Abstract
Background
Few studies address concurrent exposures to common household allergens, specific allergen sensitization and childhood asthma morbidity.
Objective
To identify levels of allergen exposures that trigger asthma exacerbations in sensitized individuals.
Methods
We sampled homes for common indoor allergens (fungi, dust mites (Der p 1, Der f 1), cat (Fel d 1), dog (Can f 1) and cockroach (Bla g 1)) for levels associated with respiratory responses among school-aged children with asthma (N=1233) in a month-long study. Blood samples for allergy testing and samples of airborne fungi and settled dust were collected at enrollment. Symptoms and medication use were recorded on calendars. Combined effects of specific allergen sensitization and level of exposure on wheeze, persistent cough, rescue medication use and a 5-level asthma severity score were examined using ordered logistic regression.
Results
Children sensitized and exposed to any Penicillium experienced increased risk of wheeze (odds ratio [OR] 2.12 95% confidence interval [CI] 1.12, 4.04), persistent cough (OR 2.01 95% CI 1.05, 3.85) and higher asthma severity score (OR 1.99 95% CI 1.06, 3.72) compared to those not sensitized or sensitized but unexposed. Children sensitized and exposed to pet allergen were at significantly increased risk of wheeze (by 39% and 53% for Fel d 1 > 0.12 µg/g and Can f 1>1.2 µg/g, respectively). Increased rescue medication use was significantly associated with sensitization and exposure to Der p 1 > 0.10 µg/g (by 47%) and Fel d 1>0.12 µg/g (by 32%).
Conclusion
Asthmatic children sensitized and exposed to low levels of common household allergens Penicillium, Der p 1, Fel d 1 and Can f 1 are at significant risk for increased morbidity.
Keywords: Asthma, Mold, Dust mites, Pet allergens, Wheezing
1. Introduction
Both general atopy and degree of specific sensitization are prominent features of asthma phenotypes (Fitzpatrick et al., 2011). Commonly encountered indoor triggers of asthma include allergens (e.g., from airborne fungi (Gent et al., 2002; Turyk et al., 2006), dust mites (Gehring et al., 2001), pets (Gehring et al., 2001), cockroach (Gruchalla et al., 2005)), respiratory irritants (e.g., from cigarette smoking and other home combustion sources such as gas stoves) (Belanger et al., 2006) as well as respiratory viruses (Miller, 2010).
Levels of common outdoor molds (e.g., Alternaria and Cladosporium) observed indoors are strongly influenced by their numbers outdoors (Platts-Mills et al., 1987). In New England highest outdoor levels of Cladosporium are found in spring to late fall and very little is present during the winter, while Penicillium shows much lower seasonal variability (Ren et al., 1999). In fact, Penicillium can survive in house dust (a reservoir for fungal spores) for 5 years (Scott et al., 2004). The presence of moisture in a home often leads to an infestation of fungi (Bush and Portnoy, 2001).
The amount of antigen measured in household dust depends on presence of water damage, dampness, climate, pet ownership and location (i.e., urban, suburban, rural) (Gehring et al., 2004; Gent et al., 2009). The most commonly encountered indoor dust allergens in the Northeastern US are house dust mite allergens (Dermatophagoides pteronyssinus [Der p] and D. farinae [Der f]), cat (Felis domesticus [Fel d]), dog (Canis familiaris [Can f]), and cockroach (Blattella germanica [Bla g]) (Leaderer et al., 2002).
Although there are many studies on the association of common household exposures and asthma symptoms, our goal was to address concurrent household exposures, specific allergen sensitization and childhood asthma morbidity. In the present study of school-age children with asthma (n=1233) living in urban and suburban Connecticut and Western Massachusetts, we tested for specific allergic sensitivity to common indoor allergens: fungi (Penicillium and Cladosporium), dust mites (Der p 1, Der f 1), cat (Fel d 1), dog (Can f 1) and cockroach (Bla g 1). In order to identify levels of household allergen exposures that trigger asthma exacerbations in sensitized individuals, we sampled homes for common allergens, then assessed the association between specific allergic status, level of household exposure to specific allergens and asthma severity as measured by days of wheeze, persistent cough, rescue medication use, and an asthma severity score for the month immediately following allergy testing and sample collection.
2. Materials and methods
2.1. Study population
Families with an asthmatic child were recruited through schools in Connecticut and the Springfield and Worcester areas of Massachusetts to enroll in the parent study which was a prospective investigation of the effect of nitrogen dioxide (NO2) on asthma severity. Children were eligible if they were age 5–10, had a caregiver who spoke English, and had active asthma defined as two of the following: physician diagnosis, asthma symptoms within the past 12 months (wheeze, persistent cough, chest tightness, shortness of breath), and/or use of prescription asthma medication within the past 12 months. Enrollment was contingent on completion of successful blood sample collection for allergy testing. From 2006–2009, 1642 eligible children were identified and 1401 enrolled in the parent study. Included in the present analysis were participants with complete information on health outcome measures during the first, one-month monitoring period, allergy test results and successful sampling of fungal and dust allergen levels in their homes (N=1233). The Human Investigation Committee of Yale University, New Haven, Connecticut, approved this study. All respondents (primary caregivers of study subjects) gave written consent and all children age 7 and older gave assent.
2.2. Data collection
At the home visit, the respondent provided demographic information (self-identified race/ethnicity, respondent education level in years, housing type); information about the child’s home environment (reported smoking, pets, presence of visible mold/mildew in the home); and medical history. The respondent was given a calendar for recording the child’s daily asthma symptoms and medication use.
By design each monitoring period was one-month long (actual mean (standard deviation [SD]) 32 (6.9) days, median 30 days), at the end of which a research assistant phoned to collect daily symptoms (wheeze, persistent cough, shortness of breath, chest tightness), night symptoms and use of asthma medication (short-acting rescue medications and/or maintenance [controller] medications including inhaled or systemic steroids, cromolyn sodium, leukotriene inhibitors).
2.3. Allergy testing
Blood samples were collected at the time of enrollment. Serum was analyzed using the UniCAP® system for a 10 allergen-specific IgE panel including fungi (Penicillium notatum [properly known as P. chrysogenum], Cladosporium herbarum), dust mite (Der p 1, Der f 1), cat (Fel d 1), dog (Can f 1), cockroach (Bla g 1), meadow grass (Kentucky blue, Poa pratensis), ragweed (Ambrosia elatior), and egg as well as total IgE. General atopy was defined as a total IgE greater than age-adjusted levels (Mayo Clinic, 2006) or a positive response (specific IgEZ0.35 kilo units per liter [kU/L]) to one or more of the specific allergen panel.
2.4. Environmental sampling
Environmental samples were collected once during the study at the time of the enrollment home visit. Indoor airborne fungal propagules were collected using a Burkard Portable Sampler (Burkard Manufacturing Co. Ltd., Rickmansworth, UK) in the main living area. The research assistant obtained a single sample using a plate with dichloran-18% glycerol (DG-18) agar, and a sampler air collection rate of 20 liters per minute [L/min] for 1 min. Samples were brought to the study laboratory for incubation at 25 °C for approximately 7 days after which the resulting fungal colonies were identified, enumerated and reported as colony-forming units per cubic meter (CFU/m3).
Dust samples were collected once at the time of the home visit from the floor and furniture of the main living area following a protocol described previously (Gent et al., 2002, 2009; Belanger et al., 2003). Samples were assayed by enzyme-linked immunosorbent assay (ELISA) for allergens including Der p1, Der f 1, Fel d 1, Can f 1, and Bla g 1 (Gent et al., 2009). Results were recorded as micrograms per gram (µg/g) for dust mite and pet allergens, and as units of allergen per gram (U/g) for cockroach allergen.
Indoor NO2 for this analysis was measured for one month using two passive monitors (Palmes tubes) (Palmes et al., 1976) placed by a research assistant at the time of enrollment —one in the main room and the other in the child’s bedroom. Respondents were contacted by phone at the end of the monitoring period and instructed to remove, cap, and return the samplers to the study center in a prepaid mailer. Level of indoor NO2 (in parts per billion [ppb]) for each home was calculated as the mean of the two samplers.
2.5. Health outcome variables
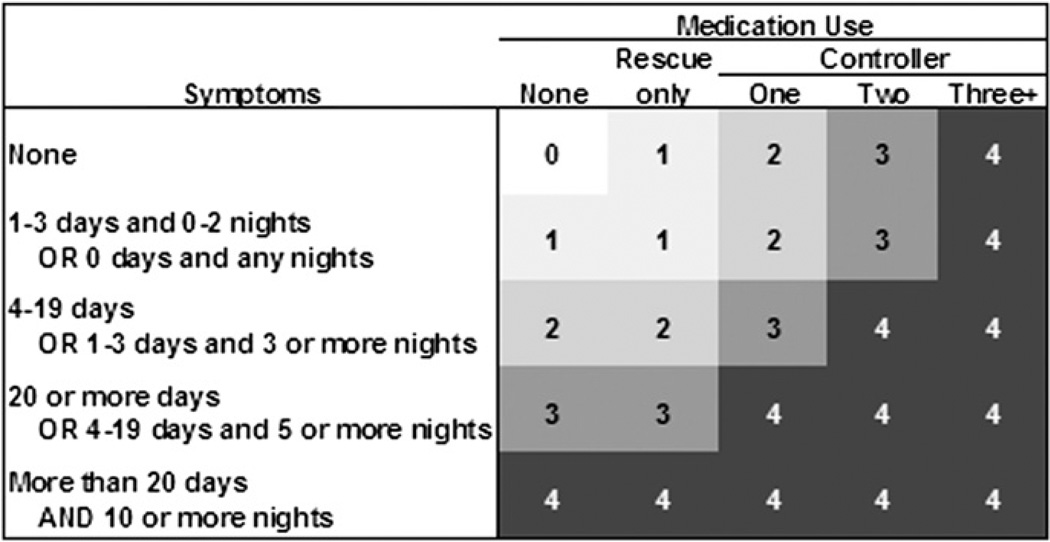
Days of wheeze, persistent cough and rescue medication use were standardized to a 28-day monitoring period and categorized as no symptoms or inhaler use, 1–4 days, or >4 days. Additionally, a 5-level asthma severity score based on the Global Initiative for Asthma Guidelines (US Department of Health and Human Services, 2002) was calculated as illustrated in Fig. 1 using total number of days and/or nights with symptoms standardized to a 28-day monitoring period, together with maintenance medication use. Symptom and medication use were combined to create a score that reflects the dependence of symptom frequency on level of medication (Fig.1).
Fig. 1.
Five-level asthma severity score, based on the Global Initiative for Asthma Guidelines (US Department of Health and Human Services, 2002), is shown as numbers in shaded area in body of figure: 0 (no symptoms or medication), 1 (intermittent), 2 (mild persistent), 3 (moderate persistent), 4 (severe persistent). Scores are calculated as a combination of frequency of symptoms during the day (wheeze, persistent cough, shortness of breath, or chest tightness) as well as night symptoms, and medication use (rescue and controller) during the month-long observation period. (See Section 2.).
2.6. Exposure variables
Distributions of fungi and dust allergen levels were highly skewed and were subjected to a natural log transformation (of CFU/m3+1 for fungi and values below detection recoded to level of detection for dust allergens) then grouped evenly into categories above detection no smaller than 20% of the total distribution. This resulted in exposure-concentration categories of two (Penicillium, Bla g 1), three (Cladosporium, Der p 1, Der f 1, Can f 1), or five (Fel d 1). For each allergen, a binary variable was created that included allergen-specific sensitization and a critical level of allergen-specific exposure —an approach similar to that used by Lewis et al. (2002). This variable was constructed such that “1” indicated that the child exhibited a specific sensitivity AND the allergen was present in the home at or above the critical concentration (i.e., “exposed”), and “0” indicated the child did not have a specific allergic sensitivity OR the allergen was below the critical level (“unexposed”). The critical level for Penicillium and Bla g 1 was at the level of detection since there were only two categories in the distribution. For the remaining exposure variables, each category boundary was used in succession to explore associations with health outcomes. Critical concentrations for these allergens were: at the level of detection for Der p 1 and Fel d 1, 148 CFU/m3 for Cladosporium, 2.1 µg/g for Der f 1, and 1.2 µg/g for Can f 1.
2.7. Statistical analysis
Ordered logistic regression analyses, using a cumulative logit approach, were used to examine unadjusted associations between covariates and health outcomes and household allergen levels. Covariates included: personal variables (gender, age, maintenance medication use during the month of observation, general atopy); a socioeconomic status variable (housing type); other household exposures (smoking in the home, indoor NO2 levels); and season of sampling. Other socioeconomic variables were considered (mother’s education and ethnicity), but were excluded due to high correlation with multifamily housing (Spearman r=−0.50 and 0.46, respectively).
Ordered logistic regression analyses, using a cumulative logit approach, were also used to examine the association between each categorized health outcome and an exposure variable representing a combination of categorized allergen level and specific allergic status for Penicillium, Cladosporium, Der p 1, Der f 1, Fel d 1, Can f 1, and Bla g 1. Final models for wheeze, persistent cough, and rescue medication use were adjusted for gender, age, maintenance medication use, general atopic status, housing type, smoking, indoor NO2 concentration, and season. Models for the asthma severity score were adjusted for all of the above covariates except maintenance medication use because it is used in constructing the severity score (see Fig. 1). The proportional odds assumption was evaluated using the score test on unadjusted models. Fit of the fully adjusted models were evaluated using a Pearson goodness-of-fit statistic.
3. Results
Characteristics of the study population are shown in Table 1. Over half of the children used maintenance medication (53%), two-thirds were atopic, nearly half (45%) lived in multifamily housing, and season of sampling was evenly divided between warm (48%) and cool (52%) months. The distributions of daily symptoms, rescue medication use and asthma severity are shown in Table 2. Nearly half of the subjects (46%) experienced wheeze, nearly two-thirds (63%) experienced persistent cough, over half (56%) reported use of rescue medication, and 75% experienced mild, moderate, or severe persistent asthma (Fig.1) during the month-long monitoring period.
Table 1.
Personal characteristics of children participating in month long study (N=1233). (Connecticut and Massachusetts 2007–2009).
| Characteristics | N (%) or Mean±SD |
|---|---|
| Gender | |
| Male | 726(58.9) |
| Female | 507(41.1) |
| Age(yrs) | 7.4±1.7 |
| Maintenance medication use | |
| No | 579(47.0) |
| Yes | 654(53.0) |
| General atopya | |
| No | 423(34.3) |
| Yes | 810(65.7) |
| Ethnicity | |
| Whiteb | 488(39.6) |
| Black | 239(19.4) |
| Hispanic | 444(36.0) |
| Mixed and other | 62(5.0) |
| Mother’s education(yrs) | |
| <12 | 211(17.1) |
| 12–15 | 660(53.6) |
| ≥16 | 361(29.3) |
| Housing type | |
| Single family | 678(55.0) |
| Multifamily | 555(45.0) |
| Smoking in the home | |
| No | 1092(89.9) |
| Yes | 123(10.1) |
| Indoor nitrogen dioxide(NO2,ppb)c | 10.6±.96 |
| Season of sampling | |
| Warm(May–Oct) | 587(47.6) |
| Cool(Nov–Apr) | 646(52.4) |
SD standard deviation
Atopy defined as a total IgE greater than age-adjusted levels (Mayo Clinic, 2006) or a positive response to one or more of the panel of 10-allergens tested: fungi (Penicillium notatum, Cladosporium herbarum), dust mite (Der f 1, Der p 1), cat (Fel d 1), dog (Can f 1), cockroach (Bla g 1), meadow grass (Kentucky blue, Poa pratensis), ragweed (Ambrosia elatior), egg.
Includes White (n=453) and Asian (n=35).
N=1186 completed samples.
Table 2.
Distribution of days of wheeze, persistent cough, rescue medication use and asthma severity score during a one-month observation period. (N=1233 children) (Connecticut and Massachusetts 2007–2009).
| Health out come | N(%) |
|---|---|
| Wheeze(days) | |
| 0 | 664(53.9) |
| 1–4 | 380(30.8) |
| >4 | 189(15.3) |
| Persistent cough(days) | |
| 0 | 451(36.7) |
| 1–4 | 417(33.9) |
| >4 | 361(29.4) |
| Rescue medication use(days) | |
| 0 | 536(43.7) |
| 1–4 | 389(31.7) |
| >4 | 303(24.6) |
| Asthma severity(score)a | |
| 0 No symptoms | 182(17.8) |
| 1 Intermittent | 121(9.8) |
| 2 Mild Persistent | 316(25.6) |
| 3 Moderate persistent | 302(24.5) |
| 4 Severe persistent | 312(25.3) |
Five-level asthma severity score based on the Global Initiative for Asthma Guidelines (US Department of Health and Human Services, 2002). Scores are based on a combination of frequency of symptoms during the day (wheeze, persistent cough, shortness of breath, chest tightness), night symptoms and medication use (rescue and controller) during the month-long observation period. (See Section 2 and Fig. 1.)
Table 3 displays the results from ordered logistic regression analyses of unadjusted associations between covariates and health outcomes. The 95% confidence intervals for odds ratios for all of the following did not include the null: increased risk of wheeze associated with maintenance medication use, positive allergic status, living in multifamily housing, smoking in the home, and cooler months; increased risk of persistent cough associated with younger ages, maintenance medication use and cooler months; increased risk of short-acting inhaler use associated with maintenance medication use, multifamily housing, higher levels of indoor NO2 and cooler months; and finally, increased risk of higher asthma severity score associated with cooler months.
Table 3.
Associationsa of covariates with health outcome variables. (N=1233 children in Connecticut and Massachusetts 2007–2009).
| Covariate | Wheeze OR(95%CI) |
Persistent cough OR(95%CI) |
Rescue medication use OR(95%CI) |
Asthma severity score OR(95%CI) |
|---|---|---|---|---|
| Gender | ||||
| Male | 1.00 | 1.00 | 1.00 | 1.00 |
| Female | 0.98(0.78,1.22) | 0.97(0.79,1.20) | 1.14(0.92,1.41) | 0.99(0.81,1.21) |
| Age(1 yr increase) | 1.04(0.98,1.11) | 0.88(0.83,0.93) | 1.00(0.94,1.06) | 0.97(0.91,1.02) |
| Maintenance medication use | ||||
| No | 1.00 | 1.00 | 1.00 | |
| Yes | 1.70(1.37,2.12) | 2.07(1.68,2.55) | 2.32(1.88,2.88) | |
| General atopyb | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.47(1.17,1.86) | 0.89(0.72,1.11) | 1.07(0.86,1.34) | 0.99(0.80,1.22) |
| Housing type | ||||
| Single family | 1.00 | 1.00 | 1.00 | 1.00 |
| Multifamily | 1.47(1.19,1.82) | 0.94(0.76,1.15) | 1.54(1.25,1.90) | 0.89(0.73,1.08) |
| Smoking in the home | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.81(1.28,2.56) | 0.84(0.59,1.18) | 1.27(0.90,1.79) | 0.88(0.63,1.23) |
| Indoor nitrogen dioxide(10 ppb increase) | 1.10(0.98,1.22) | 1.04(0.93,1.16) | 1.15(1.03,1.30) | 1.07(0.96,1.19) |
| Season of sampling | ||||
| Warm(May-Oct) | 1.00 | 1.00 | 1.00 | 1.00 |
| Cool(Nov-Apr) | 1.25(1.01,1.55) | 1.58(1.29,1.95) | 1.37(1.11,1.69) | 1.41(1.16,1.72) |
Odds ratios (OR) and 95% confidence intervals (95% CI) for ordered logistic regression models for 3-level outcome variables for days of wheeze, persistent cough, rescue medication use (categorized as 0,1–4, >4 days) or 5-level asthma severity score (0 no symptoms, no medication use, 1 intermittent, 2 mild persistent, 3 moderate persistent, 4 severe persistent) during a one-month observation period. Separate models were run for each outcome and covariate.
Atopy defined as a total IgE greater than age-adjusted levels (Mayo Clinic, 2006) or a positive response to one or more of the panel of10-allergens tested: fungi (Penicillium notatum, Cladosporium herbarum), dust mite (Der f1, Der p1), cat (Fel d1), dog (Can f1), cockroach (Bla g1), meadow grass (Kentucky blue, Poa pratensis), ragweed (Ambrosia elatior), egg.
Summary statistics for the two fungi and five dust allergens are shown in Table 4. Penicillium was found in 33% of 1,233 homes and Cladosporium in 65%. Among the additional 61 genera identified from environmental samples (data not shown), the most common were Basidiomycetes (45%), unidentified yeasts (27%), Alternaria (19%) and Rodotorula (13%). No fungal colonies were detected in 6% of the samples (data not shown). More dust samples contained detectable levels of Der f 1 (67%) than Der p 1 (54%) and were significantly associated with each other (χ2, p<0.0001), as well as with both pet allergens (χ2, p<0.0001; data not shown). Detectable levels of Fel d 1 and Can f 1 were found in 84% and 72% of the homes, respectively, and measurable levels of both pet allergens were found in 65% of homes (data not shown). Fel d 1 and Can f 1 allergen concentrations were significantly associated with each other (χ2, p<0.0001; data not shown). Bla g 1, found in 34% of the samples, was significantly associated with low levels of Der f 1 (χ2, p=0.002) and high levels of Fel d 1 (χ2, p<0.0001; data not shown).
Table 4.
Distribution of household levels of airborne fungi and dust allergens (Connecticut and Massachusetts 2007–2009).
| House hold exposures | N | Detect able N(%) | Mean(SD)a | P50 | P75 | Max | IQR |
|---|---|---|---|---|---|---|---|
| Fungi(CFU/m3) | 1233 | ||||||
| Penicillium | 409(33.2) | 4.8(10.3) | 1 | 50 | 13,400 | 50 | |
| Cladosporium | 805(65.3) | 27.1(12.7) | 50 | 200 | 8350 | 200 | |
| Dust(µg/g) | |||||||
| Der p 1 | 1214 | 662(54.5) | 0.32(4.6) | 0.17 | 0.61 | 55.8 | 6.0 |
| Der f1 | 1210 | 815(67.4) | 0.81(7.0) | 0.66 | 4.20 | 483.8 | 41.9 |
| Fel d1 | 1211 | 1013(83.6) | 1.40(7.2) | 1.30 | 5.10 | 1032.2 | 23.7 |
| Can f1 | 1215 | 879(72.3) | 0.82(6.8) | 0.50 | 2.90 | 3176.0 | 23.9 |
| Bla g 1(U/g) | 1211 | 415(34.2) | 0.83(2.1) | 0.60 | 0.67 | 212.6 | 1.1 |
SD standard deviation; P50 median; P75 75th percentile; Max maximum; IQR interquartile range
Antilog of geometric mean (SD).
Categorized concentration levels of fungi and dust allergens are shown in Table 5, Part A. Results of ordered logistic regression analysis of unadjusted associations between covariates and categorized allergen exposures are shown in Supplementary Tables S1 (fungi and dust mite allergens) and S2 (pet and cockroach allergens). With the exception of Penicillium, housing type was associated with levels of all allergens levels measured in homes. Higher levels of indoor NO2 were associated with higher levels of fungi, detectable levels of Bla g 1, and lower levels of Der f 1 and Can f 1. Sampling in cooler months was associated with higher levels of dust mites allergens, pet allergens, and lower levels of Cladosporium.
Table 5.
Household allergens: exposure categories (A) and allergen specific sensitization status (B). (N=1233 children; Connecticut and Massachusetts 2007–2009).
| A. Exposure Categories |
B. Specific Sensitization |
||
|---|---|---|---|
| Allergen concentration | N (%) | No [N (%)] | Yes [N (%)] |
| Fungi (CFU/m3) | |||
| Penicillium | 1126 (91.8) | 100 (8.2) | |
| 0 | 820 (67.0) | 754 | 66 |
| >0 | 406 (33.0) | 372 | 34 |
| Cladosporium | 1128 (91.9) | 99 (8.1) | |
| 0 | 425 (34.6) | 391 | 34 |
| 0 to ≤148 | 373 (30.4) | 347 | 26 |
| >148 | 429 (35.0) | 390 | 39 |
| Dust (µg/g) | |||
| Der p 1 | 675 (56.0) | 531 (44.0) | |
| ≤0.10 | 552 (45.8) | 339 | 213 |
| 0.10 to ≤0.60 | 342 (28.4) | 179 | 163 |
| >0.60 | 312 (25.9) | 157 | 155 |
| Der f 1 | 692 (57.8) | 505 (42.1) | |
| ≤0.10 | 393 (32.8) | 239 | 154 |
| 0.10 to ≤2.1 | 403 (33.7) | 229 | 174 |
| >2.1 | 401 (33.5) | 224 | 177 |
| Fel d 1 | 814 (67.4) | 394 (32.6) | |
| ≤0.12 | 228 (18.9) | 163 | 65 |
| 0.12 to ≤0.65 | 254 (21.0) | 172 | 82 |
| 0.65 to ≤1.9 | 244 (20.2) | 157 | 87 |
| 1.9 to ≤7.8 | 239 (19.8) | 164 | 75 |
| >7.8 | 243 (20.1) | 158 | 85 |
| Can f 1 | 753 (62.2) | 457 (37.8) | |
| ≤0.12 | 350 (28.9) | 222 | 128 |
| 0.12 to ≤1.2 | 438 (36.2) | 268 | 170 |
| >1.2 | 422 (34.9) | 263 | 159 |
| Bla g 1 (U/g) | 989 (82.6) | 208 (17.4) | |
| ≤0.60 | 884 (73.8) | 745 | 139 |
| >0.60 | 313 (26.2) | 244 | 69 |
Specific allergen sensitization test results are shown in Table 5, Part B. Sensitization to Penicillium or Cladosporium was found in 8% of children and 6% tested positive to both (data not shown). Fewer than half of the children were sensitized to dust mite allergens Der p 1 (44%) or Der f 1 (42%) and 41% were sensitized to both (data not shown). Slightly more children were sensitized to Can f 1 (38%) than to Fel d 1 (33%) and 29% were sensitized to both (data not shown). Specific sensitization to Bla g 1 was found in 17% of the children.
The combined effect of sensitization and exposure to specific household allergens on asthma severity was explored with cumulative logistic regression adjusted for selected covariates (Table 6). Children who were both sensitized and exposed to detectable levels of Penicillium were twice as likely to experience increased days of wheeze, persistent cough and a higher asthma severity score than children who were either not sensitized or unexposed. The combination of specific sensitivity and exposure to Cladosporium was not significantly associated with health outcomes at any level. Combined exposure and sensitivity to detectable levels of Der p 1, was significantly associated with a 47% increased likelihood of rescue medication use. A similar association was seen for the asthma severity score, but only for the highest level of Der p 1 (40.60 µg/g, odds ratio [OR] 1.52 95% confidence interval [CI] 1.10, 2.10, data not shown). Combined sensitization and exposure to detectable levels of Fel d 1 was significantly associated with a 39% increased likelihood of more days of wheeze and a 32% increased likelihood of more rescue medication use compared to children not sensitized or unexposed. Sensitization and exposure to Can f 1 above 1.2 mg/g was associated with an increased risk of wheeze. No significant associations were found for sensitization and any exposure level for Der f 1 or Bla g 1.
Table 6.
Odds ratios (OR) and 95% confidence intervals (95% CI) for adjusted cumulative logistic regression modelsa examining the effect of sensitization and exposure to specific household allergens on asthma morbidity. (Connecticut and Massachusetts 2007–2009).
| Allergens combined exposure and sensitization status |
N (%) | Wheezeb OR (95% CI) |
Persistent coughb OR (95% CI) |
Rescue medication useb OR (95% CI) |
Asthma severity scoreb OR (95% CI) |
|---|---|---|---|---|---|
| Penicilium | |||||
| ≤LOD (0 CFU/m3) OR not allergic | 1192 (97.2) | 1.00 | 1.00 | 1.00 | 1.00 |
| >LOD AND allergic | 34(2.8) | 2.12 (1.12, 4.04) | 2.01 (1.05, 3.85) | 1.68 (0.88, 3.20) | 1.99 (1.06, 3.72) |
| Cladosporium | |||||
| ≤148 CFU/m3 OR not allergic | 1162 (94.7) | 1.00 | 1.00 | 1.00 | 1.00 |
| >148 CFU/m3 AND allergic | 65 (5.3) | 1.22 (0.66, 2.26) | 0.98 (0.54, 1.80) | 0.69 (0.37, 1.29) | 1.58 (0.88, 2.83) |
| Der p 1 | |||||
| ≤0.10 µg/gOR not allergic | 888 (73.6) | 1.00 | 1.00 | 1.00 | 1.00 |
| >0.10 µg/gAND allergic | 318 (26.4) | 1.26 (0.95, 1.67) | 1.18 (0.90, 1.55) | 1.47 (1.11, 1.94) | 1.19 (0.92, 1.55) |
| Der f 1 | |||||
| ≤2.1 µg/gOR not allergic | 1043 (85.5) | 1.00 | 1.00 | 1.00 | 1.00 |
| >2.1 µg/gAND allergic | 177 (14.5) | 0.89 (0.63, 1.24) | 0.90 (0.65, 1.25) | 1.09 (0.78, 1.51) | 1.28 (0.94, 1.74) |
| Fel d 1 | |||||
| ≤0.12 µg/gOR not allergic | 879 (72.8) | 1.00 | 1.00 | 1.00 | 1.00 |
| >0.12 µg/gAND allergic | 329 (27.2) | 1.39 (1.05, 1.84) | 0.89 (0.68, 1.17) | 1.32 (1.01, 1.74) | 1.14 (0.88, 1.47) |
| Can f 1 | |||||
| ≤1.2 µg/gOR not allergic | 1051 (86.9) | 1.00 | 1.00 | 1.00 | 1.00 |
| >1.2 µg/gAND allergic | 159 (13.1) | 1.53 (1.09, 2.15) | 1.11 (0.80, 1.56) | 1.15 (0.82, 1.62) | 1.15 (0.83, 1.58) |
| Bla g 1 | |||||
| ≤0.60 U/g OR not allergic | 1128 (94.2) | 1.00 | 1.00 | 1.00 | 1.00 |
| >0.60 U/g AND allergic | 69 (5.8) | 1.11 (0.68, 1.82) | 0.70 (0.43, 1.15) | 1.19 (0.73, 1.93) | 0.87 (0.54, 1.38) |
LOD level of detection
Outcomes include 3-level variables for days of wheeze, persistent cough, rescue medication use and a 5-level asthma severity score during a one-month observation period. Separate models were run for each outcome and exposure. Models for wheeze, persistent cough, rescue medication use (categorized as 0, 1–4, >4 days) include gender, age (yrs), maintenance medication use (no, yes), general atopic status (none, any), housing type (single, multifamily), smoking in the home (no, yes), indoor NO2 concentration (ppb), season of sampling (warm, cool months). Models for asthma severity score (0 no symptoms, no medication use, 1 intermittent, 2 mild persistent, 3 moderate persistent, 4 severe persistent) include all of the above covariates except maintenance medication use. The fit of all final, fully adjusted models was good (Pearson goodness-of-fit test, p>0.05).
The proportional odds assumption was met for all outcomes and all exposures (score test p>0.05), with the exception of the models for wheeze with Der f 1 and Fel d 1 (p=0.004 for both). Reducing wheeze from 3 categories to 2 (any vs. none) in adjusted models produced results similar to those shown above. In the model for exposure and sensitization to Der f 1, the confidence interval continued to include the null. In the model for Fel d 1, being sensitized and exposed predicted a 54% increased risk of any wheeze (95% CI 1.14, 2.08).
4. Discussion
In the current study we found that for asthmatic children sensitized to Penicillium, exposure to any Penicillium in the home doubles the risk of having more days of wheeze, persistent cough and of having a higher (worse) asthma severity score than asthmatic children who are not sensitized or unexposed. We also found that children sensitized and exposed to pet allergen were at significantly increased risk of wheeze (by 39% and 53% for Fel d 1 >0.12 µg/g, Can f 1>1.2 µg/g, respectively), and that increased rescue medication use was significantly associated with sensitization and exposure to Der p 1>0.10 µg/g (by 47%) and Fel d 1 >0.12 µg/g (by 32%).
We have reported similar results from two previous studies in our region: one examining peak expiratory flow variability (PEFV) in asthmatic children (Bundy et al., 2009) and one following respiratory symptoms in infants at risk for asthma (Gent et al., 2002). Among 225 asthmatic children monitored for PEFV, and in models controlling for general atopic status, exposure to any detectable levels of household Penicillium was associated with a greater than two-fold increased risk of mean PEFV of 18.5% or more (OR 2.39 95% CI 1.19, 4.81) (Bundy et al., 2009). In an earlier study, infants (N=880) exposed to levels of Penicillium of 1,000 CFU/m3 or more measured in the home at the time of enrollment, were at double the risk for increased rates of wheeze (rate ratio [RR] 2.15 95% CI 1.34, 3.46) and persistent cough (RR 2.06 95% CI 1.31, 3.24) during the first year of life (Gent et al., 2002).
Two similar birth cohort studies in our region found similar results. In Boston, a study of infants (N=499) at risk for asthma followed for lower respiratory tract illness during the first year of life found that airborne levels of Penicillium greater than 189 CFU/m3 (90th percentile) measured in the home at the time of enrollment were associated with nearly double the risk of increased rates of lower respiratory tract illness (RR 1.73 95% CI 1.23, 2.43) (Stark et al., 2003). In a more recent study in upstate New York, infants (N=103) at risk for asthma exposed to airborne levels of Penicillium of 120 CFU/m3 or more were at significant risk of experiencing any wheeze during the first year of life (OR 6.18 95% CI 1.34, 28.46) (Rosenbaum et al., 2010). Levels of Penicillium found in our study were similar to those found else-where in our region, e.g., the 90th percentile was 150 CFU/m3. However, we found that any detectable level (>0 CFU/m3) of Penicillium was significantly associated with respiratory effects among sensitized individuals.
We did not find any associations between sensitization and exposure to Cladosporium and asthma outcomes. This is consistent with all studies mentioned above: none found associations between household airborne levels of Cladosporium and respiratory symptoms (Bundy et al., 2009; Gent et al., 2002; Rosenbaum et al., 2010) or lower respiratory tract illness (Stark et al., 2003), which may suggest that unlike Penicillium, exposure to Cladosporium at levels typically encountered in homes in the Northeastern US is not a significant risk factor for asthma exacerbations in children. The predominant species of Cladosporium are C. sphaer-ospermum and C. cladosporiodes (Bensch et al., 2010) but the allergy panel included a test for sensitivity to C. herbarum which mainly occurs on plants and plant debris outdoors. Identification of predominant indoor species and examination of potential species-specific health effects remains to be done.
Penicillium chrysogenum sensu lato (synonymous with P. notatum on the allergen panel) was found to be the predominant species indoors in several studies (Andersen et al., 2011; Scott et al., 2004). Penicillium can appear as a pale greenish spot on building material and can also grow on fabric, leather, wood and paper products that have been exposed to water damage or high humidity. The growing mold, which is not easy to identify on sight, often releases volatile organic compounds creating a musty smell (Elke et al., 1999). In the current study, we did not find significant associations between respondent’s report of (1) mold or mildew or (2) water leaks or water damage, and any of the measured levels of mold (data not shown). Nor did we find associations between these reports of mold or water damage and increased risk of respiratory symptoms or asthma severity (data not shown). In reviews of the literature on damp indoor spaces and health (Mendell et al., 2011), and in a recent review (Tischer et al., 2011a) and meta-analysis (Tischer et al., 2011b) limited to studies conducted in Europe, there is strong evidence of associations between visible mold (reported, not measured) and asthma symptoms in children. Our negative findings between reported mold and health outcomes may reflect the difficulty in identifying the presence of mold in our region rather than a true lack of association.
Since the early 1990s, the level of dust mite exposure considered to be a “threshold” for sensitization and morbidity is 2 µg/g (Platts-Mills et al., 1997; Rosenstreich et al., 1997). Household dust mite levels exceeding 2 µg/g were found in 15% (Der p 1) and 35% (Der f 1) of our study homes, compared to 70% of homes in an Australian study (Peat et al., 1996) and 46% of homes in a British study (Sporik et al., 1990). Although we did observe an association between level of Der p 1 sensitization, many sensitized subjects lived in homes with levels below detection. We also found an association between sensitization, current exposure and risk of increased asthma morbidity: children both sensitized and exposed to Der p 1 were at significantly greater risk for increased rescue medication use (for any detectable level of exposure) and higher asthma severity score (OR 1.52 95% CI 1.10, 2.10) for exposures to levels >0.60 µg/g (data not shown). Our results support the argument that exposure to dust mites in the home is strongly associated not only with sensitization, but also with severity of asthma (Platts-Mills et al., 2009) and at levels lower than 2 µg/g.
Exposure to pets is associated with respiratory symptoms (Chen et al., 2010), but studies that look at the combined effects of sensitization and exposure on asthma morbidity are rare. In our study, we found children who were both sensitized and exposed to any detectable levels of Fel d 1 (>0.12 µg/g) were at greater risk for wheeze and increased use of rescue medication. We found children both sensitized and exposed to dog allergen at levels above 1.2 µg/g were at 53% greater risk of wheeze. Most studies in a systematic review of the literature since 2000 on the role of household pets in allergy and asthma (Chen et al., 2010), use report of cat or dog ownership as the exposure measure. In our analyses, when cat or dog ownership is substituted for “exposed,” we found no significant relationships with exposure/sensitization status and any of the health outcomes (data not shown). Although there are significant associations between pet allergens sampled in our study homes and pet ownership (p<0.0001, data not shown), the relationship is not perfect. Twenty percent of the homes had levels of Fel d 1>7.8 µg/g, and of these, 51% did not report cats in the home. Likewise, 30% of the homes had levels of Can f 1 > 1.2 µg/g, and 36% of these did not report dog ownership. Resuspension rates of both of these allergens is high, especially for cat dander (Raja et al., 2010), and aerodynamic characteristics of both Fel d 1 and Can f 1 make it easy for them to be carried into areas where no pets are in residence (Custovic et al., 1996), therefore, it is not surprising that measured allergen level is a more accurate way to classify exposure (any vs none) than reported pet ownership. In our earlier study of children with asthma, we found that measured exposure (to levels above detection) and sensitization to Can f 1, but not to Fel d 1 were significantly associated with asthma morbidity (as measured by use of controller medication for ≥9 months during the year of follow-up) (Gent et al., 2009). Another study of asthmatic children where levels of pet allergen were measured found no associations between level of exposure and wheezing in analyses controlling for any atopic sensitization (El-Sharif et al., 2006). In contrast, two studies of adult asthmatics did find associations between risk of wheezing and specific sensitization/exposure status for measured levels of Fel d 1 (Gehring et al., 2001; Lewis et al., 2002).
Levels of Bla g 1 measured in homes in our study region (made up exclusively of small cities and suburbs) were very low: only 26% of the homes had levels above detection (0.60 U/g). As with dust mite allergen, in spite of these low levels, there was a significant association between any Bla g 1 detected and specific sensitization. We did not, however, find any significantly increased respiratory health risk for sensitized individuals exposed to cockroach allergen. In contrast, significant associations between asthma morbidity and exposure to Bla g 1, are reported in a study of low income asthmatic children conducted in Chicago where the median Bla g 1 level is 38 U/g (Turyk et al., 2006).
Study strengths include concurrent assessment in a large number of asthmatic children of sensitization to seven specific allergens, measurement of household levels of these same seven allergens, and assessment of asthma severity during the month immediately following allergy testing and sample collection. The study was limited to the seven allergens that were both sampled in the home and included on the allergy panel, but these allergens represent some of the most commonly encountered household allergens in our study area (Ren et al., 1999; Leaderer et al., 2002). Other potential limitations would include those inherent in the environmental sampling methodologies used: cultures of airborne spores for fungi and assays of settled dust for non-mold allergens. Airborne fungi samples can miss rare species, but given our focus on assessing levels of two of the more common household molds, this was not a problem. Both collection methods would be affected by recent vacuuming activity on the part of the homeowner, although at the time of scheduling, respondents were asked not to use their vacuums before the enrollment visit. Viable samples were collected in the homes of nearly 90% of those enrolled.
We assessed exposures to common household allergens, current sensitization status to the same allergens, and asthma morbidity. We found that children with asthma living in New England who are sensitized and exposed to low levels of household allergens Penicillium (>0 CFU/m3), Der p 1 (>0.10 µg/g), Fel d 1 (>0.12 µg/g) and Can f 1 (>1.2 µg/g) are at significant risk for increased asthma morbidity. No significant health outcome associations were found between sensitization and exposure to dust mite allergen Der f 1 or cockroach allergen Bla g 1. Our results suggest that reductions in levels of pet allergens, dust mites and visible mold would reduce morbidity in atopic children with asthma.
Supplementary Material
Acknowledgments
Funding Source
This work was supported by a grant from the National Institutes of Health: ES05410.
Human Investigation Committee Approval
The Human Investigation Committee of Yale University School of Medicine reviewed and approved this research (HIC Protocol no. 0504027631).
Abbreviations
- CFU/m3
colony forming units per cubic meter
- DG-18
dichloran-18% glycerol agar
- NO2
nitrogen dioxide
- OR
odds ratio
- RR
rate ratio
- CI
confidence interval
Footnotes
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.envres.2012.07.005.
Contributor Information
Janneane F. Gent, Email: janneane.gent@yale.edu.
Julie M. Kezik, Email: julie.colburn@yale.edu.
Melissa E. Hill, Email: melissa.hill@yale.edu.
Eling Tsai, Email: tsai.umiami@gmail.com.
De-Wei Li, Email: DeWei.Li@ct.gov.
Brian P. Leaderer, Email: brian.leaderer@yale.edu.
References
- Andersen B, Frisvad JC, Sondergaard I, Rasmussen IS, Larsen LS. Associations between fungal species and water-damaged building materials. Appl. Environ. Microbiol. 2011;77:4180–4188. doi: 10.1128/AEM.02513-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger K, Beckett W, Triche E, Bracken MB, Holford T, Ren P, et al. Symptoms of wheeze and persistent cough in the first year of life: associations with indoor allergens, air contaminants, and maternal history of asthma. Am. J. Epidemiol. 2003;158:195–202. doi: 10.1093/aje/kwg148. [DOI] [PubMed] [Google Scholar]
- Belanger K, Gent JF, Triche EW, Bracken MB, Leaderer BP. Association of indoor nitrogen dioxide exposure with respiratory symptoms in children with asthma. Am. J. Respir. Crit. Care Med. 2006;173:297–303. doi: 10.1164/rccm.200408-1123OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch K, Groenewald JZ, Dijksterhuis J, Starink-Willemse M, Andersen B, Summerell BA, et al. Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales) Stud. Mycol. 2010;67:1–94. doi: 10.3114/sim.2010.67.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundy KW, Gent JF, Beckett W, Bracken MB, Belanger K, Triche E, et al. Household airborne Penicillium associated with peak expiratory flow variability in asthmatic children. Ann. Allergy Asthma Immunol. 2009;103:26–30. doi: 10.1016/S1081-1206(10)60139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush RK, Portnoy JM. The role and abatement of fungal allergens in allergic diseases. J. Allergy Clin. Immunol. 2001;107:S430–S440. doi: 10.1067/mai.2001.113669. [DOI] [PubMed] [Google Scholar]
- Chen CM, Tischer C, Schnappinger M, Heinrich J. The role of cats and dogs in asthma and allergy—a systematic review. Int. J. Hyg. Environ. Health. 2010;213:1–31. doi: 10.1016/j.ijheh.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Custovic A, Green R, Taggart SCO, Smith A, Pickering CAC, Chapman MD, et al. Domestic allergens in public places. II: dog (Can f 1) and cockroach (Bla g 2) allergens in dust and mite, cat, dog and cockroach allergens in the air in public buildings. Clin. Exp. Allergy. 1996;26:1246–1252. [PubMed] [Google Scholar]
- El-Sharif N, Douwes J, Hoet P, Nemery B. Childhood asthma and indoor aeroallergens and endotoxin in Palestine: a case-control study. J. Asthma. 2006;43:241–247. doi: 10.1080/02770900600567122. [DOI] [PubMed] [Google Scholar]
- Elke K, Begerow J, Oppermann H, Kramer U, Jermann E, Dunemann L. Determination of selected microbial volatile organic compounds by diffusive sampling and dual-column capillary GC-FID—a new feasible approach for the detection of an exposure to indoor mould fungi? J. Environ. Monit. 1999;1:445–452. doi: 10.1039/a903034d. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, et al. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J. Allergy Clin. Immunol. 2011;127:382–389. doi: 10.1016/j.jaci.2010.11.015. e1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring U, Heinrich J, Jacob B, Richter K, Fahlbusch B, Schlenvoigt G, et al. Respiratory symptoms in relation to indoor exposure to mite and cat allergens and endotoxins. Eur. Respir. J. 2001;18:555–563. doi: 10.1183/09031936.01.00096801. [DOI] [PubMed] [Google Scholar]
- Gehring U, Triche E, van Strien RT, Belanger K, Holford T, Gold DR, et al. Prediction of residential pet and cockroach allergen levels using questionnaire information. Environ. Health Perspect. 2004;112:834–839. doi: 10.1289/ehp.6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gent JF, Belanger K, Triche EW, Bracken MB, Beckett WS, Leaderer BP. Association of pediatric asthma severity with exposure to common household dust allergens. Environ. Res. 2009;109:768–774. doi: 10.1016/j.envres.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gent JF, Ren P, Belanger K, Triche E, Bracken MB, Holford TR, et al. Levels of household mold associated with respiratory symptoms in the first year of life in a cohort at risk for asthma. Environ. Health Perspect. 2002;110:A781–A786. doi: 10.1289/ehp.021100781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruchalla RS, Pongracic J, Plaut M, Evans R, 3rd, Visness CM, Walter M, et al. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J. Allergy Clin. Immunol. 2005;115:478–485. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Leaderer BP, Belanger K, Triche E, Holford T, Gold DR, Kim Y, et al. Dust mite, cockroach, cat, and dog allergen concentrations in homes of asthmatic children in the northeastern United States: impact of socioeconomic factors and population density. Environ. Health Perspect. 2002;110:419–425. doi: 10.1289/ehp.02110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SA, Weiss ST, Platts-Mills TAE, Burge H, Gold DR. The role of indoor allergen sensitization and exposure in causing morbidity in women with asthma. Am. J. Respir. Crit. Care Med. 2002;165:961–966. doi: 10.1164/ajrccm.165.7.2103044. [DOI] [PubMed] [Google Scholar]
- Mayo Clinic. [(accessed 05.04.11)];Immunoglobulin E (IgE), Serum. 2006 Available at: < http://mayomedicallaboratories.com/test-catalog/pring.pht?unit_code=8159S>.
- Mendell MJ, Mirer AG, Cheung K, Tong M, Douwes J. Respiratory and allergic health effects of dampness, mold, and dampness-related agents: a review of the epidemiologic evidence. Environ. Health Perspect. 2011;119:748–756. doi: 10.1289/ehp.1002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KE. New human rhinovirus species and their significance in asthma exacerbation and air remodeling. Immunol. Allergy Clin. North Am. 2010;30:541–552. doi: 10.1016/j.iac.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmes ED, Gunnison AF, DiMattio J, Tomczyk C. Personal sampler for nitrogen dioxide. Am. Ind. Hyg. Assoc. J. 1976;37:570–577. doi: 10.1080/0002889768507522. [DOI] [PubMed] [Google Scholar]
- Peat JK, Tovey E, Toelle BG, Haby MM, Gray EJ, Mahmic A, et al. House dust mite allergens. A major risk factor for childhood asthma in Australia. Am. J. Respir. Crit. Care Med. 1996;153:141–146. doi: 10.1164/ajrccm.153.1.8542107. [DOI] [PubMed] [Google Scholar]
- Platts-Mills TAE, Erwin EA, Heymann PW, Woodfolk JA. Pro: the evidence for a causal role of dust mites in asthma. Am. J. Respir. Crit. Care Med. 2009;180:109–113. doi: 10.1164/rccm.200811-1756PR. discussion 120–121. [DOI] [PubMed] [Google Scholar]
- Platts-Mills TAE, Hayden ML, Chapman MD, Wilkins SR. Seasonal variation in dust mite and grass-pollen allergens in dust from the houses of patients with asthma. J. Allergy Clin. Immunol. 1987;79:781–791. doi: 10.1016/0091-6749(87)90211-9. [DOI] [PubMed] [Google Scholar]
- Platts-Mills TAE, Vervloet D, Thomas WR, Aalberse RC, Chapman MD. Indoor allergens and asthma: report of the Third International Workshop. J. Allergy Clin. Immunol. 1997;100:S2–S24. doi: 10.1016/s0091-6749(97)70292-6. [DOI] [PubMed] [Google Scholar]
- Raja S, Xu Y, Ferro AR, Jaques PA, Hopke PK. Resuspension of indoor aeroallergens and relationship to lung inflammation in asthmatic children. Environ. Int. 2010;36:8–14. doi: 10.1016/j.envint.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Ren P, Jankun TM, Leaderer BP. Comparisons of seasonal fungal prevalence in indoor and outdoor air and in house dusts of dwellings in one Northeast American county. J. Exposure Anal. Environ. Epidemiol. 1999;9:560–568. doi: 10.1038/sj.jea.7500061. [DOI] [PubMed] [Google Scholar]
- Rosenbaum PF, Crawford JA, Anagnost SE, Wang CJK, Hunt A, Anbar RD, et al. Indoor airborne fungi and wheeze in the first year of life among a cohort of infants at risk for asthma. J. Exposure Sci. Environ. Epidemiol. 2010;20:503–515. doi: 10.1038/jes.2009.27. [DOI] [PubMed] [Google Scholar]
- Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N. Engl. J. Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- Scott J, Untereiner WA, Wong B, Straus NA, Malloch D. Genotypic variation in Penicillium chysogenum from indoor environments. Mycologia. 2004;96:1095–1105. [PubMed] [Google Scholar]
- Sporik R, Holgate ST, Platts-Mills TAE, Cogswell JJ. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study. N. Engl. J. Med. 1990;323:502–507. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- Stark PC, Burge HA, Ryan LM, Milton DK, Gold DR. Fungal levels in the home and lower respiratory tract illnesses in the first year of life. Am. J. Respir. Crit. Care Med. 2003;168:232–237. doi: 10.1164/rccm.200207-730OC. [DOI] [PubMed] [Google Scholar]
- Tischer C, Chen CM, Heinrich J. Association between domestic mould and mould components, and asthma and allergy in children: a systematic review. Eur. Respir. J. 2011a;38:812–824. doi: 10.1183/09031936.00184010. [DOI] [PubMed] [Google Scholar]
- Tischer CG, Hohmann C, Thiering E, Herbarth O, Muller A, Henderson J, et al. Meta-analysis of mould and dampness exposure on asthma and allergy in eight European birth cohorts: an ENRIECO initiative. Allergy. 2011b;66:1570–1579. doi: 10.1111/j.1398-9995.2011.02712.x. [DOI] [PubMed] [Google Scholar]
- Turyk M, Curtis L, Scheff P, Contraras A, Coover L, Hernandez E, et al. Environmental allergens and asthma morbidity in low-income children. J. Asthma. 2006;43:453–457. doi: 10.1080/02770900600758333. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. [(accessed 01.11.11)];Global Initiative for Asthma, Global Strategy for Asthma Management and Prevention. 2002 Available at: < http://www.ginasthma.org/Guidelines/guidelines-2002-original1%3a-work shop-report%2c-global-strategy-for-asthma-management-and-prevention.html>.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.