Abstract
Primary cilia are antenna-like sensory organelles protruding from the plasma membrane. Defects in ciliogenesis cause diverse genetic disorders. NDR2 was identified as the causal gene for a canine ciliopathy, early retinal degeneration, but its role in ciliogenesis remains unknown. Ciliary membranes are generated by transport and fusion of Golgi-derived vesicles to the pericentrosome, a process requiring Rab11-mediated recruitment of Rabin8, a GDP–GTP exchange factor (GEF) for Rab8, and subsequent Rab8 activation and Rabin8 binding to Sec15, a component of the exocyst that mediates vesicle tethering. This study shows that NDR2 phosphorylates Rabin8 at Ser-272 and defects in this phosphorylation impair preciliary membrane assembly and ciliogenesis, resulting in accumulation of Rabin8-/Rab11-containing vesicles at the pericentrosome. Rabin8 binds to and colocalizes with GTP-bound Rab11 and phosphatidylserine (PS) on pericentrosomal vesicles. The phospho-mimetic S272E mutation of Rabin8 decreases affinity for PS but increases affinity for Sec15. These results suggest that NDR2-mediated Rabin8 phosphorylation is crucial for ciliogenesis by triggering the switch in binding specificity of Rabin8 from PS to Sec15, thereby promoting local activation of Rab8 and ciliary membrane formation.
Keywords: ciliogenesis, NDR kinase, phosphatidylserine, Rabin8, Sec15
Introduction
Primary cilia are antenna-like sensory organelles protruding from the plasma membrane of many growth-arrested cells. Primary cilia consist of a microtubule-based axoneme, extending from the mother centriole-derived basal body, and a ciliary membrane surrounding the axoneme. Various signalling receptors and ion channels are enriched on ciliary membranes, allowing primary cilia to sense and transmit many chemical and mechanical cues from outside of the cell (Goetz and Anderson, 2010). Since primary cilia are required for development and homeostasis of many tissues, defects in primary cilium formation or function cause diverse genetic disorders, including Bardet–Biedl syndrome (BBS), polycystic kidney disease, and retinal degeneration (Fliegauf et al, 2007; Gerdes et al, 2009).
Vesicular trafficking to the vicinity of the centrosome plays a key role in ciliary membrane formation (Sorokin, 1962; Kim et al, 2010). In the initial step of ciliogenesis, Golgi- and recycling endosome-derived vesicles are recruited to the distal end of the mother centriole to generate the ‘ciliary vesicle’. As the axoneme grows, the ciliary vesicle elongates to form the bilayered ‘ciliary sheath’ enveloping the growing axoneme. Finally, the outer membrane of the ciliary sheath fuses with the plasma membrane, resulting in the emergence of cilium on the cell surface (Rohatgi and Snell, 2010). Recent studies have demonstrated that a signalling cascade, composed of Rab11, Rabin8 (a GDP–GTP exchange factor (GEF) for Rab8), and Rab8, plays a pivotal role in vesicle transport and fusion during ciliary membrane biogenesis (Hattula et al, 2002; Nachury et al, 2007; Yoshimura et al, 2007). Rab11, Rabin8, and Rab8 are the mammalian orthologs of the budding yeast Ypt31p/32p, Sec2p, and Sec4p, respectively. In yeast, Ypt31p/32p recruits Sec2p, a GEF for Sec4p, and then activated Sec4p promotes the docking and fusion of Golgi-derived vesicles to the plasma membrane of the bud for polarized cell growth (Walch-Solimena et al, 1997; Ortiz et al, 2002; Medkova et al, 2006). Similarly, in mammals, GTP-bound Rab11 binds to Rabin8 and activates its GEF activity towards Rab8; this cascade is required for directional vesicle trafficking to the centrosome and preciliary membrane assembly (Knödler et al, 2010; Bryant et al, 2010; Westlake et al, 2011). A recent study further revealed that Rabin8 transiently accumulates on the pericentrosome (Westlake et al, 2011), implicating Rabin8 in the initial step of ciliary membrane assembly; however, how vesicle trafficking and fusion are regulated at the onset of ciliogenesis remains elusive.
The exocyst is an octameric protein complex involved in the tethering and docking of vesicles to the plasma membrane and the centrosome (Heider and Munson, 2012; Hehnly et al, 2012). Recent studies have shown that Sec15, a component of the exocyst complex, is required for primary cilium formation (Feng et al, 2012). Sec15 is a downstream effector of Rab11 and Rab8, and can bind to Rabin8 (Wu et al, 2005; Feng et al, 2012), suggesting that the interplays between Rab11, Rabin8, Rab8, and Sec15 play a role in vesicle tethering and fusion in ciliary membrane formation. In yeast, Sec15p competitively binds to Sec2p with Ypt32p, and phosphatidylinositol 4-phosphate (PI4P) inhibits Sec15p binding to Sec2p; hence, the reduction in the level of PI4P causes a switch in Sec2p-binding partner from Ypt32p to Sec15p (Mizuno-Yamasaki et al, 2010). Some Sec2p mutants exhibit decreased affinity for Ypt32p but increased affinity for Sec15p (Mizuno-Yamasaki et al, 2010). Similar to yeast Sec2p, a Rabin8 mutant exhibits decreased affinity for Rab11 and increased affinity for Sec15 (Feng et al, 2012). Based on these findings, it has been postulated that Rabin8 can switch its binding specificity from Rab11 to Sec15 and this switch is crucial for vesicle trafficking and tethering during ciliary membrane formation; however, the mechanism physiologically triggering this switch, as well as the role of phospholipids in this switch in mammalian cells, remains unknown.
NDR1 and NDR2 (also known as STK38 and STK38L) are members of the NDR/LATS subfamily of serine/threonine kinases, which are highly conserved in eukaryotes (Hergovich et al, 2006; Emoto, 2011). Mammalian NDRs and their orthologs (including fruitfly Trc and budding yeast Cbk1p) are implicated in the regulation of polarized cell growth, dendrite and epidermal morphogenesis, centrosome duplication, mitotic chromosome alignment, and tumour suppression (Emoto et al, 2004; Chiba et al, 2009; Cornils et al, 2010). Recently, NDR2 was identified as the causal gene for a canine ciliopathy, early retinal degeneration (erd), implicating NDR2 in cilium formation (Goldstein et al, 2010; Berta et al, 2011). Knowledge of the signalling downstream of NDRs is limited. In budding yeast, Cbk1p was shown to phosphorylate Sec2p and thereby regulate the polarized transport of Golgi-derived vesicles (Kurischko et al, 2008). Very recently, NDRs were shown to phosphorylate Rabin8 and regulate dendrite arborization and spine development in rat hippocampal neurons (Ultanir et al, 2012). Since Rabin8 plays a crucial role in ciliogenesis, we hypothesized that NDR-mediated Rabin8 phosphorylation serves to control ciliogenesis.
This study provides evidence that NDR2 phosphorylates Rabin8 at Ser-272 and that this phosphorylation event is crucial for ciliogenesis. We show that Rabin8 binds to GTP-bound Rab11 and phosphatidylserine (PS), and that the phospho-mimetic mutation of Rabin8 decreases binding affinity for PS but increases affinity for Sec15. We propose that NDR2-mediated Rabin8 phosphorylation triggers the switch in binding specificity from PS to Sec15 and thereby promotes local activation of Rab8 and ciliary membrane formation.
Results
NDR kinases phosphorylate Rabin8 at Ser-272
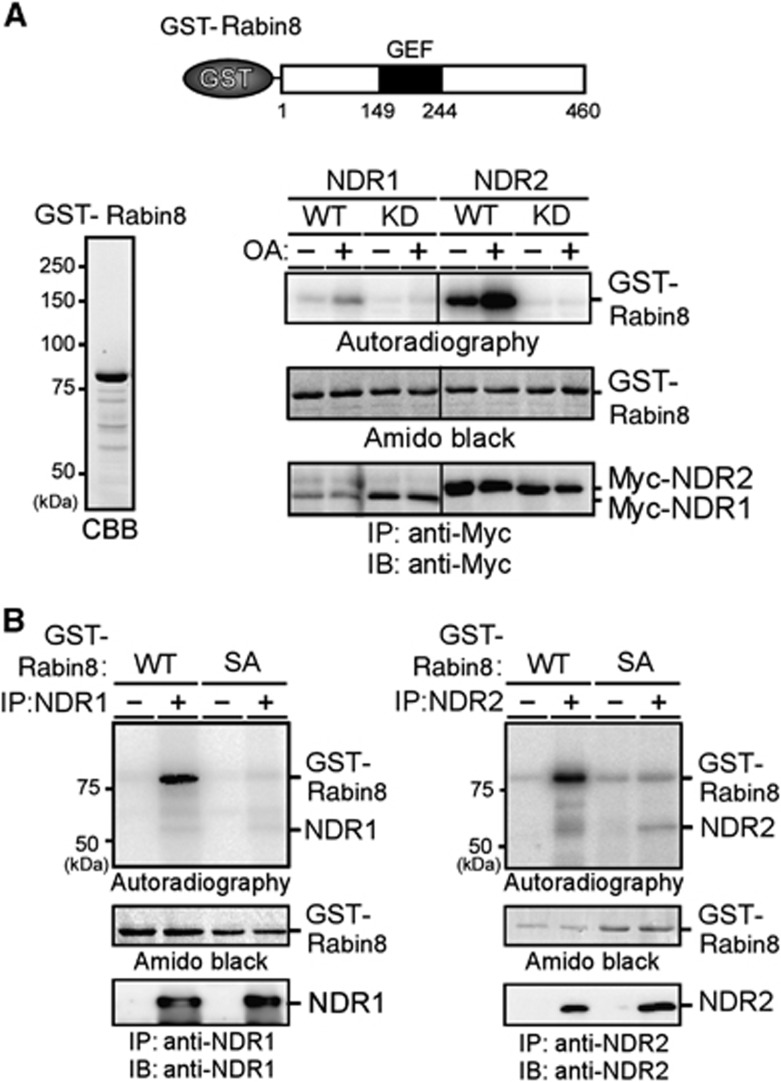
To determine whether NDR kinases (NDR1 and NDR2) phosphorylate Rabin8, Myc-tagged NDR1 and NDR2 were expressed in COS-7 cells, immunopurified with anti-Myc antibody, and then subjected to in vitro kinase assays using GST-Rabin8 as a substrate. Wild-type (WT) NDR1 and NDR2, but not their kinase-dead (KD) mutants, effectively phosphorylated Rabin8 (Figure 1A). The level of phosphorylation was elevated when COS-7 cells expressing Myc-NDRs were exposed to okadaic acid (OA), an inhibitor of protein phosphatase 2A known to increase the kinase activity of NDRs (Millward et al, 1999). Rabin8 contains the sequence HTRNKS272 adjacent to the C terminus of the GEF domain (amino acids 149–244). This sequence is consistent with the consensus motif of NDR substrates (HxRxxS/T) (Mazanka et al, 2008) and is conserved across vertebrate Rabin8 proteins. We therefore constructed the Rabin8 point mutant S272A, in which Ser-272 was replaced by alanine. In vitro kinase assays revealed that NDR1 and NDR2 effectively phosphorylated Rabin8(WT), but not Rabin8(S272A) (Figure 1B), indicating that NDR1 and NDR2 preferentially phosphorylate Rabin8 at Ser-272. Phosphorylation of Ser-272 by NDR2 was also shown by immunoblot analysis using the antibody specific to Ser-272-phosphorylated Rabin8 (Supplementary Figure S1A–C). NDRs effectively phosphorylated Rabin8, to the extent similar to histone H1, a generally used substrate (Supplementary Figure S1D).
Figure 1.
NDR kinases phosphorylate Rabin8 at Ser-272. (A) NDRs phosphorylate Rabin8. GST-Rabin8 was purified from E. coli (left panel). Myc-NDR1/2(WT) or KD mutants, which were expressed in COS-7 cells untreated or treated with OA, were immunoprecipitated and subjected to in vitro kinase assays using GST-Rabin8 as a substrate. Schematic structure of GST-Rabin8 is shown. Black box indicates the coiled–coil domain, which probably functions as a GEF domain. (B) NDRs phosphorylate Rabin8 at Ser-272. NDR1 (left panel) and NDR2 (right panel) were immunoprecipitated and subjected to in vitro kinase assays with GST-Rabin8(WT or S272A), as in (A).
Source data for this figure is available on the online supplementary information page.
NDR2-mediated Rabin8 phosphorylation is crucial for ciliogenesis
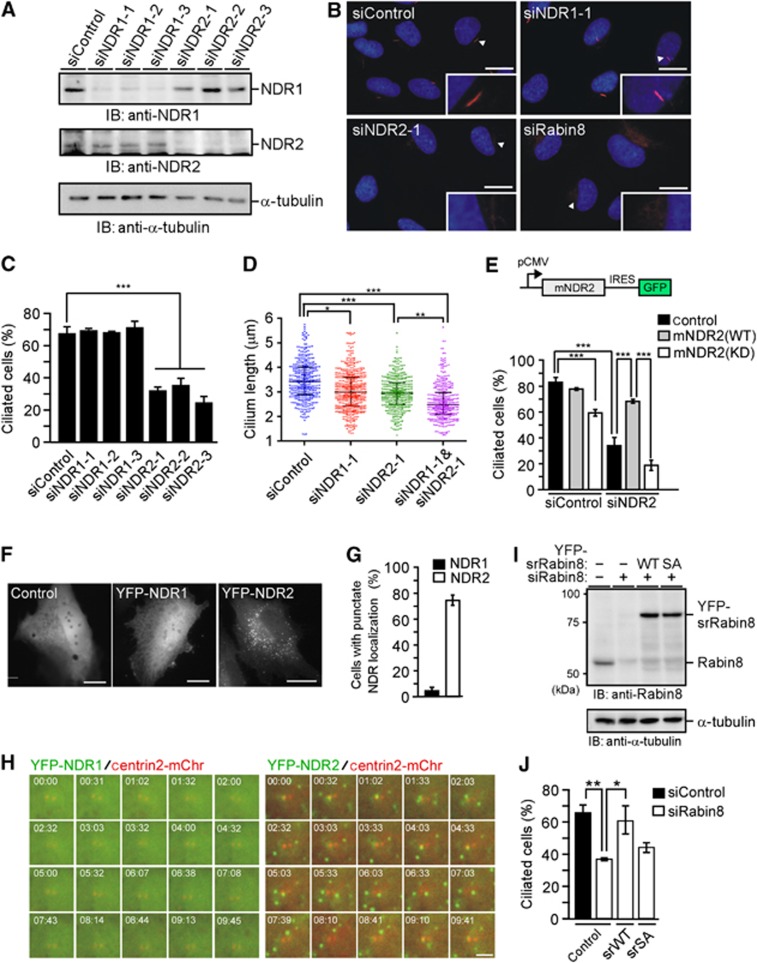
To explore the role of NDRs in ciliogenesis, the effect of NDR1 or NDR2 depletion on ciliogenesis was assessed in human telomerase reverse transcriptase (hTERT)-immortalized retinal pigmented epithelium (RPE)1 cell lines (hereafter referred to as RPE1 cells). RPE1 cells transfected with NDR1 or NDR2 siRNA were cultured for 48 h, and then subjected to serum starvation for 48 h, after which the number of ciliated cells was counted by staining acetyl (Ac)-tubulin. Immunoblot analyses revealed that transfection of NDR1 or NDR2 siRNA specifically suppressed the expression of each target protein in RPE1 cells (Figure 2A). Exposure to three independent NDR2 siRNAs significantly decreased the number of ciliated cells compared to control siRNA (Figure 2B and C; Supplementary Figure S2A). In contrast, NDR1 siRNAs had only a limited effect on ciliogenesis; the number of ciliated cells was slightly decreased only when we used the higher doses of NDR1 siRNA (Figure 2B and C; Supplementary Figure S2A and B). The length of cilia that were formed in NDR1- or NDR2-depleted cells was shorter than that in control cells (Figure 2D). Double knockdown of NDR1 and NDR2 slightly decreased the number of ciliated cells and the length of cilia, compared with the effect of NDR2 single knockdown (Figure 2D; Supplementary Figure S2C). These results indicate that NDR2 plays a preferential role in primary cilium formation whereas NDR1 appears to have a limited role. Inhibition of ciliation by NDR2 siRNA was significantly blocked in cells expressing siRNA-resistant (sr) mouse NDR2(WT), but not in cells expressing mouse kinase-dead NDR2(KD) (Figure 2E), indicating that NDR2 siRNA inhibits ciliogenesis by suppressing endogenous NDR2 expression and that the kinase activity of NDR2 is required for ciliogenesis. Expression of NDR2(KD) decreased the number of ciliated cells (Figure 2E), indicating that NDR2(KD) acts as a dominant-negative mutant for ciliogenesis.
Figure 2.
NDR2-mediated Rabin8 phosphorylation is crucial for ciliogenesis. (A) Effect of siRNAs on NDR1/2 expression. RPE1 cells were transfected with control, NDR1, or NDR2 siRNAs (25 nM), and cultured for 72 h. Cell lysates were analysed by immunoblotting with anti-NDR1 or anti-NDR2 antibody. (B) Effect of NDR1/2 or Rabin8 depletion on ciliogenesis. RPE1 cells transfected with siRNAs (25 nM) were cultured for 48 h and serum-starved for 48 h. Cells were stained for Ac-tubulin (red) and DAPI (blue). Scale bar, 10 μm; insets magnification, × 2.7. (C) Quantification of the number of ciliated cells. Data are means±s.e.m. (n=4; >100 cells per experiment, one-way ANOVA followed by Turkey test). ***P<0.001 compared with control siRNA. (D) Effect of NDR1/2 depletion on the cilium length (n=7 in control and NDR1 siRNA cells; n=6 in NDR2 siRNA cells; n=5 in NDR1/NDR2 double siRNA cells; >50 axonemes per experiment). Scatter plots show the median, 25th and 75th percentiles. Dots indicate individual data points. *P<0.05; **P<0.01; ***P<0.001, by paired t-test. (E) Kinase activity of NDR2 is required for ciliogenesis. RPE1 cells stably expressing control vector or sr mouse (m) NDR2(WT or KD) were transfected with NDR2 siRNA, cultured for 36 h and serum-starved for 36 h. Data are means±s.e.m. (n=3; >100 cells per experiment). ***P<0.001, by one-way ANOVA followed by Turkey test. (F) Localization of NDR1 and NDR2. RPE1 cells were transfected with control YFP, YFP-NDR1, or -NDR2, fixed and imaged by YFP fluorescence. Bar, 20 μm. (G) Quantification of the number of cells with punctate NDR localization. Data are means±s.e.m. (n=3; >40 cells per experiment). (H) Time-lapse fluorescent images of YFP-NDR1/2 and mCherry-centrin2 in serum-starved RPE1 cells. See also Supplementary Movie S1 and S2. Bar, 10 μm. (I) Expression levels of Rabin8 and YFP-sr-Rabin8(WT or S272A). RPE1 cells or those stably expressing YFP-sr-Rabin8(WT or S272A) were transfected with Rabin8 siRNA. Cell lysates were analysed by anti-Rabin8 immunoblotting. (J) Effect of S272A mutation on ciliogenesis. RPE1 cells or those stably expressing YFP-sr-Rabin8(WT or S272A) were transfected with Rabin8 siRNA and treated as in (C). Data are means±s.e.m. (n=3; >100 cells per experiment). *P<0.05, **P<0.01, by one-way ANOVA followed by Turkey test.
Source data for this figure is available on the online supplementary information page.
To explore how NDR2 has a more active role in ciliogenesis than NDR1, we analysed subcellular localization of YFP- or Myc-tagged NDR1 and NDR2 in RPE1 cells. As reported in other cells (Devroe et al, 2004), NDR2 exhibited a vesicular localization in the cytoplasm, whereas NDR1 was distributed diffusely in the cytoplasm and the nucleus in RPE1 cells (Figure 2F and G; Supplementary Figure S2D). Time-lapse fluorescence analysis of serum-starved RPE1 cells revealed that vesicular YFP-NDR2 signals were often associated with the centriole (Figure 2H; Supplementary Movies S1 and S2). Thus, the functional difference between NDR2 and NDR1 in ciliogenesis is likely due to their distinct subcellular localization.
NDR2 depletion had no apparent effect on the localization of centriolar proteins, including Cep170, centrin, cenexin, and ninein (Supplementary Figure S2E), indicating that NDR2 depletion does not affect structural organization and maturation of the centrosome. Additionally, Ki-67 staining revealed that most NDR2-depleted cells were in growth arrest after serum starvation (Supplementary Figure S2F), indicating that the inhibition of ciliogenesis by NDR2 depletion is not due to cell cycle perturbation.
To examine the role of Rabin8 phosphorylation in ciliogenesis, the effect of replacing endogenous Rabin8 with the S272A mutant was assessed. RPE1 cell lines stably expressing sr-YFP-Rabin8 or the S272A mutant were exposed to Rabin8 siRNA to deplete endogenous Rabin8. Rabin8 siRNA suppressed the expression of endogenous Rabin8 and the expression levels of YFP-sr-Rabin8 proteins were comparable to that of endogenous Rabin8 in control cells (Figure 2I). Exposure to Rabin8 siRNA significantly decreased the number of ciliated cells (Figure 2B and J); however, Rabin8 siRNA-induced inhibition of ciliation was significantly blocked in cells expressing sr-Rabin8(WT), but not in cells expressing the S272A mutant (Figure 2J). These results indicate that Rabin8 siRNA inhibits ciliogenesis by suppressing endogenous Rabin8 expression and that the S272A mutant fails to substitute for the function of endogenous Rabin8. Together, these results demonstrate that NDR-mediated Rabin8 phosphorylation at Ser-272 is crucial for ciliogenesis.
Non-phosphorylatable Rabin8 mutant or NDR2 depletion causes the accumulation of Rabin8 on the pericentrosome
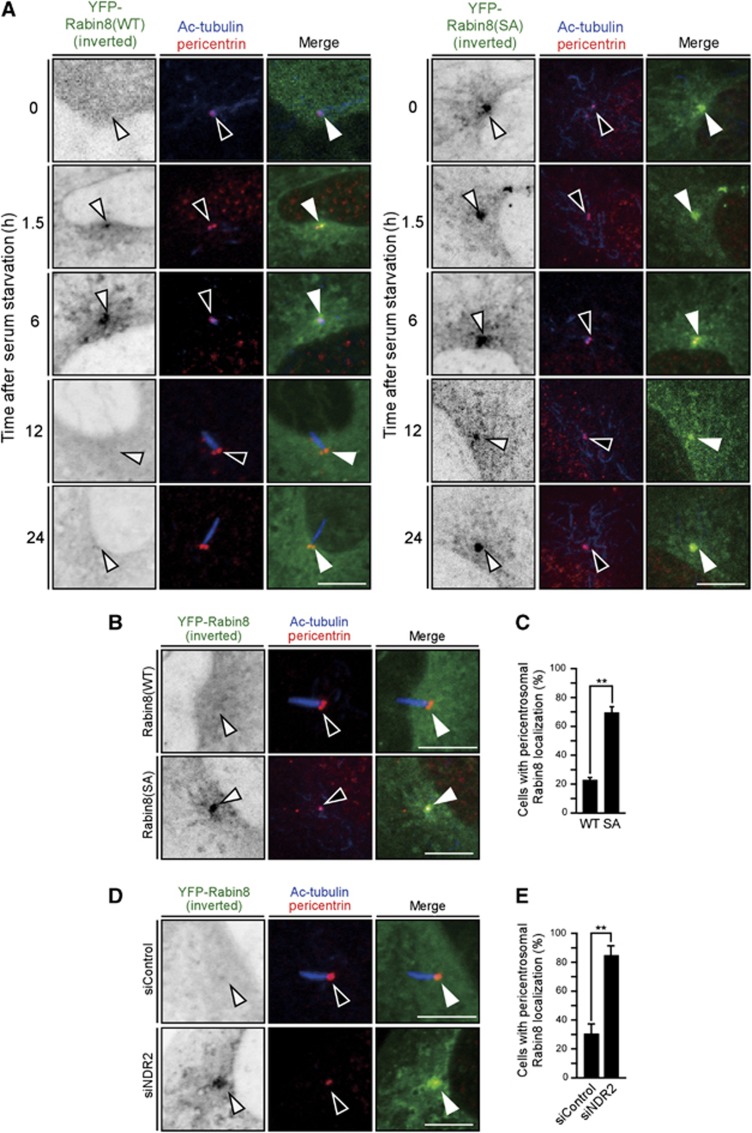
A recent study showed that, after serum starvation, Rabin8 transiently accumulates on pericentrosomal vesicles and then disperses prior to cilium formation (Westlake et al, 2011). When the localization of YFP-Rabin8, together with pericentrin (a marker of centrosomes) and Ac-tubulin (a marker of cilium), was analysed in RPE1 cells, Rabin8 was distributed diffusely in the cytoplasm in serum-fed cells but accumulated on the pericentrosome 1.5–6 h after serum starvation, and then dispersed 12 and 24 h after serum starvation (Figure 3A). The number of cells with pericentrosomal localization of YFP-Rabin8 increased 1–6 h after serum starvation and then gradually decreased; conversely, the number of ciliated cells (as measured by Ac-tubulin staining) gradually increased from 6–24 h after serum starvation (Supplementary Figure S3A). Thus, as reported (Westlake et al, 2011), Rabin8 transiently accumulates on the pericentrosome and then disperses as cilium formation proceeds.
Figure 3.
Non-phosphorylatable mutation of Rabin8 or depletion of NDR2 causes pericentrosomal accumulation of Rabin8. (A) Time course of Rabin8 localization after serum starvation. RPE1 cells stably expressing YFP-Rabin8(WT or S272A) were serum-starved. At the indicated times, cells were fixed and imaged by YFP fluorescence (inverted images or green) and pericentrin (red) and Ac-tubulin (blue) immunostaining. Arrowheads indicate the position of the centrosome. Bar, 10 μm. (B) S272A mutation causes pericentrosomal accumulation of Rabin8. RPE1 cells transfected with YFP-Rabin8(WT or S272A) were serum-starved for 24 h. Cells were imaged as in (A). Bar, 10 μm. (C) Quantification of the number of cells with pericentrosomal localization of YFP-Rabin8. (D) NDR2 depletion causes pericentrosomal accumulation of Rabin8. RPE1 cells transfected with YFP-Rabin8(WT) and control or NDR2 siRNA were serum-starved for 24 h. Cells were imaged as in (A). Bar, 10 μm. (E) Quantification of the number of cells with pericentrosomal localization of YFP-Rabin8. In (C) and (E), data are means±s.e.m. (n=3; >100 cells per experiment). **P<0.01, by paired t-test.
To examine whether phosphorylation of Rabin8 at Ser-272 is involved in localization, YFP-Rabin8 or the S272A mutant was expressed in RPE1 cells and localization was analysed 24 h after serum starvation. At that time point, YFP-Rabin8(WT) was dispersed diffusely throughout the cytoplasm in most cells, but the S272A mutant was retained on pericentrosomal vesicles (Figure 3B). The number of cells with pericentrosomal localization of YFP-Rabin8(S272A) was higher than that obtained with YFP-Rabin8(WT) (Figure 3C). We also analysed time-dependent changes in the localization of YFP-Rabin8(S272A) after serum starvation in RPE1 cells. In contrast to Rabin8(WT), Rabin8(S272A) almost constantly accumulated on the pericentrosome, regardless of time after serum starvation (Figure 3A; Supplementary Figure S3B). Accumulation of Rabin8(S272A) even in cells before serum starvation suggests the possibility that Rabin8 is usually transported to the centrosome and recycled into the cytoplasm and that Ser-272 phosphorylation is involved in the recycling of Rabin8 from the centrosome in serum-fed cells. The effect of NDR2 depletion on YFP-Rabin8 localization was also analysed. Importantly, depletion of NDR2 also caused pericentrosomal accumulation of YFP-Rabin8(WT) 24 h after serum starvation (Figure 3D). Quantitative analysis showed that the number of cells with pericentrosomal localization of YFP-Rabin8(WT) in NDR2-depleted cells was higher than that observed in control cells (Figure 3E). Thus, mutation of Rabin8 to the non-phosphorylatable S272A or depletion of NDR2 caused the accumulation of Rabin8 on the pericentrosome, which suggests that NDR2-mediated Rabin8 phosphorylation at Ser-272 is involved in the regulation of Rabin8 localization on the pericentrosome.
Rabin8 binds to GTP-bound Rab11 and colocalizes with Rab11-GTP-containing vesicles
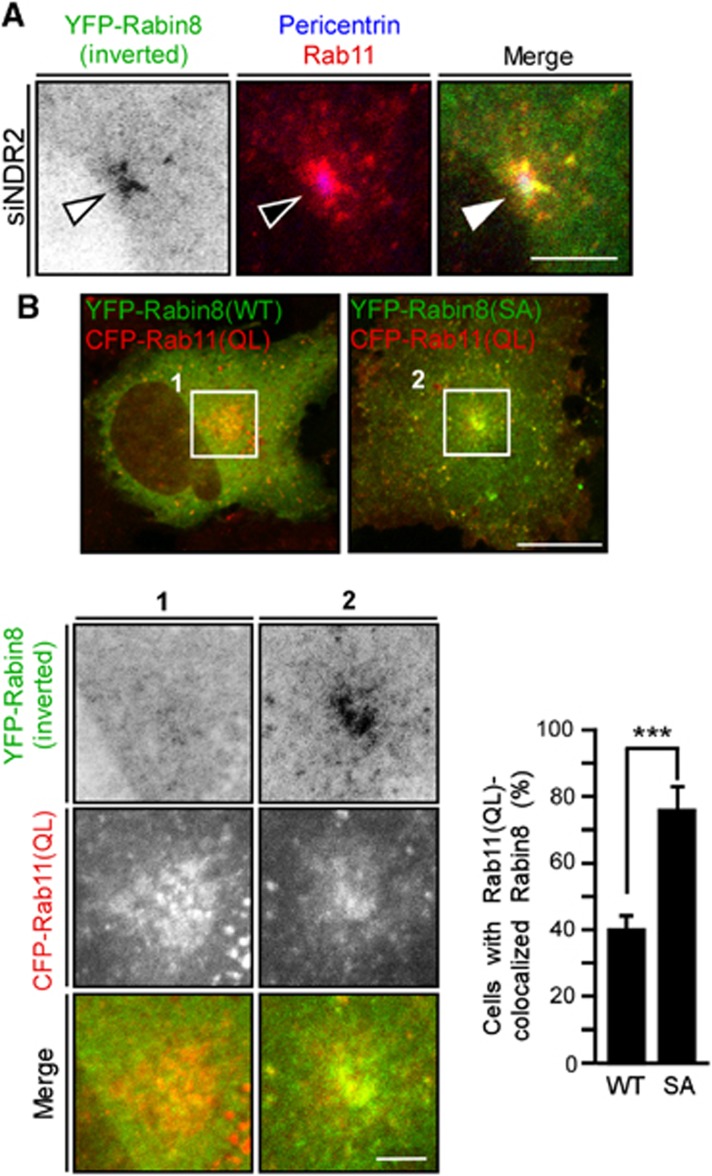
Rabin8 binds to GTP-bound Rab11 and this binding event is required for Rabin8 trafficking to the pericentrosome. As reported (Knödler et al, 2010), Rabin8 bound to Rab11(WT) and GTP hydrolysis-deficient Rab11(QL), but not to GDP-locked Rab11(SN) (Supplementary Figure S4A). Rabin8 colocalized with Rab11(WT) or Rab11(QL) on pericentrosomal and endosomal vesicles but diffusely distributed in the cytoplasm in cells expressing Rab11(SN) (Supplementary Figure S4B). Thus, GTP-bound Rab11 is required for the pericentrosomal localization of Rabin8. In NDR2-depleted cells, Rabin8 and Rab11 colocalized and accumulated on pericentrosomal vesicles (Figure 4A), indicating that Rabin8-containing pericentrosomal vesicles contain Rab11. When YFP-Rabin8(WT) or the S272A mutant was coexpressed with CFP-Rab11(QL), Rabin8(S272A) was more abundantly accumulated on the Rab11(QL)-containing vesicles around the centrosome than Rabin8(WT) (Figure 4B). These results indicate that the defect in Rabin8 phosphorylation leads to accumulation of Rabin8/Rab11-bearing vesicles around the centrosome. We examined whether Rabin8 phosphorylation affects its Rab11-binding affinity. GST pull-down assays showed that Rab11(QL) bound to Rabin8(WT) and to the S272A and S272E mutants to a similar extent (Supplementary Figure S4C), indicating that Rabin8 phosphorylation has no apparent effect on its binding to Rab11-GTP, at least in in vitro binding assays.
Figure 4.
Colocalization of Rabin8 with Rab11. (A) Rabin8 and Rab11 colocalize on pericentrosomal vesicles in NDR2-depleted cells. RPE1 cells were transfected with YFP-Rabin8(WT) and NDR2 siRNA and serum-starved. Cells were imaged by YFP fluorescence (green) and Rab11 (red) and pericentrin (blue) staining. Bar, 10 μm. (B) Rabin8(S272A) colocalizes with Rab11(QL) on pericentrosomal vesicles. RPE1 cells were cotransfected with YFP-Rabin8(WT or S272A) (green) and CFP-Rab11(QL) (red). Bar, 20 μm (top panel) and 5 μm (bottom panel). Right panel shows the quantification of the number of cells with YFP-Rabin8 colocalization with CFP-Rab11(QL) on pericentrosomal vesicles. Data are means±s.e.m. (n=3; >50 cells per experiment). ***P<0.001, by paired t-test.
Rab11 is anchored to the lipid membrane via C-terminal geranylgeranyl modifications. We examined whether this modification affects the binding ability of Rab11 to Rabin8, using a mutant Rab11(QL, 2CA) that is defective in geranylgeranyl modification. When the post-nuclear supernatants of cells expressing Rab11(QL) or Rab11(QL, 2CA) were subjected to coimmunoprecipitation assays with Rabin8, Rabin8 coprecipitated Rab11(QL) and Rab11(QL, 2CA) to a similar extent (Supplementary Figure S4D), indicating that the C-terminal lipid modification of Rab11 does not affect its binding to Rabin8.
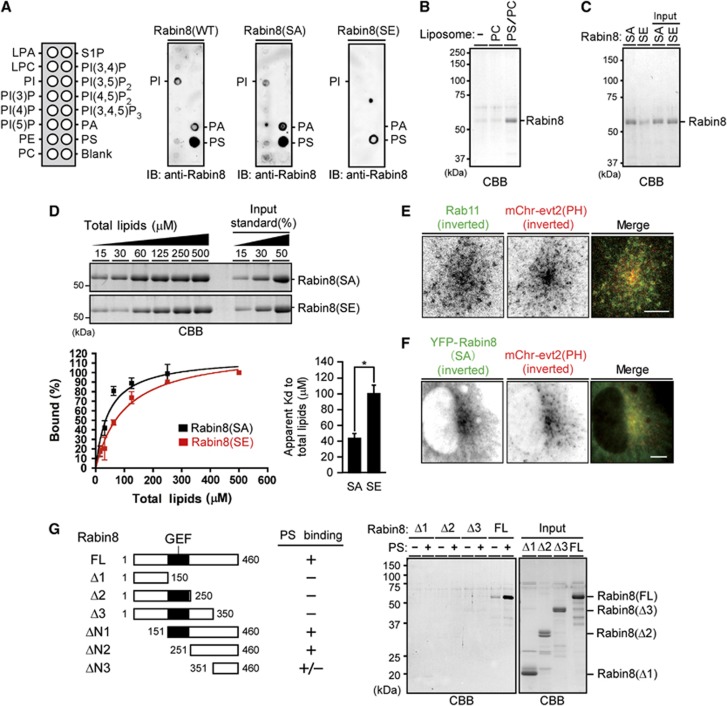
Rabin8 binds to PS and this binding is weakened by Ser-272 phosphorylation
Several lines of evidence indicate that Rab GTPases and phospholipids function cooperatively to define membrane identity and that the coincidence of both types of molecules controls the specificity of protein recruitment (Santiago-Tirado and Bretscher, 2011; Jean and Kiger, 2012). We examined whether Rabin8 binds to any specific phospholipid. Rabin8(WT) and the S272A and S272E mutants were purified and subjected to phospholipid blot and cosedimentation assays. Interestingly, phospholipid blot analysis revealed that Rabin8(WT) and Rabin8(S272A) bound strongly to PS and weakly to phosphatidic acid (PA) and phosphatidylinositol (PI), while Rabin8(S272E) displayed weaker binding to PS (Figure 5A). Phospholipid-cosedimentation assays using phospholipid-containing multilamellar vesicles also revealed that Rabin8(WT) bound to PS-containing vesicles, but not to control phosphatidylcholine (PC) vesicles (Figure 5B; Supplementary Figure S5A). Rabin8(S272A) bound to PS vesicles more tightly than Rabin8(S272E) (Figure 5C). Quantitative analysis showed that the apparent Kd value of Rabin8(S272E) to total lipids of PS vesicles was 2.3-fold higher than that of Rabin8(S272A) (Figure 5D). Under the conditions used, yeast Sec2p did not bind to PS vesicles (Supplementary Figure S5B). These results indicate that Rabin8 preferentially binds to PS and that the PS-binding ability of Rabin8 is weakened by Ser-272 phosphorylation.
Figure 5.
Rabin8 binds to and colocalizes with PS, and the phospho-mimic mutation of Rabin8 inhibits binding to PS. (A) Phospholipid blot assays. Rabin8 or the S272A and S272E mutants were purified from Sf-21 cells, incubated with phospholipid blots, and immunoblotted with anti-Rabin8 antibody. (B, C) Phospholipid-cosedimentation assays using multilamellar vesicles. In (B), purified Rabin8 was incubated with multilamellar vesicles composed of PC (100%) or PS/PC (15:85 molar ratio). In (C), purified Rabin8(S272A or S272E) mutants were incubated with multilamellar vesicles composed of PS/PC (15:85 molar ratio). After centrifugation, pellets were analysed by CBB staining. (D) Quantitative analysis of Rabin8 binding to PS vesicles. Purified Rabin8(S272A or S272E) was incubated with various amounts of multilamellar vesicles composed of PS/PC (15:85 molar ratio) and analysed, as in (C). The apparent dissociation constants (Kd) for the binding of Rabin8(S272A) and Rabin8(S272E) to total lipids of PS/PC vesicles were calculated. Data are means±s.e.m. (n=3). *P<0.05, by paired t-test. (E) Colocalization of Rab11 and PS. RPE1 cells transfected with mCherry-evt2(PH) were analysed by Rab11 staining (green) and mCherry fluorescence (red). Bar, 10 μm. (F) Colocalization of Rabin8(S272A) and PS on pericentrosomal vesicles. RPE1 cells transfected with YFP-Rabin8(S272A) and mCherry-evt2(PH) were serum-starved and imaged by YFP (green) and mCherry (red) fluorescence. Bar, 10 μm. (G) Mapping of the region of Rabin8 required for PS binding. Rabin8 deletion mutants purified from Sf-21 cells were subjected to phospholipid-cosedimentation assays, as in (C).
Source data for this figure is available on the online supplementary information page.
PS is enriched on the cytosolic surfaces of plasma membranes, recycling endosomes, and trans-Golgi network, and controls protein localization in cells (Yeung et al, 2008; Fairn et al, 2011; Uchida et al, 2011). Using the mCherry-tagged PH domain of evectin-2 [evt2(PH)], which specifically binds to PS (Uchida et al, 2011), the localization of PS together with Rab11 or Rabin8(S272A) was analysed in serum-starved RPE1 cells. Whereas mCherry-evt2(PH) was localized on the vesicular structures throughout the cytoplasm, Rab11 and Rabin8(S272A) colocalized with mCherry-evt2(PH) on pericentrosomal vesicles (Figure 5E and F; Supplementary Figure S6 (bottom panel)), indicating that Rabin8 localizes on PS/Rab11-containing pericentrosomal vesicles. Since the S272E mutation weakens the ability of Rabin8 to bind to PS, Ser-272 phosphorylation probably reduces the association of Rabin8 with PS/Rab11-containing vesicles.
Next, we analysed the region of Rabin8 required for binding PS. Cosedimentation assays using PS-containing multilamellar vesicles revealed that full-length Rabin8 bound to PS, but the C-terminally truncated mutants (Δ1, Δ2, or Δ3) did not (Figure 5G). The ΔN1 and ΔN2 mutants bound to PS, but the ΔN3 mutant bound to PS only weakly (Figure 5G; Supplementary Figure S5C and D). These results indicate that the C-terminal 351–460 region is required but not sufficient for effective binding to PS, and that the C-terminal ΔN2 region (amino acids 251–460) is a minimum PS-binding domain of Rabin8 at this moment. When YFP-Rabin8(WT) and its mutants were coexpressed with CFP-Rab11(QL) and mCherry-evt2(PH) in RPE1 cells, Rabin8(WT) and the S272A mutant were colocalized with Rab11(QL) and evt2(PH) around the centrosome, but colocalization of Rabin8 deletion mutants (including ΔN2) with Rab11(QL) and evt2(PH) was scarcely detectable (Supplementary Figure S6). Thus, the C-terminal ΔN2 region is not sufficient for effective localization of Rabin8 on the PS/Rab11-containing vesicles in cells and the N-terminal region appears to be involved in its vesicular localization. Additionally, ΔN2 and Δ3 localized on the centrosome (Supplementary Figure S6), suggesting that the common overlapping region (amino acids 251–350) is involved in the localization of Rabin8 to the centrosome.
Ser-272 phosphorylation promotes Rabin8 binding to Sec15
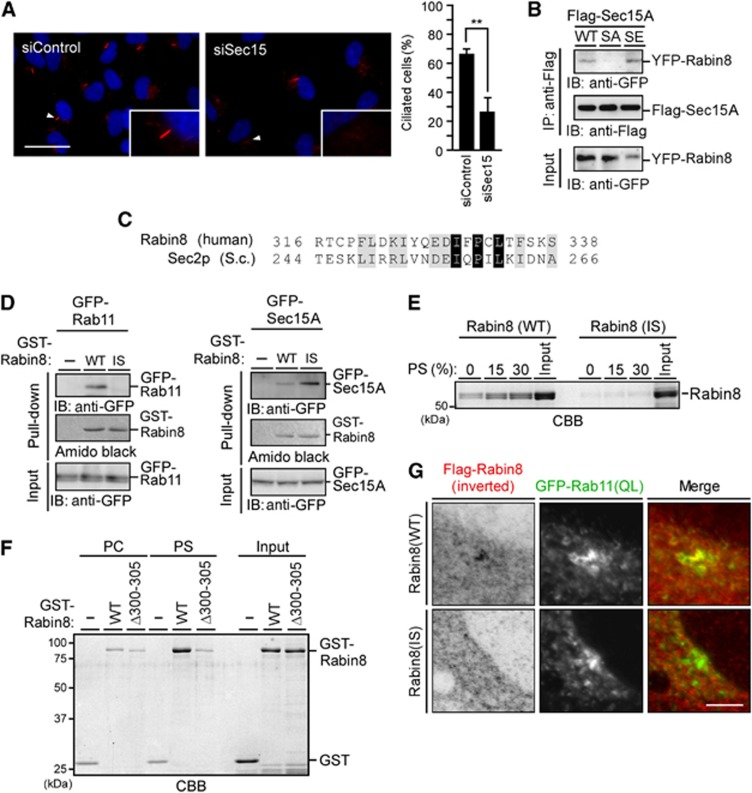
The exocyst component Sec15 is an effector of Rab11 and Rab8, and binds to Rabin8, implicating Sec15 in ciliogenesis (Wu et al, 2005; Feng et al, 2012). We examined the effect of Sec15 depletion on cilium formation in serum-starved RPE1 cells. Depletion of both Sec15A and Sec15B significantly decreased the number of ciliated cells (Figure 6A), indicating that Sec15 plays a critical role in ciliogenesis. Several yeast Sec2p mutations decreased the binding affinity for Ypt32p but increased affinity for Sec15p (Mizuno-Yamasaki et al, 2010). Similarly, a Rabin8 deletion mutant, Δ300–305, has decreased binding affinity for Rab11 but increased affinity for Sec15 (Feng et al, 2012). These results suggest that these mutations of Sec2p and Rabin8 alter binding specificity from Ypt32p/Rab11 to Sec15p/Sec15. Whereas yeast Sec2p-binding specificity was controlled by changing the level of PI4P (Mizuno-Yamasaki et al, 2010), how Rabin8-binding specificity is altered from Rab11 to Sec15 under physiological conditions remains unknown. We hypothesized that Ser-272 phosphorylation may affect the binding specificity of Rabin8. When Flag-tagged Sec15A was coexpressed with YFP-Rabin8(WT) or the S272A or S272E mutant and immunoprecipitated with anti-Flag antibody, Rabin8(WT) and the S272E mutant coprecipitated with Flag-Sec15A, but the S272A mutant did not (Figure 6B). This indicates that Ser-272 phosphorylation is required for Rabin8 binding to Sec15. Since the S272E mutation decreased binding to PS and increased binding to Sec15, Ser-272 phosphorylation is likely involved in the switch of Rabin8 from a form that preferentially binds PS to a form that preferentially binds Sec15.
Figure 6.
Phospho-mimic mutation of Rabin8 promotes binding to Sec15 and characterization of Rabin8(I329S) mutant. (A) Effect of Sec15 depletion on ciliogenesis. RPE1 cells transfected with Sec15A and Sec15B siRNAs were cultured for 48 h in growth medium and serum-starved for 48 h. Cells were fixed and stained for Ac-tubulin (red) and DNA (blue). Scale bar, 20 μm; insets magnification, × 2.7. Right panel shows the quantification of the number of ciliated cells. Data are means±s.e.m. (n=3; >100 cells per experiment). **P<0.01, by paired t-test. (B) The Rabin8 S272E mutant has increased binding ability to Sec15. RPE1 cells stably expressing Rabin8 (WT, S272A, or S272E) were transfected with Flag-Sec15. Cell lysates were immunoprecipitated with anti-Flag antibody and then analysed by anti-GFP immunoblotting. (C) Alignment of the sequences of human Rabin8 and yeast Sec2p. Black and grey boxes indicate identical and homologous residues, respectively. (D) Rabin8(I329S) has a lower affinity for Rab11 and a higher affinity for Sec15. GFP-Rab11 or GFP-Sec15 expressed in 293T cells was subjected to GST pull-down assays using GST (−) or GST-Rabin8(WT or I329S). (E) Rabin8(I329S) has decreased affinity for PS. Rabin8(WT or I329S) purified from Sf-21 cells was incubated with multilamellar vesicles composed of different PS/PC ratios and analysed as in Figure 5B. (F) Rabin8(Δ300–305) has decreased affinity for PS. GST-Rabin8(WT or Δ300–305) purified from Sf-21 cells was incubated with multilamellar vesicles composed of PS/PC (15:85 molar ratio) and analysed as in Figure 5B. (G) Rabin8(I329S) does not accumulate on the pericentrosome. RPE1 cells cotransfected with Flag-Rabin8(WT or I329S) and GFP-Rab11(QL) were serum-starved for 3 h. Cells were analysed by anti-Flag staining and GFP fluorescence. Bar, 10 μm.
Source data for this figure is available on the online supplementary information page.
Mutations of Rabin8 switch binding specificity from PS to Sec15
To examine whether the mutation of Rabin8 switches the binding specificity from PS/Rab11 to Sec15, Rabin8 mutants were constructed and their ability to bind to PS/Rab11 and Sec15 was analysed. Although the sequence conservation is low between Rabin8 and Sec2p in the region C-terminal to the GEF domains, a weak sequence similarity was observed around Ile-257 of Sec2p, the residue previously reported to be involved in the switch of Sec2p (Mizuno-Yamasaki et al, 2010) (Figure 6C). The Rabin8 I329S point mutant was constructed as an analogue of Sec2p(I257S) and analysed for its ability to bind to Rab11(QL), PS, and Sec15. Similar to Sec2p(I257S), Rabin8(I329S) lost the ability to bind to Rab11(QL) and exhibited increased binding to Sec15 (Figure 6D). Intriguingly, Rabin8(I329S) also lost PS-binding ability, as measured by phospholipid-cosedimentation assays (Figure 6E). In addition, Rabin8(Δ300–305), which was reported to bind to Sec15 but not to Rab11(QL) (Feng et al, 2012), did not bind to PS multilamellar vesicles (Figure 6F). Thus, both the Rabin8 I329S and Δ300–305 mutants exhibited decreased binding affinity for Rab11 and PS, and increased affinity for Sec15, which may reflect a switch in Rabin8 from a Rab11-/PS-binding form to a Sec15-binding form. Rabin8(I329S) was distributed diffusely in the cytoplasm and did not colocalize with Rab11(QL) on pericentrosomal vesicles in RPE1 cells (Figure 6G), indicating that binding to Rab11 and/or PS is required for Rabin8 recruitment and trafficking to the pericentrosome.
Rabin8 phosphorylation does not affect Rab8-GEF activity
Rabin8 is a GEF for Rab8 (Hattula et al, 2002). To assess whether Rabin8 phosphorylation at Ser-272 affects its GDP–GTP exchange activity, the exchange activity of Rabin8(WT) and that of the S272A and S272E mutants were measured. GST-Rabin8 and mutants were expressed in insect cells, purified, and used after cleavage of GST (Supplementary Figure S7A). Purified Rab8 was preloaded with [3H]GDP and the time course of nucleotide release was measured in the presence or absence of Rabin8. Rabin8(WT) and the S272A and S272E mutants stimulated GDP release from Rab8 to a similar extent (Supplementary Figure S7B), indicating that Ser-272 phosphorylation does not affect Rabin8 GEF activity.
Discussion
Ciliary membrane formation depends on the trafficking and fusion of vesicles to the distal end of the mother centriole (Sorokin, 1962; Kim et al, 2010; Rohatgi and Snell, 2010). In this study, we provide evidence that NDR2-mediated Rabin8 phosphorylation plays a crucial role in ciliogenesis by regulating the process of vesicular trafficking and tethering to the pericentrosome.
Consistent with previous studies (Knödler et al, 2010; Westlake et al, 2011), we showed that Rabin8 binds to GTP-bound Rab11 and this binding event is required for Rabin8 recruitment and trafficking to pericentrosomal vesicles. However, in vitro binding assays showed that purified Rab11(QL) exhibits very weak binding affinity for Rabin8, with a Kd value of ∼54 μM (Feng et al, 2012), which suggests that Rab11-GTP alone may not be sufficient for the effective recruitment of Rabin8 to vesicular membranes and that membrane components may assist the binding of Rabin8 to Rab11-containing vesicles. In this respect, previous studies demonstrated that Rab GTPases and phospholipids cooperatively function to recruit selective proteins (Santiago-Tirado and Bretscher, 2011; Jean and Kiger, 2012). This study showed that Rabin8 preferentially binds to PS. PS is most concentrated in recycling endosomes and the trans-Golgi network among intracellular organelles, where Rab11 is highly concentrated (Fairn et al, 2011; Uchida et al, 2011). Using the PH domain of evectin-2, we showed colocalization of Rabin8, Rab11, and PS on pericentrosomal vesicles, suggesting that PS and Rab11 colocalize and function cooperatively to recruit Rabin8 to Rab11-/PS-containing vesicles and to transport it to the pericentrosome. In accordance with this, two Rabin8 mutants, Rabin8(I329S) and Rabin8(Δ300–305), which exhibit decreased binding affinity for Rab11 and PS, were diffusely distributed in the cytoplasm and did not accumulate on the pericentrosome. Thus, the recruitment and trafficking of Rabin8 appears to be regulated by Rab11 and PS.
In yeast, Sec2p is recruited to secretory vesicles by interacting with Ypt32p and PI4P, and then switches its binding partner from Ypt32p to Sec15p as the PI4P level on vesicles declines (Mizuno-Yamasaki et al, 2010). Several Sec2p mutations decrease binding to Ypt32p and increase binding to Sec15p (Mizuno-Yamasaki et al, 2010). Two Rabin8 mutants, Δ300–305 and I329S, exhibit decreased binding to Rab11 and PS, and increased binding to Sec15. Therefore, similar to yeast Sec2p, Rabin8 may switch its conformation from a Rab11-/PS-binding form to a Sec15-binding form. Intriguingly, the S272E mutant had decreased binding to PS and increased binding to Sec15, compared to the S272A mutant, which suggests that Ser-272 phosphorylation induces the switch of Rabin8 from a PS-binding form to a Sec15-binding form. This switch probably contributes to the transfer of Rabin8 from Rab11-/PS-containing vesicles to Sec15-containing exocysts.
Based on our observations, we propose a model for the role of Rabin8 phosphorylation in the trafficking, tethering, and fusion of vesicles for ciliary membrane formation (Supplementary Figure S8). Rabin8 is initially recruited to the Rab11-/PS-bearing vesicles, which become transported to the pericentrosome. Rabin8 converts Rab8-GDP to active Rab8-GTP. Since the exocyst localizes on the distal end of the mother centriole (Hehnly et al, 2012), these vesicles are anchored to the distal end of the mother centriole via the interaction between Rab8-GTP (or Rab11-GTP) and Sec15. Rabin8 is then phosphorylated by NDR2 and transferred from the Rab11/PS vesicles to Sec15 (exocyst) on the mother centriole. The interaction between Rabin8 and Cep164 (a centriolar protein that was recently identified as a Rabin8 interactor) (Schmidt et al, 2012) may also contribute to anchor Rabin8 on the centriole. Since Sec15 is an effector of Rab8, the interaction between Rabin8 (Rab8 GEF) and Sec15 (Rab8 effector) can induce a positive feedback loop, by which local activation of Rab8 is attainable. Given the critical role of Rab8 in ciliary membrane formation (Nachury et al, 2007; Westlake et al, 2011), Rabin8-mediated local activation of Rab8 on the distal end of the mother centriole probably promotes the tethering of precursor vesicles to the mother centriole as well as the tethering and fusion between precursor vesicles, which leads to ‘ciliary vesicle’ formation and expansion. Defects in Rabin8 phosphorylation probably block the transfer of Rabin8 to the centriole and local activation of Rab8, resulting in the inhibition of tethering and fusion of vesicles for ciliogenesis. It could be that the phosphorylation event is also required as the initial step for the release of Rabin8 from the centrosome and its recycling, although the mechanism of Rabin8 release from the centrosome has yet to be elucidated.
Previous studies showed that Rabin8 binds to Rab11 and Sec15 through amino acids 262–331 (Knödler et al, 2010; Feng et al, 2012). Since the deletion of the C-terminal 332–460 region of Rabin8 enhances the binding of Sec15 and weakens the binding of Rab11, the C-terminal region seems to play an autoinhibitory role for Sec15 binding and to promote Rab11 binding (Feng et al, 2012). Because Rabin8 mutants (Δ300–305 and I329S) displaying a higher affinity for Sec15 and a lower affinity for Rab11/PS have mutations within amino acids 262–331, these mutations may cause a conformational change that relieves the autoinhibitory interaction of the C-terminal region, allowing binding to Sec15, and concomitantly disrupt binding to Rab11/PS. We showed that the C-terminal region (amino acids 351–460) of Rabin8 is required but not sufficient for binding to PS, which further supports a model in which 262–331 region acts cooperatively with the C-terminal region to bind to PS. Since the Ser-272 phosphorylation site also exists in the 262–331 region, it is possible that Ser-272 phosphorylation destroys the intramolecular interaction between the 262–331 region and the C-terminal region, thereby promoting the binding of Sec15 and inhibiting the binding of PS. More detailed biochemical and structural analyses are required to understand the molecular mechanisms by which Rabin8 interacts with Rab11, PS, and Sec15, and by which Rabin8 phosphorylation regulates binding specificity.
NDR kinases are activated downstream of MST kinases (termed Hippo in Drosophila) (Hergovich et al, 2006; Emoto, 2011). The MST (Hippo) pathway plays crucial roles in restraining cell proliferation, controlling organ size, and suppressing tumour progression (Zeng and Hong, 2008). In Drosophila, the Hippo pathway is activated by signalling events triggered by cell–cell contacts (Grusche et al, 2010). Since the primary cilium is formed in response to growth-inhibitory signals, such as serum starvation and cell–cell contact, the upstream regulators of the MST pathway may be involved in primary ciliogenesis via NDR activation.
Defects in primary cilium formation impair cell sensitivity to external signals in various tissues and can cause diverse genetic disorders (Fliegauf et al, 2007; Gerdes et al, 2009). In the retina, defects in ciliogenesis-related genes cause aberrant accumulation of rhodopsin-bearing vesicles at the base of the connecting cilium and impair rhodopsin transport to the outer segment of photoreceptor cells, resulting in retinal degeneration (Fliegauf et al, 2007). Recently, NDR2 (also called STK38L) was identified as the causal gene for canine erd, the genetic disorder corresponding to the human ciliopathy Leber congenital amaurosis (Goldstein et al, 2010; Berta et al, 2011), which may reflect the crucial role of NDR2 in vesicle transport in the retinal connecting cilium.
In budding yeast, Cbk1p phosphorylates Sec2p and regulates polarized transport of Golgi-derived vesicles, although the phosphorylation site has not been determined (Kurischko et al, 2008). In addition, during meiotic sporulation in fission yeast, Golgi-derived vesicles are transported to the spindle pole body (a functional analogue of the centrosome) and fuse with each other to form the forespore membrane. This process seemingly resembles ciliary membrane formation; in both processes, vesicles accumulate and fuse in the vicinity of the spindle pole body or centrosome. Intriguingly, NDR-related kinase Mug27/Slk1 as well as the Rabin8 ortholog Spo13 contribute to forespore membrane formation in fission yeast (Shimoda, 2004; Ohtaka et al, 2008; Perez-Hidalgo et al, 2008; Yan et al, 2008; Yan and Neiman, 2010), which suggests an evolutionarily conserved role for NDR-related kinases in vesicle trafficking and fusion. Very recently, NDR kinases were shown to phosphorylate proteins involved in vesicle trafficking, including Rabin8 and adaptor-associated kinase (AAK1), and to be implicated in dendrite branching and spine development (Ultanir et al, 2012). Together with our findings, NDR family kinases appear to have a function, conserved from yeast to humans, in regulating intracellular membrane biogenesis by phosphorylating Rabin8 and other substrates related to vesicle trafficking and fusion.
In conclusion, we provide evidence that NDR2-mediated Rabin8 phosphorylation is crucial for ciliogenesis and that this phosphorylation event triggers a switch in the binding preference of Rabin8 from PS to Sec15, thereby regulating the trafficking, tethering, and fusion of pericentrosomal vesicles in ciliary membrane formation. Our findings will help to understand better the molecular mechanisms and the signalling pathways regulating ciliogenesis and causing ciliopathies. Future studies will further uncover the roles of NDR kinases in vesicle trafficking and fusion.
Materials and methods
Reagents and antibodies
OA was purchased from Sigma. NDR1 antibody was prepared as described previously (Chiba et al, 2009). Rabbit polyclonal antibodies specific to NDR2 were raised against the peptide CSDILQPVPNTTEPDYKS (MBL). Rabbit antibodies specific to Rabin8 were raised against recombinant Rabin8 purified from Escherichia coli. These antibodies were purified using antigen-conjugated columns. Other antibodies were purchased as follows: rabbit polyclonal antibodies against pericentrin (Babco) and GFP (Molecular Probes), and mouse monoclonal antibodies against Myc (Roche; 9E10), GST (Cell Signalling), Flag (Sigma; M2), acetylated α-tubulin (Sigma; 6-11B-1), α-tubulin (Sigma; GTU-88), γ-tubulin (Sigma; B-5-1-2), and Rab11 (BD Pharmingen; 47/Rab11). The secondary antibodies used in this study were FITC-labelled anti-rabbit IgG (Chemicon), rhodamine-labelled anti-mouse IgG (Chemicon), and Alexa Fluor 488- or 633-labelled anti-mouse or rabbit IgG (Molecular Probes).
Plasmid construction and siRNA duplex
The cDNAs coding for mouse NDR1 (STK38), NDR2 (STK38L), Rab8a, Rab11a, and Sec15A were PCR-amplified from a mouse cDNA library. Rabin8 cDNA (FLJ25115) and centrin2 (FLJ92207) were purchased from the National Institute of Technology and Evaluation, Kisarazu, Japan. PCR-amplified cDNA fragments were subcloned into the pCMV-Flag, FPC1-Myc, pEYFP-C1, pEGFP-C1, pmCherry-C1 (Clontech), pGEX-6P1 (GE Healthcare), and pFastBac1 (Invitrogen) expression vectors. The cDNA plasmids for Rabin8 and NDR1/2 mutants were constructed using the Quick Change site-directed mutagenesis kit (Stratagene). The Stealth siRNAs targeting human NDR1, NDR2, Rabin8, and Sec15A/B were purchased from Invitrogen. The siRNA sequences are shown in Supplementary data. Control Stealth siRNA was also purchased from Invitrogen. For rescue experiments, mouse NDR2 (WT or KD) cDNA (containing four distinct bases from human NDR2 within the siRNA sequence) was subcloned into a pIRES-AcGFP1 vector (Clontech). To construct plasmids for sr-Rabin8, five bases in the targeting sequences were mutated by PCR-based mutagenesis.
Cell culture, transfection, and assay for primary cilium formation
hTERT-RPE1 (referred to as RPE1) cells were cultured in DMEM/F12 supplemented with 10% FCS. Transfection was performed using FuGene-HD (Promega). RPE1 cells stably expressing YFP-sr-Rabin8 or sr-NDR2 were generated by plasmid transfection followed by selection with G418. For analysing the effect of siRNAs on cilia formation, 0.5–1.0 × 105 cells plated on cover slips in 35 mm plates were transfected with 12.5–100 nM (usually 25 nM) siRNAs using Lipofectamine RNAiMax (Invitrogen). Cells were shifted from 10 to 0.2% serum 24–48 h after transfection and then fixed 72–96 h post transfection.
In vitro kinase assay
NDRs were immunoprecipitated with anti-Myc, anti-NDR1, or anti-NDR2 antibody. Immunoprecipitates were washed three times with wash buffer A (20 mM Tris–HCl, pH 7.5, 300 mM NaCl, 10% glycerol, 1% NP-40, 50 mM NaF, 10 mM Na3VO4, 10 μg/ml leupeptin, and 1 mM DTT), twice with wash buffer B (buffer A containing 150 mM NaCl), and then once with kinase buffer (20 mM Tris–HCl, pH 7.5, 1 mM DTT, and 10 mM MgCl2). After centrifugation, kinase reactions were carried out in 20 μl of kinase buffer containing 50 mM ATP, 2.5 μCi [γ-32P] ATP, and 2 μg GST-Rabin8 (purified from E. coli) at 30°C for 60 min. The reaction mixture was separated by SDS–PAGE and analysed by autoradiography to measure 32P-labelled GST-Rabin8.
Purification of recombinant proteins
GST-tagged Rabin8, Rab8a, and Rab11 were expressed in Sf-21 cells using the Bac-to-Bac baculovirus expression system (Invitrogen) and purified with glutathione-Sepharose (GE Healthcare) as described previously (Kurita et al, 2008). Non-tagged proteins were purified by cleaving GST-tagged proteins with 20 units of PreScission Protease (GE Healthcare). GST-tagged Rabin8 was expressed in E. coli at 16°C overnight with 20 μM IPTG and purified by the same method as that used for proteins from insect cells.
Protein–lipid-binding assay
Recombinant Rabin8 or mutants were purified from Sf-21 cells. Phospholipid blots were performed using 0.5 μg/ml of purified Rabin8 and PIP strips (Echeron Biosciences). Phospholipid-cosedimentation assays using phospholipid-containing multilamellar vesicles were performed as described (Fukuda et al, 1996). Briefly, multilamellar vesicles composed of PC or PS/PC were prepared in 50 mM HEPES-KOH (pH 7.2) and 100 mM NaCl by sonication, collected by centrifugation, and equilibrated with 50 mM HEPES-KOH (pH 7.2). Rabin8 was incubated with multilamellar vesicles corresponding to 160 μg of phospholipid in 500 μl HEPES buffer for 15 min at room temperature. After centrifugation at 12 000 g for 10 min at room temperature, the pellets were washed with HEPES buffer and then extracted with ice-cold acetone for 30 min at −20°C. The pellets obtained by centrifugation at 12 000 g at 4°C were subjected to SDS–PAGE.
Immunostaining and fluorescence microscopy
For cell staining, RPE1 cells grown on coverslips were washed with PBS and fixed with ice-cold methanol for 10 min or 4% paraformaldehyde at 37°C for 15 min, followed by permeabilization with 0.1% Triton X-100 for 5 min. For endogenous Rab11 staining, cells were fixed with trichloroacetic acid for 15 min at 4°C, followed by permeabilization with 0.1% Triton X-100 for 5 min, then subjected to staining with the antibody diluted with Can-Get-Signal immunostain (Toyobo, Osaka, Japan). Fluorescent images were obtained using a LSM710 confocal microscope (Carl Zeiss) equipped with a Plan Apochromat × 63 oil immersion objective lens (NA 1.4) and processed using ImageJ software. Time-lapse fluorescent images were taken using a Leica DMIRBE fluorescent microsope, equipped with a PL Apo 63 × oil objective lens (NA 1.3) and a cooled charge-coupled device camera (CoolSNAP HQ; Roper Scientific) driven by Metamorph imaging software (Molecular devices).
Supplementary Material
Acknowledgments
We thank Y Kanno for technical assistance, H Nakanishi and M Matsuyama for providing hTERT-RPE1 cells, S Fukai for providing Sec2p cDNA, and K Ohashi, S Kanno, A Yasui, T Inoue, T Kobayashi, and K Tanaka for valuable comments. This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and Uehara Memorial Foundation.
Author contributions: SC and YA designed and performed experiments. YH performed experiments. MF provided several cDNAs, antibodies, and intellectual input. SC and KM wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Berta AI, Boesze-Battaglia K, Genini S, Goldstein O, O'Brien PJ, Szel A, Acland GM, Beltran WA, Aguirre GD (2011) Photoreceptor cell death, proliferation and formation of hybrid rod/S-cone photoreceptors in the degenerating STK38L mutant retina. PLoS One 6: e24074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Datta A, Rodriguez-Fraticelli AE, Peränen J, Martin-Belmonte F, Mostov KE (2010) A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol 12: 1035–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S, Ikeda M, Katsunuma K, Ohashi K, Mizuno K (2009) MST2- and Furry-mediated activation of NDR1 kinase is critical for precise alignment of mitotic chromosomes. Curr Biol 19: 675–681 [DOI] [PubMed] [Google Scholar]
- Cornils H, Stegert MR, Hergovich A, Hynx D, Schmitz D, Dirnhofer S, Hemmings BA (2010) Ablation of the kinase NDR1 predisposes mice to the development of T cell lymphoma. Sci Signal 3: ra47. [DOI] [PubMed] [Google Scholar]
- Devroe E, Erdjument-Bromage H, Tempst P, Silver PA (2004) Human Mob proteins regulate the NDR1 and NDR2 serine-threonine kinases. J Biol Chem 279: 24444–24451 [DOI] [PubMed] [Google Scholar]
- Emoto K (2011) The growing role of the Hippo-NDR kinase signalling in neuronal development and disease. J Biochem 150: 133–141 [DOI] [PubMed] [Google Scholar]
- Emoto K, He Y, Ye B, Grueber WB, Adler PN, Jan LY, Jan YN (2004) Control of dendritic branching and tiling by the tricornered-kinase/furry signaling pathway in Drosophila sensory neurons. Cell 119: 245–256 [DOI] [PubMed] [Google Scholar]
- Fairn GD, Schieber NL, Ariotti N, Murphy S, Kuerschner L, Webb RI, Grinstein S, Parton RG (2011) High-resolution mapping reveals topologically distinct cellular pools of phosphatidylserine. J Cell Biol 194: 257–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Knödler A, Ren J, Zhang J, Zhang X, Hong Y, Huang S, Peränen J, Guo W (2012) A Rab8 guanine nucleotide exchange factor-effector interaction network regulates primary ciliogenesis. J Biol Chem 287: 15602–15609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegauf M, Benzing T, Omran H (2007) When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol 8: 880–893 [DOI] [PubMed] [Google Scholar]
- Fukuda M, Kojima T, Mikoshiba K (1996) Phospholipid composition dependence of Ca2+-dependent phospholipid binding to the C2A domain of synaptotagmin IV. J Biol Chem 271: 8430–8434 [DOI] [PubMed] [Google Scholar]
- Gerdes JM, Davis EE, Katsanis N (2009) The vertebrate primary cilium in development, homeostasis, and disease. Cell 137: 32–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV (2010) The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet 11: 331–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein O, Kukekova AV, Aguirre GD, Acland GM (2010) Exonic SINE insertion in STK38L causes canine early retinal degeneration (erd). Genomics 96: 362–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusche FA, Richardson HE, Harvey KF (2010) Upstream regulation of the Hippo size control pathway. Curr Biol 20: R574–R582 [DOI] [PubMed] [Google Scholar]
- Hattula K, Furuhjelm J, Arffman A, Peränen J (2002) A Rab8-specific GDP/GTP exchange factor is involved in actin remodeling and polarized membrane transport. Mol Biol Cell 13: 3268–3280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehnly H, Chen CT, Powers CM, Liu HL, Doxsey S (2012) The centrosome regulates the Rab11-dependent recycling endosome pathway at appendages of the mother centriole. Curr Biol 22: 1944–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider MR, Munson M (2012) Exorcising the exocyst complex. Traffic 13: 898–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergovich A, Stegert MR, Schmitz D, Hemmings BA (2006) NDR kinases regulate essential cell processes from yeast to humans. Nat Rev Mol Cell Biol 7: 253–264 [DOI] [PubMed] [Google Scholar]
- Jean S, Kiger AA (2012) Coordination between RAB GTPase and phosphoinositide regulation and functions. Nat Rev Mol Cell Biol 13: 463–470 [DOI] [PubMed] [Google Scholar]
- Kim J, Lee JE, Heynen-Genel S, Suyama E, Ono K, Lee K, Ideker T, Aza-Blanc P, Gleeson JG (2010) Functional genomic screen for modulators of ciliogenesis and cilium length. Nature 464: 1048–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knödler A, Feng S, Zhang J, Zhang X, Das A, Peränen J, Guo W (2010) Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci USA 107: 6346–6351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurischko C, Kuravi VK, Wannissorn N, Nazarov PA, Husain M, Zhang C, Shokat KM, McCaffery JM, Luca FC (2008) The yeast LATS/Ndr kinase Cbk1 regulates growth via Golgi-dependent glycosylation and secretion. Mol Biol Cell 19: 5559–5578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita S, Watanabe Y, Gunji E, Ohashi K, Mizuno K (2008) Molecular dissection of the mechanisms of substrate recognition and F-actin-mediated activation of cofilin-phosphatase Slingshot-1. J Biol Chem 283: 32542–32552 [DOI] [PubMed] [Google Scholar]
- Mazanka E, Alexander J, Yeh BJ, Charoenpong P, Lowery DM, Yaffe M, Weiss EL (2008) The NDR/LATS family kinase Cbk1 directly controls transcriptional asymmetry. PLoS Biol 6: e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medkova M, France YE, Coleman J, Novick P (2006) The rab exchange factor Sec2p reversibly associates with the exocyst. Mol Biol Cell 17: 2757–2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward TA, Hess D, Hemmings BA (1999) Ndr protein kinase is regulated by phosphorylation on two conserved sequence motifs. J Biol Chem 274: 33847–33850 [DOI] [PubMed] [Google Scholar]
- Mizuno-Yamasaki E, Medkova M, Coleman J, Novick P (2010) Phosphatidylinositol 4-phosphate controls both membrane recruitment and a regulatory switch of the Rab GEF Sec2p. Dev Cell 18: 828–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peränen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK (2007) A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129: 1201–1213 [DOI] [PubMed] [Google Scholar]
- Ohtaka A, Okuzaki D, Nojima H (2008) Mug27 is a meiosis-specific protein knase that functions in fission yeast meiosis II and sporulation. J Cell Sci 121: 1547–1558 [DOI] [PubMed] [Google Scholar]
- Ortiz D, Medkova M, Walch-Solimena C, Novick P (2002) Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J Cell Biol 157: 1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Hidalgo L, Rozalen AE, Martin-Castellanos C, Moreno S (2008) Slk1 is a meiosis-specific Sid2-related kinase that coordinates meiotic nuclear division with growth of the forespore membrane. J Cell Sci 121: 1383–1392 [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Snell WJ (2010) The ciliary membrane. Curr Opin Cell Biol 22: 541–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Tirado FH, Bretscher A (2011) Membrane-trafficking sorting hubs: cooperation between PI4P and small GTPases at the trans-Golgi network. Trends Cell Biol 21: 515–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KN, Kuhns S, Neuner A, Hub B, Zentgraf H, Pereira G (2012) Cep164 mediates vesicular docking to the mother centriole during early steps of ciliogenesis. J Cell Biol 199: 1083–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda C (2004) Forespore membrane assembly in yeast: coordinating SPBs and membrane trafficking. J Cell Sci 117: 389–396 [DOI] [PubMed] [Google Scholar]
- Sorokin S (1962) Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol 15: 363–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Hasegawa J, Chinnapen D, Inoue T, Okazaki S, Kato R, Wakatsuki S, Misaki R, Koike M, Uchiyama Y, Iemura S, Natsume T, Kuwahara R, Nakagawa T, Nishikawa K, Mukai K, Miyoshi E, Taniguchi N, Sheff D, Lencer WI et al. (2011) Intracellular phosphatidylserine is essential for retrograde membrane traffic through endosomes. Proc Natl Acad Sci USA 108: 15846–15851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ultanir SK, Hertz NT, Li G, Ge WP, Burlingame AL, Pleasure SJ, Shokat KM, Jan LY, Jan YN (2012) Chemical genetic identification of NDR1/2 kinase substrates AAK1 and Rabin8 uncovers their roles in dendrite arborization and spine development. Neuron 73: 1127–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena C, Collins RN, Novick PJ (1997) Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J Cell Biol 137: 1495–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlake CJ, Baye LM, Nachury MV, Wright KJ, Ervin KE, Phu L, Chalouni C, Beck JS, Kirkpatrick DS, Slusarski DC, Sheffield VC, Scheller RH, Jackson PK (2011) Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci USA 108: 2759–2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Mehta SQ, Pichaud F, Bellen HJ, Quiocho FA (2005) Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat Struct Mol Biol 12: 879–885 [DOI] [PubMed] [Google Scholar]
- Yan HJ, Ge W, Chew TG, Chow JY, McCollum D, Neiman AM, Balasubramanian MK (2008) The meiosis-specific Sid2p-related protein Slk1p regulates forespore membrane assembly in fission yeast. Mol Biol Cell 19: 3676–3690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HJ, Neiman AM (2010) A guanine nucleotide exchange factor is a component of the meiotic spindle pole body in Schizosaccharomyces pombe. Mol Biol Cell 21: 1272–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung T, Gilbert GE, Shi J, Silvius J, Kapus A, Grinstein S (2008) Membrane phosphatidylserine regulates surface charge and protein localization. Science 319: 210–213 [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Egerer J, Fuchs E, Haas AK, Barr FA (2007) Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol 178: 363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q, Hong W (2008) The emerging role of the Hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell 13: 188–192 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.