacol. 2007;71:628–634. doi: 10.1124/mol.106.030288. [DOI] [PubMed] [Google Scholar]
- Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91:733–794. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- Xin B, Wang H. Multiple sequence variations in SLC5A1 gene are associated with glucose-galactose malabsorption in a large cohort of Old Order Amish. Clin.Genet. 2011;79:86–91. doi: 10.1111/j.1399-0004.2010.01440.x. [DOI] [PubMed] [Google Scholar]
- Yu L, Lv JC, Zhou XJ, Zhu L, Hou P, Zhang H. Abnormal expression and dysfunction of novel SGLT2 mutations identified in familial renal glucosuria patients. Hum.Genet. 2011;129:335–344. doi: 10.1007/s00439-010-0927-z. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ng CM, List JF, Pfister M. Synergy between scientific advancement and technological innovation, illustrated by a mechanism-based model characterizing sodium- glucose cotransporter-2 inhibition. J.Clin.Pharmacol. 2010;50:113S–120S. doi: 10.1177/0091270010376974. [DOI] [PubMed] [Google Scholar]