Abstract
Development of the nephron tubules, the functional units of the kidney, requires the differentiation of a renal progenitor population of mesenchymal cells to epithelial cells. This process requires an intricate balance between self-renewal and differentiation of the renal progenitor pool. Sall1 is a transcription factor necessary for renal development which is expressed in renal progenitor cells (cap mesenchyme). Sall1 recruits the Nucleosome Remodeling and Deacetylase (NuRD) chromatin remodeling complex to regulate gene transcription. We deleted Mi2β, a component of the NuRD complex, in cap mesenchyme (CM) to examine its role in progenitor cells during kidney development. These mutants displayed significant renal hypoplasia with a marked reduction in nephrons. Markers of renal progenitor cells, Six2 and Cited1 were significantly depleted and progenitor cell proliferation was reduced. We also demonstrated that Sall1 and Mi2β exhibited a strong in vivo genetic interaction in the developing kidney. Together these findings indicate that Sall1 and NuRD act cooperatively to maintain CM progenitor cells.
Keywords: Renal progenitor cells, Kidney development, Sall1, Nucleosome, Remodeling and Deacetylase (NuRD), Mi2beta, CHD4
Introduction
Formation of the kidney requires a delicate balance between progenitor cell self-renewal and differentiation. These opposing forces are initiated by invasion of the ureteric bud (UB) into the metanephric mesenchyme (MM) at mouse embryonic (E) day 10.5 and continue until nephrogenesis ceases. If this balance is disrupted so that growth is impaired, then the kidney will not achieve the full complement of nephrons (renal hypoplasia), the tubules that comprise the functional units of this organ. Renal hypoplasia is a common cause of childhood kidney failure and can lead to hypertension in otherwise asymptomatic adults (Keller et al., 2003). Conversely, if progenitor cell proliferation is uncontrolled and differentiation is arrested, Wilms’ tumor, an embryonic tumor of the kidney results (Kreidberg and Hartwig, 2008).
Recent gene expression studies have refined our understanding of the renal progenitor compartment (Boyle et al., 2008; Kobayashi et al., 2008). These important studies demonstrate that Six2 and Cited1 define a population of self-renewing progenitors that aggregate around the tips of the UB, called “cap” mesenchyme (CM). These CM cells are induced to undergo the first step in forming the tubular segments of the nephron via a mesenchymal- to-epithelial transition, giving rise to the renal vesicle (Costantini and Kopan, 2010; Dressler, 2009). As kidney development proceeds, the UB undergoes branching morphogenesis and induces CM at each tip, an iterative process that gives rise to the ~10–12,000 nephrons in a mouse kidney or ~500,000 in humans (Puelles et al., 2011). At the same time, a subset of CM cells proliferates to maintain the progenitor pool until an appropriate number of nephrons is formed and nephrogenesis is complete. Numerous studies using genetically engineered mice, metanephric organ culture and other model organisms have identified genes that regulate the maintenance and expansion of renal progenitors or promote differentiation (Dressler, 2009). However, the molecular mechanisms that regulate cell fate decisions to balance these processes throughout the period of nephron formation are incompletely understood.
Balancing the timely induction of differentiation versus proliferation and growth of renal progenitors requires tight regulation of gene expression programs. This is accomplished by the coordinated action of sequence specific transcription factors and enzymes that modify chromatin, thereby regulating DNA accessibility for transcription of specific genes. Sall1 encodes a multi-zinc finger transcription factor essential for kidney development as its genetic deficiency results in bilateral renal agenesis or severe hypodysplasia in mice (Nishinakamura et al., 2001). Mutations in human SALL1 cause the autosomal dominant Townes–Brocks Syndrome which is characterized by multi-organ defects including renal hypoplasia, deafness, limb deformities and imperforate anus (Kohlhase et al., 1998). Sall1 is expressed in the CM in the Six2-positive multipotent progenitor cells that give rise to all segments of the nephron, except the UB-derived collecting ducts and the mesangial and endothelial cells of the glomeruli (Osafune et al., 2006). Unlike Six2, Sall1 expression is maintained in renal vesicles, and comma- and S-shaped bodies, structures that are precursors of the mature neprhon epithelial tubules. Sall1 deficient CM is competent to differentiate into nephrons in vitro, but colony sizes are significantly reduced compared with wild type renal progenitors (Osafune et al., 2006). This suggests that Sall1 is required to maintain or expand progenitor cells rather than providing an instructive signal for differentiation. However, the molecular mechanism by which Sall1 maintains renal progenitor cells is not known.
Proteins that act on chromatin are thought to be recruited to specific genomic regions by sequence specific DNA binding factors. Sall1 is a potent transcriptional repressor that interacts with the Nucleosome Remodeling and Deacetylase (NuRD) complex via a conserved 12-amino acid motif to regulate gene expression (Kiefer et al., 2002; Lauberth et al., 2007; Lauberth and Rauchman, 2006). NuRD is a multi-protein complex that contains both histone deacetylase (HDAC) activity and ATP-dependent nucleosome remodeling activity due to Mi2β. Recent studies establish a role for NuRD in embryonic stem (ES) cell pluripotency (Kaji et al., 2006; Reynolds et al., 2012) and in differentiation of progenitor cells in complex self-renewing epithelia (e.g. skin) and in the hematopoetic system (Kashiwagi et al., 2007; Yoshida et al., 2008). Specifically, Mi2β is a key regulator of progenitor cell self-renewal and multi-lineage restriction of hematopoetic stem cells and T lymphocytes (Williams et al., 2004). Evidence that Sall1 and NuRD interact to regulate gene expression led us to hypothesize an important role for Mi2–NuRD during kidney development. To test this we examined the consequences of Mi2β deletion in CM renal progenitors, a cell population that also expresses Sall1.
We deleted Mi2β in CM cells and show that these mutants displayed significant renal hypoplasia with a marked reduction in nephrons. Proteins which are expressed in renal progenitor cells, Six2, Cited1, and Pax2 were significantly depleted and progenitor cell proliferation was reduced. We found that Sall1 and Mi2β exhibited a strong in vivo genetic interaction in the developing kidney. Together these findings indicate that Sall1 and NuRD act cooperatively to maintain CM progenitor cells.
Materials and methods
Mouse strains
The Six2Cre:GFPTg/+ BAC transgenic strain, conditional Mi2βfl/fl, and Sall1+/− mice have been described previously (Kobayashi et al., 2008; Nishinakamura et al., 2001; Williams et al., 2004). Double heterozygous Six2Cre:GFPTg/+, Mi2βfl/+ male mice were mated to Mi2βfl/fl and Sall1+/− Mi2βfl/fl female mice to create Mi2βΔ/Δ, Sall1+/− Mi2βΔ/+, and Sall1+/− Mi2βΔ/Δ mutant embryos. Wild type ICR/CD-1 (Harlan) mice were used for co-immunoprecipitation assays. Female mice were checked for presence of a mucosal plug and noon on the day of detection was considered to be E0.5. Pregnant females were euthanized at the appropriate developmental stages and a C-section was performed to remove embryos. These studies were performed under the auspices of St. Louis University and St. Louis VA Medical Center animal care guidelines.
Quantitative real-time PCR
RNA was isolated from embryonic kidneys at E12.5 using the RNeasy RNA isolation kit (Qiagen) according to the manufacturer’s protocol. cDNA was prepared using the High Capacity RNA-to-cDNA kit (InVitrogen) and sequence-specific PCR primer sets were designed by Primer Express 3.0 (PE Applied Biosystems). qPCR analyses of NuRD components Mta 1/2/3, Mbd 2/3, and Mi2 α/β was performed using a 7300 Real-Time PCR Instrument and SYBR Green reagent (Applied Biosystems). The thermal cycling parameters were as follows: 50 °C for 2 min, 95 °C for 10 min, 40 cycles of 15 s at 95 °C, and 1 min at 60 °C. The dissociation curve for each primer pair confirmed a single reaction product. Reactions were performed in triplicate using samples from three independent embryos. The amount of each amplification product was determined relative to a standard curve of input cDNA. The following primer sets were used for qPCR analysis: Mta1 (5′-CGTGCTCCGGTATCTTGAG-3′ and 5′-CGCTTCTTCATGTTGCCATC-3′), Mta2 (5′-TTTCCGGCGAAGGGATATTTC-3′ and 5′-GCTTCAGTTGATGTCTCTGCT-3′), Mta3 (5′-CCCAGAGCGCCCTCCTA-3′ and 5′-CTCTGCAGTCGGGCTGG-3′), Mbd2 (5′-GAAGAAGGAGGAAGTGATCCG-3′ and 5′-ACTAGGCATCATCTTGCCG-3′), Mbd3 (5′-GGAAAGGGAAGAAGTGCCC-3′ and 5′-GTTCATCAACATCTTTCCGGT-3′), Mi2α (5′-CCGCACTATGCGGAGATC-3′ and 5′-TCTGGATGTTCATCTCATCC-3′), and Mi2β (5′-CCTAAGTTTGCAGAGATGGAAGA-3′ and 5′-TCCTGAATCTCCACGTCCTG-3′).
Tissue section analysis
E12.5–16.5 kidneys were fixed, sectioned, and stained with Hematoxylin and Eosin as described previously (Kiefer et al., 2003). Immunofluorescence was performed on 10 µm frozen sections using rabbit anti-Sall1(Kiefer et al., 2002), mouse anti-Mi2β (1:500, Abcam), mouse anti-NCAM (1:300, Abcam), rabbit anti-WT1 (1:1000, Santa Cruz) biotin -LTL (1:200, Vector), rat anti-E-cadherin (1:1000, Abcam), rabbit anti-pHH3 (1:1000, Fisher), rabbit anti-Cited1 (1:500, Abcam), rabbit anti-Six2 (1:500, Lifespan Biosciences), rabbit anti-Pax2 (1:2000, Covance), rabbit anti-Lef1 (1:250, Cell Signaling), mouse anti-Lhx1 (1:50, Developmental Studies Hybridoma Bank) and mouse anti-cytokeratin (1:1000, Lifespan Biosciences). Antibody reactivity was detected using Alexa 488-labeled anti-rabbit (1:400, Invitrogen), Cy3-labeled anti-rat (1:2000, Jackson ImmunoResearch) or Cy3-labeled anti-mouse (1:2000, Jackson ImmunoResearch), and mounted in Mowiol (Polysciences) as previously described (Kiefer et al., 2002). Sections were incubated without primary antibody to control for non-specific staining of secondary antibodies. 10 µm frozen sections were also used for analysis of apoptosis using the ApopTag Red In Situ Apoptosis Detection kit (Millipore) according to the manufacturer’s protocol.
Whole mount immunofluoresence and in situ hybridization
E12.5 kidneys were dissected as described above from wild type and Mi2βΔ/Δ mice and fixed for 30 min in 4% PFA. Kidneys were blocked in PBS plus 1% Triton and then incubated overnight at 4 °C in primary antibody mouse anti-cytokeratin (1:400). Kidneys were washed and probed with secondary anti-mouse Cy3 (1:1000). Whole-mount in situ hybridization was performed using a digoxigenin-labeled antisense riboprobe for Ret (nucleotides 818–1222) as previously described (Kiefer et al., 2008). After incubation with digoxigenin-alkaline phosphatase antibody (1:2500), signal was visualized using the alkaline phosphatase substrate BM purple (Roche, Indianapolis, IN, USA).
Quantification of UB branching
10 µm sections of wild type and Mi2βΔ/Δ kidneys harvested from three independent embryos at E12.5, E13.5, E14.5, and E15.5 were immunostained for cytokeratin. Total UB tips were counted on at least five 20× sections from each kidney for each stage and genotype. Results were reported as the average number of tips per section. Statistical analysis using standard t test was performed.
Quantification of proliferation and apoptosis
10 µm sections of kidneys from two independent wild type and Mi2β mutant embryos at E12.5, 13.5, and 14.5 were co-stained with anti-Sall1 and anti-pHH3 as stated above. Double Sall1/pHH3-positive cells surrounding UB tips were counted and related to the total number of Sall1-positive cells surrounding that tip. Results are reported as the percentage of double Sall1/pHH3-positive cells divided by the total number of Sall1-positive cells ± SD.
Results
The nucleosome remodeling and deacetylase complex is expressed in the developing kidney
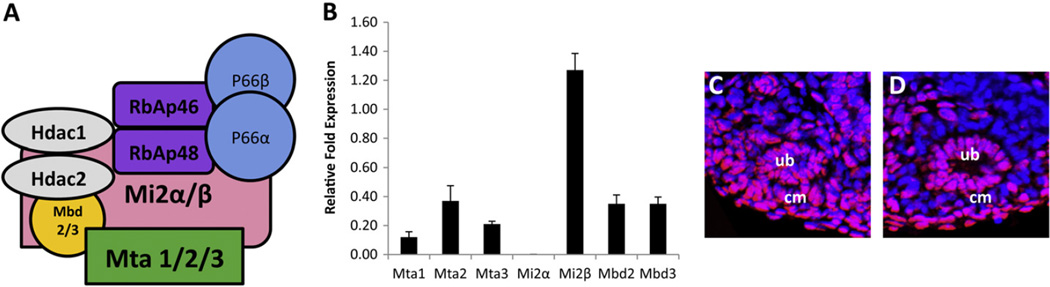
The Mi2/NuRD complex is a multi-protein complex that regulates transcriptional activation or repression through its actions on chromatin (Xue et al., 1998; Zhang et al., 1999). It is made up of histone deacetylases (HDAC) 1/2, retinoblastoma associated proteins (RbAp) 46/48, methyl CpG binding domain (MBD) proteins 2/3, metastasis tumor associated (Mta) proteins 1/2/3, and chromodomain-helicase-DNA-binding (CHD) protein 3/4, also known and referred to here as Mi2 α and β (Fig. 1A). The NuRD complex components are evolutionarily conserved and broadly expressed in many tissue types (McDonel et al., 2009). However, several of its components (Mbd, Mta, and Mi2) exist as multiple isoforms that form distinct complexes with non-overlapping functions (Bowen et al., 2004).We thus determined which components are expressed in the developing kidney. We examined the mRNA levels of Mta 1/2/3, Mi2 α/β, and Mbd 2/3 in E12.5 wild-type kidneys by RT-PCR. Mta 1, 2, and 3, and Mbd 2 and 3 were expressed at similar levels relative to the housekeeping gene ribosomal protein L19 (Rpl19). Only one isoform of Mi2 (Mi2β) was expressed at relatively high levels (Fig. 1B). RbAp46/48, Hdac1/2, and p66 have previously been shown to be expressed in the developing kidney (Chen et al., 2011; Guan et al., 1998); therefore all NuRD components are expressed, except Mi2α, allowing for the existence of multiple unique NuRD complexes; however the only ATPase component is Mi2β.
Fig. 1.
Nucleosome remodeling and deacetylase complex is expressed in the developing kidney. (A) Schematic of the NuRD components. (B) mRNA levels of NuRD components Mta 1/2/3, Mbd 2/3, and Mi2 α/β were measured in E12.5 kidneys. (C–D) Immunofluorescence analysis of Mi2β in E13.5 wild type and Mi2βΔ/Δ kidneys. Mi2β is detected in CM in wild type (C) kidneys. In Six2-Cretg/+, Mi2βfl/fl mutants (D), Mi2β protein expression was significantly and specifically reduced in CM surrounding UB tips and its derivative structures.
Conditional deletion of Mi2β from cap mesenchyme results in renal hypoplasia
Mi2β was detected in all compartments of the kidney including the population of self-renewing renal progenitors in the cap mesenchyme [CM] (Fig. 1C). Since Mi2–NuRD has been implicated in regulating differentiation of progenitor cells in the hematopoetic system (Yoshida et al., 2008), we hypothesized that Mi2β would be required in renal progenitor cells. To test this, we used a Six2-Cre:GFP BAC transgenic mouse (Six2Cre) to specifically delete Mi2β from the CM (Kobayashi et al., 2008). Expression of the Six2 transgene is restricted to the CM cells and pre-tubular aggregates which give rise to all epithelial components of the nephron segments except collecting ducts (Kobayashi et al., 2008; Oliver et al., 1995).
Deletion of Mi2β using Cre recombinase under control of the Six2 promoter was efficient as shown by marked reduction of Mi2β protein expression in the CM cells surrounding the UB tips (Fig. 1C, D). Reduced expression was also observed in forming renal vesicles and further differentiated structures such as comma- and S-shaped bodies that are derived from CM (data not shown). UB expression of Mi2β was not affected confirming that the Six2Cre transgene is specifically active in the CM.
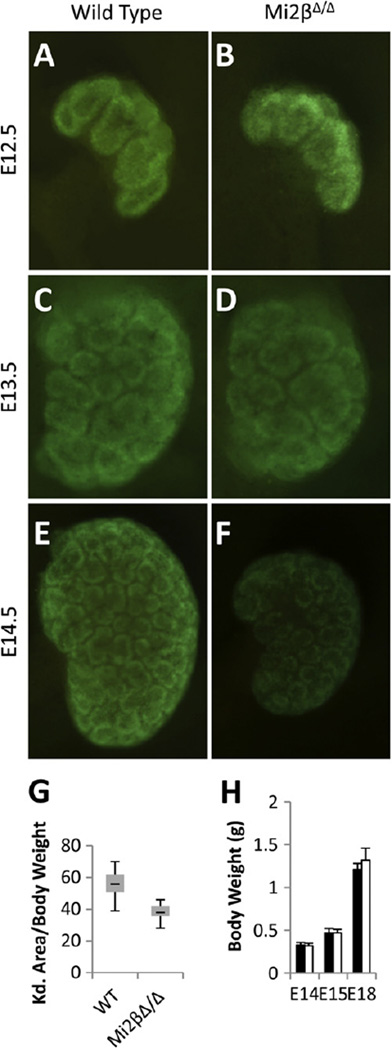
We analyzed kidneys in which Mi2β was deleted from the CM at several stages of embryonic development. There were no significant differences observed at E12.5–13.5 (Fig. 2A–D). Kidneys of both wild-type and Mi2βΔ/Δ mice were similar in size and Six2-positive cap mesenchyme formed as marked by GFP expression from the Six2 transgene. However, at E14.5, kidneys were significantly hypoplastic (27/30 kidneys; n = 15 embryos) compared to wild-type littermates (Fig. 2E, F). Additionally, GFP expression was reduced in Mi2βΔ/Δ kidneys at E14.5, suggesting a decrease in the number of Six2-positive CM cells at this stage (Fig. 2E, F). To quantify hypoplasia, we calculated the area of the kidney by measuring the width and height and related this to the overall weight of the embryo. The average kidney size to body weight ratio for Mi2βΔ/Δ mice (38.0 ± 5.95) was significantly smaller (p < 0.001) than that of wild-type kidneys (56.5 ± 7.27) (Fig. 2G). Body weights for wild-type and homozygous mutants were identical for all stages examined, confirming that the renal hypoplasia was not due to developmental delay affecting the overall growth of the embryo (Fig. 2H).
Fig. 2.
Deletion of Mi2β from cap mesenchyme cells results in renal hypoplasia. (A–F) Whole kidney images of GFP expressed from the Cre:GFP fusion protein under control of the Six2 BAC transgene in wild type and Mi2βΔ/Δ embryos at E12.5 (A, B), E13.5 (C, D), and E14.5 (E, F). Both wild type and Mi2βΔ/Δ kidneys were similar in size at E12.5 and E13.5 (A–D). Hypoplasia and reduction of GFP expression was evident at E14.5 in Mi2βΔ/Δ kidneys compared to wild type littermates (E, F). (G) Quantification of hypoplasia by relating kidney area to body weight of the embryo over a developmental stage period of E13.5 to E18.5. Deletion of Mi2β resulted in renal hypoplasia in 90% of mutants with an average 33% reduction in the area/body weight ratio. (H) Average body weights of wild-type and Mi2βΔ/Δ embryos. Body weights of wild type (black bars) and Mi2βΔ/Δ mutant (white bars) embryos were indistinguishable throughout development.
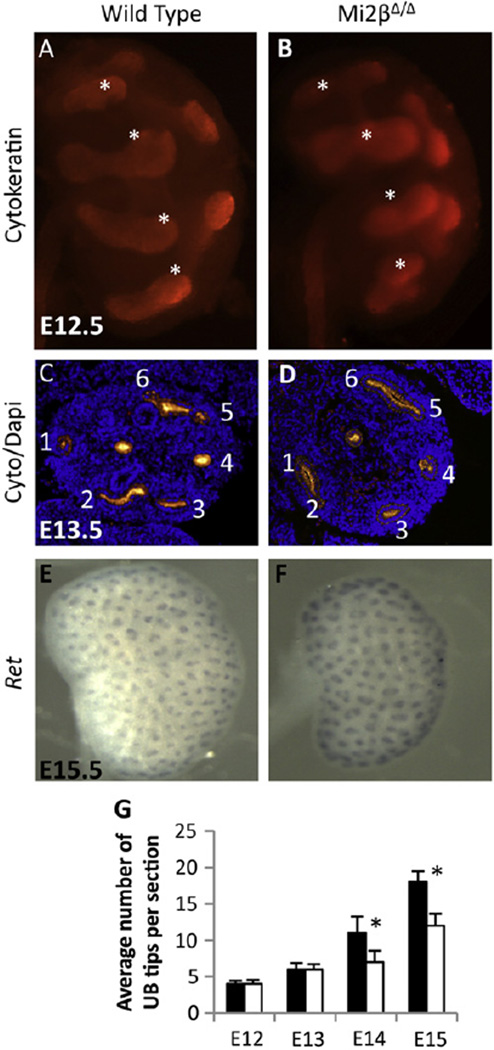
To quantify branching, we counted the number of UB tips per section identified by cytokeratin immunostaining at E12.5–15.5 (Fig. 3C). There was no significant (p = 0.27) difference in the average number of tips per section for wild type and Mi2βΔ/Δ mutants at E12.5 (4 ± 0.45 vs. 4 ± 0.55) and E13.5 (6 ± 0.87 vs. 6 ± 0.71). However, there was a significant (p < 0.001) reduction in the average number of UB tips per section at E14.5 in Mi2βΔ/Δ mutants compared to the wild type (8 ± 2.12 vs. 11 ± 2.25) and E15.5 (12 ± 1.67 vs. 18 ± 1.48). At E15.5, when Mi2βΔ/Δ kidneys are notably hypoplastic, expression of Ret mRNA at UB tips was unchanged in mutant kidneys (Fig. 3D, E). Together, these results (Figs. 2 and 3) show that renal hypoplasia is first apparent in the Mi2βΔ/Δ mutant at E14.5. At this stage, there is depletion of Six2-positive CM and reduced UB branching. Because reduced UB branching could be secondary to loss of CM, we hypothesized that defects in the CM prior to E14.5 represent the primary cause of the phenotype.
Fig. 3.
Ureteric bud branching is not altered at early stages in Mi2βΔ/Δ kidneys. (A–D) Immunostaining of the UB with cytokeratin. At E12.5 and E13.5 UB branching was similar in wild type (A, C) and Mi2βΔ/Δ (B, D) kidneys. (E and F) Whole mount in situ hybridization of Ret in E15.5 wild type (E) and Mi2βΔ/Δ (F) kidneys. Ret expression at UB tips is preserved in Mi2βΔ/Δ kidneys. (G) Quantification of UB branching as measured by tip counting of sections from E12.5–E15.5 wild type (black bars) and Mi2βΔ/Δ (white bars) kidneys. At E12.5 and E13.5, there was no significant difference (p = 0.27) in the average number of UB tips per section in wild type (4 ± 0.45 and 6 ± 0.87) and Mi2βΔ/Δ (4 ± 0.55 and 6 ± 0.71). A significant reduction (p < 0.001) in the average number of UB tips per section was observed in Mi2βΔ/Δ kidneys compared to wild type kidneys at E14.5 (8 ± 2.12 vs. 11 ± 2.12) and E15.5 (12 ± 1.67 vs. 18 ± 1.48).
Renal progenitor cells are depleted in Mi2β mutants
To further investigate the mechanism of renal hypoplasia, we examined several proteins known to be expressed in the renal progenitor cells. We analyzed the expression of Cited1 and Six2, two genes specific for the proliferating progenitor population (CM) of the developing kidney, at both E12.5 and E14.5. Six2 is also expressed in pre-tubular aggregates (Self et al., 2006), while Cited1 expression is restricted to a subpopulation of Six2-expressing cap mesenchyme cells that constitute the self-renewing progenitors, but is absent from induced mesenchyme/pre-tubular aggregates (Plisov et al., 2005). Pax2 is expressed in CM, early differentiating epithelial structures, and the branching ureteric bud epithelium (Dressler et al., 1990). Sall1 is highly expressed in CM cells and its expression persists in differentiating structures (Nishinakamura et al., 2001).
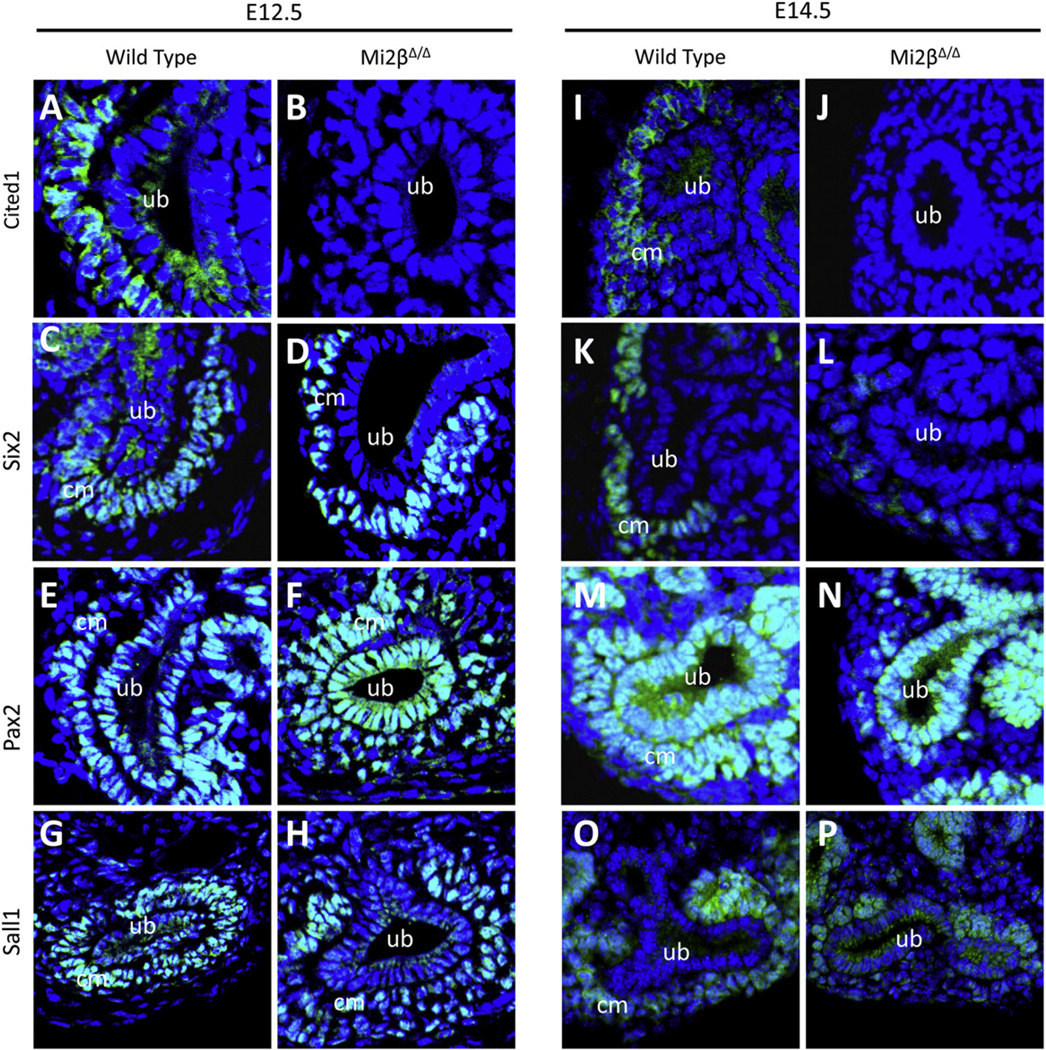
At E12.5, Cited1 was expressed in the cap mesenchyme surrounding UB tips in wild-type kidneys (Fig. 4A), however, it was not detected in Mi2βΔ/Δ kidneys (Fig. 4B). Six2 was expressed in both wild-type and Mi2βΔ/Δ kidneys in CM, indicating that CM does indeed initially form in these mutant kidneys (Fig. 4C, D), consistent with preserved GFP expression from Six2Cre in the mutant (Fig. 2B). Pax2 was also expressed in cap mesenchyme as well as branching UB in both wild-type and Mi2βΔ/Δ kidneys (Fig. 4E, F). Like Six2 and Pax2, Sall1 expression was maintained in the CM cells of Mi2β mutant kidneys compared to wild type kidneys (Fig. 4G, H). Thus, while expression of several proteins was preserved in CM at E12.5, Cited1 was markedly reduced, suggesting a defect in CM prior to when renal hypoplasia is evident.
Fig. 4.
Progenitor cell markers are decreased in Mi2β mutants. Immunofluorescence analysis of protein expression at E12.5 and E14.5 of Cited1 (A, B, I, J), Six2 (C, D, K, L), Pax2 (E, F, M, N), and Sall1 (G, H, O, P). (A, B, I, and J) Cited1 was expressed in CM cells surrounding UB tips at E12.5 and E14.5 in wild type kidneys (A, I), however it was not detected in Mi2βΔ/Δ kidneys at either developmental stage (B, J). (C, D, K, and L) At E12.5, Six2 was expressed in CM cells surrounding UB tips in both wild type and Mi2βΔ/Δ kidneys (C, D), however there was a marked reduction in the number of Six2-positive cells at E14.5 in Mi2βΔ/Δ kidneys compared to wild type (K,L). (E, F, M, and N) Pax2 immunostaining was detected in ureteric bud (UB) branches as well as CM cells at E12.5 in both wild type and Mi2βΔ/Δ kidneys (E, F). Its expression in UB was maintained in E14.5 wild type and Mi2βΔ/Δ kidneys, however there were fewer Pax2-positive cells surrounding UB tips in Mi2βΔ/Δ kidneys compared to wild type at this stage (M, N). (G, H, O, and P) Sall1 expression was evident in CM cells surrounding UB tips in both wild type and Mi2βΔ/Δ kidneys at E12.5 (G, H); however like Six2 and Pax2, there was a reduction in the number of Sall1-positive cells surrounding UB tips in E14.5 Mi2βΔ/Δ kidneys compared to wild type (O,P).
At E14.5, both Cited1 and Six2 continued to be expressed in the cap mesenchyme surrounding UB tips in wild-type tissues (Fig. 4I, K). In Mi2β mutants, Cited1 expression remained undetectable in these cells (Fig. 4J). Six2 expression was reduced overall and the number of Six2-positive CM cells was diminished (Fig. 4L). Like Six2, Pax2 expression in CM of Mi2β mutants was reduced compared to wild-type kidneys (Fig. 4M, N). In contrast, Pax2 expression was maintained in the UB of Mi2β mutant kidneys. Consistent with this, there was also a reduction in the number of Sall1-positive cells surrounding the UB tips of Mi2βΔ/Δ mutant kidneys (Fig. 4O, P). Together, these results support the conclusion that renal progenitor cells form at the initial stage of kidney development in Mi2β mutants, however their progressive depletion contributes substantially to renal hypoplasia.
Proliferation but not apoptosis is decreased in cap mesenchyme of Mi2β mutants
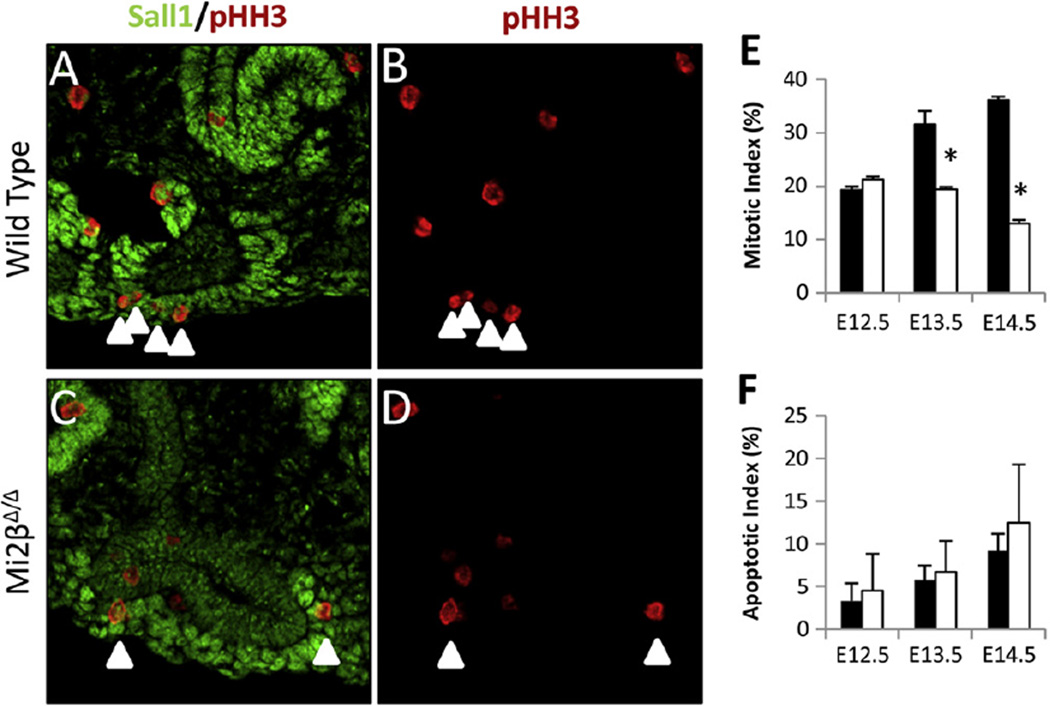
Depletion of CM can be caused by an increase in apoptosis or a decrease in proliferation. We thus analyzed whether apoptosis or proliferation was altered in the CM lacking Mi2β at E12.5–E14.5. We quantified proliferation by immunostaining for phospho-histone H3 (pHH3) (Fig. 5A–E). The number of pHH3/Sall1 double positive cells was related to the total number of Sall1-positive cells surrounding UB tips in wild-type and Mi2βΔ/Δ mutant kidneys. At E12.5, there was no significant difference (p = 0.5) in the mitotic index of wild type (19 ± 0.7%), and Mi2β mutant kidneys (21 ± 0.6%). However, at E13.5 there was significant reduction (p = 0.01) in the mitotic index in Mi2βΔ/Δ kidneys (19 ± 0.5%) compared with wild type controls (32 ± 2.5%). A similar significant reduction (p < 0.001) in the percentage of pHH3-positive cells of Mi2βΔ/Δ kidneys (13 ± 0.7%) compared to the wild type (36 ± 0.7%) was also evident at E14.5 (Fig. 5E).
Fig. 5.
Proliferation but not apoptosis is decreased in Mi2β mutants. (A–D) Proliferation of Sall1-positive CM cells as determined by pHH3 staining at E13.5. Arrowheads indicate CM cells that were scored as pHH3-positive. Sall1/pHH3 merged (A, C) and pHH3 staining alone (B, D) are shown. (E) Double-positive Sall1 (green) and pHH3 (red) CM cells were counted and expressed as a percentage of the total number of Sall1-positive CM cells surrounding UB tips in E12.5–E14.5 wild type (black bars) and Mi2βΔ/Δ kidneys (white bars). At E12.5 proliferation of CM cells was similar in wild type (19 ± 0.7%) and Mi2βΔ/Δ (21 ± 0.6%) [n = 2, total of 104 cells counted in wild type and 66 cells in Mi2βΔ/Δ kidneys. However, at E13.5 the percentage of proliferating cells was significantly decreased in Mi2βΔ/Δ kidneys (19 ± 0.5%) compared with wild type littermates (A, B) (32 ± 2.5%) [n = 2, total of 250 cells counted in the wild type and 221 in Mi2βΔ/Δ kidneys]. Reduction in cell proliferation persisted at E14.5 in Mi2βΔ/Δ kidneys (13 ± 0.7%) compared to wild type (36 ± 0.7%) [n = 3, total of 208 cells counted for wild type and 200 cells for Mi2βΔ/Δ kidneys]. (F) Apoptosis of Sall1-positive CM cells as measured by TUNEL assay (see Supplementary Fig. 1S) in E12.5, E13.5, and E14.5 wild type (black bars) and Mi2βΔ/Δ kidneys (white bars). There was no significant difference in TUNEL-positive CM cells between wild-type and Mi2βΔ/Δ kidneys.
Apoptosis was measured by TUNEL assay in wild-type and Mi2βΔ/Δ kidney sections in a similar fashion as proliferation. There was no significant difference (p = 0.5) in the number of cells undergoing apoptosis in wild-type and Mi2βΔ/Δ kidneys at E12.5, E13.5, and E14.5 (Fig. 5F and Supplementary Fig. 1S). These results indicate that deficiency of Mi2β in renal progenitor cells leads to decreased cell proliferation and does not significantly affect apoptosis. Importantly, the defect in cell proliferation was detected at E13.5, preceding significant overt renal hypoplasia and prior to a reduction in UB branching, suggesting it is causative. This decrease in cell proliferation would be expected to have a profound effect on the size of the progenitor pool as the kidney grows.
Differentiated epithelial structures form but are reduced in number in Mi2β mutants
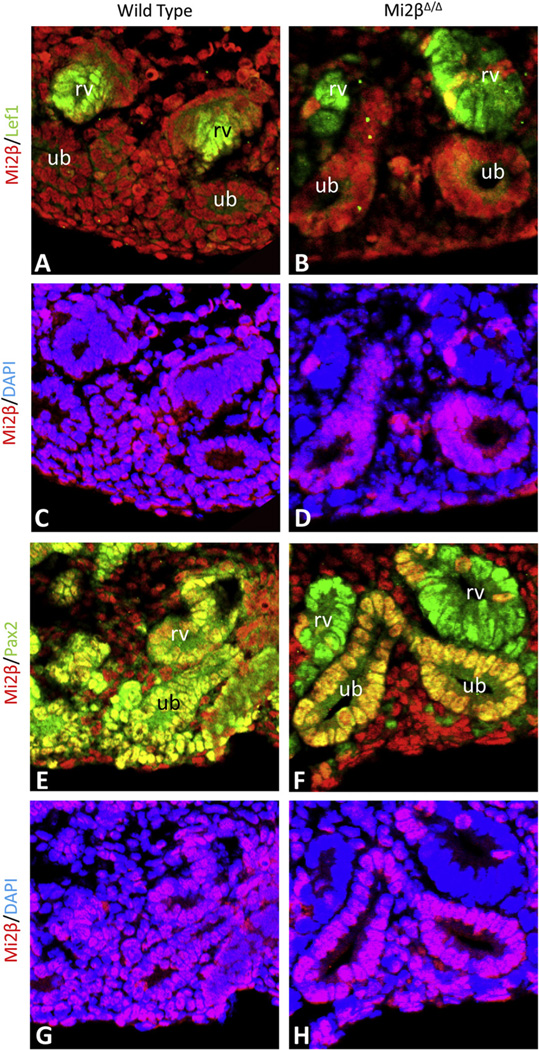
Since the cells of the CM undergo mesenchymal-to-epithelial transition [MET] (Kispert et al., 1998; Stark et al., 1994a) to form the tubular segments of the nephron (Carroll et al., 2005), we next analyzed whether that process was affected in Mi2βΔ/Δ kidneys. We tested whether the MET occurred in the absence of Mi2β by co-immunostaining wild type and Mi2βΔ/Δ kidneys for Mi2β and proteins expressed in CM-derived differentiated structures. At E15.5, renal vesicles co-expressed Mi2β and Lef1 in wild type kidneys (Fig. 6A, C). While Lef1-positive structures were detected in Mi2βΔ/Δ kidneys, they lacked Mi2β expression (Fig. 6B, D). Pax2 and Mi2β were present in the CM, UB, and differentiated structures of wild type kidneys (Fig. 6E,G), In contrast, in Mi2β mutants, Mi2β was only detected in Pax2-positive UB of Mi2βΔ/Δ kidneys, but was absent from renal vesicles (Fig. 6F, H). Expression of Wnt4, a gene required for MET (Stark et al., 1994b), was also detected by whole mount in situ hybridization at E14 in Mi2βΔ/Δ mutants (Fig. 2S). Together, these results indicate that Mi2β deficient CM cells are capable of undergoing MET to form Lef1- and Pax2-positve renal vesicles.
Fig. 6.
Differentiation of CM cells occurs despite the lack of Mi2β. (A–H) Identification of renal vesicles in wild type and Mi2βΔ/Δ kidneys at E15.5 by immunostaining. Co-localization of Mi2β (red) and Lef1 (green) was observed in renal vesicles of wild-type (A, C) kidneys. However, Lef1-positve renal vesicles in Mi2βΔ/Δ kidneys (B) lacked Mi2β expression (D). Pax2 and Mi2β were co-expressed in the CM, UB, and differentiated structures of wild type kidneys (E, G), In Mi2βΔ/Δ kidneys, Pax2-positive renal vesicles (F) did not express Mi2β (H). In contrast, Mi2β expression was retained in the UB and stroma (A, D, F, H) in conditional mutant kidneys in which Mi2β is deleted in CM by Six2-Cre.
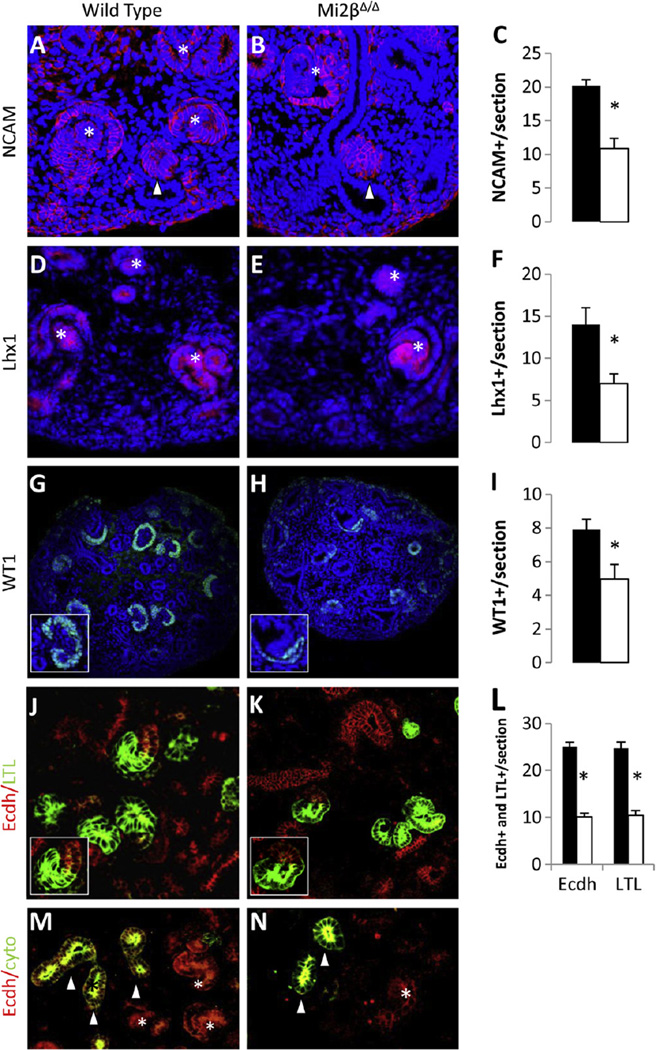
Renal vesicles undergo morphological transitions and segmentation into comma-and S-shaped bodies, and ultimately form all of the nephron segments except the collecting duct (Faa et al., 2012). To determine if Mi2β deficient renal vesicles can form more mature structures, we analyzed proteins that delineate the stages of nephron development at E15.5 including: (1) NCAM and Lhx1, which mark early renal vesicles and comma- and S-shaped bodies (Fujii et al., 1994; Klein et al., 1988), (2) WT1, which has restricted expression to the developing podocytes in S-shaped bodies (Kreidberg, 2003), (3) LTL, which binds specifically to proximal tubular segments, and (4) E-cadherin, which is expressed in cytokeratin negative distal tubules.
In Mi2βΔ/Δ kidneys, the number of NCAM-positive structures was significantly reduced (10.67 ± 1.52) compared to wild-type (20.25 ± 0.84) (Fig. 7A–C). We noted a similar reduction in the number of Lhx1-postive differentiated structures in the Mi2βΔ/Δ kidneys (7 ± 1.15) compared to wild type (14 ± 2) (Fig. 7D–F). Fewer WT1-positive podocyte structures were formed in Mi2β mutants (4.97 ± 0.86) compared to the wild-type (7.91 ± 0.61) (Fig. 7G–I). WT1-positive structures were smaller and morphologically abnormal in the mutant (Fig. 7G, H inserts). Mi2βΔ/Δ kidneys were capable of forming proximal and distal tubules as marked by E-cadherin and LTL (Fig. 7K, inset) which were comparable to those seen in wild type (Fig. 7J, inset). However, there were a significant decrease in the number of both LTL-positive proximal tubules (10.43 ± 1.02 vs. 24.71 ± 0.75) and E-cadherin-positive/cytokeratin-negative distal tubules (10.13 ± 1.33 vs. 25 ± 1) in Mi2βΔ/Δ kidneys compared to wild-type (Fig. 7J–N). Taken together, these results indicate that while all nephron segments can form in Mi2β mutants, there is a proportional decrease in nephron induction that is likely due predominantly to the progressive depletion of CM progenitor cells.
Fig. 7.
Differentiated epithelial structures are reduced in number in Mi2βΔ/Δ kidneys. (A–N) Immunofluoresence and quantification of differentiated structures in wild type and Mi2βΔ/Δ kidneys at E15.5. NCAM (red) staining of wild type (A) and Mi2βΔ/Δ kidneys (B) revealed a decreased number of renal vesicles (arrowheads) and later differentiated comma- and S-shaped bodies (asterisks) in Mi2βΔ/Δ kidneys (C). A similar reduction (F) was noted in the number of Lhx1-positive (red) differentiated structures in Mi2βΔ/Δ kidneys (E) compared to wild type (D). WT1 (green) staining in wild type (G) and mutants (H) indicates that fewer glomeruli formed in Mi2βΔ/Δ kidneys (I). Glomeruli that formed in mutants are smaller and showed an abnormal arrangement of the podocyte layer (G, H insets). Staining of LTL (green) and E-cadherin (red) in wild type (J) and mutants (K) indicates that proximal and distal nephron segments formed (J, K insets) in Mi2β mutants, but their numbers were reduced (L). E-cadherin-positive distal tubules were distinguished from UB epithelium by co-staining with cytokeratin which stains only UB (M, N).
Sall1 and NuRD genetically interact during kidney development
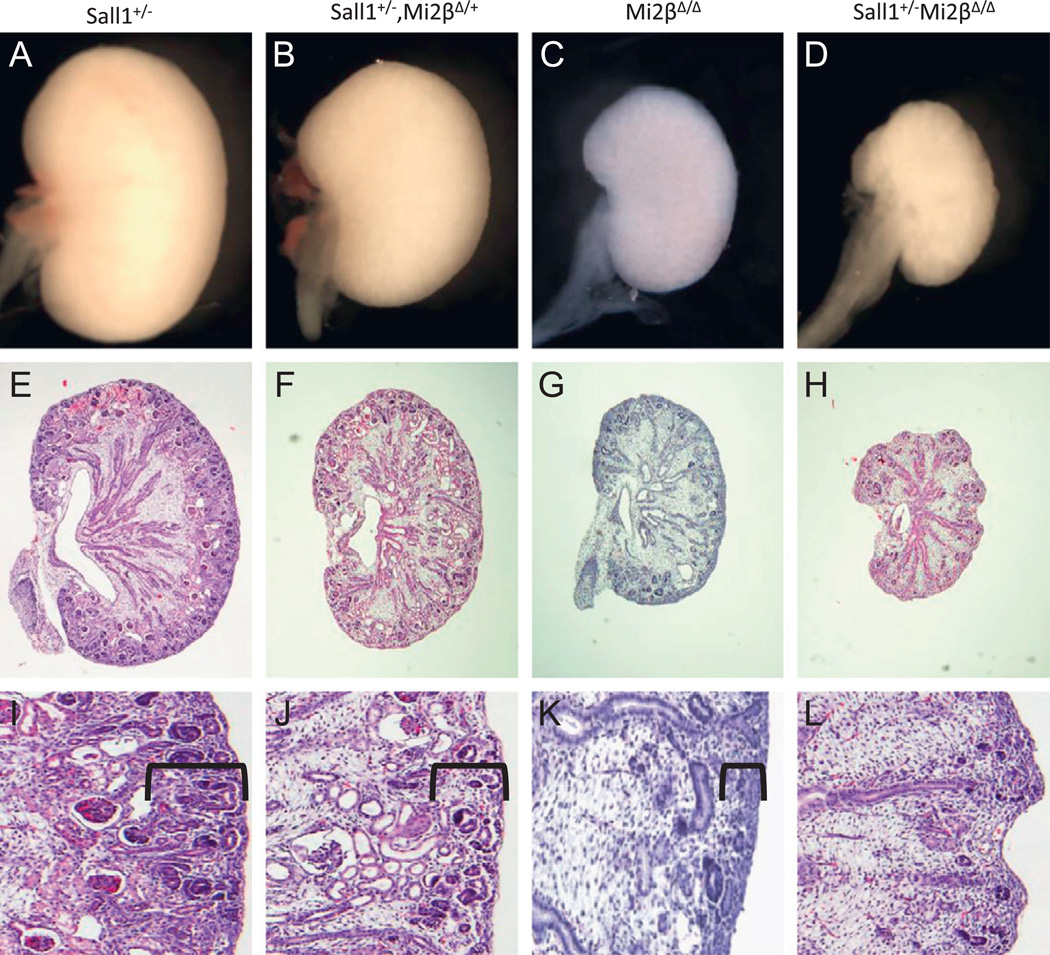
We have previously demonstrated that Sall1 and Mi2–NuRD associate to regulate developmental gene expression in Xenopus embryos and in a cell culture model (Lauberth et al., 2007; Lauberth and Rauchman, 2006). We thus hypothesized that NuRD’s role in developing kidney would involve its interaction with the transcription factor Sall1, an essential gene in nephrogenesis. To test for a functional interaction in vivo, we examined whether the Mi2β mutant phenotype was sensitive to a genetic reduction of Sall1 dose by crossing Mi2β mutants with Sall1+/− heterozygous mice. Both Sall1 and Mi2β single heterozygous mutant mice had normal kidneys. Average kidney area to body weight ratios for Sall1+/− (57.5 ± 1.32) and Mi2βΔ/+ (54.7 ± 1.53) were not significantly different than wild-type littermates (57.9 ± 1.32). However, double heterozygous Sall1+/− Mi2βΔ/+ mice exhibited significant renal hypoplasia. The average kidney area to body weight ratio for double heterozygotes (41.8 ± 1.04) was significantly smaller than wild type and single heterozygous mice, and comparable to Mi2βΔ/Δ homozygous mutants (38.4 ± 1.63) (Table 1 and Fig. 8B–D).
Table 1.
Average kidney size to body weight ratio for Sall1/Mi2β compound mutants.
| Genotype | N | Avg. KS/BW |
|---|---|---|
| WT | 16 | 57.9 ± 1.32 |
| Sall1+/− | 12 | 57.5 ± 1.32 |
| Mi2βΔ/+ | 12 | 54.7 ± 1.54 |
| Sall1+/−Mi2βΔ/+ | 14 | 41.8 ± 1.04* |
| Mi2βΔ/Δ | 13 | 38.4 ± 1.63* |
| Sall1+/−Mi2βΔ/Δ | 6 | 18 ± 0.29** |
P < 0.01
P < 0.001.
Fig. 8.
Sall1 and Mi2β genetically interact during embryonic kidney development. (A–D) E15.5 kidneys dissected from genetic crosses between Sall1+/−, Mi2βfl/+ and Six2-Cretg/+, Mi2βfl/+. Renal hypoplasia was seen in Sall1+/−/Mi2βΔ/+ double heterozygotes (B) and even more prominently in Mi2βΔ/Δ (C) and Sall1+/−, Mi2βΔ/Δ (D) compared to Sall1+/− (A) and wild type (data not shown) mice. (E–L) Histological analysis of Sall1/Mi2β compound mutants. Thickness of the nephrogenic zone (bracket) was reduced in Sall1+/−/ Mi2βΔ/+ double heterozygous kidneys (F, J); The loss of nephrogenic zone in Mi2βΔ/Δ kidneys was more severe (G, K). In Sall1+/−, Mi2βΔ/Δ mutant kidneys the nephrogenic zone was not detected at this stage and there were rare differentiated structures (H, L). Sall1+/− kidneys had a normal nephrogenic zone with many epithelial nephron precursor structures (E, I).
Deletion of both Mi2β alleles from the CM cells in the Sall1 heterozygous mutant background further worsened the kidney phenotype. Renal hypoplasia in these mutant mice was considerably more severe than that of Sall1, Mi2β double heterozygous or Mi2βΔ/Δ homozygous mutants alone (average kidney area to body weight of 18 ± 0.29) (Fig. 8E).
We performed histological staining of these compound mutants to further characterize the renal hypoplasia. Examination of E15.5 Sall1+/− Mi2βΔ/+ kidneys revealed a moderate reduction in the thickness of the nephrogenic zone and fewer differentiated structures compared to wild-type and Sall1+/− controls (Fig. 8F, J). Some tubules and glomeruli exhibited cystic dilation, indicating abnormalities in the differentiation process. Histological analysis of Mi2βΔ/Δ kidneys revealed a slightly more severe reduction in the size of the nephrogenic zone compared to Sall1+/− Mi2βΔ/+ kidneys (Fig. 8G, K). In Sall1+/−Mi2βΔ/Δ mutant kidneys, no demarcated nephrogenic zone existed, with near complete loss of CM and presence of only rare differentiated structures (Fig. 8H, L). The renal medulla formed in these compound mutants with a reduction in size that is comparable to the overall decrease in the kidney. These results demonstrate a strong, dose dependent genetic interaction between Sall1 and Mi2β, suggesting that they act cooperatively to regulate kidney development.
Discussion
These results provide the first demonstration that Mi2β, a key component of the NuRD complex is required for normal kidney development. Specific deletion of Mi2β in CM affects the proliferation of renal progenitor cells leading to renal hypoplasia. Mi2β and Sall1 display a strong genetic interaction indicating that they cooperate in formation of the kidney.
Kidney development is an iterative process leading to induction of thousands of individual functional units, called nephrons. Thus, growth of the kidney depends on a dramatic expansion of the progenitor pool. Our studies provide insight into the control of this process by showing that Mi2β is required for maintenance and expansion of these cells. In the absence of Mi2β, the ability for sustained or extended proliferative capacity necessary for development of a normal complement of nephrons was critically dependent on the presence of Mi2β. This conclusion is supported by several lines of evidence showing that the CM defects preceded the depletion of these progenitor cells and the development of an overt renal hypoplasia phenotype first noted at E14.5. These observations include specific loss of Cited1 expression in CM at E12.5 (Fig. 4B), reduced CM cell proliferation at E13.5 (Fig. 5E), with no detectable increase in apoptosis (Fig. 5F). In addition, UB branching was not reduced in the mutant at E13.5 (Fig. 3C). Together these results support the conclusion that the loss of proliferative capacity of Mi2β deficient CM is a principal cause of severe hypoplasia in these mutants.
In addition to the CM, Mi2β is expressed broadly in the developing kidney, including various stages of nephron precursors, and specific deletion of Mi2β in CM removes this gene from all nephron structures derived from these progenitor cells. Thus, Mi2β could have functions at several steps of kidney development, apart from a critical role in CM growth, which could contribute to renal hypoplasia. We addressed this possibility by examining nephron formation in Mi2β mutants. We observed a severe reduction of nephron number in Mi2β mutants, resulting in peri-natal lethality. This was associated with a reduction in renal vesicle formation, the earliest morphological precursor of the nephron, and by a proportionate decrease in all the more terminally differentiated segments of the nephron (Fig. 7). Our studies show that Lef1-positive renal vesicles (RVs) that formed in the mutant were composed of Mi2β deficient cells (Fig. 6B, D), indicating that Mi2β is not required for mesenchymal-to-epithelial transition (MET). We cannot exclude the possibility, however, that the efficiency of MET is impaired by loss of Mi2β and thereby contributed to reduced RV formation. Our studies also revealed that podocyte precursors, as well as proximal and distal tubules formed, albeit in reduced numbers, in the Mi2β mutants. This suggests that segmentation of the nephron was not overtly impaired by loss of Mi2β. Overall, these analyses of nephron formation are consistent with our model that reduced renal progenitor cell proliferation is the predominant cause of renal hypoplasia due to deletion of Mi2β in CM by Six2-Cre.
However, our studies may have also uncovered a specific role for Mi2β in the proper differentiation of nephrons. While Wt1-positive glomeruli formed in Mi2β deficient kidneys, the podocyte layer was disorganized (Fig. 7H, inset). This suggests that Mi2β may be required for the developmental transition from the precursor epithelial structure (renal vesicle) to formation of the most proximal segment of the nephron (glomerulus). Sall1 is highly expressed in renal vesicles and its interaction with Mi2β in this structure, rather than in CM, could account for the patterning defect we observed in podocytes. Consistent with this idea, Mi2β, Sall1 compound heterozygotes also displayed evidence of abnormal nephron development, including cystic dilatation of glomeruli and renal tubules (Fig. 8K). These compound mutants died of kidney failure at 6–8 weeks indicating the severity of this defect. Future studies using other informative Cre strains could be used to bypass the requirement of Mi2β in CM and more precisely define its role in segment or cell type specific specification of renal epithelial cells.
Six2, a gene that is required to maintain renal progenitors, was reduced in Mi2β deficient CM. In Six2 mutant kidneys, premature differentiation and apoptosis of CM is thought to account for progenitor cell depletion (Self et al., 2006). Mi2β mutants did not display premature differentiation (Fig. 4E–H and data not shown) or increased apoptosis. In contrast, the profound reduction in cell proliferation that we observed in Mi2β mutant kidneys can likely account for the progressive depletion of CM, suggesting a different molecular mechanism than the one proposed for Six2. Recent studies show that canonical Wnt signaling is required for maintenance and proliferation of progenitor cells (Karner et al., 2011). Since both Mi2–NuRD and Sall1 have been shown to modulate Wnt activity (Kiefer et al., 2010; Moon, 2004; Sato et al., 2004), we speculate that their cooperative action in the kidney could regulate renal progenitors through this pathway.
While Mi2 has been postulated to have effects independent (Kunert and Brehm, 2009) of its association with the NuRD complex, it is likely that its role in renal progenitor cells is NuRD-dependent. We have previously shown that Sall1 physically associates with NuRD to directly regulate gene expression (Lauberth et al., 2007; Lauberth and Rauchman, 2006). Analysis of Sall1+/−; Mi2βΔ/Δ compound mutants showed a more profound degree of renal hypodysplasia, accompanied by a severe depletion of CM, indicating that they act cooperatively to maintain renal progenitors. The strong genetic interaction in the kidney between Sall1 and Mi2β described in this study likely relates to function of the NuRD complex. In support of this conclusion, we have recently found that disruption of the Sall1-NuRD interaction in vivo leads to severe renal hypodysplasia (Denner & Rauchman, manuscript in preparation). NuRD has been shown to influence gene expression at the level of chromatin either through its direct association with DNA, binding of pre-existing histone modifications or through its recruitment of sequence-specific DNA binding factors such as Ikaros, Fog1 and Bcl11a (Cismasiu et al., 2005; Hong et al., 2005; Lai and Wade, 2011). Elegant studies from the Georgopoulos laboratory suggest a model whereby proper targeting of the Mi2–NuRD complex through its interaction with lineage specific DNA binding factors, such as Ikaros, is essential for the balance between growth and differentiation of progenitors (Zhang et al., 2012). In ES cells, Sall4 and NuRD are found in a macromolecular complex that has been shown to regulate pluripotency and lineage determination (Liang et al., 2008). Our studies raise the intriguing possibility that Sall1-NuRD similarly act together to control specific target genes in renal progenitors that affect the timely induction of renal vesicles versus the decision to proliferate and thereby expand the progenitor pool. Future genome-wide analyses of Sall1 and NuRD chromatin binding and transcriptional profiling will be needed to define the epigenetic regulation of nephron induction versus progenitor cell self-renewal in developing kidney.
Renal hypodysplasia, similar to that observed in Mi2β, Sall1 compound heterozygous mutants, is a common birth defect that accounts for −40% of childhood renal failure (Cain et al., 2010). Thus it is important to identify genetic pathways that lead to these disorders and that could be manipulated therapeutically to increase nephron formation. HDAC inhibitors and agents targeting other chromatin modifying enzymes are now being tested for the treatment of various diseases, especially cancers. Understanding the role of chromatin remodeling complexes in the epigenetic regulation of pathways that control nephron number has the potential to produce novel approaches to these common birth defects.
Supplementary Material
Acknowledgments
Thank you to Lynn Robbins for technical support and to Dr. Susan Kiefer and Dr. Jeannine Basta for critical analysis of the manuscript. Dr. Katia Georgopoulos (Massachusetts General Hospital, Charlestown, MA) kindly provided Mi2β flox mice. This work was supported by an Established Investigator Award to M. Rauchman from the American Heart Association.
Footnotes
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.ydbio.2012.11.018.
References
- Bowen NJ, Fujita N, Kajita M, Wade PA. Mi-2/NuRD: multiple complexes for many purposes. Biochim. Biophys. Acta. 2004;1677:52–57. doi: 10.1016/j.bbaexp.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Boyle S, Misfeldt A, Chandler KJ, Deal KK, Southard-Smith EM, Mortlock DP, Baldwin HS, de Caestecker M. Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev. Biol. 2008;313:234–245. doi: 10.1016/j.ydbio.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain JE, Di Giovanni V, Smeeton J, Rosenblum ND. Genetics of renal hypoplasia: insights into the mechanisms controlling nephron endowment. Pediatr. Res. 2010;68:91–98. doi: 10.1203/PDR.0b013e3181e35a88. [DOI] [PubMed] [Google Scholar]
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev. Cell. 2005;9:283–292. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Chen S, Bellew C, Yao X, Stefkova J, Dipp S, Saifudeen Z, Bachvarov D, El-Dahr SS. Histone deacetylase (HDAC) activity is critical for embryonic kidney gene expression, growth, and differentiation. J. Biol. Chem. 2011;286:32775–32789. doi: 10.1074/jbc.M111.248278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cismasiu VB, Adamo K, Gecewicz J, Duque J, Lin Q, Avram D. BCL11B functionally associates with the NuRD complex in T lymphocytes to repress targeted promoter. Oncogene. 2005;24:6753–6764. doi: 10.1038/sj.onc.1208904. [DOI] [PubMed] [Google Scholar]
- Costantini F, Kopan R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev. Cell. 2010;18:698–712. doi: 10.1016/j.devcel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler GR. Advances in early kidney specification, development and patterning. Development. 2009;136:3863–3874. doi: 10.1242/dev.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler GR, Deutsch U, Chowdhury K, Nornes HO, Gruss P. Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development. 1990;109:787–795. doi: 10.1242/dev.109.4.787. [DOI] [PubMed] [Google Scholar]
- Faa G, Gerosa C, Fanni D, Monga G, Zaffanello M, Van Eyken P, Fanos V. Morphogenesis and molecular mechanisms involved in human kidney development. J. Cell Physiol. 2012;227:1257–1268. doi: 10.1002/jcp.22985. [DOI] [PubMed] [Google Scholar]
- Fujii T, Pichel JG, Taira M, Toyama R, Dawid IB, Westphal H. Expression patterns of the murine LIM class homeobox gene lim1 in the developing brain and excretory system. Dev. Dyn. 1994;199:73–83. doi: 10.1002/aja.1001990108. [DOI] [PubMed] [Google Scholar]
- Guan LS, Rauchman M, Wang ZY. Induction of Rb-associated protein (RbAp46) by Wilms’ tumor suppressor WT1 mediates growth inhibition. J. Biol. Chem. 1998;273:27047–27050. doi: 10.1074/jbc.273.42.27047. [DOI] [PubMed] [Google Scholar]
- Hong W, Nakazawa M, Chen YY, Kori R, Vakoc CR, Rakowski C, Blobel GA. FOG-1 recruits the NuRD repressor complex to mediate transcriptional repression by GATA-1. EMBO J. 2005;24:2367–2378. doi: 10.1038/sj.emboj.7600703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji K, Caballero IM, MacLeod R, Nichols J, Wilson VA, Hendrich B. The NuRD component Mbd3 is required for pluripotency of embryonic stem cells. Nat. Cell Biol. 2006;8:285–292. doi: 10.1038/ncb1372. [DOI] [PubMed] [Google Scholar]
- Karner CM, Das A, Ma Z, Self M, Chen C, Lum L, Oliver G, Carroll TJ. Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development. 2011;138:1247–1257. doi: 10.1242/dev.057646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi M, Morgan BA, Georgopoulos K. The chromatin remodeler Mi-2beta is required for establishment of the basal epidermis and normal differentiation of its progeny. Development. 2007;134:1571–1582. doi: 10.1242/dev.001750. [DOI] [PubMed] [Google Scholar]
- Keller G, Zimmer G, Mall G, Ritz E, Amann K. Nephron number in patients with primary hypertension. N. Engl. J. Med. 2003;348:101–108. doi: 10.1056/NEJMoa020549. [DOI] [PubMed] [Google Scholar]
- Kiefer SM, McDill BW, Yang J, Rauchman M. Murine Sall1 represses transcription by recruiting a histone deacetylase complex. J. Biol. Chem. 2002;277:14869–14876. doi: 10.1074/jbc.M200052200. [DOI] [PubMed] [Google Scholar]
- Kiefer SM, Ohlemiller KK, Yang J, McDill BW, Kohlhase J, Rauchman M. Expression of a truncated Sall1 transcriptional repressor is responsible for Townes–Brocks syndrome birth defects. Hum. Mol. Genet. 2003;12:2221–2227. doi: 10.1093/hmg/ddg233. [DOI] [PubMed] [Google Scholar]
- Kiefer SM, Robbins L, Barina A, Zhang Z, Rauchman M. SALL1 truncated protein expression in Townes–Brocks syndrome leads to ectopic expression of downstream genes. Hum. Mutat. 2008;29:1133–1140. doi: 10.1002/humu.20759. [DOI] [PubMed] [Google Scholar]
- Kiefer SM, Robbins L, Stumpff KM, Lin C, Ma L, Rauchman M. Sall1-dependent signals affect Wnt signaling and ureter tip fate to initiate kidney development. Development. 2010;137:3099–3106. doi: 10.1242/dev.037812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kispert A, Vainio S, McMahon AP. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development. 1998;125:4225–4234. doi: 10.1242/dev.125.21.4225. [DOI] [PubMed] [Google Scholar]
- Klein G, Langegger M, Goridis C, Ekblom P. Neural cell adhesion molecules during embryonic induction and development of the kidney. Development. 1988;102:749–761. doi: 10.1242/dev.102.4.749. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3:169–181. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhase J, Wischermann A, Reichenbach H, Froster U, Engel W. Mutations in the SALL1 putative transcription factor gene cause Townes–Brocks syndrome. Nat. Genet. 1998;18:81–83. doi: 10.1038/ng0198-81. [DOI] [PubMed] [Google Scholar]
- Kreidberg JA. Podocyte differentiation and glomerulogenesis. J. Am. Soc. Nephrol. 2003;14:806–814. doi: 10.1097/01.asn.0000054887.42550.14. [DOI] [PubMed] [Google Scholar]
- Kreidberg JA, Hartwig S. Wilms’ tumor-1: a riddle wrapped in a mystery, inside a kidney. Kidney Int. 2008;74:411–412. doi: 10.1038/ki.2008.307. [DOI] [PubMed] [Google Scholar]
- Kunert N, Brehm A. Novel Mi-2 related ATP-dependent chromatin remodelers. Epigenetics. 2009;4:209–211. doi: 10.4161/epi.8933. [DOI] [PubMed] [Google Scholar]
- Lai AY, Wade PA. Cancer biology and NuRD: a multifaceted chromatin remodelling complex. Nat. Rev. Cancer. 2011;11:588–596. doi: 10.1038/nrc3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauberth SM, Bilyeu AC, Firulli BA, Kroll KL, Rauchman M. A phosphomimetic mutation in the Sall1 repression motif disrupts recruitment of the nucleosome remodeling and deacetylase complex and repression of Gbx2. J. Biol. Chem. 2007;282:34858–34868. doi: 10.1074/jbc.M703702200. [DOI] [PubMed] [Google Scholar]
- Lauberth SM, Rauchman M. A conserved 12-amino acid motif in Sall1 recruits the nucleosome remodeling and deacetylase corepressor complex. J. Biol. Chem. 2006;281:23922–23931. doi: 10.1074/jbc.M513461200. [DOI] [PubMed] [Google Scholar]
- Liang J, Wan M, Zhang Y, Gu P, Xin H, Jung SY, Qin J, Wong J, Cooney AJ, Liu D, Songyang Z. Nanog and Oct4 associate with unique transcriptional repression complexes in embryonic stem cells. Nat. Cell Biol. 2008;10:731–739. doi: 10.1038/ncb1736. [DOI] [PubMed] [Google Scholar]
- McDonel P, Costello I, Hendrich B. Keeping things quiet: roles of NuRD and Sin3 co-repressor complexes during mammalian development. Int. J. Biochem. Cell Biol. 2009;41:108–116. doi: 10.1016/j.biocel.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RT. Teaching resource. Canonical Wnt/beta-catenin signaling. Sci. STKE. 2004;2004 doi: 10.1126/stke.2402004tr5. (tr5). [DOI] [PubMed] [Google Scholar]
- Nishinakamura R, Matsumoto Y, Nakao K, Nakamura K, Sato A, Copeland NG, Gilbert DJ, Jenkins NA, Scully S, Lacey DL, Katsuki M, Asashima M, Yokota T. Murine homolog of SALL1 is essential for ureteric bud invasion in kidney development. Development. 2001;128:3105–3115. doi: 10.1242/dev.128.16.3105. [DOI] [PubMed] [Google Scholar]
- Oliver G, Wehr R, Jenkins NA, Copeland NG, Cheyette BN, Hartenstein V, Zipursky SL, Gruss P. Homeobox genes and connective tissue patterning. Development. 1995;121:693–705. doi: 10.1242/dev.121.3.693. [DOI] [PubMed] [Google Scholar]
- Osafune K, Takasato M, Kispert A, Asashima M, Nishinakamura R. Identification of multipotent progenitors in the embryonic mouse kidney by a novel colony-forming assay. Development. 2006;133:151–161. doi: 10.1242/dev.02174. [DOI] [PubMed] [Google Scholar]
- Plisov S, Tsang M, Shi G, Boyle S, Yoshino K, Dunwoodie SL, Dawid IB, Shioda T, Perantoni AO, de Caestecker MP. Cited1 is a bifunctional transcriptional cofactor that regulates early nephronic patterning. J. Am. Soc. Nephrol. 2005;16:1632–1644. doi: 10.1681/ASN.2004060476. [DOI] [PubMed] [Google Scholar]
- Puelles VG, Hoy WE, Hughson MD, Diouf B, Douglas-Denton RN, Bertram JF. Glomerular number and size variability and risk for kidney disease. Curr. Opin. Nephrol. Hypertens. 2011;20:7–15. doi: 10.1097/MNH.0b013e3283410a7d. [DOI] [PubMed] [Google Scholar]
- Reynolds N, Salmon-Divon M, Dvinge H, Hynes-Allen A, Balasooriya G, Leaford D, Behrens A, Bertone P, Hendrich B. NuRD-mediated deacetylation of H3K27 facilitates recruitment of Polycomb Repressive Complex 2 to direct gene repression. EMBO J. 2012;31:593–605. doi: 10.1038/emboj.2011.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Kishida S, Tanaka T, Kikuchi A, Kodama T, Asashima M, Nishinakamura R. Sall1, a causative gene for Townes–Brocks syndrome, enhances the canonical Wnt signaling by localizing to heterochromatin. Biochem. Biophys. Res. Commun. 2004;319:103–113. doi: 10.1016/j.bbrc.2004.04.156. [DOI] [PubMed] [Google Scholar]
- Self M, Lagutin OV, Bowling B, Hendrix J, Cai Y, Dressler GR, Oliver G. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J. 2006;25:5214–5228. doi: 10.1038/sj.emboj.7601381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994a;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994b;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- Williams CJ, Naito T, Arco PG, Seavitt JR, Cashman SM, De Souza B, Qi X, Keables P, Von Andrian UH, Georgopoulos K. The chromatin remodeler Mi-2beta is required for CD4 expression and T cell development. Immunity. 2004;20:719–733. doi: 10.1016/j.immuni.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Xue Y, Wong J, Moreno GT, Young MK, Cote J, Wang W. NURD, a novel complex with both ATP-dependent chromatin-remodeling and histone deacetylase activities. Mol. Cell. 1998;2:851–861. doi: 10.1016/s1097-2765(00)80299-3. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Hazan I, Zhang J, Ng SY, Naito T, Snippert HJ, Heller EJ, Qi X, Lawton LN, Williams CJ, Georgopoulos K. The role of the chromatin remodeler Mi-2beta in hematopoietic stem cell self-renewal and multilineage differentiation. Genes Dev. 2008;22:1174–1189. doi: 10.1101/gad.1642808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Jackson AF, Naito T, Dose M, Seavitt J, Liu F, Heller EJ, Kashiwagi M, Yoshida T, Gounari F, Petrie HT, Georgopoulos K. Harnessing of the nucleosome-remodeling-deacetylase complex controls lymphocyte development and prevents leukemogenesis. Nat. Immunol. 2012;13:86–94. doi: 10.1038/ni.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ng HH, Erdjument-Bromage H, Tempst P, Bird A, Reinberg D. Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev. 1999;13:1924–1935. doi: 10.1101/gad.13.15.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.