Abstract
The conserved kinases Mps1 and Ipl1/Aurora B are critical for enabling chromosomes to attach to microtubules such that partner chromosomes will be segregated correctly from each other, but the precise roles of these kinases have been unclear. Here, imaging of live yeast cells was performed to elucidate the stages of chromosome-microtubule interactions, and their regulation by Ipl1 and Mps1, through meiosis I. Ipl1 was found to release kinetochore-microtubule (kMT) associations following meiotic entry, liberating chromosomes to begin homologous pairing. Surprisingly, most chromosome pairs were found to begin their spindle interactions with incorrect kMT attachments. Ipl1 released these improper connections while Mps1 triggered the formation of new force-generating microtubule attachments. This microtubule release and reattachment cycle can prevent catastrophic chromosome segregation errors in meiosis.
In order to be segregated properly, partner chromosomes must attach to microtubules that emanate from opposite poles of the spindle – a configuration referred to as bi-oriented. Chromosomes become bi-oriented in a multi-step process (fig. S1A) that involves the release and re-attachment of kMT connections that would lead to chromosome segregation errors (reviewed in (1)). A surveillance mechanism, the spindle checkpoint, delays progression out of metaphase until incorrect kinetochore-microtubule (kMT) connections become corrected (2). Meiosis I, which involves the segregation of homologous chromosomes, presents extra challenges to the bi-orientation machinery (fig. S1). Ipl1 (Aurora B in mammals) and Mps1 are implicated in several aspects of chromosome segregation in meiosis and mitosis (3–6). Their failures lead to the generation of aneuploid cells. Paradoxically, tumor cells with abnormal chromosome compositions can be especially sensitive to their inactivation (7, 8). Although both kinases are required to biorient chromosomes, their functional relationship is unclear. Aurora B is known to destabilize kMT associations. Mps1 has been suggested to modulate Aurora B activity in mammals, and both to couple and sever kMT connections in yeast (1, 9).
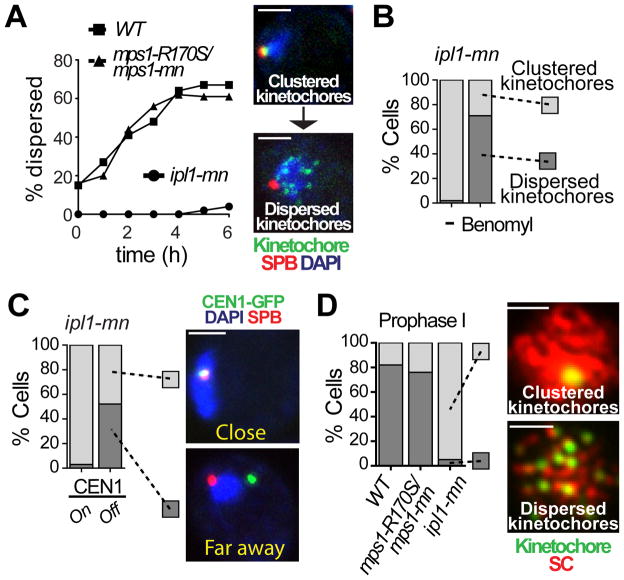
Mps1 has been difficult to study as it is because it is involved in multiple functions (4). Here we have taken advantage of a separation-of-function allele (mps1-R170S) to determine the role of Mps1 in meiotic chromosome segregation (fig. S2A). This allele exhibited only mild defects during mitosis (consistent with a defective spindle checkpoint) but catastrophic failures during meiosis (fig. S2B and C). The mps1-R170S mutation is in a region outside of the kinase domain implicated in chromosome bi-orientation, presumably by mediating interactions between the kinase and key substrates (10). In fact, mps1-R170S mutants, like ipl1 mutants, errantly moved both homologous partners to the same pole at meiosis I (meiosis I non-disjunction, or NDJ) in over half the meioses (fig. S2D). This elevated NDJ was not due to failure to form kMT attachments (fig. S3). To explain this defect, we investigated kMT connections, which are regulated at two points in meiosis I (fig. S1A). The first occurs as cells enter meiosis with their centromeres clustered near the single spindle pole body (SPB, the microtubule organizing center). This cluster, termed the Rabl cluster (reviewed in (11)), disperses in prophase, as homologous chromosomes begin pairing (12, 13). ipl1-mn (“mn” for meiotic-null) mutants, in contrast to mps1-R170S, and two other mps1 mutant alleles (fig. S4A), exhibited a striking phenotype: centromeres never dispersed from the SPB (Fig. 1A). In mitosis, Ipl1 triggers kMT release by phosphorylating target proteins. Here, blocking Ipl1 kinase activity prevented release of the cluster (fig. S4B). In ipl1 mutants, the cluster could be released by de-stabilizing microtubules (Fig. 1B) or by disrupting the kinetochore, by inducing transcription through the centromere (14) (Fig. 1C) or depleting a kinetochore protein (fig. S4C), demonstrating the Rabl cluster is maintained by kMTs. The failure of ipl1 mutants to release clustered centromeres was not due to a delay in meiotic progression because the cells exhibited hallmarks of meiotic progression while the Rabl persisted (Fig. 1D and below). Thus Ipl1, but not Mps1, mediates programmed release of the Rabl cluster by reversing kMT attachments.
Fig. 1.
Ipl1 is necessary to release centromeres from the SPB in meiotic prophase. (A) Dispersion of kinetochores was monitored in wild-type (square), ipl1-mn (circle), and mps1-R170S/mps1-mn (triangle) cells carrying SPB (Spc42-DsRed) and kinetochore (Mtw1-GFP) markers. Cells with one SPB and dispersed kinetochores were counted (n ≥ 100). T=0 represents the time at which cells were switched to sporulation medium. (B) As in (A) ipl1-mn diploid cells with clustered (light grey) or dispersed kinetochores (dark grey) were analyzed (n ≥ 100) with or without addition benomyl. (C) Separation of CEN1-GFP from the SPBs (Spc42-DsRed) was monitored in ipl1-mn cells with or without inactivation of CEN1 (see Methods). Separation was scored as either close to (<0.75μm, light grey) or far away from the SPB (≥0.75μm, dark grey) (n≥30). (D) Cells with Zip1 staining were scored for having clustered (light grey) or dispersed (dark grey) kinetochores 3 hours after meiotic induction (n≥38). Cells were stained with antibodies against GFP (MTW1-GFP; green) and Zip1 (red). Scale bar: 2μm.
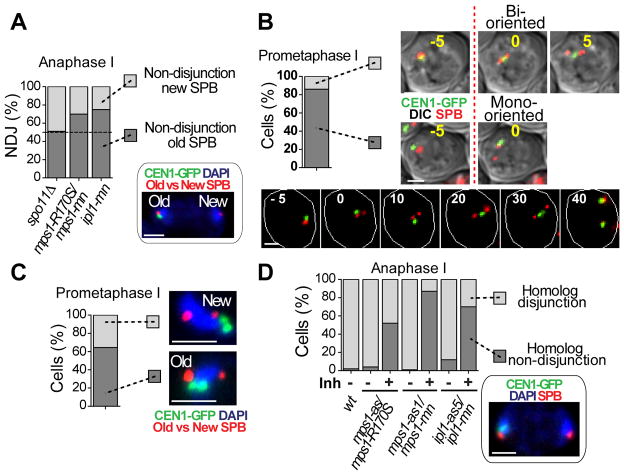
The failure of ipl1 mutants to release the Rabl cluster predicts that their meiotic non-disjunctions will be towards this “old” SPB. Tagging SPBs with a fluorescent protein allowed old and new SPBs to be distinguished (fig. S5). Randomly segregating chromosomes (spo11) non-disjoined equally, but ipl1 non-disjoined to the old SPB (Fig. 2A). Surprisingly this was also true of mps1 mutants. This result could be explained if, in wild-type cells, most attachments of chromosomes were monopolar, and to the older SPB, and were then corrected by Mps1. Indeed, we found that in 85% of cells, when new spindles formed, both homologs were mono-oriented and were biased for attachment to the older pole (Fig. 2B and C). Thus in wild-type cells, most chromosome pairs begin pro-metaphase in a monopolar configuration, which is then corrected. We found there is a higher density of microtubules radiating from the old pole of new spindles, which may explain this bias (fig. S6). To investigate the roles of Ipl1 and Mps1 in re-orienting monopolar attachments, we used ipl1-as5 or mps1-as1 alleles that could be inactivated with inhibitors after prophase (fig. S7). Inactivation of either Ipl1 or Mps1 lead to very high NDJ (70% and 90% respectively, Fig. 2D), which correlates well with the proportion of chromosomes that begin metaphase mono-oriented (85%) (Fig. 2B), and is consistent with roles for both in reorientating chromosomes. An alternate explanation, that in these mutants homologous chromosomes cannot be separated was eliminated (fig. S8).
Fig. 2.
Chromosomes begin pro-metaphase with monopolar spindle attachments. (A) NDJ of CEN1 towards the new (light grey) or old SPB (dark grey) was determined in anaphase I cells (n≥60) homozygous for CEN1-GFP and expressing Spc42-RedStar, which differentiates old from new SPBs (fig. S5). An example of NDJ to the old SPB is shown. (B) The frequency of mono- or bipolar orientation of CEN1-GFP on newly formed spindles (Spc42-DsRed) was observed by time-lapse imaging (5 min intervals). The first frame with a bipolar spindle is t=0. Cells with CEN1-GFP overlapping one pole were scored as mono-oriented (dark grey) and between poles as bi-oriented (light grey) (n=29). (C) Association of mono-oriented CEN1-GFP with the old (dark grey) or new SPB (light grey) measured in diploid cells carrying GAL4-ER, GAL-NDT80, homozygous CEN1-GFP, and SPC42-RedStar following release from prophase arrest (β-estradiol addition) (fig. S5). Cells with short spindles were scored (0.75–1.5μm) (n=104). (D) Non-disjunction following inactivation of Ipl1-as5 or Mps1-as1. Cells were released from prophase in the presence or absence of inhibitor. Disjunction (light grey) or NDJ (dark grey) of CEN1-GFP was scored at anaphase I. Scale bar: 2μm.
In wild-type meioses the spindle is short and the chromosomes become bi-oriented quickly (Fig. 2B), making it difficult to monitor re-orientation events. Thus we monitored reorientation in spo11Δ mutants, which have longer spindles (15) and do not tether their homologous partners with chiasmata. Because these univalent chromosomes can attach to only one microtubule (16), we reasoned they should exhibit cycles of microtubule attachment and detachment in futile attempts to biorient, optimizing our opportunity to quantify this process (fig. S9A). In both controls and ipl1 mutants, upon the exit from prophase, the dispersed centromeres migrated toward the SPBs before spindle formation (Fig. 3A–B). In contrast, mps1 mutants were defective in this movement. In wild-type cells once the spindle was formed, the centromeres traversed from one pole to pole (Fig. 3A, C) at a rate of about 2 microns/minute (Fig. 3E); similar to the 1.5 microns/minute estimated for kinetochores with end-on attachments to microtubules in mitotic cells (17). The centromeres also made “trial” excursions, in which they left one pole, lingered, and then returned (Fig. 3A). Both mps1 and ipl1 mutants exhibited very few traverses (Fig. 3A, C). In ipl1 cells, once centromeres arrived at a pole they remained there indefinitely (Fig. 3E). In contrast in mps1 mutants, the centromeres were released from the SPB and began excursions as efficiently as wild-type controls (Fig. 3E) but they could not efficiently traverse the spindle (Fig. 3C), instead making futile trial excursions (Fig. 3F). In rare cases when centromeres traversed the spindle, the traverses were slow and featured back-and-forth movements and pauses characteristic of kinetochores with lateral microtubule attachments (17) (fig. S9 and S10). In mps1 mutants, many kinetochores in each cell failed to migrate completely to the poles before anaphase (fig. S11). Nonetheless, these kinetochores remained close to the poles as they separated at anaphase and were not left at the mid-zone as lagging chromosomes, suggesting that mps1 mutants are proficient in forming kMT attachments.
Fig. 3.

Ipl1 and Mps1 have different roles in re-orientation. (A–F) spo11Δ (=WT), spo11Δ mps1-as1/mps1-mn (=mps1) or spo11Δ ipl1-as5/ipl1-mn (=ipl1) cells with one CEN1-GFP tagged chromosome and expressing Spc42-DsRed were observed by time-lapse imaging during meiosis at 2 min (A–B, E) or 45 sec intervals (C–D, F) following release from prophase arrest, with or without inhibitor (fig. S7). (A) Images from representative cells (t=0 represents first frame with SPB separation). (B) Cells in which CEN1 migrated to SPBs before spindle formation. (C) After release from the SPB, complete migrations of CEN1 to the opposite SPB were quantified as traverses. (D) The speed for crossing the spindle was evaluated for each individual traverse (n=55 for WT, n=19 for mps1-as1). Below: an mps1-as1 mutant showing back-and-forth movements. Scale bar: 2μm. (E) Dwell-time of CEN1 at SPBs. (F) Migrations away from and then back to the same SPB (trials).
Similar assays were used to evaluate the behavior of homologous chromosome pairs tethered by chiasmata (SPO11). As in the single chromosome assay (above), mps1 mutants were proficient in releasing mono-polar attachments but chromosome pairs could not move efficiently across the spindle or bi-orient (fig. S12–S13). A comparison of mps1 mutants to a spindle checkpoint mutant revealed that the migration defects of mps1 mutants are not due to a loss of the spindle checkpoint (fig. S12–S13).
The chromosome behavior in the mps1 mutants was characteristic of a failure to convert side-on attachments, which in yeast appear unable to support rapid, processive, movements (17), into end-on kMT attachments, which do. A hallmark of end-on attachment formation is the association of the Duo/Dam complex with the outer kinetochore (18). If mps1 mutants are defective in forming end-on attachments then Dam1 should exhibit reduced co-localization with kinetochores. Indeed, the mps1 cells were significantly reduced in co-localization of Dam1 with kinetochores (Fig. 4A).
Fig. 4.
Mps1 is necessary to generate end-on attachment during meiosis I. (A) Co-localization of Mtw1-RFP and Dam1-GFP were observed in spread nuclei from WT or mps1-as1/mps1-mn (=mps1-as1) chromosome spreads after release from prophase arrest with addition of inhibitor (fig. S7). The proportion of chromosomes (Mtw1) located between the SPBs with Dam1 over-lapping staining (white arrow) was determined. The difference between WT and mps1 mutant is significant (Fischer’s test, p<0.001). Scale bar: 2μm. (B) Model showing kinetochore behaviors in meiosis and the roles of Ipl1 and Mps1.
We conclude that Ipl1 and Mps1 act sequentially. Ipl1 is required for releasing kMT attachments while Mps1 is required to promote new force-generating end-on attachments that allow released chromosomes to be moved back towards the opposite pole (Fig. 4B). Prior work has suggested that Aurora B and Mps1 have shared phenotypes because they regulate each other (19). The fact that ipl1 mutants have no dramatic defect in poleward migration and mps1 mutants are able to release kMT attachments efficiently suggests that, at least in budding yeast meiosis, each kinase can perform its function independently of the other, a conclusion supported by the finding that, in mitosis, the kinase activity of each does not appear dependent on the other (20). However, we cannot eliminate the possibility that cross phosphorylation might enhance these functions, or promote activities beyond those reported here.
In yeast, Mps1 is known to phosphorylate multiple proteins that could have roles in kMT function, including the kinetochore components Dam1, Ndc80, Cnn1, and Spc105 (9, 21–23), but how phosphorylation of these potential targets contributes to meiotic bi-orientation remains an unanswered question. Mammalian meiotic chromosomes also experience multiple incorrect attachments that must be corrected by Mps1 and Aurora B (3, 24). The elucidation of the manner in which Mps1 promotes bi-orientation in meiosis, and the identification of a conserved region of the Mps1 kinase critical for this activity should refine the search for its key targets in this process.
Supplementary Material
Acknowledgments
We thank S. Jaspersen for critically reading the manuscript, K. Benjamin, S. Biggins, M. Dresser, M. Knop, K. Nasmyth, and A. Straight, for providing strains and reagents, and current and past laboratory members for reagents, and discussions of our work. P.D.S. was supported in part by NIH training grant (GM-07135). This work was supported by March of Dimes Birth Defects Foundation grant (FY98.409/FY99.617) to M.W., and NSF grant 0950005 and NIH grant GM087377 to D.D. Data used in this paper are available in the supplementary materials.
References and Notes
- 1.Tanaka TU. Kinetochore-microtubule interactions: steps towards bi-orientation. EMBO J. 2010;29:4070. doi: 10.1038/emboj.2010.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nezi L, Musacchio A. Sister chromatid tension and the spindle assembly checkpoint. Curr Opin Cell Biol. 2009;21:785. doi: 10.1016/j.ceb.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Hached K, et al. Mps1 at kinetochores is essential for female mouse meiosis I. Development. 2011;138:2261. doi: 10.1242/dev.061317. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, Winey M. The MPS1 Family of Protein Kinases. Annual Review of Biochemistry. 2012;81:561. doi: 10.1146/annurev-biochem-061611-090435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marston AL, Amon A. Meiosis: cell-cycle controls shuffle and deal. Nat Rev Mol Cell Biol. 2004;5:983. doi: 10.1038/nrm1526. [DOI] [PubMed] [Google Scholar]
- 6.Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol. 2007;8:798. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- 7.Colombo R, et al. Targeting the mitotic checkpoint for cancer therapy with NMS-P715, an inhibitor of MPS1 kinase. Cancer Res. 2010;70:10255. doi: 10.1158/0008-5472.CAN-10-2101. [DOI] [PubMed] [Google Scholar]
- 8.Lens SM, Voest EE, Medema RH. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat Rev Cancer. 2010;10:825. doi: 10.1038/nrc2964. [DOI] [PubMed] [Google Scholar]
- 9.Shimogawa MM, et al. Mps1 phosphorylation of Dam1 couples kinetochores to microtubule plus ends at metaphase. Curr Biol. 2006;16:1489. doi: 10.1016/j.cub.2006.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Araki Y, et al. N-terminal regions of Mps1 kinase determine functional bifurcation. J Cell Biol. 2010;189:41. doi: 10.1083/jcb.200910027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowan CR, Carlton PM, Cande WZ. The Polar Arrangement of Telomeres in Interphase and Meiosis. Rabl Organization and the Bouquet. Plant Physiology. 2001;125:532. doi: 10.1104/pp.125.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi A, Ogawa H, Kohno K, Gasser SM, Hiraoka Y. Meiotic behaviours of chromosomes and microtubules in budding yeast: relocalization of centromeres and telomeres during meiotic prophase. Genes Cells. 1998;3:587. doi: 10.1046/j.1365-2443.1998.00215.x. [DOI] [PubMed] [Google Scholar]
- 13.Jin Q-w, Trelles-Sticken E, Scherthan H, Loidl J. Yeast Nuclei Display Prominent Centromere Clustering That Is Reduced in Nondividing Cells and in Meiotic Prophase. The Journal of Cell Biology. 1998;141:21. doi: 10.1083/jcb.141.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill A, Bloom K. Genetic manipulation of centromere function. Mol Cell Biol. 1987;7:2397. doi: 10.1128/mcb.7.7.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shonn MA, McCarroll R, Murray AW. Requirement of the spindle checkpoint for proper chromosome segregation in budding yeast meiosis. Science. 2000;289:300. doi: 10.1126/science.289.5477.300. [DOI] [PubMed] [Google Scholar]
- 16.Winey M, Morgan GP, Straight PD, Giddings TH, Jr, Mastronarde DN. Three-dimensional ultrastructure of Saccharomyces cerevisiae meiotic spindles. Molecular biology of the cell. 2005;16:1178. doi: 10.1091/mbc.E04-09-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka K, Kitamura E, Kitamura Y, Tanaka TU. Molecular mechanisms of microtubule-dependent kinetochore transport toward spindle poles. J Cell Biol. 2007;178:269. doi: 10.1083/jcb.200702141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maure JF, et al. The Ndc80 loop region facilitates formation of kinetochore attachment to the dynamic microtubule plus end. Curr Biol. 2011;21:207. doi: 10.1016/j.cub.2010.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lan W, Cleveland DW. A chemical tool box defines mitotic and interphase roles for Mps1 kinase. J Cell Biol. 2010;190:21. doi: 10.1083/jcb.201006080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maure JF, Kitamura E, Tanaka TU. Mps1 kinase promotes sister-kinetochore bi-orientation by a tension-dependent mechanism. Curr Biol. 2007;17:2175. doi: 10.1016/j.cub.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bock LJ, et al. Cnn1 inhibits the interactions between the KMN complexes of the yeast kinetochore. Nat Cell Biol. 2012;14:614. doi: 10.1038/ncb2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kemmler S, et al. Mimicking Ndc80 phosphorylation triggers spindle assembly checkpoint signalling. EMBO J. 2009;28:1099. doi: 10.1038/emboj.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.London N, Ceto S, Ranish JA, Biggins S. Phosphoregulation of Spc105 by Mps1 and PP1 regulates Bub1 localization to kinetochores. Curr Biology. 2012;22:900. doi: 10.1016/j.cub.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitajima TS, Ohsugi M, Ellenberg J. Complete kinetochore tracking reveals error-prone homologous chromosome biorientation in mammalian oocytes. Cell. 2011;146:568. doi: 10.1016/j.cell.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 25.Dresser ME, Ewing DJ, Harwell SN, Coody D, Conrad MN. Nonhomologous synapsis and reduced crossing over in a heterozygous paracentric inversion in Saccharomyces cerevisiae. Genetics. 1994;138:633. doi: 10.1093/genetics/138.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burke DDD, Stearns T. Methods in Yeast Genetics. Cold Spring Harb Laboratory Press. 2000 [Google Scholar]
- 27.Hochwagen A, et al. Novel response to microtubule perturbation in meiosis. Mol Cell Biol. 2005;25:4767. doi: 10.1128/MCB.25.11.4767-4781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janke C, et al. A versatile toolbox for PCR-based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast. 2004;21:947. doi: 10.1002/yea.1142. [DOI] [PubMed] [Google Scholar]
- 29.Longtine MS, et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 30.Toth A, et al. Functional genomics identifies monopolin: a kinetochore protein required for segregation of homologs during meiosis i. Cell. 2000;103:1155. doi: 10.1016/s0092-8674(00)00217-8. [DOI] [PubMed] [Google Scholar]
- 31.Benjamin KR, Zhang C, Shokat KM, Herskowitz I. Control of landmark events in meiosis by the CDK Cdc28 and the meiosis-specific kinase Ime2. Genes Dev. 2003;17:1524. doi: 10.1101/gad.1101503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinsky BA, Kung C, Shokat KM, Biggins S. The Ipl1-Aurora protein kinase activates the spindle checkpoint by creating unattached kinetochores. Nat Cell Biol. 2006;8:78. doi: 10.1038/ncb1341. [DOI] [PubMed] [Google Scholar]
- 33.Jones MH, et al. Chemical genetics reveals a role for Mps1 kinase in kinetochore attachment during mitosis. Curr Biol. 2005;15:160. doi: 10.1016/j.cub.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Grandin N, Reed SI. Differential function and expression of Saccharomyces cerevisiae B-type cyclins in mitosis and meiosis. Mol Cell Biol. 1993;13:2113. doi: 10.1128/mcb.13.4.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obeso D, Dawson DS. Temporal Characterization of Homology-Independent Centromere Coupling in Meiotic Prophase. PLoS One. 2010;5:e10336. doi: 10.1371/journal.pone.0010336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitamura E, Tanaka K, Kitamura Y, Tanaka TU. Kinetochore microtubule interaction during S phase in Saccharomyces cerevisiae. Genes Dev. 2007;21:3319. doi: 10.1101/gad.449407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akiyoshi B, et al. Tension directly stabilizes reconstituted kinetochore-microtubule attachments. Nature. 2010;468:576. doi: 10.1038/nature09594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riedel CG, et al. Protein phosphatase 2A protects centromeric sister chromatid cohesion during meiosis I. Nature. 2006;441:53. doi: 10.1038/nature04664. [DOI] [PubMed] [Google Scholar]
- 39.Gilliland WD, Wayson SM, Hawley RS. The meiotic defects of mutants in the Drosophila mps1 gene reveal a critical role of Mps1 in the segregation of achiasmate homologs. Curr Biol. 2005;15:672. doi: 10.1016/j.cub.2005.02.062. [DOI] [PubMed] [Google Scholar]
- 40.Poss KD, Nechiporuk A, Stringer KF, Lee C, Keating MT. Germ cell aneuploidy in zebrafish with mutations in the mitotic checkpoint gene mps1. Genes Dev. 2004;18:1527. doi: 10.1101/gad.1182604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castillo AR, Meehl JB, Morgan G, Schutz-Geschwender A, Winey M. The yeast protein kinase Mps1p is required for assembly of the integral spindle pole body component Spc42p. J Cell Biol. 2002;156:453. doi: 10.1083/jcb.200111025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He X, Rines DR, Espelin CW, Sorger PK. Molecular analysis of kinetochore-microtubule attachment in budding yeast. Cell. 2001;106:195. doi: 10.1016/s0092-8674(01)00438-x. [DOI] [PubMed] [Google Scholar]
- 43.Gladstone MN, Obeso D, Chuong H, Dawson DS. The synaptonemal complex protein Zip1 promotes bi-orientation of centromeres at meiosis I. PLoS Genet. 2009;5:e1000771. doi: 10.1371/journal.pgen.1000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goetsch L, Byers B. Meiotic cytology of Saccharomyces cerevisiae in protoplast lysates. Mol Gen Genet. 1982;187:54. doi: 10.1007/BF00384383. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka TU, et al. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell. 2002;108:317. doi: 10.1016/s0092-8674(02)00633-5. [DOI] [PubMed] [Google Scholar]
- 46.Taxis C, et al. Spore number control and breeding in Saccharomyces cerevisiae. The Journal of Cell Biology. 2005;171:627. doi: 10.1083/jcb.200507168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carlile TM, Amon A. Meiosis I is established through division-specific translational control of a cyclin. Cell. 2008;133:280. doi: 10.1016/j.cell.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheslock PS, Kemp BJ, Boumil RM, Dawson DS. The roles of MAD1, MAD2 and MAD3 in meiotic progression and the segregation of nonexchange chromosomes. Nature Genetics. 2005;37:756. doi: 10.1038/ng1588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.