Abstract
Dendritic cells (DC) are required for priming antigen-specific T cells and acquired immunity to many important human pathogens, including Mycobacteriuim tuberculosis (TB) and influenza. However, inappropriate priming of auto-reactive T cells is linked with autoimmune disease. Understanding the molecular mechanisms that regulate the priming and activation of naïve T cells is critical for development of new improved vaccines and understanding the pathogenesis of autoimmune diseases. The serine/threonine kinase IKKα (CHUK) has previously been shown to have anti-inflammatory activity and inhibit innate immunity. Here, we show that IKKα is required in DC for priming antigen-specific T cells and acquired immunity to the human pathogen Listeria monocytogenes. We describe a new role for IKKα in regulation of IRF3 activity and the functional maturation of DC. This presents a unique role for IKKα in dampening inflammation while simultaneously promoting adaptive immunity that could have important implications for the development of new vaccine adjuvants and treatment of autoimmune diseases.
Keywords: dendritic cells, I kappa B Kinase alpha, interferon regulatory factor 3, T-cell priming
Introduction
Our immune system can be broadly divided into innate and adaptive mechanisms, the latter providing immunological memory to protect against secondary infection. In the past two decades, the critical role of the innate immune response in shaping acquired immunity has been established on both a cellular and molecular basis (Iwasaki and Medzhitov, 2010). Two key discoveries have driven developments in this field; the identification of Toll-like receptors (TLRs) expressed on innate immune cells that recognize microbial-associated molecular patterns (Takeuchi and Akira, 2010), and the characterization of dendritic cells (DC) that acquire antigens and prime naïve T cells (Steinman, 2008). T cell-mediated immunity (CMI) is critical for protection against many important infectious diseases in humans including TB and influenza. CMI is maintained by antigen-specific T cells and initiated by DC-mediated T-cell priming (Steinman, 2008; Iwasaki and Medzhitov, 2010). In the case of exogenous antigens, DC process and present peptides on MHC II molecules to naïve CD4+ helper T cells (TH), endogenous or viral antigens are presented on MHC I molecules to CD8+ cytotoxic T cells (CTLs). TH1 cells produce interferon (IFN) γ, which is critical for immunity to intracellular pathogens including TB and viruses through the activation of macrophages and CTLs. Whereas TH2 cells produce interleukin (IL)-4 and promote B cell-mediated antibody responses.
Effective T-cell priming requires three signals: antigen presentation to a specific T-cell receptor (TCR), co-stimulation of the antigen-presenting cell (APC) by T-cell ligands, and cytokine production by the APC (Steinman, 2008; Iwasaki and Medzhitov, 2010). The third signal is usually triggered by pattern recognition receptors (PRRs), including TLRs, expressed by the APC that detect tissue injury or infection (Takeuchi and Akira, 2010), and is required to trigger the functional maturation of the APC (Sporri and Reis e Sousa, 2005). TH1 cells require IL-12 production by the APC, which is triggered by engagement of specific TLRs that detect bacterial or viral infection (Manicassamy and Pulendran, 2009). There are 12 TLRs in the mouse and 10 in the human, TLR1–9 are conserved in both species (Takeuchi and Akira, 2010). Each TLR responds to a different class of molecule and triggers specific cellular responses, this is attributed to the recruitment of different signalling adaptors (Takeuchi and Akira, 2010). MyD88 is utilized by all TLRs except TLR3, which responds to double-stranded RNA (dsRNA) from virus-infected cells through a different adaptor called TRIF. TLR4 is the only receptor that recruits both MyD88 and TRIF, both of which are required for cytokine production in response to TLR4 signalling. MyD88 drives inflammatory cytokine production through NF-κB activation (Dev et al, 2011), while TRIF mediates activation of IFN regulatory factor 3 (IRF3) and expression of type I IFN (IFNα/β) (Honda and Taniguchi, 2006). MyD88-dependent signalling is critical for priming TH1 cell responses (Schnare et al, 2001), but the contribution of TRIF-dependent IRF3 activation is less clear. TLR7 and TLR9 also trigger type I IFN production, in response to ssRNA or CpG-DNA, respectively, but through a MyD88-dependent pathway leading to activation of IRF7 or IRF1 (Takeuchi and Akira, 2010), and not IRF3.
Type I IFN production is usually considered in the context of innate anti-viral immunity, however TLR4, 3, 7 and 9 agonists are potent adjuvants for TH1-mediated immune responses and have the conserved property of inducing type I IFN (Manicassamy and Pulendran, 2009), suggesting a potentially important role for type I IFN in TH1 cell priming.
NF-κB is the prototypical pro-inflammatory transcription factor (Dev et al, 2011), and MyD88-mediated NF-κB activation is dependent on the IκB kinase complex (IKK) (Zandi et al, 1997), which leads to phosphorylation and degradation of the endogenous inhibitors of NF-κB (IκBs). The canonical IKK complex consists of three subunits: IKKα (IKK1; CHUK), IKKβ (IKK2) and IKKγ (NEMO). IKKα and IKKβ are serine/threonine kinases, but only IKKβ is required for IκB phosphorylation and canonical NF-κB activation (Hacker and Karin, 2006). IKKγ is an ubiquitin binding protein that links the IKK complex with upstream signalling pathways and is critical for canonical NF-κB activation. The role of IKKα in the canonical IKK complex is still unclear, IKKα is not required for canonical NF-κB activation (Hacker and Karin, 2006), but several studies have shown a role for IKKα in negative regulation of NF-κB activity by a variety of different mechanisms (Lawrence et al, 2005; Li et al, 2005; Liu et al, 2007; Shembade et al, 2011). However, IKKα is required for an alternative NF-κB pathway through the phosphorylation and processing of p100 leading to activation of p52/RelB heterodimers (Senftleben et al, 2001). Alternative NF-κB signalling is independent of IKKβ and IKKγ but is instead mediated by NF-κB inducing kinase (NIK; MAP3K14), which phosphorylates and activates IKKα (Senftleben et al, 2001). Genetic studies in mice clearly show that the NIK-IKKα-p100 pathway is required for lymphoid organogenesis and B-cell development (Bonizzi and Karin, 2004). Components of alternative NF-κB signalling, namely NIK and RelB, have also been implicated in DC development and function (Wu et al, 1998; Hofmann et al, 2011), although the signalling pathways involved have not been delineated.
Recent studies have shown that IKKα is required for MyD88-dependent type I IFN expression in FLT3-derived plasmacytoid DC (pDC) and conventional DC (cDC), through phosphorylation of IRF7 and IRF1, respectively (Hoshino et al, 2006, 2010). But the role of IKKα in DC function and T-cell priming has not been addressed. We previously showed that IKKα attenuates innate immunity through downregulation of NF-κB-mediated transcription in macrophages (Lawrence et al, 2005). Here, we evaluated the role of IKKα in acquired immunity and priming antigen-specific T cells. Using knock-in mice that express a mutant form of IKKα that cannot be activated (IKKαAA) (Cao et al, 2001), we show that IKKα activation in the haematopoietic compartment is critical for acquired immunity to the facultative intracellular pathogen; Listeria monocytogenes. Although innate resistance to primary L. monocytogenes infection was enhanced in IkkαAA/AA mice, they failed to develop T-cell memory and showed increased susceptibility to secondary infection. IKKα was required in DC for IRF3-dependent IFNβ and IL-12 expression and priming of naïve CD4+ T cells.
Our studies show that IKKα plays reciprocal roles in innate and adaptive immunity, limiting non-specific inflammation while promoting acquired antigen-specific immunity. This unique role for IKKα in the transition from innate to adaptive immunity may have important implications for vaccine development and treatment of infectious diseases.
Results
IKKα is required for priming antigen-specific T cells in vivo
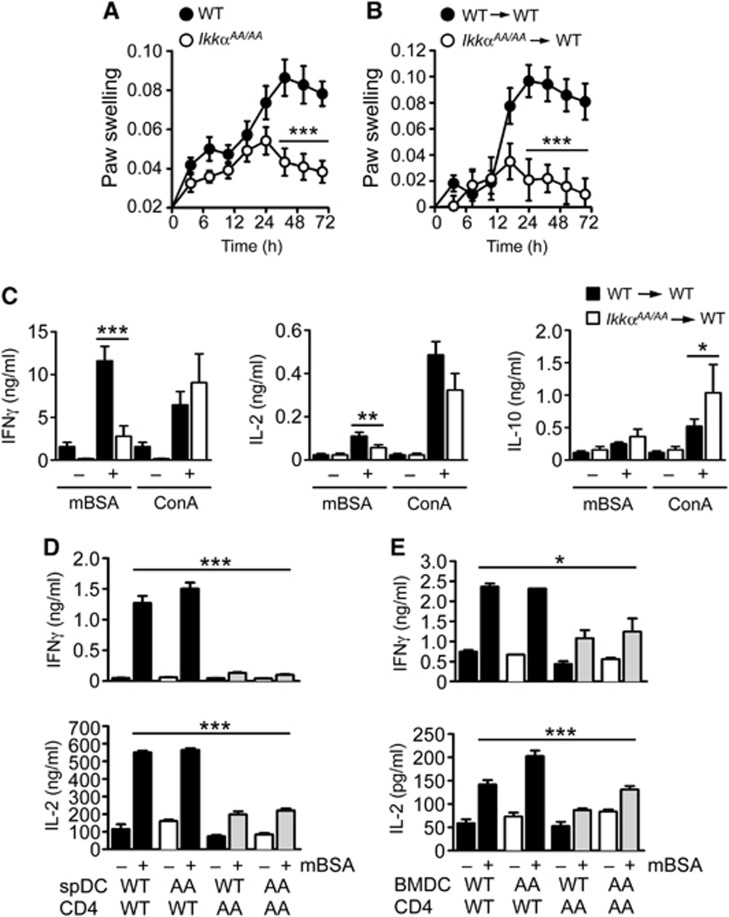
Delayed-type (IV) hypersensitivity (DTH) is a classic model of CMI. Methylated BSA (mBSA) is commonly used as an antigen in animal models of DTH and provokes a strong TH1-mediated IFNγ response (Asquith et al, 2009). We evaluated the role of IKKα in CMI using a mouse model of mBSA-DTH; IkkαAA/AA mice (Cao et al, 2001) and littermate controls (WT) were immunized with mBSA in Freunds’ complete adjuvant (mBSA/CFA), by intradermal (i.d.) injection. Two weeks after immunization, mBSA was injected into the right paw to provoke an antigen-specific immune reaction, PBS was injected into the left paw as a control, paw swelling was measured daily to monitor the inflammatory reaction. Figure 1A shows a substantial decrease in the response to mBSA after immunization of IkkαAA/AA mice. Due to the requirement for IKKα in lymphotoxin β (LTβ) signalling in radiation-resistant stromal cells, IkkαAA/AA mice have defects in lymph node (LN) and spleen development (Bonizzi et al, 2004). To determine if this role for IKKα was responsible for the defect in DTH, we conducted bone marrow adoptive transfer experiments. C57Bl6/Ly5.1 transgenic mice were lethally irradiated and reconstituted with bone marrow cells from IkkαAA/AA or WT mice (Ly5.2), LN and spleen micro-architecture were normal in IkkαAA/AA chimeric mice (data not shown) (Bonizzi et al, 2004). However, the mice remained unresponsive to mBSA challenge (Figure 1B). Therefore, IKKα activation in the haematopoietic compartment mediates DTH.
Figure 1.
IKKα is required for CD4+ T-cell priming in vivo. (A) IkkαAA/AA and littermate control mice (WT), and radiation chimeras generated with WT and IkkαAA/AA bone marrow cells (B), were immunized intradermally (i.d.) with CFA/mBSA. Fourteen days after immunization, mice were injected with mBSA in the left footpad, PBS was injected into the right footpad as a control. Paw swelling was measured at the indicated time points by plethysmography as an index of antigen (mBSA)-induced inflammation. Data are represented as mean±s.e.m. of n=10, statistical analysis was performed with Mann–Whitney test; ***P≤0.001. (C) Splenocytes from CFA/mBSA-immunized WT and IkkαAA/AA chimeric mice were collected after 14 days and re-stimulated in vitro with mBSA or concanavalin A (ConA); IFNγ, IL-2 and IL-10 were measured in culture supernatants by ELISA after 72 h. Data are represented as mean±s.e.m. of n=10, statistical analysis was performed with Mann–Whitney test; *P≤0.05, **P≤0.005, ***P≤0.001. (D) CD4+ T cells were isolated from lymph nodes (LN) and spleen of CFA/mBSA-immunized WT and IkkαAA/AA (AA) mice after 14 days; T cells were co-cultured with CD11c+ DC isolated from spleens of naïve WT and IkkαAA/AA mice (spDC; D), or bone marrow-derived DC (BMDC; E), in the presence or absence of mBSA. IFNγ and IL-2 were measured in culture supernatants by ELISA after 72 h. Data are represented as mean±s.e.m. of n=6, statistical analysis was performed with Mann–Whitney test; *P≤0.05, ***P≤0.001.
To check the priming of endogenous mBSA-specific T cells in IkkαAA/AA mice, we performed ex vivo antigen re-call assays. Antigen (mBSA)-specific IFNγ and IL-2 production by splenocytes from mBSA/CFA-immunized IkkαAA/AA chimeric mice was severely impaired, with no effect on IL-10 production or non-specific activation of T cells with concanavalin A (ConA) (Figure 1C). There were no defects in activation of T cells from IkkαAA/AA mice in response to TCR cross-linking with anti-CD3/CD28 antibodies (Supplementary Figure S1), indicating that IKKα had no intrinsic role in antigen-mediated T-cell activation. To test priming of naïve T-cell responses in IkkαAA/AA mice, we purified CD4+ T cells from spleen and LNs of mBSA/CFA-immunized mice and co-cultured them with naïve splenic CD11c+ DC or bone marrow-derived DC (BMDC) from either WT or IkkαAA/AA mice, in the presence of mBSA. DC from WT or IkkαAA/AA mice were able to trigger IFNγ and IL-2 production by CD4+ T cells from mBSA/CFA-immunized WT mice, but CD4+ T cells from mBSA/CFA-immunized IkkαAA/AA mice were unresponsive even in the presence of WT DC (Figure 1D and E). These data suggested a defect in T-cell priming rather than antigen presentation and activation.
IKKα is required for acquired immunity to infection
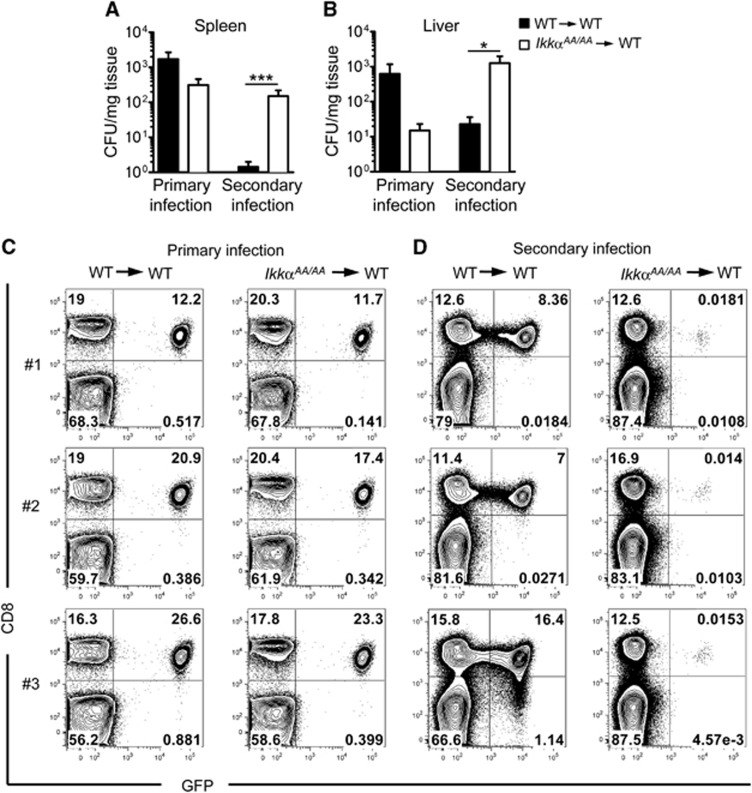
To investigate the role of IKKα in acquired immunity in the context of infection, we used the human pathogen L. monocytogenes (Lm). Lm is a facultative intracellular bacteria that is normally controlled by cell-mediated immune responses but can cause fatal listeriosis in immune-compromised individuals (Lecuit, 2007). Lm infection in mice is widely used as a model to study the cellular and molecular basis of CMI (Pamer, 2004); acquired immunity to Lm requires CD4+ T cell-dependent development of memory CD8+ T cells (Sun and Bevan, 2003). We infected IkkαAA/AA and WT bone marrow chimeric mice intravenously (i.v.) with 104 colony forming units (CFU) of a recombinant Lm strain engineered to express chicken ovalbumin (Lm-OVA). IkkαAA/AA chimeras showed increased resistance to primary Lm-OVA infection with reduced bacterial load in spleen and liver after 5 days (Figure 2A and B), however, upon secondary infection after 35 days with a high dose of bacteria (106 CFU), IkkαAA/AA chimeric mice showed impaired protection with significantly increased bacterial load in spleen and liver (Figure 2A and B). To test the role of IKKα in the priming and activation of protective Lm-specific CD8+ T cells, we injected mice with naïve CD8+ T cells that specifically recognize OVA peptide in the context of MHC I (OT-I cells) before primary infection with Lm-OVA. We measured the expansion of OT-I cells in the spleen of infected mice 7 days after infection. IkkαAA/AA chimeric mice showed no impairment in expansion of OT-I cells after primary infection (Figure 2C). However, upon secondary infection 35 days later, OT-I cells failed to re-expand in IkkαAA/AA chimeric mice compared to mice reconstituted with WT bone marrow (Figure 2D). Re-call assays ex vivo with splenocytes and OT-I-specific OVA peptide showed defective expansion of OVA-specific CD8+ T cells correlated with severely reduced numbers of IFNγ producing cells (Supplementary Figure S2). These data suggest that IKKα plays an important role in the development and/or maintenance of memory CD8+ T cells, and thus acquired immunity to infection.
Figure 2.
IKKα is required for acquired immunity to L. monocytogenes and CD8+ T-cell memory. WT and IkkαAA/AA chimeric mice were infected intravenously (i.v.) with 104 CFU L. monocytogenes expressing chicken ovalbumin (Lm-OVA), spleens (A) and liver (B) were collected after 7 days for CFU measurements (primary infection). In parallel experiments, mice received a second infection with 106 CFU Lm-OVA 35 days after primary infection and CFU was determined in spleen and liver after a further 5 days (secondary infection). Data are represented as mean±s.e.m. of n=8–16, statistical analysis was performed with Mann–Whitney test; *P≤0.05, ***P≤0.001. (C) 104 CD8+ OT-I.EGFP cells were adoptively transferred to WT and IkkαAA/AA chimeric mice prior to infection with Lm-OVA (104 CFU i.v.), expansion of OVA-specific OT-I cells was measured by FACS analysis of spleens 7 days post infection (primary infection; C). In parallel experiments, mice received a second infection with 106 CFU Lm-OVA after 35 days, spleens were collected after a further 5 days for FACS analysis of OT-I cells (secondary infection; D). Representative data are shown from n=6 mice.
IKKα activation regulates the functional maturation of DC
Priming of naïve antigen-specific T cells is the defining property of DC (Steinman, 2008), whereas both DC and macrophages are able to trigger antigen-specific T-cell activation (Hume, 2008). Our data showed a defect in CD4+ T-cell priming in IkkαAA/AA mice that is intrinsic to the APC compartment and likely reflects a role of IKKα in DC. It was also shown that DC are critical for priming protective CD8+ T cells and acquired immunity to Lm infection in mice (Jung et al, 2002). We next assessed the role of IKKα specifically in DC function. Effective T-cell priming by DC requires three signals (Manicassamy and Pulendran, 2009): (1) Antigen presentation by MHC to a specific TCR. (2) Co-stimulation by cognate ligand-receptor interactions between the DC and T cell. (3) Cytokine production by the DC, usually provoked by TLR stimulation or a ‘danger’ signal that triggers DC functional maturation. In the case of TH1-mediated IFNγ responses, the critical cytokine produced by DC is IL-12 (Manicassamy and Pulendran, 2009).
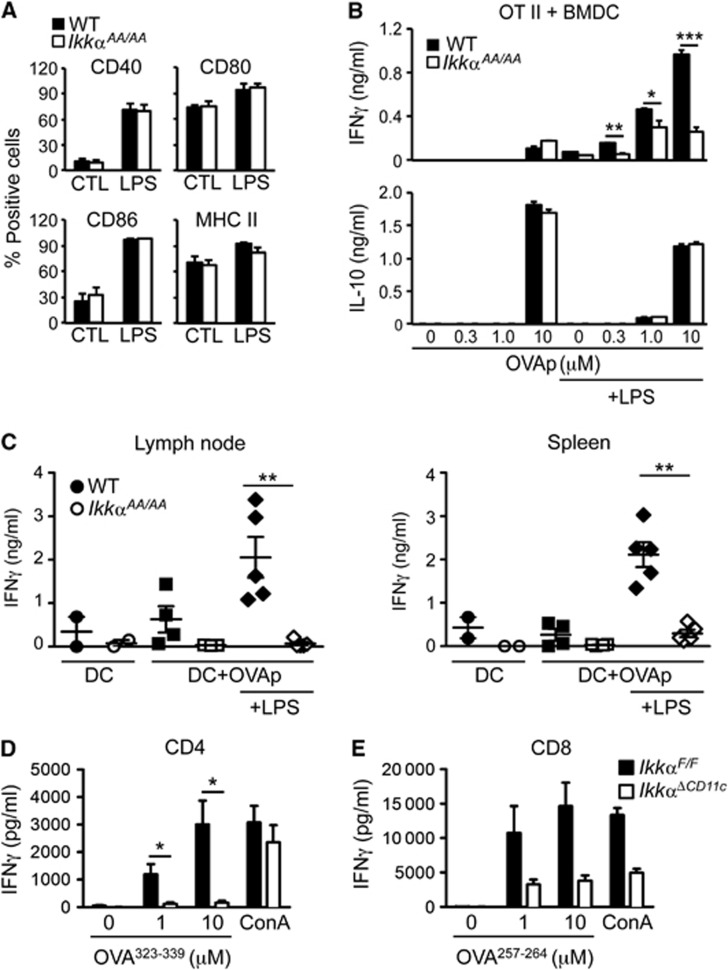
FACS analysis of DC populations in spleen of IkkαAA/AA mice showed no overt differences in number or phenotype compared with littermate controls (Supplementary Figure S3). Furthermore, LPS-induced maturation of GM-CSF-derived BMDC from IkkαAA/AA mice appeared normal based on MHC II and co-stimulatory molecule expression (Figure 3A). Similar results were obtained with FLT3-derived cDC (data not shown). In a functional assay of T-cell priming, using naïve CD4+ T cells from OT-II transgenic mice, LPS-stimulated BMDC from IkkαAA/AA mice showed a profound impairment of TH1 cell priming in the presence of MHC II-specific OVA peptide, measured by IFNγ production (Figure 3B). However, LPS-independent antigen-induced IL-10 production remained unchanged. To test if the observed defect could prevent T-cell priming in vivo, we used an adoptive transfer approach. BMDC derived from WT or IkkαAA/AA mice were loaded with MHC II-restricted OVA peptide (OVAp) in the presence or absence of LPS and injected into the footpad of naïve WT mice. Seven days after injection of antigen-loaded DC the draining popliteal LN and spleen was collected from recipient mice and single-cell suspensions prepared for re-call responses to OVAp ex vivo. Mice immunized with IkkαAA/AA BMDC in the presence of LPS showed severely impaired IFNγ responses compared with WT BMDC (Figure 3C), in keeping with an intrinsic defect in DC-mediated TH1 cell priming. FACS analysis of antigen-loaded DC prior to adoptive transfer confirmed no difference in activation markers and MHC II expression (Supplementary Figure S4). Finally, to confirm that IKKα activity in DC was required for T-cell priming in vivo, we used mice with a targeted deletion of IKKα in CD11c+ cells. Mice expressing a ‘floxed’ Ikkα gene (IkkαF/F) (Liu et al, 2008) were bred with transgenic mice expressing Cre recombinase from the CD11c promoter (CD11c-Cre) (Caton et al, 2007). FACS analysis of splenic DC populations in IkkαΔCD11c mice and littermate controls (IkkαF/F) revealed no obvious differences in number or phenotype (Supplementary Figure S5A), in keeping with data from IkkαAA/AA mice. To test T-cell priming in vivo, we transferred naïve T cells from OT-I (CD8+) or OT-II (CD4+) TCR transgenic mice, to IkkαΔCD11c and IkkαF/F mice before immunization with OVA/CFA by i.d. injection at the base of the tail. Draining inguinal LN was collected after 5 days for antigen re-call assay. LN cell suspensions were re-stimulated with OVAp specific for MHC I or MHC II, respectively, and IFNγ production measured in cell culture supernatants by ELISA. IFNγ production by both CD4+ and CD8+ OVA-specific T cells was significantly impaired in IkkαΔCD11c mice compared to controls (Figure 3D and E). These data demonstrate that IKKα expression and activity is required specifically in DC for priming antigen-specific CD4+ and CD8+ T-cell responses in vivo.
Figure 3.
IKKα is required for TLR-induced functional maturation of DC. (A) FACS analysis of BMDC from WT and IkkαAA/AA mice after LPS (100 ng/ml)-induced maturation for 24 h. Data are represented as mean±s.e.m. of n=3. (B) BMDC from WT and IkkαAA/AA mice were loaded with MHC II-restricted OVA peptide (OVA323–339; OVAp) with and without LPS stimulation for 24 h before co-culture with naïve CD4+ OT-II T cells. IFNγ and IL-10 were measured in culture supernatants after 72 h. Data are represented as mean±s.e.m. of n=4, statistical analysis was performed with Student’s t-test; *P≤0.05, **P≤0.005, ***P≤0.001. (C) BMDC from WT and IkkαAA/AA mice were loaded with OVAp in the presence and absence of LPS for 24 h, 2.5 × 105 DC were injected into the footpad of naïve WT mice. Seven days after DC injection, the popliteal LN and spleen were collected and single-cell suspensions prepared for antigen (OVAp) re-call assay in vitro; measured by IFNγ production in culture supernatants after 72 h. Data are represented as mean±s.e.m. of n=5, statistical analysis was performed with Mann–Whitney test; **P<0.005. (D, E) 106 OVA-specific CD4+ (OT-II; D) or CD8+ (OT-I; E) T cells were adoptively transferred to IkkαΔCD11c and IkkαF/F mice, before immunization with CFA/OVA i.d. The inguinal LN was collected after 5 days and single-cell suspensions prepared for antigen re-call assays with MHC II (OVA323–339) and MHC I (OVA257–264) specific peptides; IFNγ production was measured in culture supernatants after 72 h. Data are represented as mean±s.e.m. of n=3–6, statistical analysis was performed with Mann–Whitney test; *P<0.05.
IKKα regulates IRF3-dependent IFNβ and IL-12 production by DC
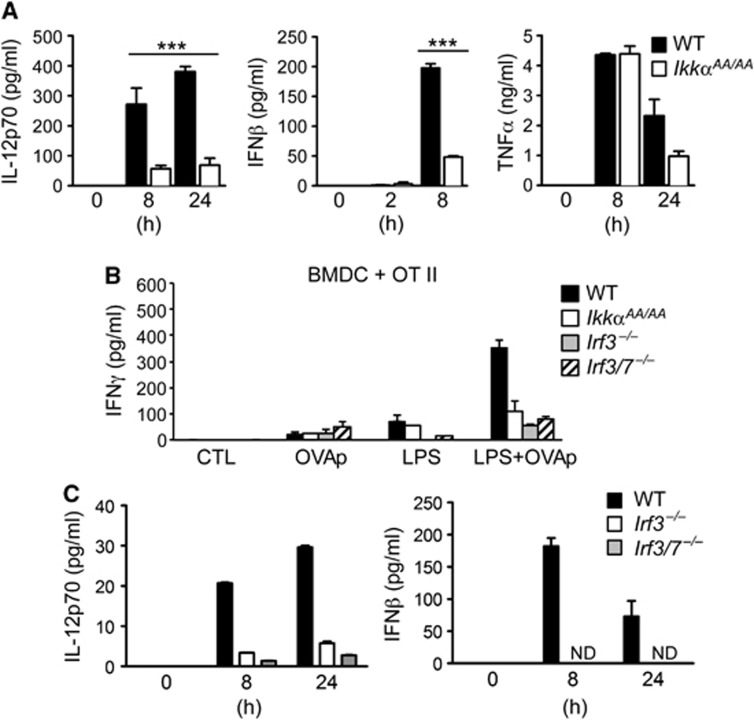
We next measured TLR-induced cytokine production by BMDC from IkkαAA/AA and WT mice; IkkαAA/AA DC showed a specific defect in IL-12 and IFNβ production in response to the TLR4-ligand LPS, whereas TNF-α production was unaltered (Figure 4A). This was confirmed at the mRNA level by quantitative real-time PCR analysis (qRT–PCR; Supplementary Figure S6). TLR-induced IL-12 expression by DC is a critical factor for priming TH1 responses (Manicassamy and Pulendran, 2009); IFNβ expression is also linked to the functional maturation of DC, at least in part through autocrine/paracrine regulation of IL-12 expression (Gautier et al, 2005). Another important signal for cytokine production by DC is T cell-mediated co-stimulation; in particular, CD40 activation on DC by CD40L (CD154) expressed by activated T cells is an important signal for IL-12 production and TH1 cell priming (Schulz et al, 2000). CD40 triggers activation of alternative NF-κB signalling through IKKα in B cells (Senftleben et al, 2001), therefore we tested the role of IKKα in CD40-induced IL-12 expression in BMDC. However, we found no difference in CD40L-induced IL-12 production by IkkαAA/AA BMDC compared to WT controls (Supplementary Figure S7).
Figure 4.
IKKα is required for IRF3-dependent functional maturation of DC. (A) BMDC from WT and IkkαAA/AA mice were stimulated with LPS and cytokine production measured in culture supernatants by ELISA at the indicated time points. Data are represented as mean±s.e.m. of n=4, statistical analysis was performed with Student’s t-test; ***P≤0.001. (B) BMDC from WT, IRF3, IRF3+IRF7 knockout (Irf3−/−; Irf3/7−/−) and IkkαAA/AA mice were loaded with MHC II-restricted OVA peptide (OVA323–339; OVAp) with and without LPS stimulation for 24 h before co-culture with naïve CD4+ OT-II T cells. IFNγ was measured in culture supernatants after 72 h. (C) WT, Irf3−/− and Irf3/7−/− BMDC were stimulated with LPS and cytokine production measured in culture supernatants by ELISA at the indicated time points. Data are represented as mean±s.e.m. of n=3.
TLR4-induced IL-12 and IFNβ expression in DC is regulated by MyD88-dependent NF-κB activation, and TRIF-mediated IRF3 activation (Yamamoto et al, 2003). Despite its critical role in IFNβ expression, the role of IRF3 in the functional maturation of DC is not well established. To determine if IRF3 in DC was required for T-cell priming, we derived BMDC from IRF3 knockout mice (Irf3−/−) and performed parallel experiments as done with IkkαAA/AA BMDC. Although Irf3−/− BMDC showed no defects in LPS-induced MHC II and co-stimulatory molecule expression (Supplementary Figure S8), they were unable to prime OT-II T cells in the presence of OVAp, measured by IFNγ production (Figure 4B), displaying a similar phenotype to IkkαAA/AA BMDC. As expected, IFNβ and IL-12 expression was profoundly inhibited in BMDC from IRF3 knockout mice (Figure 4C), confirming that TLR4-induced IFNβ/IL-12 expression is critically dependent on IRF3. Furthermore, there was no additive effect in BMDC from mice deficient in both IRF3 and IRF7 (Irf3/Irf7−/−), suggesting a minimal role for IRF7 in this context. These data demonstrate a striking overlap between the functions of IRF3 and IKKα in DC that suggested that IKKα may regulate IRF3 activity.
IKKα-mediated IFNβ and IL-12 expression is independent of NF-κB activation
IKKα is a component of the canonical IKK complex regulating NF-κB activation in response to TLR signalling (Karin and Ben-Neriah, 2000; Takeuchi and Akira, 2010). However, previous studies have established no role for IKKα in TLR4-mediated activation of NF-κB signalling (Senftleben et al, 2001; Lawrence et al, 2005). In fact, several studies have shown that IKKα can attenuate NF-κB activation (Lawrence et al, 2005; Li et al, 2005; Liu et al, 2007; Shembade et al, 2011). LPS-induced NF-κB activation was unaffected in BMDC from IkkαAA/AA mice, as measured by phosphorylation and degradation of IκBα (Supplementary Figure S9A), as was expression of the canonical NF-κB target gene Tnfa (Figure 4A; Supplementary Figure S6). IKKα is critical for the alternative NF-κB signalling pathway, which depends on the processing of p100 (NFκB2) to p52 (Senftleben et al, 2001). This pathway requires the inducible degradation of the E3 ligase TRAF3, leading to stabilization of NIK and subsequent phosphorylation of IKKα (Vallabhapurapu et al, 2008). The mutation in IkkαAA/AA mice prevents the phosphorylation of IKKα by NIK in this pathway (Senftleben et al, 2001). We found no evidence for activation of alternative NF-κB signalling in LPS-stimulated BMDC, with no changes in TRAF3, NIK or p100/p52 expression in either WT of IkkαAA/AA cells (Supplementary Figure S9B). Furthermore, BMDC from NIK knockout mice (Nik−/−) showed no defects in LPS-induced IFNβ or IL-12 production (Supplementary Figure S9C). To confirm that the phenotype of IkkαAA/AA DC was not due to defects in alternative NF-κB signalling, we compared LPS-induced IL-12 and IFNβ gene expression in BMDC derived from Nik−/− or Nfkb2−/− mice, and mice that constitutively express active p52 due to a p100 truncation (p100ΔGRR; Nfkb2Δ/Δ) (Ishikawa et al, 1997). Nfkb2−/−, Nfkb2Δ/Δ, or Nik−/− BMDC showed no obvious defects in LPS-induced IL-12/IFNβ (Supplementary Figure S10). These data showed that IKKα-mediated IFNβ and IL-12 expression was independent of either canonical or alternative NF-κB signalling.
TRIF-mediated TAK1 activation is required for IFNβ expression in DC
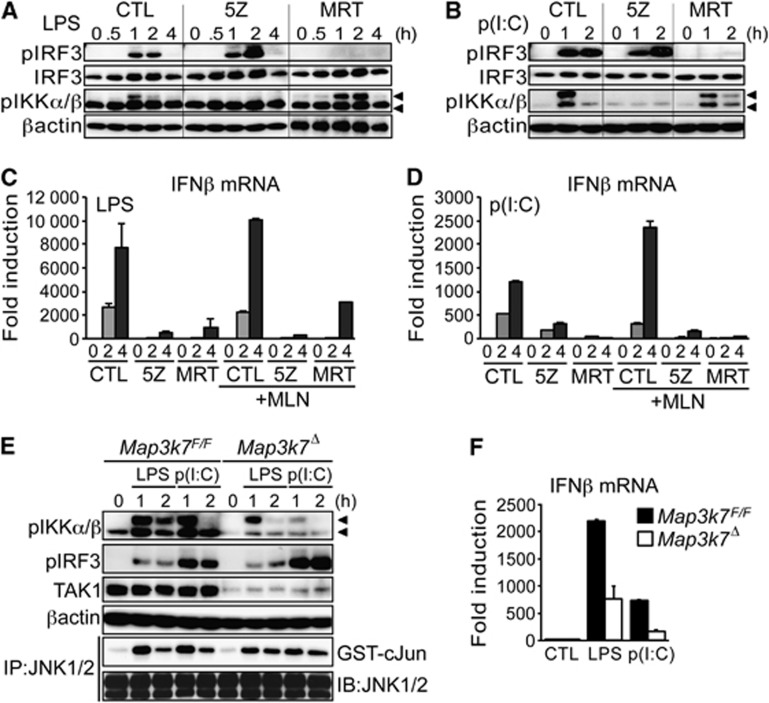
MyD88-dependent TLR4 signalling triggers TAK1-mediated phosphorylation of IKKβ on two serine residues in its activation loop (Ser176/180) (Karin and Ben-Neriah, 2000), that are equivalent to the sites in IKKα (Ser177/181) phosphorylated by NIK (Senftleben et al, 2001). The TRIF adaptor protein was previously shown to recruit TAK1 in response to TLR3 signalling (Jiang et al, 2004). Since NIK was not required for IFNβ expression in BMDC, we examined the possible involvement of TAK1 in this process. To examine the role of TAK1 in TRIF-mediated IFNβ expression, we stimulated BMDC with LPS or dsRNA (poly(dI:dC)) in the presence or absence of a TAK1 inhibitor; 5Z-7-oxozeaenol (5Z) (Ninomiya-Tsuji et al, 2003). In parallel, we used an IKKε/TBK1 inhibitor MRT67307 (MRT) that blocks TRIF-mediated activation of IRF3 (Gleason et al, 2011). Treatment of BMDC with 5Z blocked inducible phosphorylation of IKKα/β, detected with an antibody that recognizes phosphorylation of both kinases in their respective activation loops (Figure 5A). As expected, 5Z did not affect Ser396 phosphorylation of IRF3 which is mediated by IKKε/TBK1 (Zhao et al, 2007). In contrast, the IKKε/TBK1 inhibitor (MRT) blocked IRF3 phosphorylation but did not affect IKKα/β phosphorylation (Figure 5A and B). However, both 5Z and MRT effectively inhibited LPS or dsRNA-induced IFNβ expression in BMDC (Figure 5C and D). These data suggest a requirement for TAK1 in TRIF-dependent IKKα/β activation and IFNβ expression in BMDC, but not IKKε/TBK1-mediated IRF3 phosphorylation. As expected, IKKε/TBK1-mediated Ser396 phosphorylation of IRF3 was also unaffected in IkkαAA/AA BMDC (Supplementary Figure S11), despite inhibition of IFNβ expression.
Figure 5.
TAK1-mediated IKKα/β activation is required for IRF3-dependent IFNβ expression in DC. BMDC were stimulated with LPS or dsRNA (p(I:C); 25 μg/ml) in the presence of 1 μM 5Z-7-oxozeaenol (5Z), 2 μM MRT67307 (MRT), or vehicle alone (CTL). (A, B) Immunoblot (IB) analysis of IKKα/β (pIKKα/β; 85 kDa/89 kDa) and IRF3 phosphorylation (Ser396; pIRF3), βactin and IRF3 expression were used as loading controls. (C, D) BMDC were treated as above in the presence or absence of 1 μM MLN4926 (MLN), RNA was extracted at the indicated time points for qRT–PCR analysis of IFNβ mRNA expression; data are represented as fold induction over control, normalized to cyclophillin (CPH) mRNA. (E, F) BMDC were generated from Map3k7F/F and Map3k7F/F.CreER mice and treated with 4 μM tamoxifen (OHT) for 48 h, before stimulation with LPS or dsRNA as described above. Protein extracts were prepared at the indicated time points and phosphorylation of IKKα/β and IRF3 was measured by IB analysis; JNK activity was measured by IP kinase assay using recombinant GST-cJun as a substrate (E). In parallel experiments, RNA was extracted at 4 h and IFNβ expression measured by qRT–PCR (F). Representative data from at least two independent experiments are shown. qRT–PCR data are presented as mean±s.e.m. of three replicates.
Source data for this figure is available on the online supplementary information page.
TAK1 is well established to regulate both IKK and JNK activation downstream of TLR4. However, we found no change in LPS or dsRNA-induced JNK activation in IkkαAA/AA BMDC (Supplementary Figure S12A), indicating that reduced IFNβ expression in IkkαAA/AA DC is not attributed to effects on JNK activation. Furthermore, a specific JNK inhibitor had only a partial effect on LPS and dsRNA-induced IFNβ expression in BMDC (Supplementary Figure S12B), whereas the TAK1 inhibitor almost completely blocked IFNβ induction (Figure 5B and C). These data suggest that TAK1-mediated JNK activation only partially contributes to IFNβ expression and TAK1-mediated IKKα/β activation also has a significant role.
Next, we treated BMDC with the NEDDylation inhibitor MLN4924 (MLN) to block poly-ubiquitination and degradation of phosphorylated IκBα, preventing activation of NF-κB downstream of TAK1-mediated IKKα/β activation. Despite effectively blocking IκBα degradation (data not shown), MLN did not inhibit IFNβ induction in BMDC stimulated with either LPS or dsRNA (Figure 5C and D). Furthermore, even in the presence of MLN, 5Z effectively inhibited IFNβ expression (Figure 5C and D), demonstrating that TAK1-mediated regulation of IFNβ induction was independent of NF-κB activation. Using a specific IKKβ inhibitor (BI605906) (Pauls et al, 2012), we confirmed that IKKβ-mediated NF-κB activation was not required for TRIF-dependent IFNβ expression in BMDC (Supplementary Figure S13). Finally, to confirm the results with the TAK1 inhibitor we generated TAK1 (Map3k7)-deficient BMDC from Map3k7F/F mice expressing tamoxifen-inducible Cre recombinase (CreER) (Wang et al, 2012). BMDC from Map3k7F/F-CreER mice were treated with tamoxifen (4-OHT) to induce deletion of TAK1 followed by stimulation with LPS or dsRNA. TAK1 deletion (Map3k7Δ) in BMDC inhibited the phosphorylation of IKKα/β in response to both LPS and dsRNA, but did not affect Ser 396 phosphorylation of IRF3 (Figure 5E), recapitulating the effects of the TAK1 inhibitor (Figure 5A and B). Furthermore, TAK1 deletion in BMDC inhibited both LPS and dsRNA-induced IFNβ expression (Figure 5F), again in keeping with the results of the TAK1 inhibitor (Figure 5C and D). Collectively, these data suggest a requirement for TAK1-mediated IKKα activation in IRF3-dependent IFNβ expression in BMDC.
IKKα regulates IRF3-mediated transcription downstream of IKKε/TBK1
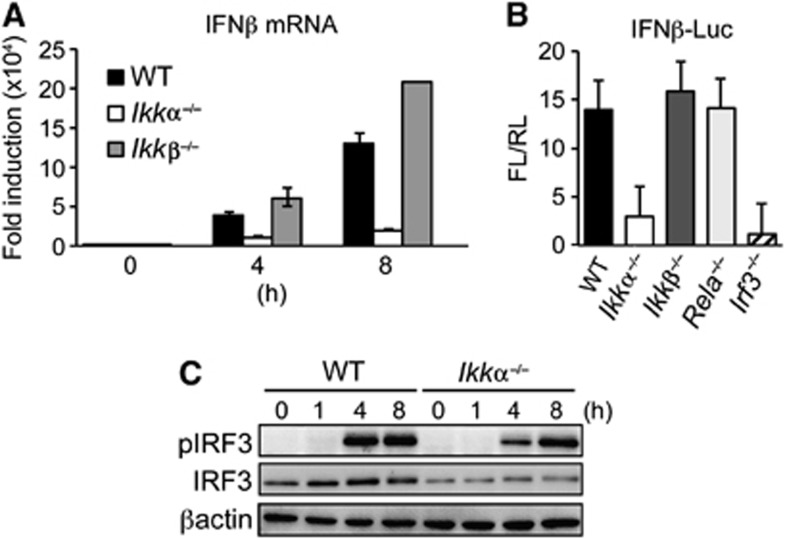
To further explore the role of IKKα in IRF3-mediated IFNβ expression, we established an IFNβ-promoter reporter assay in mouse embryonic fibroblasts (MEF). In keeping with data from IkkαAA/AA BMDC, endogenous IFNβ expression in response to dsRNA transfection, which triggers IKKε/TBK1-mediated IRF3 activation (Zhao et al, 2007), was blocked in IKKα-deficient cells (Ikkα−/−) but was unaffected by IKKβ deletion (Ikkβ−/−; Figure 6A). IFNβ-promoter activity was also IKKα and IRF3 dependent, but unaffected by IKKβ or RelA deletion (Figure 6B), demonstrating that IFNβ induction in MEF was not dependent on NF-κB activation. Furthermore, IKKε/TBK1-mediated Ser396 phosphorylation of IRF3 was not significantly impaired in Ikkα−/− cells (Figure 6C), suggesting that IKKα may regulate IRF3-mediated IFNβ expression downstream of IKKε/TBK1 activation.
Figure 6.
IFNβ-promoter activity is IKKα dependent. (A) WT, Ikkα−/− and Ikkβ−/− MEF cells were transfected with 1 μg/ml dsRNA (p(I:C)) and IFNβ mRNA expression measured at the indicated time points by qRT–PCR, data are expressed as fold induction over control normalized to CPH expression. (B) WT, Ikkα−/−, Ikkβ−/−, Rela−/− and Irf3−/− MEF cells were co-transfected with an IFNβ-promoter reporter vector expressing firefly luciferase (IFNβ-Luc; FL) and a constitutive renilla-luciferase reporter (RL); IFNβ-promoter activity was measured by dual luciferase assay 6 h after p(I:C) stimulation, data are expressed as fold induction of FL activity normalized to RL (FL/RL). Experiments were performed in triplicate and data represented as mean±s.e.m. of three independent experiments. (C) IB analysis of IRF3 Ser396 phosphorylation (pIRF3) in WT and Ikkα−/− MEF cells after p(I:C) stimulation at the indicated time points. Representative data from three independent experiments are shown.
Source data for this figure is available on the online supplementary information page.
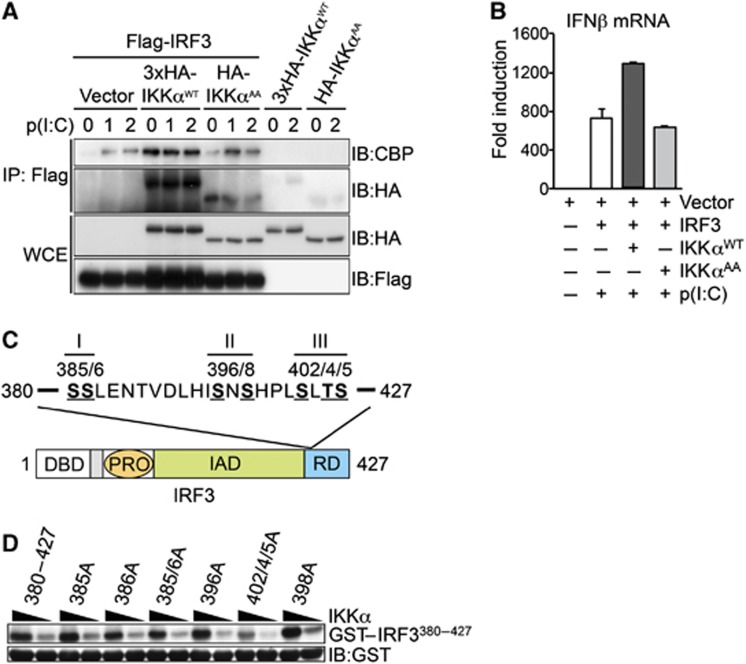
To further explore the role of IKKα in regulation of IRF3 activity, we performed co-transfection experiments in HEK293T cells that stably express TLR3. Co-expression of wild-type IKKα (IKKαWT) and IRF3 resulted in a strong interaction detected by co-immunoprecipitation (co-IP) (Figure 7A), this interaction was significantly reduced when the IKKαAA mutant was co-expressed with IRF3. Furthermore, the co-expression of IKKα, but not the IKKαAA mutant, increased the association of IRF3 with the transcriptional co-activator CBP (Figure 7A). The increased recruitment of CBP after overexpression of IKKα was also associated with increased transcription of endogenous IFNβ mRNA (Figure 7B). The inability of IKKαAA to promote CBP recruitment or increased IFNβ transcription indicates the requirement for IKKα kinase activity.
Figure 7.
IKKα regulates IRF3-medaited transcription downstream of IKKε/TBK1-mediated IRF3 phosphorylation. (A) HEK293-TLR3 cells were transfected with cDNA vectors expressing Flag-IRF3, HA-IKKαWT and HA-IKKαAA, in the presence of absence of p(I:C) as indicated. Protein extracts were prepared and immunoprecipitation (IP) of IRF3 was performed with anti-Flag antibody, co-IP of HA-IKKα and endogenous CBP was detected by IB analysis. Expression of Flag-IRF3, HA-IKKαWT and HA-IKKαAA was measured in total protein extracts (WCE) by IB. (B) In parallel experiments, RNA was extracted at 4 h and IFNβ expression measured by qRT–PCR. Representative data from at least two independent experiments are shown; qRT–PCR data are presented as mean±s.e.m. of three replicates. (C) Schematic representation of the seven potential phosphorylation sites in the C-terminal regulatory domain of IRF3 organized into three clusters: I—Ser385/Ser386; II—Ser396/Ser398; III—Ser402/Thr404/Ser405. DBD, DNA-binding domain; PRO, proline-rich domain; IAD, auto-inhibitory domain; RD, regulatory domain. (D) GST-IRF3380–427 and various mutant peptides were expressed and purified from bacteria and incubated with recombinant active IKKα in the presence of γ-32P-ATP, IKKα-mediated phosphorylation of GST-IRF3 peptides was quantified by autoradiography. IB analysis of GST was used a loading control for IRF3 substrate. Representative data from two independent experiments are shown.
Source data for this figure is available on the online supplementary information page.
The rate-limiting step in IRF3 activation is the inducible phosphorylation of Ser396 in the C-terminal regulatory domain by IKKε/TBK1 (Lin et al, 1999). However, this domain (aa 380–427) contains a total of seven residues that are targets for inducible phosphorylation and contribute to IRF3 activity (Lin et al, 1999; Mori et al, 2004) (Figure 7C). To test if the regulatory domain of IRF3 was a target for IKKα-mediated phosphorylation, we prepared recombinant GST-IRF3 fusion protein encompassing the C-terminal regulatory domain (GST-IRF3380–427) and various Ser-Ala point mutants, and performed in vitro kinase assays with active IKKα. IRF3380–427 was strongly phosphorylated by IKKα in vitro and this phosphorylation was significantly reduced by mutation of Ser402/404/405 to Ala (Figure 7D), however, mutation of the other putative target residues including Ser396 and Ser386 did not affect IKKα-mediated phosphorylation. These data suggest that direct phosphorylation of IRF3 by IKKα could regulate IRF3-mediated transcription through enhanced CBP recruitment.
Discussion
Here, we describe a new role for the kinase IKKα (CHUK) in DC required for priming antigen-specific T cells. Bone marrow chimeras generated from transgenic mice that express a mutant form of IKKα (IkkαAA/AA) showed impaired DTH in vivo, which is mediated by antigen-specific TH1 cells. Further experiments demonstrated impaired TH1 cell priming in IkkαAA/AA mice. This is distinguished from the function of IKKα in lymphoid organogenesis, which is intrinsic to radiation-resistant stromal cells through the alternative NF-κB pathway (Senftleben et al, 2001; Bonizzi et al, 2004).
We show that IKKα is required for acquired immunity to the facultative intracellular pathogen Listeria monocytogenes (Lm). Innate immunity to primary Lm infection was enhanced in IkkαAA/AA mice; however, acquired immunity to secondary infection was severely impaired, which is dependent on the development of protective CD8+ memory T cells (Pamer, 2004). Usually the innate immune response is tightly coupled to development of acquired immunity, these data suggest that IKKα has the unusual property of uncoupling innate and adaptive immunity; inhibiting innate non-specific immunity while enhancing antigen-specific acquired immunity.
It was previously shown that IKKα has anti-inflammatory activity by attenuating NF-κB activation in macrophages (Lawrence et al, 2005; Li et al, 2005; Liu et al, 2007). In addition, macrophages from IkkαAA/AA mice have increased resistance to apoptosis, and consequently enhanced anti-microbial activity, upon infection with Streptococcus agalactiae (Lawrence et al, 2005). Macrophage apoptosis also plays an important role in resistance to primary Lm infection in mice (Stockinger et al, 2009). The anti-inflammatory and pro-apoptotic functions of IKKα in macrophages are likely to explain the increased clearance of Lm after primary infection in IkkαAA/AA mice.
In contrast, acquired immunity to Lm in mice requires the development of CD8+ memory T cells. Expansion of Lm-specific CD8+ T cells was normal upon primary infection of IkkαAA/AA mice. However, Lm-specific CD8+ T cells in IkkαAA/AA mice failed to respond to secondary infection, suggesting impaired development or survival of CD8+ memory T cells. The expansion of pathogen-specific CD8+ T cells upon primary Lm infection is independent of CD4+ T-cell help, but the survival of protective CD8+ memory T cells critically requires MHC II-restricted CD4+ T cells (Sun and Bevan, 2003). We show that CD4+ T-cell priming is defective in IkkαAA/AA mice, which may result in defective CD8+ T-cell memory. Other factors that directly influence the programming and survival of CD8+ memory T cells include; antigen dose, co-stimulation and cytokine production (Pamer, 2004). However, further experiments are required to establish the role of IKKα in these additional processes that may contribute to the development and maintenance of T-cell memory.
We present several lines of evidence that show IKKα activation in DC is required for T-cell priming; DC derived from IkkαAA/AA mice cannot prime naïve CD4+ T cells in vitro to produce IFNγ, and antigen-loaded IkkαAA/AA DC adoptively transferred to naïve mice show similar defects in TH1 cell priming in vivo. Furthermore, targeted deletion of IKKα in CD11c+ DC impairs priming of both CD4+ and CD8+ T cells after immunization in vivo. Naïve T-cell priming by DC requires three signals (Steinman, 2008); antigen presentation, co-stimulation and cytokine production. DC derived from IkkαAA/AA mice showed no defects in antigen-presentation or co-stimulatory molecule expression, but significantly reduced IFNβ and IL-12 expression in response to TLR stimulation. Other cytokines such as TNF-α and IL-10 are expressed normally. TLR-mediated cytokine production by DC is required for T-cell priming in the context of infection, in the case of TH1-mediated IFNγ responses the critical cytokine is IL-12 (Manicassamy and Pulendran, 2009). Type I IFN has also been shown to be an important cytokine for DC maturation and function (Honda et al, 2003), at least in part through promoting the increased expression of IL-12 in DC (Gautier et al, 2005). Thus, the ability of adjuvants to induce IFNα/β is an important facet for priming TH1 immunity (Sugiyama et al, 2008; Manicassamy and Pulendran, 2009).
IKKα was previously shown to regulate MyD88-dependent type I IFN production by pDC and conventional CD8+ DC (cDC), in response to TLR7 and TLR9 ligands (Hoshino et al, 2006, 2010). TLR4 and TLR3-mediated IFNβ expression is dependent on TRIF and IRF3 activation (Yamamoto et al, 2003). IRF3 is also critical for IL-12 expression in response to TLR4 stimulation (Ramirez-Carrozzi et al, 2009). However, the role of IRF3 in DC maturation and function has not been established. We showed that IRF3-deficient DC have profoundly impaired ability to prime naïve CD4+ T cells in the presence of LPS, which was associated with severely reduced IFNβ and IL-12 expression. Thus, the roles of IRF3 and IKKα in DC phenotype show a striking correlation. Interestingly, LPS-induced IFNβ expression in DC was independent of NIK, suggesting another upstream kinase regulates IKKα activation in this context. TAK1 mediates IKKβ activation in response to MyD88 signalling (Wang et al, 2001), and TRIF has also been shown to recruit TAK1 (Jiang et al, 2004). Therefore, TAK1 is a likely candidate to trigger IKKα activation in TRIF-mediated TLR signalling. We showed that TRIF-mediated IKKα/β phosphorylation and IFNβ expression in DC was blocked by a TAK1 inhibitor (Ninomiya-Tsuji et al, 2003), or targeted deletion of the TAK1 gene (Map3k7), however, IFNβ induction was not dependent on IKKβ-mediated NF-κB activation. This suggested that TAK1-mediated IKKα activation might regulate IRF3 activity independently of NF-κB.
IRF3 activation is triggered by IKKε/TBK1-mediated phosphorylation on Ser396 (Sharma et al, 2003); however, Ser396 phosphorylation was not affected by TAK1 inhibition. These data suggested that TAK1-mediated IKKα activation may regulate IRF3-dependent IFNβ expression downstream of IKKε/TBK1 activation in DC. Although Ser396 phosphorylation is required, it is not sufficient for full IRF3 activity (Mori et al, 2004). There are in fact 7 C-terminal Ser residues in IRF3 that are targets for inducible phosphorylation and regulate IRF3 activation (Hiscott and Lin, 2005), but it is not clear if IKKε/TBK1 is responsible for subsequent phosphorylation events or other kinase activities are required. To further explore the role of IKKα in IRF3 activity, we performed IFNβ-promoter activity assays in embryonic fibroblasts (MEF) from various knockout mice; Ikkα−/− MEF showed substantially reduced ability to drive IFNβ-promoter activity in response to dsRNA, despite little effect on Ser396 phosphorylation, implying that an IKKα-dependent signal is required for IRF3-mediated transcription downstream of IKKε/TBK1 activation.
In co-transfection experiments, we showed a direct interaction between IKKα and IRF3, which was associated with the increased recruitment of the transcriptional co-activator CBP, suggesting that IKKα directly regulates IRF3 transcriptional activity. We also showed IKKα strongly phosphorylates Ser402/404/405 in the C-terminal regulatory domain of IRF3 in vitro, phosphorylation of this cluster of residues has previously been shown to be important for IRF3 activation and IFNβ expression in response to viral infection (Lin et al, 1998). These data suggest that IKKα-mediated phosphorylation of IRF3 promotes CBP recruitment and transcriptional activity. Interestingly, IKKγ has been shown to be required for IKKε/TBK1-mediated Ser396 phosphorylation of IRF3 in response to viral infection (Zhao et al, 2007), this would also provide a platform to recruit both TAK1 and IKKα to the signalling complex and give access to IKKε/TBK1-phosphorylated IRF3.
In summary, IKKα has been shown to have anti-inflammatory activity through a variety of mechanisms (Lawrence et al, 2005; Li et al, 2005; Liu et al, 2007; Shembade et al, 2011); however, here we describe an important role for IKKα in priming adaptive immunity. This presents a unique role for IKKα in bridging the innate and adaptive immune systems; driving the resolution of inflammation while promoting acquired immunity. These properties of IKKα could be exploited therapeutically in several contexts; the pro-inflammatory side effects of adjuvants are a major barrier to vaccine development, adjuvants that promote IKKα activation may confer immuno-stimulatory functions without promoting excessive inflammation. On the other hand; inhibition of IKKα could have therapeutic potential in autoimmune diseases, such as rheumatoid arthritis (RA) and multiple sclerosis (MS), where targeting IKKα may block auto-antigen driven inflammation without compromising innate immunity and increasing susceptibility to opportunistic infections, the major limitation of current therapeutic approaches.
Materials and methods
Mice
IkkαAA/AA (Chuktm2Mka), Relb−/− (Relbtm1Brv), Nfkb2−/− (Nfkb2tm2Brv), Nfkb1Δ/Δ (Nfkb2tm1Brv) and Map3k7F/F.CreER (Map3k7tm1Aki/Tg(Rosa26-creERT2)) mice have been previously described (Ishikawa et al, 1997; Caamano et al, 1998, 1999; Cao et al, 2001; Wang et al, 2012). IkkαF/F mice (Chuktm1Yhu) (Liu et al, 2008) were provided by Y Hu (NICR, USA) and CD11c-Cre mice (Tg(Itgax-cre)1-1Reiz) (Caton et al, 2007) were provided by B Reizis (Columbia University, USA). Nik−/− mice (Map3k14tm1Rds) (Yin et al, 2001) were provided by R Schreiber (Washington University, USA). Irf3−/− (Bcl2l12/Irf3tm1Ttg), Irf3/7−/− (Bcl2l12/Irf3tm1Ttg/Irf7tm1Ttg) (Sato et al, 2000) mice were kindly provided by M Albert (Institute Pasteur, Paris, France). B6.SJL-Ptprca Pep3b/BoyJ (C57Bl6/Ly5.1), B6.129S7-Rag1tm1MomTg(TcraTcrb) (OT-I) and B6.129S7-Rag1tm1MomTg(TcraTcrb) (OT-II) mice were purchased from Taconic. OT-I.EGFP mice have been previously described (Bajenoff et al, 2010). All mice were housed under specific pathogen-free conditions and animal experimentation was conducted in strict accordance with good animal practice as defined by the French animal welfare bodies relative to European Convention (EEC Directive 86/609) and approved by the Direction Départmentale des Services Vétérinaires des Bouches du Rhônes.
Cell lines
Murine embryonic fibroblast (MEF) cell lines; Ikkβ−/− and Ikkα−/− have been previously described (Hu et al, 1997; Li et al, 1999). Nik−/− MEF cells were kindly provided by R Schreiber (Washington University, USA), Rela−/− MEF cells were provided by N Perkins (University of Newcastle, UK) and the Irf3−/− MEF cells were a gift from T Taniguchi (University of Tokyo, Japan).
Dendritic cells
BMDC: bone marrow cells were cultured for 7 days in DMEM containing 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin/streptomycin, 2 mM glutamine and 20 ng/ml murine GM-CSF (Peprotech). After 7 days, cells were collected and checked for CD11c and MHC II expression by FACS, these conditions routinely generated >97% CD11c+ MHC II+ cells. For splenic DC isolation, single-cell suspensions were prepared by enzymatic digestion of spleens with 50 μg/ml collagenase D, CD11c+ cells were purified by positive selection using MACS separation columns (Miltenyi Biotec) according to manufacturer’s instructions. The purity of DC populations was routinely between 96 and 99%.
T-cell priming assays
In vitro priming assay. Naïve CD4+ T cells isolated from LNs and spleens of OT-II mice using MACS negative selection columns were co-cultured with immature or LPS-activated BMDC (1:8/DC:T ratio) in presence or absence of chicken ovalbumin (OVA) peptide (OVA323–339). After 3 days, T-cell priming was measured by cytokine production in culture supernatants by ELISA.
DC adoptive transfer. BMDC were loaded with OVA323–339 (OVAp) in the presence or absence of LPS for 24 h, 2.5 × 105 OVAp-loaded DC were injected subcutaneously (s.c.) into the paw of naïve C57Bl6 mice, after 7 days popliteal LNs and spleens were collected and single-cell suspensions prepared. Cells were cultured in the presence or absence of OVA323–339 for 72 h and IFNγ production measured in culture supernatants by ELISA.
In vivo T-cell priming assay. 106 naïve CD4+ or CD8+ T cells, isolated by MACS selection from LN and spleen of OT-II or OT-I mice, respectively, were adoptively transferred to naïve mice 24 h before immunization with an emulsion of 400 μg heat-killed Mycobacterium tuberculosis H37RA (Difco, BD Bioscience) in Freunds’ incomplete adjuvant (CFA) and 50 μg OVA, by i.d. injection at the base of the tail. Five days later, inguinal LN was collected and single-cell suspension prepared, cells were cultured in the presence or absence of OT-II (OVA323–339) or OT-I (OVA257–264)-specific peptide for 72 h and IFNγ production measured in culture supernatants by ELISA.
Delayed-type (IV) hypersensitivity
Mice were immunized with 1 mg methylated bovine serum albumin (mBSA; Sigma) in CFA i.d. at the base of the tail, 14 days later 0.5 mg mBSA was injected s.c. in the right paw, and PBS alone was injected in the left paw as control. Paw swelling was measured at various time points by plethysmography. For ex vivo antigen re-call assays, splenocytes from immunized mice were stimulated with 25 μg/ml mBSA or 2.5 μg/ml ConA (Sigma).
Listeria monocytogenes infection
Recombinant L. monocytogenes expressing chicken ovalbumin (Lm-OVA) (Bajenoff et al, 2010) was grown in Brain Heart Infusion (BHI) broth to a density of 0.4 OD600 nm, equivalent to 108 CFU per ml. Bacteria in log-phase growth were washed in PBS and adjusted to the desired concentration before use. 104 naïve CD8+ T cells, purified from LN/spleen of OT-I.EGFP mice, were injected i.v. into naïve mice 24 h before primary infection with 104 CFU Lm-OVA i.v. 5 days later, liver and spleens were collected and homogenized in PBS 0.1% Triton X-100, CFU was determined by serial dilution on BHI agar plates. OT-I cell expansion was measured by FACS in the spleen 7 days after primary infection. Secondary infection with 106 CFU Lm-OVA was performed 35 days after primary infection. CFU in liver and spleen, and OT-I cell expansion was determined as described above. Re-call assays were performed with total splenocytes in the presence of 1 μM OVA257–264 and GolgiStop (BD Bioscience) for 6 h, intracellular IFNγ was measured in OT-I.EGFP cells by FACS.
Kinase assay, immunoblotting and immunoprecipitation
IKK kinase activity was measured in whole cell lysates after IP with anti-IKKγ antibody (BD Bioscience) as described previously (Lawrence et al, 2005). IKKα recovery was determined by immunoblotting (IB). IKKα kinase activity was measured with purified recombinant IKKα (Upstate Biotechnology). IB was performed on SDS–PAGE gel separated whole cell lysates. Co-IP experiments were performed on whole cell extracts prepared in cold lysis buffer containing10 mM Tris–HCl pH 8, 200 mM NaCl, 1 mM EDTA, 1 mM DTT, 0.5% NP-40, 0.5 mM PMSF and 5 μg/ml leupeptin, pepstatin and aprotinin, antibodies were pre-incubated with protein A-sepharose beads for 3 h at 4°C before incubation with protein extracts overnight.
Transfection and reporter assay
MEF or HEK293T-TLR3 (Invivogen) cells were plated in 12-well plates at 80–90% confluency and transfected with the indicated plasmids using Lipofectamine 2000 (Invitrogen), according to manufacturer’s instructions. Luciferase activity was determined with the Dual-Luciferase Reporter Assay System according to manufacturer’s instructions (Promega).
Statistical analysis
At least three independent experiments were performed, and representative data are shown unless otherwise indicated. Data are represented as mean±s.e.m. Student’s t test or Mann–Whitney test was performed where appropriate to test the statistical significance between data sets and P-values indicated.
Supplementary Material
Acknowledgments
We thank B Reizis (Columbia University, USA) for supplying CD11c-Cre mice, and M Albert (Institute Pasteur, Paris, France) for Irf3−/− and Irf3/7−/− mice. Nik−/−, Rela−/− and Irf3−/− MEF cells were kindly provided by R Schreiber (Washington University, USA), N Perkins (University of Newcastle, UK) and T Taniguchi (University of Tokyo, Japan), respectively. We thank P Cohen (University of Dundee, UK) for supplying MRT67307, MLN4924 and BI605906 inhibitors. We also thank R Lin (McGill University, CA) for providing various reagents and advice for IRF3 studies. These studies were supported by grants from L’Agence Nationale de la Recherche (ANR); ANR-09-MIEN-029-01, ANR-10-BLAN-1302-01 to TL, and institutional funding from INSERM, CNRS and the Universite Aix-Marseille. AM was supported by an FIRC fellowship and LL was supported by a fellowship from FRM. MK was supported by NIH grants: AI043477 and AI57153.
Author contributions: AM performed most of the experiments with significant contributions from MH, LL, MB and EJ; MH contributed data on Lm infection, EJ contributed data on DTH, LL and MB contributed data for Figures 5 and 7. CF and SM performed specific experiments. XW, MK, JC, HC and MBa contributed reagents. TL, AM, MH, EJ, MB and LL analysed the data. TL and AM wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Asquith DL, Miller AM, McInnes IB, Liew FY (2009) Animal models of rheumatoid arthritis. Eur J Immunol 39: 2040–2044 [DOI] [PubMed] [Google Scholar]
- Bajenoff M, Narni-Mancinelli E, Brau F, Lauvau G (2010) Visualizing early splenic memory CD8+ T cells reactivation against intracellular bacteria in the mouse. PLoS ONE 5: e11524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonizzi G, Bebien M, Otero DC, Johnson-Vroom KE, Cao Y, Vu D, Jegga AG, Aronow BJ, Ghosh G, Rickert RC, Karin M (2004) Activation of IKKalpha target genes depends on recognition of specific kappaB binding sites by RelB:p52 dimers. EMBO J 23: 4202–4210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonizzi G, Karin M (2004) The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol 25: 280–288 [DOI] [PubMed] [Google Scholar]
- Caamano J, Alexander J, Craig L, Bravo R, Hunter CA (1999) The NF-kappa B family member RelB is required for innate and adaptive immunity to Toxoplasma gondii. J Immunol 163: 4453–4461 [PubMed] [Google Scholar]
- Caamano JH, Rizzo CA, Durham SK, Barton DS, Raventos-Suarez C, Snapper CM, Bravo R (1998) Nuclear factor (NF)-kappa B2 (p100/p52) is required for normal splenic microarchitecture and B cell-mediated immune responses. J Exp Med 187: 185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Bonizzi G, Seagroves TN, Greten FR, Johnson R, Schmidt EV, Karin M (2001) IKKalpha provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell 107: 763–775 [DOI] [PubMed] [Google Scholar]
- Caton ML, Smith-Raska MR, Reizis B (2007) Notch-RBP-J signaling controls the homeostasis of CD8-dendritic cells in the spleen. J Exp Med 204: 1653–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev A, Iyer S, Razani B, Cheng G (2011) NF-kappaB and innate immunity. Curr Top Microbiol Immunol 349: 115–143 [DOI] [PubMed] [Google Scholar]
- Gautier G, Humbert M, Deauvieau F, Scuiller M, Hiscott J, Bates EE, Trinchieri G, Caux C, Garrone P (2005) A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med 201: 1435–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason CE, Ordureau A, Gourlay R, Arthur JS, Cohen P (2011) Polyubiquitin binding to optineurin is required for optimal activation of TANK-binding kinase 1 and production of interferon beta. J Biol Chem 286: 35663–35674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker H, Karin M (2006) Regulation and function of IKK and IKK-related kinases. Sci STKE 2006: re13. [DOI] [PubMed] [Google Scholar]
- Hiscott J, Lin R (2005) IRF-3 releases its inhibitions. Structure 13: 1235–1236 [DOI] [PubMed] [Google Scholar]
- Hofmann J, Mair F, Greter M, Schmidt-Supprian M, Becher B (2011) NIK signaling in dendritic cells but not in T cells is required for the development of effector T cells and cell-mediated immune responses. J Exp Med 208: 1917–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Sakaguchi S, Nakajima C, Watanabe A, Yanai H, Matsumoto M, Ohteki T, Kaisho T, Takaoka A, Akira S, Seya T, Taniguchi T (2003) Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc Natl Acad Sci USA 100: 10872–10877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Taniguchi T (2006) IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol 6: 644–658 [DOI] [PubMed] [Google Scholar]
- Hoshino K, Sasaki I, Sugiyama T, Yano T, Yamazaki C, Yasui T, Kikutani H, Kaisho T (2010) Critical role of IkappaB Kinase alpha in TLR7/9-induced type I IFN production by conventional dendritic cells. J Immunol 184: 3341–3345 [DOI] [PubMed] [Google Scholar]
- Hoshino K, Sugiyama T, Matsumoto M, Tanaka T, Saito M, Hemmi H, Ohara O, Akira S, Kaisho T (2006) IkappaB kinase-alpha is critical for interferon-alpha production induced by Toll-like receptors 7 and 9. Nature 440: 949–953 [DOI] [PubMed] [Google Scholar]
- Hu Y, Wang Y, Luo Z, Li C (1997) A new symmetrodont mammal from China and its implications for mammalian evolution. Nature 390: 137–142 [DOI] [PubMed] [Google Scholar]
- Hume DA (2008) Macrophages as APC and the dendritic cell myth. J Immunol 181: 5829–5835 [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Carrasco D, Claudio E, Ryseck RP, Bravo R (1997) Gastric hyperplasia and increased proliferative responses of lymphocytes in mice lacking the COOH-terminal ankyrin domain of NF-kappaB2. J Exp Med 186: 999–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R (2010) Regulation of adaptive immunity by the innate immune system. Science 327: 291–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Mak TW, Sen G, Li X (2004) Toll-like receptor 3-mediated activation of NF-kappaB and IRF3 diverges at Toll-IL-1 receptor domain-containing adapter inducing IFN-beta. Proc Natl Acad Sci USA 101: 3533–3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA (2002) In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 17: 211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y (2000) Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol 18: 621–663 [DOI] [PubMed] [Google Scholar]
- Lawrence T, Bebien M, Liu GY, Nizet V, Karin M (2005) IKKalpha limits macrophage NF-kappaB activation and contributes to the resolution of inflammation. Nature 434: 1138–1143 [DOI] [PubMed] [Google Scholar]
- Lecuit M (2007) Human listeriosis and animal models. Microbes Infect 9: 1216–1225 [DOI] [PubMed] [Google Scholar]
- Li Q, Lu Q, Bottero V, Estepa G, Morrison L, Mercurio F, Verma IM (2005) Enhanced NF-kappaB activation and cellular function in macrophages lacking IkappaB kinase 1 (IKK1). Proc Natl Acad Sci USA 102: 12425–12430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZW, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M (1999) The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J Exp Med 189: 1839–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Heylbroeck C, Pitha PM, Hiscott J (1998) Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol Cell Biol 18: 2986–2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Mamane Y, Hiscott J (1999) Structural and functional analysis of interferon regulatory factor 3: localization of the transactivation and autoinhibitory domains. Mol Cell Biol 19: 2465–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Xia X, Zhu F, Park E, Carbajal S, Kiguchi K, DiGiovanni J, Fischer SM, Hu Y (2008) IKKalpha is required to maintain skin homeostasis and prevent skin cancer. Cancer Cell 14: 212–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Yang Y, Chernishof V, Loo RR, Jang H, Tahk S, Yang R, Mink S, Shultz D, Bellone CJ, Loo JA, Shuai K (2007) Proinflammatory stimuli induce IKKalpha-mediated phosphorylation of PIAS1 to restrict inflammation and immunity. Cell 129: 903–914 [DOI] [PubMed] [Google Scholar]
- Manicassamy S, Pulendran B (2009) Modulation of adaptive immunity with Toll-like receptors. Semin Immunol 21: 185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Yoneyama M, Ito T, Takahashi K, Inagaki F, Fujita T (2004) Identification of Ser-386 of interferon regulatory factor 3 as critical target for inducible phosphorylation that determines activation. J Biol Chem 279: 9698–9702 [DOI] [PubMed] [Google Scholar]
- Ninomiya-Tsuji J, Kajino T, Ono K, Ohtomo T, Matsumoto M, Shiina M, Mihara M, Tsuchiya M, Matsumoto K (2003) A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J Biol Chem 278: 18485–18490 [DOI] [PubMed] [Google Scholar]
- Pamer EG (2004) Immune responses to Listeria monocytogenes. Nat Rev Immunol 4: 812–823 [DOI] [PubMed] [Google Scholar]
- Pauls E, Shpiro N, Peggie M, Young ER, Sorcek RJ, Tan L, Choi HG, Cohen P (2012) An essential role for IKKbeta in the production of type 1 interferons by plasmacytoid dendritic cells. J Biol Chem 287: 19216–19228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Carrozzi VR, Braas D, Bhatt DM, Cheng CS, Hong C, Doty KR, Black JC, Hoffmann A, Carey M, Smale ST (2009) A unifying model for the selective regulation of inducible transcription by CpG islands and nucleosome remodeling. Cell 138: 114–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N, Taniguchi T (2000) Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13: 539–548 [DOI] [PubMed] [Google Scholar]
- Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R (2001) Toll-like receptors control activation of adaptive immune responses. Nat Immunol 2: 947–950 [DOI] [PubMed] [Google Scholar]
- Schulz O, Edwards AD, Schito M, Aliberti J, Manickasingham S, Sher A, Reis e Sousa C (2000) CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity 13: 453–462 [DOI] [PubMed] [Google Scholar]
- Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, Karin M (2001) Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 293: 1495–1499 [DOI] [PubMed] [Google Scholar]
- Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J (2003) Triggering the interferon antiviral response through an IKK-related pathway. Science 300: 1148–1151 [DOI] [PubMed] [Google Scholar]
- Shembade N, Pujari R, Harhaj NS, Abbott DW, Harhaj EW (2011) The kinase IKKalpha inhibits activation of the transcription factor NF-kappaB by phosphorylating the regulatory molecule TAX1BP1. Nat Immunol 12: 834–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporri R, Reis e Sousa C (2005) Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat Immunol 6: 163–170 [DOI] [PubMed] [Google Scholar]
- Steinman RM (2008) Dendritic cells in vivo: a key target for a new vaccine science. Immunity 29: 319–324 [DOI] [PubMed] [Google Scholar]
- Stockinger S, Kastner R, Kernbauer E, Pilz A, Westermayer S, Reutterer B, Soulat D, Stengl G, Vogl C, Frenz T, Waibler Z, Taniguchi T, Rulicke T, Kalinke U, Muller M, Decker T (2009) Characterization of the interferon-producing cell in mice infected with Listeria monocytogenes. PLoS Pathog 5: e1000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Hoshino K, Saito M, Yano T, Sasaki I, Yamazaki C, Akira S, Kaisho T (2008) Immunoadjuvant effects of polyadenylic:polyuridylic acids through TLR3 and TLR7. Int Immunol 20: 1–9 [DOI] [PubMed] [Google Scholar]
- Sun JC, Bevan MJ (2003) Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300: 339–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140: 805–820 [DOI] [PubMed] [Google Scholar]
- Vallabhapurapu S, Matsuzawa A, Zhang W, Tseng PH, Keats JJ, Wang H, Vignali DA, Bergsagel PL, Karin M (2008) Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat Immunol 9: 1364–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ (2001) TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412: 346–351 [DOI] [PubMed] [Google Scholar]
- Wang Y, Huang G, Vogel P, Neale G, Reizis B, Chi H (2012) Transforming growth factor beta-activated kinase 1 (TAK1)-dependent checkpoint in the survival of dendritic cells promotes immune homeostasis and function. Proc Natl Acad Sci USA 109: E343–E352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, D'Amico A, Winkel KD, Suter M, Lo D, Shortman K (1998) RelB is essential for the development of myeloid-related CD8alpha- dendritic cells but not of lymphoid-related CD8alpha+ dendritic cells. Immunity 9: 839–847 [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S (2003) Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301: 640–643 [DOI] [PubMed] [Google Scholar]
- Yin L, Wu L, Wesche H, Arthur CD, White JM, Goeddel DV, Schreiber RD (2001) Defective lymphotoxin-beta receptor-induced NF-kappaB transcriptional activity in NIK-deficient mice. Science 291: 2162–2165 [DOI] [PubMed] [Google Scholar]
- Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M (1997) The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell 91: 243–252 [DOI] [PubMed] [Google Scholar]
- Zhao T, Yang L, Sun Q, Arguello M, Ballard DW, Hiscott J, Lin R (2007) The NEMO adaptor bridges the nuclear factor-kappaB and interferon regulatory factor signaling pathways. Nat Immunol 8: 592–600 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.