ABSTRACT
Our understanding of the interactions between normal skin microbiota and the human host has been greatly extended by recent investigations. In their recent study in mBio, A. Gioti et al. (mBio 4[1]:e00572-12, 2013) sequenced the genome of the atopic eczema-associated yeast, Malassezia sympodialis, and compared its gene content and organization with that of Malassezia globosa, a species implicated in dandruff. Their findings were also contrasted with those previously obtained for Ustilago maydis, which is a close relative but ecologically distinct plant parasite. Besides gaining additional insight into key host-specific adaptations and the particular function and molecular evolution of allergens related to atopic eczema, Gioti et al. also uncovered several lines of evidence that elegantly suggest the presence of an extant sexual cycle, with important implications in disease.
Commentary
Generally considered part of the normal skin mycobiota of humans and animals, Malassezia yeast species have gained a great deal of attention over the years for their association with innumerable dermatological disorders, namely, atopic eczema (AE), seborrheic dermatitis, and dandruff, which afflict >50% of humans on a daily basis (1, 2). Today, the genus Malassezia comprises 14 species, all dwelling on the skin of human and other warm-blooded animals and, almost invariably, dependent on lipids for growth (2). Along with Cryptococcus neoformans and Cryptococcus gattii, Malassezia species are the yeast-like basidiomycete fungi most commonly associated with human and animal infections. Yet their adaptation to humans has likely occurred independently during evolution, as they are only distantly related and do not share a recent common ancestor (Fig. 1). With the publication of the dandruff-associated Malassezia globosa genome in 2007 (3), it became clear that key adaptations to the skin environment seem to have a direct genomic basis and that, besides thriving on our skin, the fungus may also be having sex. This work also unveiled that the complexity of interactions of this unicellular eukaryotic organism with a multicellular tissue (skin) is far more intricate than anticipated, and thus a species-specific understanding of Malassezia biology is paramount to understand the wide-range complex of diseases.
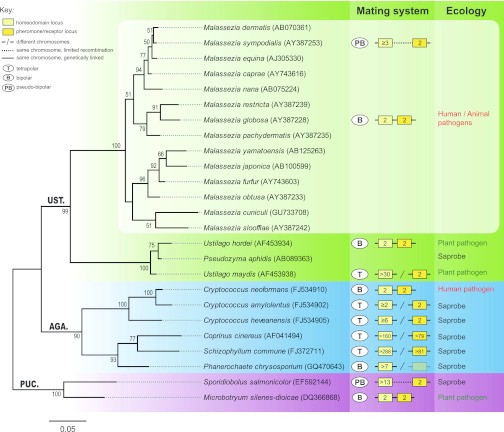
FIG 1 .
Molecular phylogeny, mating system, and ecology of Malassezia and other basidiomycete representatives. The tree is based on the nucleotide alignment of the D1/D2 domain of the LSU (large subunit) rRNA gene sequences and was inferred by maximum likelihood using the GTR (general time reversible) model and a 1,000-replicate bootstrap analysis (values of >50% are shown). Sporidiobolus salmonicolor and Microbotryum silenes-dioicae were used as an outgroup. GenBank accession numbers are shown in parentheses after species names. PUC., AGA., and UST. are abbreviations for Pucciniomycotina, Agaricomycotina, and Ustilaginomycotina, three major lineages in Basidiomycota. In the mating systems panel, numbers inside each box indicate the estimated number of alleles. All remaining features are as given in the key.
Significant advances are being made nowadays toward this goal, and the paper by Gioti et al. in this journal (4) has opened new avenues to assess the role of fungal genes in host colonization and disease. In this commentary, we will situate this report in the context of Malassezia host adaptation and speculate on specific genomic properties that may be critical to virulence and disease.
WHAT MAKES Malassezia A SPECIALIZED SKIN PATHOGEN?
From a taxonomical point of view, no one would guess that Malassezia species are specialized animal pathogens, since virtually all of their close relatives are, in fact, well-adapted plant pathogens (Fig. 1). For instance, the smut fungus Ustilago maydis is able to infect its host maize plant, establish a biotrophic interface where signal exchange and nutrient uptake between plant and fungus occur, and counteract plant defense responses using a large repertoire of secreted proteins, so-called effectors (5). These secreted proteins are generally encoded by gene clusters that are absent from all of the available Malassezia genomes (3, 4), providing the first clue that Malassezia species likely use a different approach to colonize their hosts and/or take advantage of a different “arsenal.”
With sizes of 8.9 Mb and 7.9 Mb, respectively, the genomes of M. globosa and Malassezia sympodialis are among the smallest for free-living fungi. A small genome size seems to accompany organisms with specialized niches, among which intracellular parasites like the Microsporidia constitute an extreme example of genome compaction in fungi (6, 7). Indeed, the genomes of M. globosa and M. sympodialis exhibit evidence of what may constitute a turning point toward the adaptation to the skin environment: (i) low carbohydrate-degrading capacity due to reduction of glycosyl hydrolase-encoding genes and (ii) lipid dependence for growth due to lack of a fatty acid synthase gene with concomitant expansion of lipid hydrolyzing enzymes such as secreted lipases, phospholipases, and acid sphingomyelinases that satisfy their need for fatty acids from external sources.
To cope with host defense responses, fungal pathogens have developed several strategies, the most prominent being the modification of their cell wall to evade host recognition. On the other hand, the human antifungal defense system developed key components that rely on pattern recognition receptors (PRR) to identify pathogen-associated molecular patterns (PAMPs) (8). For example, β-glucans, major components of fungal cell walls, are recognized as a PAMP by human dectin-1 (8). Chitin is also abundant in fungal cell walls, and a few studies suggest it is probably recognized as a PAMP (9, 10). Researchers have identified at least two active human chitinases (8). The comparative work of Gioti et al. (4) highlights an interesting feature with respect to chitin: four and six chitin deacetylase genes were identified in M. globosa and M. sympodialis genomes, respectively. Remarkably, in species such as Colletotrichum graminicola, the causal agent of maize anthracnose, it has been demonstrated that conversion of chitin to chitosan by chitin deacetylases occurs as a mechanism to protect invading hyphae from enzymatic hydrolysis by host chitinases and to escape host immune defense responses (11). Studies in C. neoformans have also shown that chitosan is necessary to maintain cell wall integrity and normal capsule width (12). It would be interesting to explore if gains and losses of chitin deacetylase genes throughout the fungal kingdom are related to innovations of pathogenic lifestyles. The work by Gioti et al. (4) has laid down the foundations for these studies.
Multiple Malassezia species have been associated with atopic eczema (AE), a chronic inflammatory skin disease that results from a combination of two major factors: a defective skin barrier and inappropriate immune responses caused by allergens (13). Many of these allergens have been identified in several Malassezia species (Mala genes), and at least 12 genes encoding different classes of proteins are now confirmed to be present in both M. sympodialis and M. globosa genomes. Curiously, and despite the established pairwise orthologous relationship, a high level of synonymous substitutions was detected between some of the proteins in the two species, which may indicate species-specific adaptations. Also in line with this, four genes are predicted to encode proteins similar to Mala s 7 in the M. sympodialis genome, whereas only three are present in M. globosa. As new Malassezia genome sequences become available, it will be of interest to use a phylogenetic and comparative genomics framework to elucidate if these extra gene copies resulted from species-specific gene duplications or, instead, have arisen by ancestral duplications followed by lineage-specific losses.
ARE MATING AND PATHOGENICITY IN Malassezia TWO SIDES OF THE SAME COIN?
The answer to this question may well turn out to be affirmative, but so far no sexual cycle has been observed in any of the 14 Malassezia species. Results from recent studies suggest that if the right combination of strains and culture conditions are used, sexual cycles may be induced under laboratory conditions, even in fungal species long thought to be exclusively asexual (viz. Candida albicans and Aspergillus fumigatus) (14, 15). Nevertheless, it would be naive to think of this as a straightforward task. In fact, for about 17% of the known fungal species, sexual cycles remain to be discovered despite the great deal of effort and tenacity employed to test many isolates under a broad range of culture conditions. Research in the field has, however, been spurred by the fact that for many plant or animal fungal pathogens, sexual reproduction and host infection are interconnected (16), a feature particularly important for dimorphic pathogens such as U. maydis. In this case, mating takes an essential role in their life cycle, as it initiates parasitism by a morphological and physiological transition from saprobic yeast cells to a filamentous stage that is able to infect the host plant. By analogy, Malassezia species which are close relatives (Fig. 1) may potentially complete their sexual cycle on human skin, an environment which is known to stimulate mating of C. albicans (17).
But what can we do when sex is either very rare or difficult to observe in nature? The answer may lie in the genome. Indeed, recent genome-wide studies were combined with molecular analyses to look for evidence of the ability to undergo sexual reproduction, by assessing the degree of sexual recombination in natural populations and to search for the presence of intact genes that are specifically involved in mating and sexual reproduction. Using this approach, two regions corresponding to the pheromone/receptor (P/R) and homeodomain (HD) mating type (MAT) loci were identified in both M. globosa and M. sympodialis, which seemed to be physically linked and span more than 100 kb (4). This configuration, along with the existence of only two alleles, typically defines bipolar mating systems. In contrast, tetrapolar systems typically carry biallelic P/R and multiallelic HD loci on different chromosomes, the two regions therefore segregating independently during meiosis. However, the work of Gioti et al. has brought to light compelling evidence that the MAT system of M. sympodialis, consisting of linked P/R and HD loci, in fact behaves as an intermediate mating system. Here, recombination between the two MAT regions takes place, thus yielding different mating type combinations of a biallelic P/R locus and an HD locus that is at least triallelic. These findings provide the second and independent instance of a pseudobipolar mating system which has been previously identified in the red yeast Sporidiobolus salmonicolor (18) (Fig. 1). Both bipolar and tetrapolar MAT systems occur interspersed along the basidiomycete lineage (summarized in Fig. 1), and transitions from tetrapolar to bipolar mating configurations are possibly the consequence of shifts from outbreeding to inbreeding lifestyles as species become adapted to specific niches. Accordingly, it is noteworthy that most of the well-adapted human-pathogenic fungal species have in fact bipolar MAT systems. In this scenario, it is tempting to speculate that the pseudobipolar system may constitute a transient stage in a transition from a tetrapolar to a bipolar state. However, the data from several saprobic red yeasts (18, 19) suggest that this system might not represent a short-lived mating system like those presumed to have marked the transition to the bipolar state in, e.g., C. neoformans and Ustilago hordei (20, 21). Lastly, a systematic and exhaustive identification of genes required for meiosis and signaling related to mating provided conclusive and important evidence of sexual reproduction capability in M. sympodialis.
Studies of little-explored groups of fungi, such as the one by Gioti et al., will hopefully continue to give us lessons on the evolution of multifaceted interactions between humans and microbial eukaryotes. We anticipate that advances in fungal genomics and the unprecedented analytical power of these techniques will have a tremendous impact on what we know and/or think we know about these associations, as it is undisputed that the commensal/pathogen duality still holds many hidden secrets.
Footnotes
Citation Coelho MA, Sampaio JP, Gonçalves P. 2013. Living and thriving on the skin: Malassezia genomes tell the story. mBio 4(2):e00117-13. doi:10.1128/mBio.00117-13.
REFERENCES
- 1. Saunders CW, Scheynius A, Heitman J. 2012. Malassezia fungi are specialized to live on skin and associated with dandruff, eczema, and other skin diseases. PLoS Pathog. 8:e1002701 http://dx.doi.org/10.1371/journal.ppat.1002701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gaitanis G, Magiatis P, Hantschke M, Bassukas ID, Velegraki A. 2012. The Malassezia genus in skin and systemic diseases. Clin. Microbiol. Rev. 25:106–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu J, Saunders CW, Hu P, Grant RA, Boekhout T, Kuramae EE, Kronstad JW, Deangelis YM, Reeder NL, Johnstone KR, Leland M, Fieno AM, Begley WM, Sun Y, Lacey MP, Chaudhary T, Keough T, Chu L, Sears R, Yuan B, Dawson TL., Jr 2007. Dandruff-associated Malassezia genomes reveal convergent and divergent virulence traits shared with plant and human fungal pathogens. Proc. Natl. Acad. Sci. U. S. A. 104:18730–18735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gioti A, Nystedt B, Li W, Xu J, Andersson A, Averette AF, Münch K, Wang X, Kappauf C, Kingsbury JM, Kraak B, Walker LA, Johansson HJ, Holm T, Lehtiö J, Stajich JE, Mieczkowski P, Kahmann R, Kennell JC, Cardenas ME, Lundeberg J, Saunders CW, Boekhout T, Dawson TL, Munro CA, de Groot PW, Butler G, Heitman J, Scheynius A. 2013. Genomic insights into the atopic eczema-associated skin commensal yeast Malassezia sympodialis. mBio 4(1):e00572-12 http://dx.doi.org/10.1128/mBio.00572-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brefort T, Doehlemann G, Mendoza-Mendoza A, Reissmann S, Djamei A, Kahmann R. 2009. Ustilago maydis as a pathogen. Annu. Rev. Phytopathol. 47:423–445 [DOI] [PubMed] [Google Scholar]
- 6. Cuomo CA, Desjardins CA, Bakowski MA, Goldberg J, Ma AT, Becnel JJ, Didier ES, Fan L, Heiman DI, Levin JZ, Young S, Zeng Q, Troemel ER. 2012. Microsporidian genome analysis reveals evolutionary strategies for obligate intracellular growth. Genome Res. 22:2478–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peyretaillade E, El Alaoui H, Diogon M, Polonais V, Parisot N, Biron DG, Peyret P, Delbac F. 2011. Extreme reduction and compaction of microsporidian genomes. Res. Microbiol. 162:598–606 [DOI] [PubMed] [Google Scholar]
- 8. Vega K, Kalkum M. 2012. Chitin, chitinase responses, and invasive fungal infections. Int. J. Microbiol. 2012:920459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Latgé JP. 2007. The cell wall: a carbohydrate armour for the fungal cell. Mol. Microbiol. 66:279–290 [DOI] [PubMed] [Google Scholar]
- 10. Romani L. 2011. Immunity to fungal infections. Nat. Rev. Immunol. 11:275–288 [DOI] [PubMed] [Google Scholar]
- 11. El Gueddari NE, Rauchhaus U, Moerschbacher BM, Deising HB. 2002. Developmentally regulated conversion of surface-exposed chitin to chitosan in cell walls of plant pathogenic fungi. New Phytol. 156:103–112 [Google Scholar]
- 12. Baker LG, Specht CA, Donlin MJ, Lodge JK. 2007. Chitosan, the deacetylated form of chitin, is necessary for cell wall integrity in Cryptococcus neoformans. Eukaryot. Cell 6:855–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gaitanis G, Mayser P, Scheynius A, Crameri C. 2010. Malassezia yeasts in seborrheic and atopic eczemas, p. 201–228 In Boekhout T, Guého-Kellerman E, Mayser P, Velegraki A, Malassezia and the skin. Springer-Verlag, Berlin, Germany [Google Scholar]
- 14. Bennett RJ, Johnson AD. 2003. Completion of a parasexual cycle in Candida albicans by induced chromosome loss in tetraploid strains. EMBO J. 22:2505–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O’Gorman CM, Fuller H, Dyer PS. 2009. Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature 457:471–474 [DOI] [PubMed] [Google Scholar]
- 16. Sexton AC, Howlett BJ. 2006. Parallels in fungal pathogenesis on plant and animal hosts. Eukaryot. Cell 5:1941–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lachke SA, Lockhart SR, Daniels KJ, Soll DR. 2003. Skin facilitates Candida albicans mating. Infect. Immun. 71:4970–4976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coelho MA, Sampaio JP, Goncalves P. 2010. A deviation from the bipolar-tetrapolar mating paradigm in an early diverged basidiomycete. PLoS Genet. 6:e1001052 http://dx.doi.org/10.1371/journal.pgen.1001052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coelho MA, Gonçalves P, Sampaio JP. 2011. Evidence for maintenance of sex determinants but not of sexual stages in red yeasts, a group of early diverged basidiomycetes. BMC Evol. Biol. 11:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bakkeren G, Kronstad JW. 1994. Linkage of mating-type loci distinguishes bipolar from tetrapolar mating in basidiomycetous smut fungi. Proc. Natl. Acad. Sci. U. S. A. 91:7085–7089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lengeler KB, Fox DS, Fraser JA, Allen A, Forrester K, Dietrich FS, Heitman J. 2002. Mating-type locus of Cryptococcus neoformans: a step in the evolution of sex chromosomes. Eukaryot. Cell 1:704–718 [DOI] [PMC free article] [PubMed] [Google Scholar]