Abstract
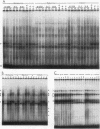
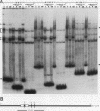
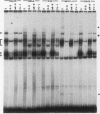
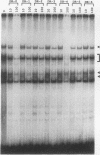
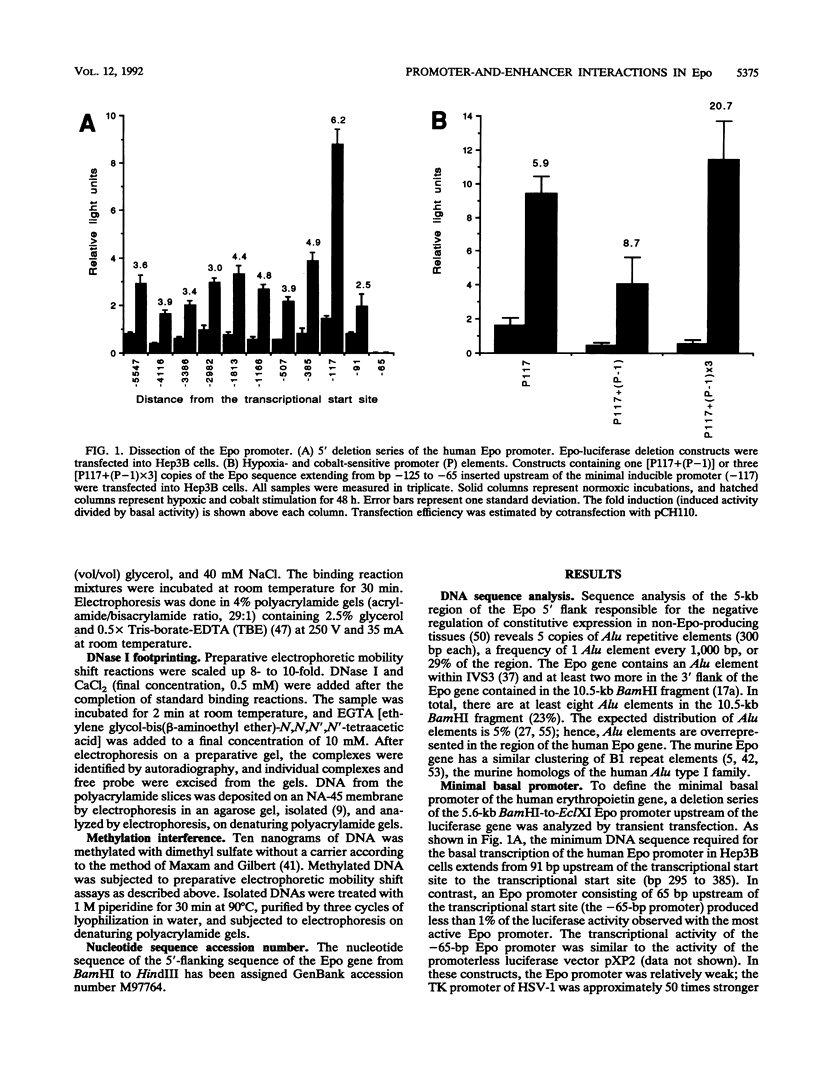
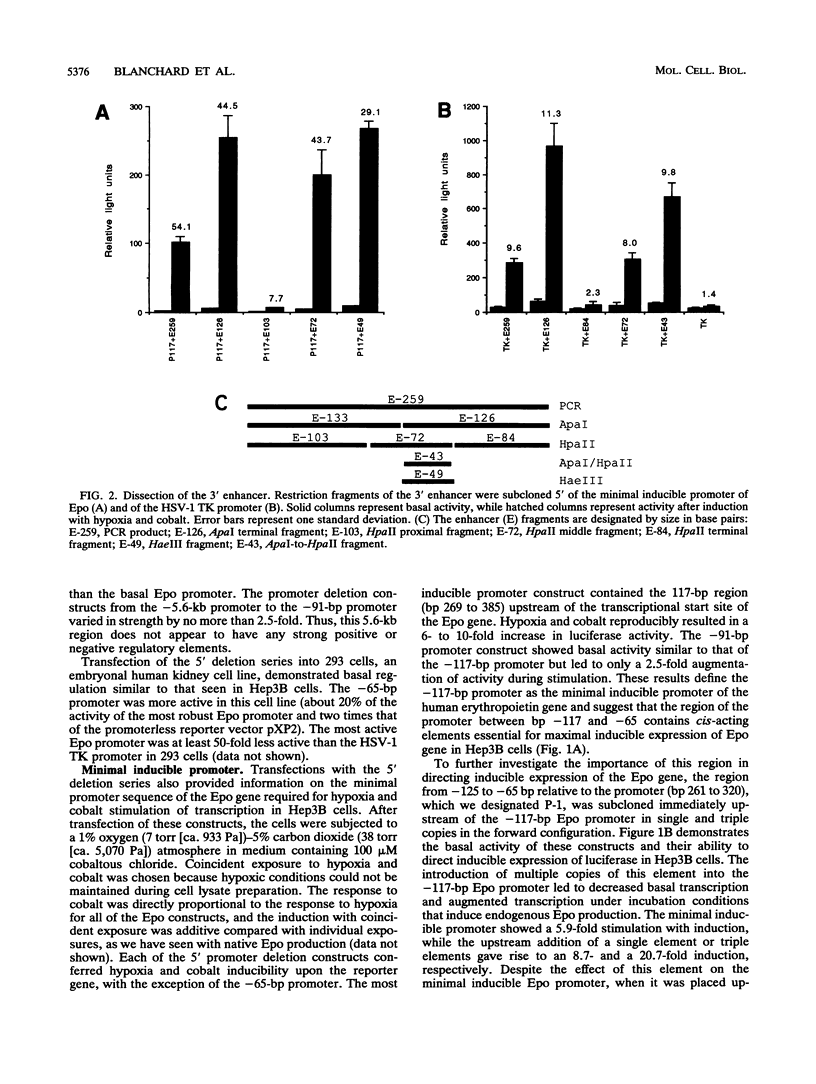
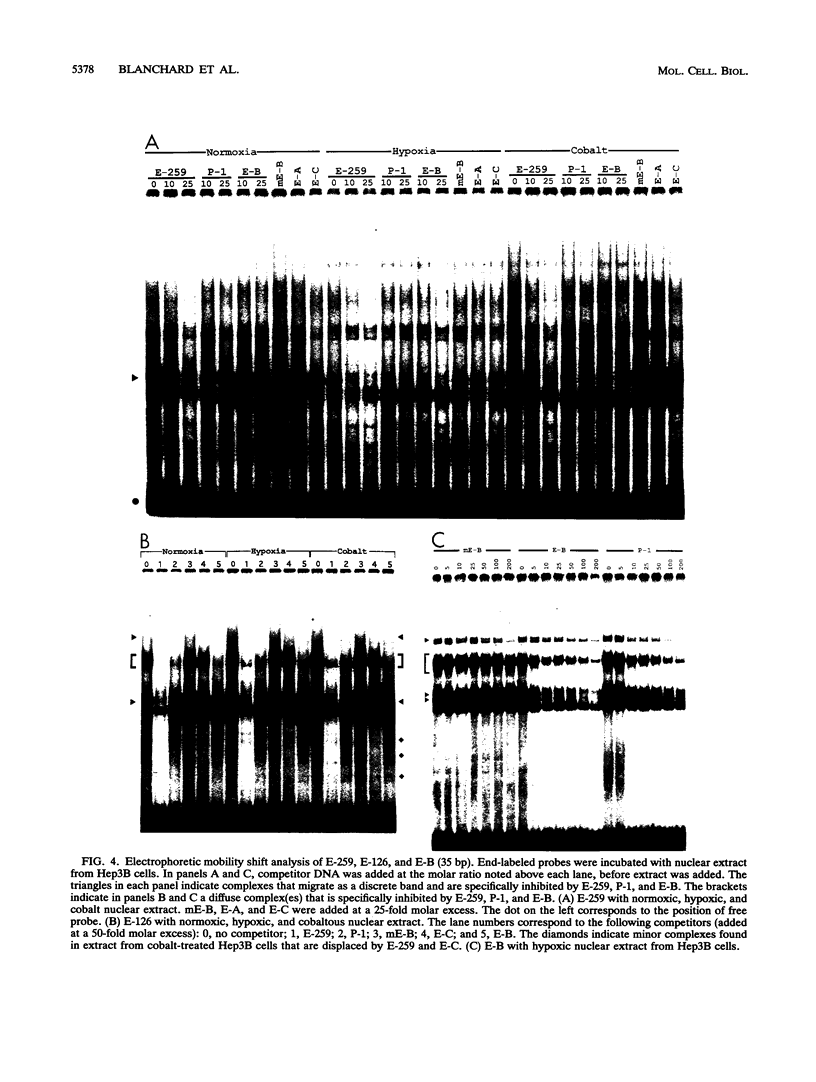
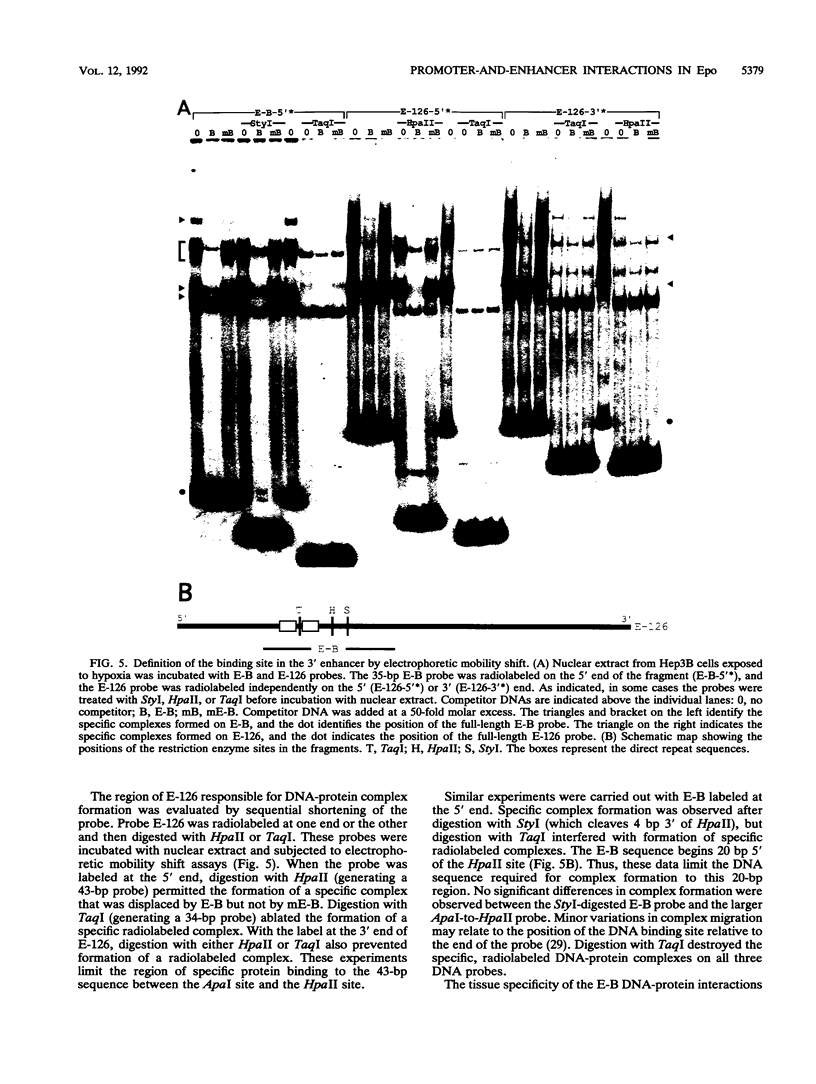
Transcription of the human erythropoietin (Epo) gene is stimulated by exposure to hypoxia and/or cobalt in whole animals and in Hep3B cells. We have systematically investigated the promoter and 3' enhancer elements necessary for this induction by transient transfection of Hep3B cells. We define a promoter region of 53 bp and an enhancer region of 43 bp that confer hypoxia and cobalt inducibility. Each element gives rise to a 6- to 10-fold induction alone. In combination they produce a 50-fold induction after stimulation, similar to the 50- to 100-fold induction of the endogenous Epo gene. Two areas of DNA sequence homology are present in these regions. We demonstrate specific DNA-protein interactions in the enhancer and the ability of the promoter element to compete with these interactions in electrophoretic mobility shift assays. DNase I footprinting and methylation interference data further refine the cis-acting element in the 43-bp enhancer to a short region containing a direct repeat of a steroid/thyroid hormone receptor response element half-site separated by a 2-bp gap. Two half-site consensus sequences are also present in the 53-bp promoter. Site-specific mutation of the half-site sequences in the enhancer destroys the functional activity of the enhancer.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagchi M. K., Tsai S. Y., Tsai M. J., O'Malley B. W. Ligand and DNA-dependent phosphorylation of human progesterone receptor in vitro. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2664–2668. doi: 10.1073/pnas.89.7.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniahmad A., Steiner C., Köhne A. C., Renkawitz R. Modular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. Cell. 1990 May 4;61(3):505–514. doi: 10.1016/0092-8674(90)90532-j. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Beck I., Ramirez S., Weinmann R., Caro J. Enhancer element at the 3'-flanking region controls transcriptional response to hypoxia in the human erythropoietin gene. J Biol Chem. 1991 Aug 25;266(24):15563–15566. [PubMed] [Google Scholar]
- Beru N., McDonald J., Goldwasser E. Activation of the erythropoietin gene due to the proximity of an expressed gene. DNA. 1989 May;8(4):253–259. doi: 10.1089/dna.1.1989.8.253. [DOI] [PubMed] [Google Scholar]
- Beru N., McDonald J., Lacombe C., Goldwasser E. Expression of the erythropoietin gene. Mol Cell Biol. 1986 Jul;6(7):2571–2575. doi: 10.1128/mcb.6.7.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beru N., Smith D., Goldwasser E. Evidence suggesting negative regulation of the erythropoietin gene by ribonucleoprotein. J Biol Chem. 1990 Aug 25;265(24):14100–14104. [PubMed] [Google Scholar]
- Brasier A. R., Tate J. E., Habener J. F. Optimized use of the firefly luciferase assay as a reporter gene in mammalian cell lines. Biotechniques. 1989 Nov-Dec;7(10):1116–1122. [PubMed] [Google Scholar]
- Costa-Giomi P., Caro J., Weinmann R. Enhancement by hypoxia of human erythropoietin gene transcription in vitro. J Biol Chem. 1990 Jun 25;265(18):10185–10188. [PubMed] [Google Scholar]
- Denner L. A., Weigel N. L., Maxwell B. L., Schrader W. T., O'Malley B. W. Regulation of progesterone receptor-mediated transcription by phosphorylation. Science. 1990 Dec 21;250(4988):1740–1743. doi: 10.1126/science.2176746. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX H. M. The effect of oxygen on the concentration of haem in invertebrates. Proc R Soc Lond B Biol Sci. 1955 Jan 27;143(911):203–214. doi: 10.1098/rspb.1955.0005. [DOI] [PubMed] [Google Scholar]
- Gilles-Gonzalez M. A., Ditta G. S., Helinski D. R. A haemoprotein with kinase activity encoded by the oxygen sensor of Rhizobium meliloti. Nature. 1991 Mar 14;350(6314):170–172. doi: 10.1038/350170a0. [DOI] [PubMed] [Google Scholar]
- Glass C. K., DiRenzo J., Kurokawa R., Han Z. H. Regulation of gene expression by retinoic acid receptors. DNA Cell Biol. 1991 Nov;10(9):623–638. doi: 10.1089/dna.1991.10.623. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Holloway J. M., Devary O. V., Rosenfeld M. G. The thyroid hormone receptor binds with opposite transcriptional effects to a common sequence motif in thyroid hormone and estrogen response elements. Cell. 1988 Jul 29;54(3):313–323. doi: 10.1016/0092-8674(88)90194-8. [DOI] [PubMed] [Google Scholar]
- Goldberg M. A., Gaut C. C., Bunn H. F. Erythropoietin mRNA levels are governed by both the rate of gene transcription and posttranscriptional events. Blood. 1991 Jan 15;77(2):271–277. [PubMed] [Google Scholar]
- Goldberg M. A., Glass G. A., Cunningham J. M., Bunn H. F. The regulated expression of erythropoietin by two human hepatoma cell lines. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7972–7976. doi: 10.1073/pnas.84.22.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husmann M., Lehmann J., Hoffmann B., Hermann T., Tzukerman M., Pfahl M. Antagonism between retinoic acid receptors. Mol Cell Biol. 1991 Aug;11(8):4097–4103. doi: 10.1128/mcb.11.8.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBSON L. O., GOLDWASSER E., FRIED W., PLZAK L. Role of the kidney in erythropoiesis. Nature. 1957 Mar 23;179(4560):633–634. doi: 10.1038/179633a0. [DOI] [PubMed] [Google Scholar]
- Jacobs K., Shoemaker C., Rudersdorf R., Neill S. D., Kaufman R. J., Mufson A., Seehra J., Jones S. S., Hewick R., Fritsch E. F. Isolation and characterization of genomic and cDNA clones of human erythropoietin. 1985 Feb 28-Mar 6Nature. 313(6005):806–810. doi: 10.1038/313806a0. [DOI] [PubMed] [Google Scholar]
- Jelinek W. R., Schmid C. W. Repetitive sequences in eukaryotic DNA and their expression. Annu Rev Biochem. 1982;51:813–844. doi: 10.1146/annurev.bi.51.070182.004121. [DOI] [PubMed] [Google Scholar]
- Jelkmann W. Erythropoietin: structure, control of production, and function. Physiol Rev. 1992 Apr;72(2):449–489. doi: 10.1152/physrev.1992.72.2.449. [DOI] [PubMed] [Google Scholar]
- Kerppola T. K., Curran T. Fos-Jun heterodimers and Jun homodimers bend DNA in opposite orientations: implications for transcription factor cooperativity. Cell. 1991 Jul 26;66(2):317–326. doi: 10.1016/0092-8674(91)90621-5. [DOI] [PubMed] [Google Scholar]
- Khosla C., Bailey J. E. Heterologous expression of a bacterial haemoglobin improves the growth properties of recombinant Escherichia coli. Nature. 1988 Feb 18;331(6157):633–635. doi: 10.1038/331633a0. [DOI] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Heyman R. A., Mangelsdorf D. J., Dyck J. A., Evans R. M. Retinoid X receptor-COUP-TF interactions modulate retinoic acid signaling. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1448–1452. doi: 10.1073/pnas.89.4.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz S. B. Erythropoietin. Blood. 1991 Feb 1;77(3):419–434. [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Ladias J. A., Karathanasis S. K. Regulation of the apolipoprotein AI gene by ARP-1, a novel member of the steroid receptor superfamily. Science. 1991 Feb 1;251(4993):561–565. doi: 10.1126/science.1899293. [DOI] [PubMed] [Google Scholar]
- Laudet V., Hänni C., Coll J., Catzeflis F., Stéhelin D. Evolution of the nuclear receptor gene superfamily. EMBO J. 1992 Mar;11(3):1003–1013. doi: 10.1002/j.1460-2075.1992.tb05139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. K., Suggs S., Lin C. H., Browne J. K., Smalling R., Egrie J. C., Chen K. K., Fox G. M., Martin F., Stabinsky Z. Cloning and expression of the human erythropoietin gene. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7580–7584. doi: 10.1073/pnas.82.22.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry C. V., Cerdán M. E., Zitomer R. S. A hypoxic consensus operator and a constitutive activation region regulate the ANB1 gene of Saccharomyces cerevisiae. Mol Cell Biol. 1990 Nov;10(11):5921–5926. doi: 10.1128/mcb.10.11.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry C. V., Weiss J. L., Walthall D. A., Zitomer R. S. Modulator sequences mediate oxygen regulation of CYC1 and a neighboring gene in yeast. Proc Natl Acad Sci U S A. 1983 Jan;80(1):151–155. doi: 10.1073/pnas.80.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry C. V., Zitomer R. S. Oxygen regulation of anaerobic and aerobic genes mediated by a common factor in yeast. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6129–6133. doi: 10.1073/pnas.81.19.6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McDonald J. D., Lin F. K., Goldwasser E. Cloning, sequencing, and evolutionary analysis of the mouse erythropoietin gene. Mol Cell Biol. 1986 Mar;6(3):842–848. doi: 10.1128/mcb.6.3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen S. K. Luciferase reporter gene vectors for analysis of promoters and enhancers. Biotechniques. 1988 May;6(5):454–458. [PubMed] [Google Scholar]
- När A. M., Boutin J. M., Lipkin S. M., Yu V. C., Holloway J. M., Glass C. K., Rosenfeld M. G. The orientation and spacing of core DNA-binding motifs dictate selective transcriptional responses to three nuclear receptors. Cell. 1991 Jun 28;65(7):1267–1279. doi: 10.1016/0092-8674(91)90021-p. [DOI] [PubMed] [Google Scholar]
- Pugh C. W., Tan C. C., Jones R. W., Ratcliffe P. J. Functional analysis of an oxygen-regulated transcriptional enhancer lying 3' to the mouse erythropoietin gene. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10553–10557. doi: 10.1073/pnas.88.23.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottman J. N., Widom R. L., Nadal-Ginard B., Mahdavi V., Karathanasis S. K. A retinoic acid-responsive element in the apolipoprotein AI gene distinguishes between two different retinoic acid response pathways. Mol Cell Biol. 1991 Jul;11(7):3814–3820. doi: 10.1128/mcb.11.7.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster S. J., Badiavas E. V., Costa-Giomi P., Weinmann R., Erslev A. J., Caro J. Stimulation of erythropoietin gene transcription during hypoxia and cobalt exposure. Blood. 1989 Jan;73(1):13–16. [PubMed] [Google Scholar]
- Semenza G. L., Dureza R. C., Traystman M. D., Gearhart J. D., Antonarakis S. E. Human erythropoietin gene expression in transgenic mice: multiple transcription initiation sites and cis-acting regulatory elements. Mol Cell Biol. 1990 Mar;10(3):930–938. doi: 10.1128/mcb.10.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G. L., Nejfelt M. K., Chi S. M., Antonarakis S. E. Hypoxia-inducible nuclear factors bind to an enhancer element located 3' to the human erythropoietin gene. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5680–5684. doi: 10.1073/pnas.88.13.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D. J., Sharp P. A., Wahli W. W., Keller M. J. A high-efficiency HeLa cell nuclear transcription extract. DNA. 1988 Jan-Feb;7(1):47–55. doi: 10.1089/dna.1988.7.47. [DOI] [PubMed] [Google Scholar]
- Shoemaker C. B., Mitsock L. D. Murine erythropoietin gene: cloning, expression, and human gene homology. Mol Cell Biol. 1986 Mar;6(3):849–858. doi: 10.1128/mcb.6.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahli W., Martinez E. Superfamily of steroid nuclear receptors: positive and negative regulators of gene expression. FASEB J. 1991 Jun;5(9):2243–2249. doi: 10.1096/fasebj.5.9.1860615. [DOI] [PubMed] [Google Scholar]
- Zanjani E. D., Poster J., Burlington H., Mann L. I., Wasserman L. R. Liver as the primary site of erythropoietin formation in the fetus. J Lab Clin Med. 1977 Mar;89(3):640–644. [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]