Abstract
Nonvolatile acid is produced from the metabolism of organic sulfur in dietary protein, and the production of organic anions during the combustion of neutral foods. Organic anion salts that are found primarily in plant foods are directly absorbed in the gastrointestinal tract and yield bicarbonate. The difference between endogenously produced nonvolatile acid and absorbed alkali precursors yields the dietary acid load, technically known as the net endogenous acid production, and must be excreted by the kidney to maintain acid-base balance. Although typically around 1 mEq/kg/day, dietary acid load is lower with greater intake of fruits and vegetables. In the setting of chronic kidney disease, a high dietary acid load invokes adaptive mechanisms to increase acid excretion despite reduced nephron number, such as increased per nephron ammoniagenesis and augmented distal acid excretion mediated by the renin-angiotensin system and endothelin-1. These adaptations may promote renal injury. Additionally, high dietary acid loads produce low-grade, subclinical acidosis that may result in bone and muscle loss. Early studies suggest that lowering the dietary acid load can improve subclinical acidosis, preserve bone and muscle, and slow decline of glomerular filtration rate in animal models and humans. Studies focusing on hard clinical outcomes are needed.
Keywords: chronic kidney disease, nutrition, metabolic acidosis, net endogenous acid production
Introduction
Diet is a major determinant of the acid load that must be excreted by the kidney to maintain acid-base balance (1). Although contemporary diets in industrialized nations are largely acid-inducing, this may not have been the case throughout the vast majority of human evolution, during which more alkalinizing foods were consumed (2-4). As a consequence, humans may be poorly adapted to contemporary acid-inducing diets and this may contribute to the pathogenesis of modern epidemics of chronic disease, including kidney disease (5). A modest body of research, including animal studies, observational epidemiology and small clinical trials, has examined the potential role of the dietary acid load in patients with chronic kidney disease (CKD). The evidence largely supports the hypothesis of a direct relationship between higher dietary acid load and CKD progression, bone loss and sarcopenia (6-9). However, due to a wide variety of techniques and terminology used to quantify the dietary acid load, it is not widely appreciated by nephrologists. In this review, we will discuss the dietary determinants of the daily acid load using simplifying terminology, as appropriate, and summarize the published literature on the role of dietary acid load in progression of CKD and CKD-related morbidity.
Net endogenous acid production and relationship to diet
Endogenous acid production
Metabolic processes generate both volatile and nonvolatile acids. Volatile acid is expired through respiration as CO2, whereas nonvolatile acid (H+) must be excreted by the kidney in the form of ammonium and titratable acid (10). The amount of nonvolatile acid produced by the body during metabolism is termed the endogenous acid production (11-13). The difference between endogenous acid production and the input of alkali absorbed in the gastrointestinal (GI) tract is the net endogenous acid production, and represents the total amount of nonvolatile acid that must be excreted to maintain daily acid-base balance (14). Although the term net endogenous acid production is favored (14), a variety of terms have been used to describe dietary acid load in the literature. A list of terms and definitions used in this review is provided in Table 1.
Table 1.
Definitions and relationships between terminology used in prior literature
| Term | Definition | Relationship to other terms† |
|---|---|---|
| Endogenous acid production (EAP) |
The sum of acid produced during oxidation of sulfur- containing protein and the generation of organic anions (OA) from neutral foods |
EAP = NEAP + GI alkali absorption |
| Gastrointestinal (GI) alkali absorption |
The net addition of alkali as the result of nutrients absorbed in the GI tract. |
GI alkali absorption = EAP − NEAP |
| Net endogenous acid production (NEAP) |
The total load of nonvolatile acid added to the body as a result of endogenous acid production and GI absorption. |
NEAP = EAP − GI alkali absorption NEAP = PRAL + OA production‡ |
| Potential renal acid load (PRAL) |
The contribution of a food or dietary pattern to net endogenous acid production. |
PRAL ≈ NEAP − OA production‡ |
| Net acid excretion (NAE) |
The total amount of acid excreted by the kidney daily | NAE ≈ NEAP NAE ≈ PRAL + OA production‡ |
all terms represented in mEq
Results from endogenous production of organic anions from neutral foods and is classicially considered diet-independent
Endogenous acid is produced when organic sulfur, found in the amino acids methionine and cysteine, is oxidized to inorganic sulfate (11, 15, 16). Additional acid is produced when neutral foods are oxidized to organic anions that are excreted in the urine, including citrate, urate, and oxalate (11, 17). This component, termed the organic anion production, is classically considered diet-independent (11), but may be augmented in response to net alkalinizing diets as a mechanism to increase base excretion (18-21). In addition to these acids that are produced endogenously from neutral foods, exogenous acids and bases are also directly absorbed in the GI tract. In particular, absorbed metabolizable organic anions, such as citrate and malate, are abundant in fruits and vegetables and undergo combustion in the body to yield bicarbonate (12-14, 17, 22). The difference in absorbed, nonmetabolizable cations and anions provides an index of the overall absorption of alkali (i.e. metabolizable organic anions) in the GI tract (13, 23). As a result, the net endogenous acid production is the sum of these biochemical reactions that can either yield or consume protons, and is dependent on diet (1, 13, 14, 17, 22-25).
Measuring dietary acid load
Several groups have derived methods to estimate the dietary acid load from measures of dietary intake (2, 14, 23-25). The most widely used methods calculate either the net endogenous acid production, or the more classically diet-dependent portion of net acid excretion, known as the potential renal acid load (Table 2) (22-24). Net endogenous acid production can be estimated either indirectly, based on the ratio of protein to potassium intake in the diet (25), or directly, using the sulfur content of foods, body weight or diet-based estimates of organic anion production and the calculated GI alkali absorption (2). Each of these intake-based estimates are limited by imprecision in the measurement of dietary intake as a result of inaccurate reporting and variation over time. Additionally, absorption of nutrients in the GI tract and the actual nutrient composition of specific foods can vary considerably across individuals and methods of preparation, but this is not accounted for by these equations (Table 2).
Table 2.
Measurements used in literature in estimation dietary acid load
| Method | Data Needed | Calculation | Strengths | Limitations |
|---|---|---|---|---|
| Endogenous Acid Production (EAP) |
Dietary intake or 24 hour urine collection |
Diet: EAP (mEq/d) = 0.75 × dietary sulfur (mEq/d) + organic anion (OA) production§ Urine: EAP (mEq/d) = urinary sulfate (mEq/d) + OA production§ |
|
|
| Gastrointestinal (GI) alkali absorption |
Dietary intake | Diet†: GI alkali absorption (mEq/d)= 0.95 × Na + 0.8 × K + 0.25 × Ca + 0.32 × Mg − 0.95 × Cl − 0.63 × P |
|
|
| Net Endogenous Acid Production (NEAP) |
Dietary intake | Direct: NEAP (mEq/d)= EAP − GI alkali absorption Indirect: NEAP (mEq/d) = 54.5 [protein (g/day)/K (mEq/d)] − 10.2 |
|
|
| Potential Renal Acid Load (PRAL) |
Dietary intake | PRAL (mEq/d) = 0.49 × protein (g/d) + 0.037 × P (mg/d) − 0.021 × K (mg/d) − 0.026 × Mg (mg/d) − 0.013 × Ca (mg/d)‡ |
|
|
| Net Acid Excretion (NAE) |
24 hour urine collection |
Direct†: NAE (mEq/d) = NH4+ + TA − HCO3− Indirect†: NAE (mEq/d) = (Cl + P + SO4 + OA§) − (Na + K + Ca + Mg) |
|
|
OA, organic anions; Na, sodium; K, potassium; Ca, calcium; Mg, magnesium; Cl, chloride; P, phosphate; NH4+, ammonium; TA, titratable acidity; HCO3−, bicarbonate; SO4, sulfate
All ions expressed as mEq/d; valence of phosphate is assumed to be 1.8
Some investigators include sodium and chloride in this calculation, but here it is ignored because they are generally balanced in the diet. Calcium is sometimes ignored due to variable GI absorption across individuals. Note dietary input variables here are expressed in different units than GI alkali absorption to be consistent with reporting in the literature.
Organic anions can be estimated from body surface area if assumed to be diet independent: OA (mEq/d)=body surface area × 41/1.73; or based on the GI alkali absorption to account for partial diet-dependence: OA (mEq/d)=32.9 + 0.15 × GI alkali absorption(2).
To surmount challenges inherent in dietary intake assessment, net endogenous acid production can be most accurately assessed as the steady-state net acid excretion measured in a 24 hour urine collection (Table 2) (14). In addition to error related to under and over-collection, in some circumstances evaluation of urinary acidification may require collection under oil, making this more difficult to perform clinically (26, 27). Furthermore, estimating dietary acid load in this way assumes acid-base equilibrium and that short term dietary intake is similar to habitual intake, which can be more directly ascertained by a food frequency questionnaire (28). Urine pH has also been proposed as a cost-effective, simple tool to monitor net endogenous acid production and may be appropriate for use in large population based studies (29). However, the relationship between net acid excretion and urine pH may not be reliable in populations with CKD, age-related renal function decline, or in those with renal tubular acidosis where distal acidification of the urine is compromised (30-33).
Foods and dietary acid load
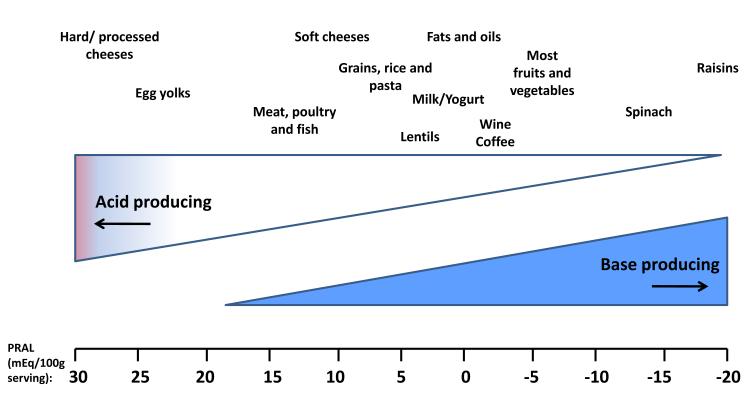
The potential renal acid load of selected common foods using the equation develop by Remer has been reported previously (Figure 1) (22). This equation does not account for differences in the sulfur content of proteins from different sources (3, 34, 35). Other estimates that directly account for sulfur, as well as the impact of dietary alkali on organic anion production, yield essentially neutral estimates for nuts and legumes (2, 5). In general, common foods that impart a high dietary acid load include cheese, meat, eggs and grains, whereas fruits and vegetables provide alkali (22, 36-39). The average American diet delivers approximately 15-17% of its energy as protein, predominantly from animal sources (40). In addition, it is low in potassium-rich fruit and vegetables (41) resulting in an average dietary acid load of approximately 1 mEq/kg/day (17). This is consistent with median estimates of dietary acid load of approximately 50-75 mEq/day reported in several general population cohorts and nearly neutral acid load in populations consuming a vegan diet (8, 24, 35-39, 42-44).
Figure 1.
Estimated acid-producing potential of selected foods. Potential renal acid load (PRAL) of selected food items (per 100g serving) is adapted from estimates performed by Remer (22), and calculated as: PRAL (mEq/d) = 0.49 × protein (g/d) + 0.037 × P (mg/d) − 0.021 × K (mg/d) − 0.026 × Mg (mg/d) − 0.013 × Ca (mg/d).
To demonstrate the net endogenous acid production of diets that are relatively enriched with plant foods (fruits, vegetables, nuts and legumes) but not vegan, we calculated the dietary acid load of three diets prescribed in the Dietary Approaches to Stop Hypertension (DASH) trial (45). The DASH study included a control diet with macronutrient and mineral content similar to average US consumption; a fruit and vegetable diet in which servings of sweets and grains were replaced with fruits and vegetables; and a combination diet that was enriched in fruits, vegetables and low fat dairy with reductions in fats, oils and meats (45). The fruit and vegetable diet yields a substantially reduced dietary acid load compared to control (net endogenous acid production of 31 versus 78 mEq/d), despite comparable protein intake (Table 3). The combination diet included a higher intake of protein than control, but also resulted in a lower dietary acid load due to more servings of fruits and vegetables (45). Recently, a small trial of patients with early CKD confirmed that augmentation of fruit and vegetable intake can lower net acid excretion by approximately a third and was comparable to administration of 0.5 mEq/kg/day of sodium bicarbonate (7). Overall these findings suggest that replacing nutrient poor, energy dense foods that are common in contemporary diets, with greater intake of fruits and vegetables could substantially lower net endogenous acid production without requiring excessive protein restriction (46, 47).
Table 3.
Estimated net endogenous acid production of diets in the Dietary Approaches to Stop Hypertension Trial scaled to 2100 kilocalories
| Control diet | Fruits and vegetable diet | Combination diet | |
|---|---|---|---|
| Net endogenous acid production- Indirect (mEq/day)† | 78.0 | 30.7 | 35.2 |
| Potential renal acid load (mEq/day)‡ | 31.8 | −23.7 | −25.4 |
| Protein (% kilocalories) | 13.8 | 15.1 | 17.9 |
| Servings of fruits and vegetables (number/day) | 3.6 | 8.5 | 9.6 |
Estimated NEAP (mEq/d) = 54.5 [protein (g/day)/K (mEq/d)] − 10.2(25)
Estimated PRAL (mEq/d) = 0.49 × protein (g/d) + 0.037 × P (mg/d) − 0.021 × K (mg/d) − 0.026 × Mg (mg/d) − 0.013 × Ca (mg/d)(22).
Phosphate (P) intake was not provided for diets and was estimated from 24 hour urinary phosphate assuming average intestinal absorption of 63%(2)
Acid excretion and development of acidosis in CKD
Metabolic acidosis is a common complication of moderate to severe CKD that results from impaired renal acid excretion (48-51). Although overt metabolic acidosis is a late complication of CKD, low-grade, subclinical metabolic acidosis likely begins early in CKD, but may be hidden by intracellular and bone buffering (52-56). In rat models of early CKD, acid loading resulted in decline of renal cortical and intramuscular pH with little change in overt measures of systemic acidosis, such as serum bicarbonate and blood pH (57). This finding suggests that the subtle differences in serum bicarbonate concentrations that result from differences in dietary acid load (44), may indicate a significant degree of underlying subclinical acidosis that could be mitigated by greater intake of base (58).
The presence of subclinical acidosis in patients with CKD is supported indirectly by a recent study in which the kidney’s response to a bolus of intravenous serum bicarbonate was evaluated in patients with stage 2 versus stage 1 CKD in the setting of a constant diet (56). Despite equivalent serum bicarbonate concentrations, the decline in renal acid excretion was blunted in participants with stage 2 versus stage 1 CKD, suggesting a total body deficit of buffer stores early in CKD that was otherwise unapparent. Administration of alkali supplements to both groups for 30 days decreased the difference between groups (56). Similar physiology is observed in older adults and closely linked to age-related renal function decline (30, 59). In older populations, reduction of the net endogenous acid production to near neutral results in small, but measurable, increases in serum bicarbonate and pH (8). Overall, the current body of literature suggests that modern dietary patterns result in low grade, subclinical metabolic acidosis in the setting of CKD and age-related renal function decline that may be “hidden” by a normal serum bicarbonate concentration.
Relationship of acidosis and dietary acid load to CKD progression
Metabolic acidosis and CKD progression
Several observational studies have demonstrated that metabolic acidosis is associated with progression of kidney disease (60-62). Consistent with these observational findings, a single-center randomized study demonstrated that amelioration of metabolic acidosis with exogenous alkali supplements slowed progression of patients with late stage CKD to dialysis dependence (63). Importantly, lower serum bicarbonate levels, even within the normal range, are also associated with faster disease progression (61), suggesting that differences in dietary acid load may underlie this observation (44).
Dietary acid load and CKD progression
In a small clinical trial of 120 patients with stage 2 hypertensive CKD and normal serum bicarbonate, the addition of sodium bicarbonate at a dose of 0.5 mEq/kg/day, resulted in a slower rate of decline in both creatinine-based and cystatin C-based estimates of glomerular filtration rate (GFR) compared to placebo over 5 years of follow-up (64). Notably, achieved serum bicarbonate levels in the treatment arm were not significantly different from placebo, but daily net acid excretion was lowered by about 15 mEq/day, reflecting the fall in net endogenous acid production. Subsequently, a similar finding was observed in participants with moderate to severe hypertensive CKD from the African American Study of Kidney Disease and Hypertension (AASK) who were consuming their free-living diets (6). In this observational study, higher estimated net endogenous acid production, based solely on diet (i.e. without the use of exogenous alkali supplements), was associated with a faster rate of decline in directly measured I125iothalamate GFR (6). This finding was present even after adjustment for serum bicarbonate and among the subset with normal serum bicarbonate concentrations. It is important to note that dietary acid load is related to protein intake, a risk factor for CKD progression that has been widely studied (65-68).
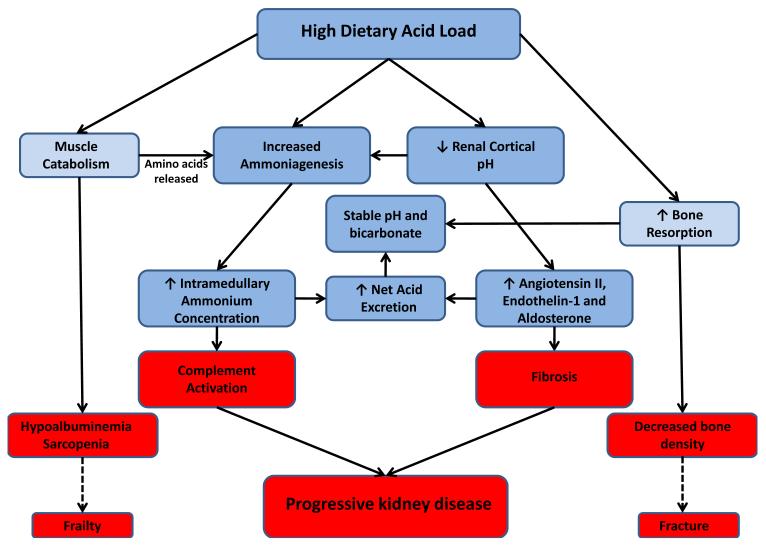
Two proposed mechanisms may underlie the associations between dietary acid load and progressive CKD, including tubular toxicity of elevated ammonium concentrations and activation of the renin-angiotensin system (56, 58, 69). With increased dietary acid load, production of ammonia is increased in the proximal tubule and H+ excretion is increased distally to augment overall acid excretion (10, 70, 71). In the setting of a reduced number of functioning nephrons in CKD, the per nephron demand for acid excretion rises dramatically, resulting in a markedly elevated rate of per nephron ammonia generation (71-73), rising intramedullary ammonium gradient (74), and increases in angiotensin II, aldosterone and endothelin-1 that promote H+ excretion (Figure 2) (70, 75-79).
Figure 2.
Proposed physiologic adaptations and consequences resulting from a high dietary acid load in the setting of chronic kidney disease. Blue boxes represent physiologic responses and red boxes their potential adverse effects. Dashed lines represent projected clinical sequelae for which current evidence is indirect.
In animal models, both acid loading and potassium deficiency augmented ammoniagenesis and resulted in activation of the alternative complement cascade (69, 80). In a rat model of early CKD, Wesson and colleagues demonstrated that acid loading increased, and base-loading decreased, angiotensin II, endothelin-1 and aldosterone-mediated renal injury (75, 81, 82). Additionally, base administration better preserved GFR and reduced kidney injury compared to acid-loaded or control animals in these and other models of CKD (58, 69, 82, 83). Subsequently, small translational studies in patients with early CKD demonstrated that lowering dietary acid load either with supplements or fruits and vegetables decreased urinary endothelin-1, aldosterone and markers of tubulointersitial injury in addition to slowing GFR decline (7, 64, 84).It is not yet known how these therapies may interact with other renoprotective strategies, such as renin-angiotensin system antagonism.
Relationship of acidosis and dietary acid load to morbidity in CKD
Metabolic acidosis and morbidity in CKD
To increase the availability of amino acid substrates for ammoniagenesis (72), metabolic acidosis stimulates muscle catabolism and inhibits albumin production through activation of the ATP-dependent ubiquitin-proteolytic pathway (85-89). Additionally, overt metabolic acidosis induces calciuria due to a combination of physiochemical effects on bone mineral and activation of osteoclastic bone resorption (90, 91). As a result of this physiology, chronic metabolic acidosis is associated with bone and muscle loss and growth restriction in children, each of which can be corrected by base administration (63, 92-97).
Dietary acid load and morbidity in CKD
These adverse physiologic consequences of overt metabolic acidosis may be present to a lesser degree in states of low grade, subclinical acidosis, such as early CKD and aging. Several studies suggest that these adverse effects may be mitigated by a reduction in the dietary acid load, although this area remains controversial (98, 99). In interventional studies, markers of bone resorption are reduced by potassium bicarbonate supplementation or consumption of the DASH diet (9, 100, 101). Observational studies have also documented an association between lower dietary acid load and improved bone density (102, 103), as well as lower rates of hip fracture in elderly women (103, 104). Importantly, adequate protein intake is important for bone health, therefore lowering acid load through greater intake of fruits and vegetables is likely to have a larger effect than protein restriction alone, but further work is needed in this area (105, 106). Finally, administration of alkali supplements to neutralize the daily acid load improves nitrogen balance in healthy elderly patients without overt metabolic acidosis (8). Most of this work has been performed in aging populations in which low grade metabolic acidosis may be present due to age-related renal function decline. It is possible that similar mechanisms underlie the elevated risk of fracture and frailty observed in CKD (107-111), but this hypothesis requires further testing.
Implications for care and research
Nutritional recommendations in CKD have focused on restriction of individual nutrients, such as dietary protein, potassium, phosphate and sodium (112). While the restriction of some nutrients, such as protein, has modest benefits in CKD (66-68), little guidance is provided regarding the intake of foods. From an acid-base perspective, a foods-based approach that considers the balance of acid-inducing and base-inducing dietary inputs is more logical and reflects consumption patterns. Greater intake of fruits and vegetables and lower intake of cereal grains, can lower dietary acid load without the need for excessive protein restriction or a large pill burden. This foods-based approach has theoretical benefits over supplement-based approaches, due to more favorable effects on blood pressure (7, 39, 43, 45) and other health benefits of fruits and vegetables (113). Risk of hyperkalemia may be greater in patients with moderate to severe CKD consuming diets high in fruits and vegetables and is an important area for future studies. Risk of hyperkalemia did not differ by dietary acid load in the AASK study (114), however, AASK only included participants with hypertensive kidney disease and these risks may be higher in diabetic patients.
CKD is a condition in which case detection and awareness are low (115). Public health strategies focusing on improving diet quality on a population level have the potential to improve CKD outcomes even in the large population of patients with early to moderate CKD who are undiagnosed or unaware. The benefits and harms of lowering dietary acid load for secondary prevention in early to moderate CKD should be rigorously tested.
Clinical Summary.
The dietary acid load is determined by the balance of acid-inducing foods, such as meats, eggs, cheese and cereal grains, and base-inducing foods, such as fruits and vegetables.
In the setting of chronic kidney disease and aging, higher dietary acid load may result in low-grade, subclinical acidosis despite a normal serum bicarbonate concentration.
Adaptations to maintain stable blood pH and augment per nephron acid excretion in the setting of chronic kidney disease may promote bone and muscle loss and further decline in glomerular filtration rate, but can be mitigated by alkali.
Studies with hard outcomes are needed to determine the safety and benefits of a foods-based approach to reducing the dietary acid load in patients with early to moderate chronic kidney disease.
Acknowledgements
J Scialla was supported by K23DK095949 from the NIDDK. CAM Anderson was supported by K01HL092595.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure: none
References
- 1.Gonick HC, Goldberg G, Mulcare D. Reexamination of the acid-ash content of several diets. Am J Clin Nutr. 1968;21(9):898–903. doi: 10.1093/ajcn/21.9.898. [DOI] [PubMed] [Google Scholar]
- 2.Sebastian A, Frassetto LA, Sellmeyer DE, Merriam RL, Morris RC., Jr. Estimation of the net acid load of the diet of ancestral preagricultural Homo sapiens and their hominid ancestors. Am J Clin Nutr. 2002;76(6):1308–1316. doi: 10.1093/ajcn/76.6.1308. [DOI] [PubMed] [Google Scholar]
- 3.Strohle A, Hahn A, Sebastian A. Estimation of the diet-dependent net acid load in 229 worldwide historically studied hunter-gatherer societies. Am J Clin Nutr. 2010;91(2):406–412. doi: 10.3945/ajcn.2009.28637. [DOI] [PubMed] [Google Scholar]
- 4.Remer T, Manz F. Paleolithic diet, sweet potato eaters, and potential renal acid load. Am J Clin Nutr. 2003;78(4):802–803. doi: 10.1093/ajcn/78.4.802. [DOI] [PubMed] [Google Scholar]
- 5.Cordain L, Eaton SB, Sebastian A, et al. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81(2):341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 6.Scialla JJ, Appel LJ, Astor BC, et al. Net endogenous acid production is associated with a faster decline in GFR in African Americans. Kidney Int. 2012;82(1):106–112. doi: 10.1038/ki.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goraya N, Simoni J, Jo C, Wesson DE. Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int. 2012;81(1):86–93. doi: 10.1038/ki.2011.313. [DOI] [PubMed] [Google Scholar]
- 8.Frassetto L, Morris RC, Jr., Sebastian A. Potassium bicarbonate reduces urinary nitrogen excretion in postmenopausal women. J Clin Endocrinol Metab. 1997;82(1):254–259. doi: 10.1210/jcem.82.1.3663. [DOI] [PubMed] [Google Scholar]
- 9.Sebastian A, Harris ST, Ottaway JH, Todd KM, Morris RC., Jr. Improved mineral balance and skeletal metabolism in postmenopausal women treated with potassium bicarbonate. N Engl J Med. 1994;330(25):1776–1781. doi: 10.1056/NEJM199406233302502. [DOI] [PubMed] [Google Scholar]
- 10.DuBose TD., Jr. Disorders of Acid-Base Balance. In: Brenner BM, editor. The Kidney. Saunders Elsevier; Philadelphia, PA: 2007. pp. 505–547. [Google Scholar]
- 11.Trilok G, Draper HH. Sources of protein-induced endogenous acid production and excretion by human adults. Calcif Tissue Int. 1989;44(5):335–338. doi: 10.1007/BF02556313. [DOI] [PubMed] [Google Scholar]
- 12.Relman AS, Lennon EJ, Lemann J., Jr. Endogenous production of fixed acid and the measurement of the net balance of acid in normal subjects. J Clin Invest. 1961;40:1621–1630. doi: 10.1172/JCI104384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lennon EJ, Lemann J., Jr. Influence of diet composition on endogenous fixed acid production. Am J Clin Nutr. 1968;21(5):451–456. doi: 10.1093/ajcn/21.5.451. [DOI] [PubMed] [Google Scholar]
- 14.Frassetto LA, Lanham-New SA, Macdonald HM, et al. Standardizing terminology for estimating the diet-dependent net acid load to the metabolic system. J Nutr. 2007;137(6):1491–1492. doi: 10.1093/jn/137.6.1491. [DOI] [PubMed] [Google Scholar]
- 15.Schuette SA, Hegsted M, Zemel MB, Linkswiler HM. Renal acid, urinary cyclic AMP, and hydroxyproline excretion as affected by level of protein, sulfur amino acid, and phosphorus intake. J Nutr. 1981;111(12):2106–2116. doi: 10.1093/jn/111.12.2106. [DOI] [PubMed] [Google Scholar]
- 16.Lemann J, Jr., Relman AS. The relation of sulfur metabolism to acid-base balance and electrolyte excretion: the effects of DL-methionine in normal man. J Clin Invest. 1959;38:2215–2223. doi: 10.1172/JCI104001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lennon EJ, Lemann J, Jr., Litzow JR. The effects of diet and stool composition on the net external acid balance of normal subjects. J Clin Invest. 1966;45(10):1601–1607. doi: 10.1172/JCI105466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hood VL, Tannen RL. Protection of acid-base balance by pH regulation of acid production. N Engl J Med. 1998;339(12):819–826. doi: 10.1056/NEJM199809173391207. [DOI] [PubMed] [Google Scholar]
- 19.Brown JC, Packer RK, Knepper MA. Role of organic anions in renal response to dietary acid and base loads. Am J Physiol. 1989;257(2 Pt 2):F170–176. doi: 10.1152/ajprenal.1989.257.2.F170. [DOI] [PubMed] [Google Scholar]
- 20.Packer RK, Curry CA, Brown KM. Urinary organic anion excretion in response to dietary acid and base loading. J Am Soc Nephrol. 1995;5(8):1624–1629. doi: 10.1681/ASN.V581624. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins AD, Dousa TP, Smith LH. Transport of citrate across renal brush border membrane: effects of dietary acid and alkali loading. Am J Physiol. 1985;249(4 Pt 2):F590–595. doi: 10.1152/ajprenal.1985.249.4.F590. [DOI] [PubMed] [Google Scholar]
- 22.Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc. 1995;95(7):791–797. doi: 10.1016/S0002-8223(95)00219-7. [DOI] [PubMed] [Google Scholar]
- 23.Remer T, Manz F. Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am J Clin Nutr. 1994;59(6):1356–1361. doi: 10.1093/ajcn/59.6.1356. [DOI] [PubMed] [Google Scholar]
- 24.Remer T, Dimitriou T, Manz F. Dietary potential renal acid load and renal net acid excretion in healthy, free-living children and adolescents. Am J Clin Nutr. 2003;77(5):1255–1260. doi: 10.1093/ajcn/77.5.1255. [DOI] [PubMed] [Google Scholar]
- 25.Frassetto LA, Todd KM, Morris RC, Jr., Sebastian A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr. 1998;68(3):576–583. doi: 10.1093/ajcn/68.3.576. [DOI] [PubMed] [Google Scholar]
- 26.Oster J, Lopez R, Perez G, Alpert H, Al-Reshaid K, Vaamonde C. The stability of pH, pCO2 and calculated [HCO3] of urine samples collected under oil. Nephron. 1988;50(4):320–324. doi: 10.1159/000185196. [DOI] [PubMed] [Google Scholar]
- 27.Yi J-H, Shin H-J, Kim S-M, Han S-W, Kim H-J, Oh M-S. Does the Exposure of Urine Samples to Air Affect Diagnostic Tests for Urine Acidification? Clin J Am Soc Nephrol. 2012;7(8):1211–1216. doi: 10.2215/CJN.03230312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bingham SA, Gill C, Welch A, et al. Validation of dietary assessment methods in the UK arm of EPIC using weighed records, and 24-hour urinary nitrogen and potassium and serum vitamin C and carotenoids as biomarkers. Int J Epidemiol. 1997;26(suppl 1):S137. doi: 10.1093/ije/26.suppl_1.s137. [DOI] [PubMed] [Google Scholar]
- 29.Welch AA, Mulligan A, Bingham SA, Khaw KT. Urine pH is an indicator of dietary acid-base load, fruit and vegetables and meat intakes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk population study. Br J Nutr. 2008;99(6):1335–1343. doi: 10.1017/S0007114507862350. [DOI] [PubMed] [Google Scholar]
- 30.Berkemeyer S, Vormann J, Gunther AL, Rylander R, Frassetto LA, Remer T. Renal net acid excretion capacity is comparable in prepubescence, adolescence, and young adulthood but falls with aging. J Am Geriatr Soc. 2008;56(8):1442–1448. doi: 10.1111/j.1532-5415.2008.01799.x. [DOI] [PubMed] [Google Scholar]
- 31.Arruda JA, Kurtzman NA. Mechanisms and classification of deranged distal urinary acidification. Am J Physiol. 1980;239(6):F515–523. doi: 10.1152/ajprenal.1980.239.6.F515. [DOI] [PubMed] [Google Scholar]
- 32.Kim S, Lee JW, Park J, et al. The urine-blood PCO gradient as a diagnostic index of H(+)-ATPase defect distal renal tubular acidosis. Kidney Int. 2004;66(2):761–767. doi: 10.1111/j.1523-1755.2004.00801.x. [DOI] [PubMed] [Google Scholar]
- 33.DuBose TD, Jr., Caflisch CR. Validation of the difference in urine and blood carbon dioxide tension during bicarbonate loading as an index of distal nephron acidification in experimental models of distal renal tubular acidosis. J Clin Invest. 1985;75(4):1116–1123. doi: 10.1172/JCI111805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scialla JJ, Appel LJ, Wolf M, et al. Plant Protein Intake is Associated With Fibroblast Growth Factor 23 and Serum Bicarbonate Levels in Patients With Chronic Kidney Disease: The Chronic Renal Insufficiency Cohort Study. J Ren Nutr. 2012;22(4):379–388. e371. doi: 10.1053/j.jrn.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strohle A, Waldmann A, Koschizke J, Leitzmann C, Hahn A. Diet-dependent net endogenous acid load of vegan diets in relation to food groups and bone health-related nutrients: results from the German Vegan Study. Ann Nutr Metab. 2011;59(2-4):117–126. doi: 10.1159/000331572. [DOI] [PubMed] [Google Scholar]
- 36.Engberink MF, Bakker SJ, Brink EJ, et al. Dietary acid load and risk of hypertension: the Rotterdam Study. Am J Clin Nutr. 2012;95(6):1438–1444. doi: 10.3945/ajcn.111.022343. [DOI] [PubMed] [Google Scholar]
- 37.Prynne CJ, Ginty F, Paul AA, et al. Dietary acid-base balance and intake of bone-related nutrients in Cambridge teenagers. Eur J Clin Nutr. 2004;58(11):1462–1471. doi: 10.1038/sj.ejcn.1602006. [DOI] [PubMed] [Google Scholar]
- 38.Gannon RH, Millward DJ, Brown JE, et al. Estimates of daily net endogenous acid production in the elderly UK population: analysis of the National Diet and Nutrition Survey (NDNS) of British adults aged 65 years and over. Br J Nutr. 2008;100(3):615–623. doi: 10.1017/S0007114508901240. [DOI] [PubMed] [Google Scholar]
- 39.Murakami K, Sasaki S, Takahashi Y, Uenishi K. Association between dietary acid-base load and cardiometabolic risk factors in young Japanese women. Br J Nutr. 2008;100(3):642–651. doi: 10.1017/S0007114508901288. [DOI] [PubMed] [Google Scholar]
- 40.Smit E, Nieto FJ, Crespo CJ, Mitchell P. Estimates of Animal and Plant Protein Intake in US Adults: Results from the Third National Health and Nutrition Examination Survey, 1988-1991. J Am Diet Assoc. 1999;99(7):813–820. doi: 10.1016/S0002-8223(99)00193-5. [DOI] [PubMed] [Google Scholar]
- 41.Rhodes D, Clemens J, Goldman J, LaComb R, Moshfegh A. 2009-2010 What We Eat In America, NHANES Tables 1-36.: Worldwide Web Site. Food Surveys Research Group; 2012. [Google Scholar]
- 42.Ausman LM, Oliver LM, Goldin BR, Woods MN, Gorbach SL, Dwyer JT. Estimated net acid excretion inversely correlates with urine pH in vegans, lacto-ovo vegetarians, and omnivores. J Ren Nutr. 2008;18(5):456–465. doi: 10.1053/j.jrn.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L, Curhan GC, Forman JP. Diet-dependent net acid load and risk of incident hypertension in United States women. Hypertension. 2009;54(4):751–755. doi: 10.1161/HYPERTENSIONAHA.109.135582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scialla JJ, Appel LJ, Astor BC, et al. Estimated net endogenous acid production and serum bicarbonate in African Americans with chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(7):1526–1532. doi: 10.2215/CJN.00150111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336(16):1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 46.Sebastian A, Sellmeyer DE, Stone KL, Cummings SR. Dietary ratio of animal to vegetable protein and rate of bone loss and risk of fracture in postmenopausal women. Am J Clin Nutr. 2001;74(3):411–412. doi: 10.1093/ajcn/74.3.411. [DOI] [PubMed] [Google Scholar]
- 47.Patterson BH, Block G, Rosenberger WF, Pee D, Kahle LL. Fruit and vegetables in the American diet: data from the NHANES II survey. Am J Public Health. 1990;80(12):1443–1449. doi: 10.2105/ajph.80.12.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsu Cy, Chertow GM. Elevations of serum phosphorus and potassium in mild to moderate chronic renal insufficiency. Nephrol Dial Transplant. 2002;17(8):1419–1425. doi: 10.1093/ndt/17.8.1419. [DOI] [PubMed] [Google Scholar]
- 49.Moranne O, Froissart M, Rossert J, et al. Timing of Onset of CKD-Related Metabolic Complications. J Am Soc Nephrol. 2009;20(1):164–171. doi: 10.1681/ASN.2008020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bowling CB, Inker LA, Gutierrez OM, et al. Age-Specific Associations of Reduced Estimated Glomerular Filtration Rate with Concurrent Chronic Kidney Disease Complications. Clin J Am Soc Nephrol. 2011;6(12):2822–2828. doi: 10.2215/CJN.06770711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kraut JA, Kurtz I. Metabolic Acidosis of CKD: Diagnosis, Clinical Characteristics, and Treatment. Am J Kidney Dis. 2005;45(6):978–993. doi: 10.1053/j.ajkd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 52.Lemann J, Jr., Lennon EJ, Goodman AD, Litzow JR, Relman AS. The Net Balance of Acid in Subjects Given Large Loads of Acid or Alkali. J Clin Invest. 1965:44507–517. doi: 10.1172/JCI105164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Litzow JR, Lemann J, Jr., Lennon EJ. The effect of treatment of acidosis on calcium balance in patients with chronic azotemic renal disease. J Clin Invest. 1967;46(2):280–286. doi: 10.1172/JCI105530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lemann J, Jr., Litzow JR, Lennon EJ. The effects of chronic acid loads in normal man: further evidence for the participation of bone mineral in the defense against chronic metabolic acidosis. J Clin Invest. 1966;45(10):1608–1614. doi: 10.1172/JCI105467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bushinsky DA. Acid-base imbalance and the skeleton. Eur J Nutr. 2001;40(5):238–244. doi: 10.1007/s394-001-8351-5. [DOI] [PubMed] [Google Scholar]
- 56.Wesson DE, Simoni J, Broglio K, Sheather S. Acid retention accompanies reduced GFR in humans and increases plasma levels of endothelin and aldosterone. Am J Physiol Renal Physiol. 2011;300(4):F830–837. doi: 10.1152/ajprenal.00587.2010. [DOI] [PubMed] [Google Scholar]
- 57.Wesson DE. Dietary acid increases blood and renal cortical acid content in rats. Am J Physiol. 1998;274(1 Pt 2):F97–103. doi: 10.1152/ajprenal.1998.274.1.F97. [DOI] [PubMed] [Google Scholar]
- 58.Wesson DE, Simoni J. Increased tissue acid mediates a progressive decline in the glomerular filtration rate of animals with reduced nephron mass. Kidney Int. 2009;75(9):929–935. doi: 10.1038/ki.2009.6. [DOI] [PubMed] [Google Scholar]
- 59.Frassetto LA, Morris RC, Jr., Sebastian A. Effect of age on blood acid-base composition in adult humans: role of age-related renal functional decline. Am J Physiol. 1996;271(6 Pt 2):F1114–1122. doi: 10.1152/ajprenal.1996.271.6.F1114. [DOI] [PubMed] [Google Scholar]
- 60.Menon V, Tighiouart H, Vaughn NS, et al. Serum Bicarbonate and Long-term Outcomes in CKD. Am J Kidney Dis. 2010;56(5):907–914. doi: 10.1053/j.ajkd.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 61.Raphael KL, Wei G, Baird BC, Greene T, Beddhu S. Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int. 2010;79(3):356–362. doi: 10.1038/ki.2010.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shah SN, Abramowitz M, Hostetter TH, Melamed ML. Serum Bicarbonate Levels and the Progression of Kidney Disease: A Cohort Study. Am J Kidney Dis. 2009;54(2):270–277. doi: 10.1053/j.ajkd.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate Supplementation Slows Progression of CKD and Improves Nutritional Status. J Am Soc Nephrol. 2009;20(9):2075–2084. doi: 10.1681/ASN.2008111205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mahajan A, Simoni J, Sheather SJ, Broglio KR, Rajab MH, Wesson DE. Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int. 2010;78(3):303–309. doi: 10.1038/ki.2010.129. [DOI] [PubMed] [Google Scholar]
- 65.Klahr S, Levey AS, Beck GJ, et al. The Effects of Dietary Protein Restriction and Blood-Pressure Control on the Progression of Chronic Renal Disease. N Engl J Med. 1994;330(13):877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 66.Levey AS, Greene T, Sarnak MJ, et al. Effect of Dietary Protein Restriction on the Progression of Kidney Disease: Long-Term Follow-Up of the Modification of Diet in Renal Disease (MDRD) Study. Am J Kidney Dis. 2006;48(6):879–888. doi: 10.1053/j.ajkd.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 67.Levey AS, Greene T, Beck GJ, et al. Dietary Protein Restriction and the Progression of Chronic Renal Disease: What Have All of the Results of the MDRD Study Shown? J Am Soc Nephrol. 1999;10(11):2426–2439. doi: 10.1681/ASN.V10112426. [DOI] [PubMed] [Google Scholar]
- 68.Pedrini MT, Levey AS, Lau J, Chalmers* TC, Wang PH. The Effect of Dietary Protein Restriction on the Progression of Diabetic and Nondiabetic Renal Diseases: A Meta-Analysis. Ann Intern Med. 1996;124(7):627–632. doi: 10.7326/0003-4819-124-7-199604010-00002. [DOI] [PubMed] [Google Scholar]
- 69.Nath KA, Hostetter MK, Hostetter TH. Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest. 1985;76(2):667–675. doi: 10.1172/JCI112020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khanna A, Simoni J, Hacker C, Duran MJ, Wesson DE. Increased endothelin activity mediates augmented distal nephron acidification induced by dietary protein. J Am Soc Nephrol. 2004;15(9):2266–2275. doi: 10.1097/01.ASN.0000138233.78329.4E. [DOI] [PubMed] [Google Scholar]
- 71.Dorhout-Mees EJ, Machado M, Slatopolsky E, Klahr S, Bricker NS. The functional adaptation of the diseased kidney : III. Ammonium excretion. J Clin Invest. 1966:45289–296. doi: 10.1172/JCI105342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tizianello A, De Ferrari G, Garibotto G, Gurreri G, Robaudo C. Renal metabolism of amino acids and ammonia in subjects with normal renal function and in patients with chronic renal insufficiency. J Clin Invest. 1980;65(5):1162–1173. doi: 10.1172/JCI109771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Busque SM, Wagner CA. Potassium restriction, high protein intake, and metabolic acidosis increase expression of the glutamine transporter SNAT3 (Slc38a3) in mouse kidney. Am J Physiol Renal Physiol. 2009;297(2):F440–450. doi: 10.1152/ajprenal.90318.2008. [DOI] [PubMed] [Google Scholar]
- 74.Packer RK, Desai SS, Hornbuckle K, Knepper MA. Role of countercurrent multiplication in renal ammonium handling: regulation of medullary ammonium accumulation. J Am Soc Nephrol. 1991;2(1):77–83. doi: 10.1681/ASN.V2177. [DOI] [PubMed] [Google Scholar]
- 75.Wesson DE, Jo C-H, Simoni J. Angiotensin II receptors mediate increased distal nephron acidification caused by acid retention. Kidney Int. 2012 doi: 10.1038/ki.2012.267. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 76.Ng HY, Chen HC, Tsai YC, Yang YK, Lee CT. Activation of intrarenal renin-angiotensin system during metabolic acidosis. Am J Nephrol. 2011;34(1):55–63. doi: 10.1159/000328742. [DOI] [PubMed] [Google Scholar]
- 77.Levine DZ, Iacovitti M, Buckman S, Hincke MT, Luck B, Fryer JN. ANG II-dependent HCO3- reabsorption in surviving rat distal tubules: expression/activation of H(+)-ATPase. Am J Physiol. 1997;272(6 Pt 2):F799–808. doi: 10.1152/ajprenal.1997.272.6.F799. [DOI] [PubMed] [Google Scholar]
- 78.Wesson DE. Endogenous endothelins mediate increased distal tubule acidification induced by dietary acid in rats. J Clin Invest. 1997;99(9):2203–2211. doi: 10.1172/JCI119393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wesson DE. Endogenous endothelins mediate increased acidification in remnant kidneys. J Am Soc Nephrol. 2001;12(9):1826–1835. doi: 10.1681/ASN.V1291826. [DOI] [PubMed] [Google Scholar]
- 80.Tolins JP, Hostetter MK, Hostetter TH. Hypokalemic nephropathy in the rat. Role of ammonia in chronic tubular injury. J Clin Invest. 1987;79(5):1447–1458. doi: 10.1172/JCI112973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Phisitkul S, Hacker C, Simoni J, Tran RM, Wesson DE. Dietary protein causes a decline in the glomerular filtration rate of the remnant kidney mediated by metabolic acidosis and endothelin receptors. Kidney Int. 2008;73(2):192–199. doi: 10.1038/sj.ki.5002647. [DOI] [PubMed] [Google Scholar]
- 82.Wesson DE, Simoni J. Acid retention during kidney failure induces endothelin and aldosterone production which lead to progressive GFR decline, a situation ameliorated by alkali diet. Kidney Int. 2010;78(11):1128–1135. doi: 10.1038/ki.2010.348. [DOI] [PubMed] [Google Scholar]
- 83.Gadola L, Noboa O, Marquez MN, et al. Calcium citrate ameliorates the progression of chronic renal injury. Kidney Int. 2004;65(4):1224–1230. doi: 10.1111/j.1523-1755.2004.00496.x. [DOI] [PubMed] [Google Scholar]
- 84.Phisitkul S, Khanna A, Simoni J, et al. Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int. 2010;77(7):617–623. doi: 10.1038/ki.2009.519. [DOI] [PubMed] [Google Scholar]
- 85.Ballmer PE, McNurlan MA, Hulter HN, Anderson SE, Garlick PJ, Krapf R. Chronic metabolic acidosis decreases albumin synthesis and induces negative nitrogen balance in humans. J Clin Invest. 1995;95(1):39–45. doi: 10.1172/JCI117668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.May RC, Kelly RA, Mitch WE. Metabolic acidosis stimulates protein degradation in rat muscle by a glucocorticoid-dependent mechanism. J Clin Invest. 1986;77(2):614–621. doi: 10.1172/JCI112344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.May RC, Kelly RA, Mitch WE. Mechanisms for defects in muscle protein metabolism in rats with chronic uremia. Influence of metabolic acidosis. J Clin Invest. 1987;79(4):1099–1103. doi: 10.1172/JCI112924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mitch WE, Medina R, Grieber S, et al. Metabolic acidosis stimulates muscle protein degradation by activating the adenosine triphosphate-dependent pathway involving ubiquitin and proteasomes. J Clin Invest. 1994;93(5):2127–2133. doi: 10.1172/JCI117208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eustace JA, Astor B, Muntner PM, Ikizler TA, Coresh J. Prevalence of acidosis and inflammation and their association with low serum albumin in chronic kidney disease. Kidney Int. 2004;65(3):1031–1040. doi: 10.1111/j.1523-1755.2004.00481.x. [DOI] [PubMed] [Google Scholar]
- 90.Krieger NS, Sessler NE, Bushinsky DA. Acidosis inhibits osteoblastic and stimulates osteoclastic activity in vitro. Am J Physiol. 1992;262(3 Pt 2):F442–448. doi: 10.1152/ajprenal.1992.262.3.F442. [DOI] [PubMed] [Google Scholar]
- 91.Bushinsky DA. Acid-base imbalance and the skeleton. European Journal of Nutrition. 2001;40(5):238–244. doi: 10.1007/s394-001-8351-5. [DOI] [PubMed] [Google Scholar]
- 92.Reaich D, Channon SM, Scrimgeour CM, Daley SE, Wilkinson R, Goodship TH. Correction of acidosis in humans with CRF decreases protein degradation and amino acid oxidation. Am J Physiol. 1993;265(2 Pt 1):E230–235. doi: 10.1152/ajpendo.1993.265.2.E230. [DOI] [PubMed] [Google Scholar]
- 93.Domrongkitchaiporn S, Pongskul C, Sirikulchayanonta V, et al. Bone histology and bone mineral density after correction of acidosis in distal renal tubular acidosis. Kidney Int. 2002;62(6):2160–2166. doi: 10.1046/j.1523-1755.2002.00656.x. [DOI] [PubMed] [Google Scholar]
- 94.Cochran M, Wilkinson R. Effect of correction of metabolic acidosis on bone mineralisation rates in patients with renal osteomalacia. Nephron. 1975;15(2):98–110. doi: 10.1159/000180501. [DOI] [PubMed] [Google Scholar]
- 95.Papadoyannakis NJ, Stefanidis CJ, McGeown M. The effect of the correction of metabolic acidosis on nitrogen and potassium balance of patients with chronic renal failure. Am J Clin Nutr. 1984;40(3):623–627. doi: 10.1093/ajcn/40.3.623. [DOI] [PubMed] [Google Scholar]
- 96.McSherry E. Acidosis and growth in nonuremic renal disease. Kidney Int. 1978;14(4):349–354. doi: 10.1038/ki.1978.135. [DOI] [PubMed] [Google Scholar]
- 97.McSherry E, Morris RC., Jr. Attainment and maintenance of normal stature with alkali therapy in infants and children with classic renal tubular acidosis. J Clin Invest. 1978;61(2):509–527. doi: 10.1172/JCI108962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McLean RR, Qiao N, Broe KE, et al. Dietary acid load is not associated with lower bone mineral density except in older men. J Nutr. 2011;141(4):588–594. doi: 10.3945/jn.110.135806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dawson-Hughes B, Harris SS. Calcium intake influences the association of protein intake with rates of bone loss in elderly men and women. Am J Clin Nutr. 2002;75(4):773–779. doi: 10.1093/ajcn/75.4.773. [DOI] [PubMed] [Google Scholar]
- 100.Maurer M, Riesen W, Muser J, Hulter HN, Krapf R. Neutralization of Western diet inhibits bone resorption independently of K intake and reduces cortisol secretion in humans. Am J Physiol Renal Physiol. 2003;284(1):F32–40. doi: 10.1152/ajprenal.00212.2002. [DOI] [PubMed] [Google Scholar]
- 101.Lin PH, Ginty F, Appel LJ, et al. The DASH diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. J Nutr. 2003;133(10):3130–3136. doi: 10.1093/jn/133.10.3130. [DOI] [PubMed] [Google Scholar]
- 102.New SA, MacDonald HM, Campbell MK, et al. Lower estimates of net endogenous noncarbonic acid production are positively associated with indexes of bone health in premenopausal and perimenopausal women. Am J Clin Nutr. 2004;79(1):131–138. doi: 10.1093/ajcn/79.1.131. [DOI] [PubMed] [Google Scholar]
- 103.Sellmeyer DE, Stone KL, Sebastian A, Cummings SR. A high ratio of dietary animal to vegetable protein increases the rate of bone loss and the risk of fracture in postmenopausal women. Study of Osteoporotic Fractures Research Group. Am J Clin Nutr. 2001;73(1):118–122. doi: 10.1093/ajcn/73.1.118. [DOI] [PubMed] [Google Scholar]
- 104.Frassetto LA, Todd KM, Morris RC, Jr., Sebastian A. Worldwide incidence of hip fracture in elderly women: relation to consumption of animal and vegetable foods. J Gerontol A Biol Sci Med Sci. 2000;55(10):M585–592. doi: 10.1093/gerona/55.10.m585. [DOI] [PubMed] [Google Scholar]
- 105.Hannan MT, Tucker KL, Dawson-Hughes B, Cupples LA, Felson DT, Kiel DP. Effect of dietary protein on bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15(12):2504–2512. doi: 10.1359/jbmr.2000.15.12.2504. [DOI] [PubMed] [Google Scholar]
- 106.Misra D, Berry SD, Broe KE, et al. Does dietary protein reduce hip fracture risk in elders? The Framingham Osteoporosis Study. Osteoporos Int. 2011;22(1):345–349. doi: 10.1007/s00198-010-1179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Roshanravan B, Khatri M, Robinson-Cohen C, et al. A Prospective Study of Frailty in Nephrology-Referred Patients With CKD. Am J Kidney Dis. 2012 doi: 10.1053/j.ajkd.2012.05.017. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wilhelm-Leen ER, Hall YN, M KT, Chertow GM. Frailty and chronic kidney disease: the Third National Health and Nutrition Evaluation Survey. Am J Med. 2009;122(7):664–671. e662. doi: 10.1016/j.amjmed.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shlipak MG, Stehman-Breen C, Fried LF, et al. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis. 2004;43(5):861–867. doi: 10.1053/j.ajkd.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 110.Nickolas TL, McMahon DJ, Shane E. Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol. 2006;17(11):3223–3232. doi: 10.1681/ASN.2005111194. [DOI] [PubMed] [Google Scholar]
- 111.Ensrud KE, Lui LY, Taylor BC, et al. Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med. 2007;167(2):133–139. doi: 10.1001/archinte.167.2.133. [DOI] [PubMed] [Google Scholar]
- 112.KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis. 2007;49(2):S12–S154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 113.Lanham-New SA. Fruit and vegetables: the unexpected natural answer to the question of osteoporosis prevention? Am J Clin Nutr. 2006;83(6):1254–1255. doi: 10.1093/ajcn/83.6.1254. [DOI] [PubMed] [Google Scholar]
- 114.Scialla JJ, Appel LJ, Astor BC, et al. Net endogenous acid production is associated with a faster decline in GFR in African Americans. Kidney Int. 2012;82(1):106–112. doi: 10.1038/ki.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Plantinga LC, Boulware LE, Coresh J, et al. Patient awareness of chronic kidney disease: trends and predictors. Arch Intern Med. 2008;168(20):2268–2275. doi: 10.1001/archinte.168.20.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]