During S phase the many thousands of replication forks involved in replicating chromosomal DNA must be co-ordinated to ensure that despite the very large quantities of DNA involved, no section of DNA is left unreplicated and no section of DNA is replicated more than once. Cells achieve this by having a distinct stage that occurs prior to S phase when replication origins are “licensed” for replication by loading complexes of the 6 minichromosome maintenance proteins, Mcm2-7, onto them1-3. During S phase, replication forks are initiated only at these licensed replication origins. As initiation occurs at each origin, the licence is removed, thereby ensuring that it fires only once in each cell cycle. In order for this system to work properly, it is essential that the licensing system is turned on as cells exit metaphase so that the DNA in newly-born cells is properly licensed, and that licensing is then turned off again before cells enter S phase, so that replicated DNA cannot become inappropriately re-licensed. We have recently uncovered an unexpected role for cyclin-dependent kinases (CDKs) and ubiquitination in the cell cycle-dependent regulation of the licensing system.
The licensing of replication origins depends on the co-ordinated activity of four proteins: the Origin Recognition Complex (ORC), Cdc6, Cdt1 and Mcm2-72-4. These proteins all bind to replication origins early in the cell cycle to form the pre-replicative complex or pre-RC. ORC, Cdc6 and Cdt1 act together to load multiple copies of Mcm2-7 onto DNA. Once Mcm2-7 have been loaded and the origin is functionally licensed, ORC, Cdc6 and Cdt1 are no longer required to maintain Mcm2-7 at the origin. Previous work on the licensing system in cell-free extracts of Xenopus eggs has shown that a small protein called geminin acts as the major inhibitor of replication licensing late in the cell cycle5,6. Geminin binds and inhibits the Cdt1 component of the licensing system5,7,8. It is therefore of interest to understand how the activity of geminin is regulated.
In a recent paper we have investigated how geminin is inactivated on exit from metaphase in cell-free extracts of Xenopus eggs9. During metaphase, Cdt1 activity is suppressed by being bound by geminin, whilst at the same time mitotic CDK activity (mainly Cdk1-cyclin B) lowers the affinity of ORC and Cdc6 for origin DNA (Figure 1, “Metaphase”). The inhibition of licensing by mitotic CDKs is consistent with a large body of data in yeasts showing that replication licensing is inhibited by CDK activity from late G1 thorough to the end of mitosis2,3. In the light of the paradigm that CDKs inhibit replication licensing, there were some surprising suggestions that Cdk1 (Cdc2), the major mitotic cyclin-dependent kinase, is required for activation of the licensing system on exit from metaphase in Xenopus10,11. We therefore performed experiments which showed that when Cdk1 activity is inhibited prior to metaphase exit, geminin fails to be inactivated and instead remains tightly bound to Cdt19. As a consequence, the licensing system remains inhibited and no DNA replication can take place. This requirement for Cdk1 to activate the licensing system explains a previously controversial observation that immunodepletion of Cdk1 (Cdc2) from metaphase extracts blocks subsequent DNA replication12.
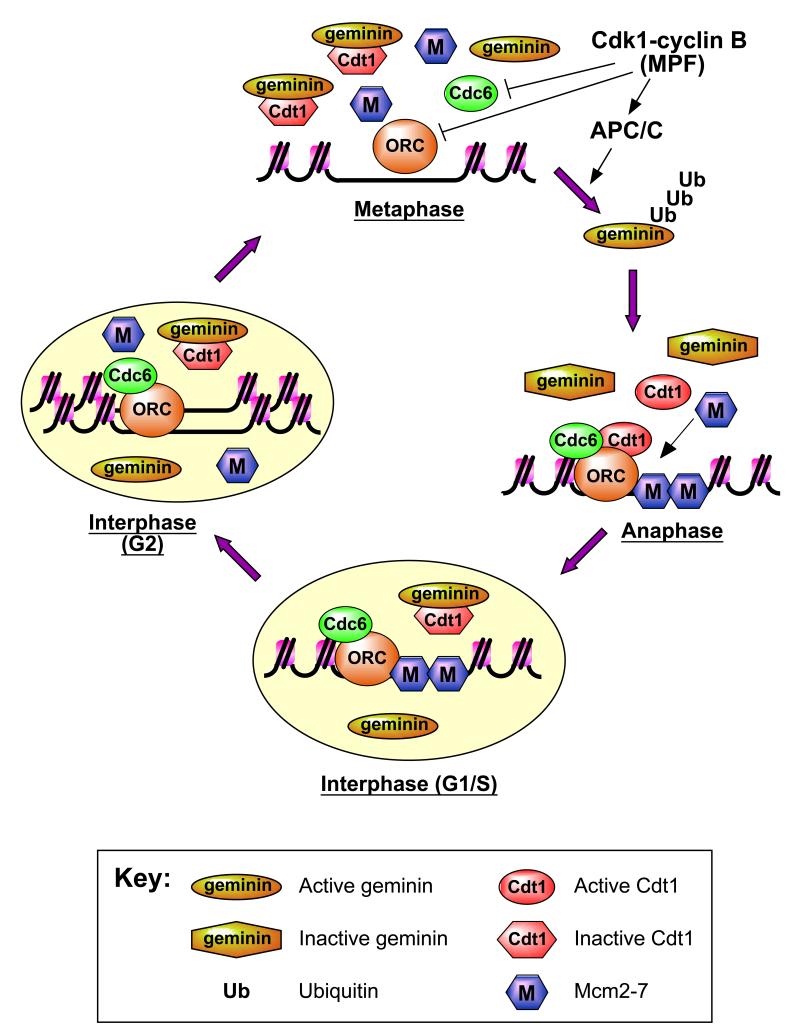
Figure 1.
A small region of chromatin surrounding a single replication origin is shown. During metaphase, geminin is active and binds Cdt1, keeping it inactive. There is an excess of geminin over Cdt1, ensuring Cdt1 inhibition is complete. Geminin in combination with Cdk1 (which reduces the affinity of ORC and Cdc6 for the origin) keeps the licensing system securely repressed. On exit from metaphase into anaphase, the APC/C becomes activated, a process which also depends on Cdk1 activity. The APC/C in turn polyubiquitinates geminin. A significant proportion of the geminin is deubiquitinated without being degraded, leaving it unable to inhibit Cdt1. The subsequent loss of Cdk1 activity during anaphase (normally due to cyclin B ubiquitination and degradation) also increases the affinity of ORC and Cdc6 for the origin. Thus the licensing system becomes rapidly activated on exit from metaphase and Mcm2-7 is loaded onto origins. On exit from anaphase into early interphase (G1/S), chromosomal DNA is assembled into an interphase nucleus. Geminin is imported into the nucleus and subsequently undergoes reactivation. Therefore no further licensing can take place within the intact nucleus. During S phase, initiation takes place at licensed origins, resulting in displacement of Mcm2-7 from origins. Because of the presence of active geminin and the lack of Cdt1 activity, replicated origins cannot be re-licensed and so DNA is not re-replicated.
Cdk1 activity is also known to be necessary (though not sufficient) for activation of the Anaphase Promoting Complex (APC/C) on exit from metaphase13. The APC/C is an E3 ubiquitin ligase that polyubiquitinates specific proteins such as cyclin B to initiate anaphase and mitotic exit14,15. Since the Cdk1-dependent inactivation of geminin only occurs on exit from metaphase, we asked whether APC/C activity is required for geminin inactivation. Using a competitive inhibitor of the APC/C and a mutant form of ubiquitin unable to form polyubiquitin chains, we showed that the APC/C was indeed required for geminin inactivation. Thus the requirement for Cdk1 in geminin inactivation can be explained by a requirement for APC/C-dependent polyubiquitination in this process.
What protein(s) need to be ubiquitinated by the APC/C in order for geminin to be inactivated? Since geminin was originally cloned in a screen for proteins ubiquitinated by the APC/C7, it was an obvious candidate to try. We showed that a mutant form of geminin which is not recognized by the APC/C does not become inactivated on mitotic exit. Whilst most proteins ubiquitinated by the APC/C are subsequently degraded by the 26S proteasome, a large proportion of geminin escapes degradation. However, we showed that the polyubiquitinated geminin is rapidly de-ubiquitinated by isopeptidases, leaving it inactive and unable to bind Cdt1 (Figure 1, “Anaphase”). Using a variety of proteasome inhibitors, we found that proteasome-mediated proteolysis was not required for geminin inactivation. Consistent with other reports16,17 we found that, surprisingly, proteasome-mediated proteolysis was required neither for mitotic exit nor for progression through S phase. In the absence of proteasome-mediated proteolysis, mitotic Cdk1 kinase activity apparently becomes down-regulated by other mechanisms16.
These results show that geminin joins an expanding collection of proteins whose activity is known to be regulated by ubiquitination independently of proteolysis18,19. In the case of geminin, polyubiquitination is only transient, but leaves the protein inactive. How this occurs is currently unclear. One possibility is that ubiquitination could permit a second inactivating modification such as phosphorylation, which is then retained when the ubiquitin is subsequently removed. 2-dimensional gels indeed show many different geminin isoforms, though none seem to correlate precisely with activity (AL and JJB, unpublished observations). An alternative possibility is that ubiquitination could force geminin into a relatively stable inactive conformation. A related question is why polyubiquitinated geminin escapes proteasome-mediated degradation. One possibility is that geminin bound to Cdt1 is specifically refractory to proteolysis; if this were the case, it would provide a simple mechanism for precisely balancing the levels of Cdt1 and geminin.
Before S phase begins, the licensing system must be turned off again in order to prevent replicated DNA from being re-licensed for a second round of replication. We have previously shown that by the beginning of S phase in the Xenopus system, geminin has become reactivated to be the major nucleoplasmic inhibitor of licensing6. Geminin is rapidly imported into interphase nuclei and the reactivation of geminin is dependent on its nuclear import6 (Figure 1, “Interphase G1/S”). Unlike the inactivation of geminin during anaphase, the reactivation of geminin following nuclear import does not require the action of CDKs. Since in the Xenopus system the initiation of replication at licensed origins depends on nuclear assembly20, the import-dependent reactivation of geminin provides an elegant way of ensuring precise chromosome replication in the short embryonic cell cycles where extensive proteolysis might be wasteful. The original experiments demonstrating the existence of a replication licensing factor showed that replicated G2 nuclei must be permeabilized prior to re-incubation in Xenopus egg extract in order for the DNA to undergo a further round of replication1,21. We can now explain these results by noting that geminin would be rapidly imported into intact nuclei and reactivated when G2 nuclei are re-incubated in Xenopus egg extract; if, however, the nuclei were permeabilized prior to re-incubation, geminin reactivation would only occur following nuclear envelope repair, thus providing time for the DNA to become re-licensed. Although our new results9 have therefore potentially provided answers to some old questions, new questions have arisen. Reactivation of geminin following nuclear import presumably involves reversal of the inactivating modification that occurs on metaphase exit. Understanding the precise nature of this change in activity is now a major challenge.
Acknowledgements
The work described here was supported by Cancer Research UK grants SP2385/0101 and C303/A3135.
References
- 1.Blow JJ, Laskey RA. A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature. 1988;332:546–548. doi: 10.1038/332546a0. [DOI] [PubMed] [Google Scholar]
- 2.Blow JJ, Hodgson B. Replication licensing - defining the proliferative state? Trends Cell Biol. 2002;12:72–78. doi: 10.1016/s0962-8924(01)02203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishitani H, Lygerou Z. Control of DNA replication licensing in a cell cycle. Genes Cells. 2002;7:523–534. doi: 10.1046/j.1365-2443.2002.00544.x. [DOI] [PubMed] [Google Scholar]
- 4.Gillespie PJ, Li A, Blow JJ. Reconstitution of licensed replication origins on Xenopus sperm nuclei using purified proteins. BMC Biochem. 2001;2:15. doi: 10.1186/1471-2091-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tada S, Li A, Maiorano D, Mechali M, Blow JJ. Repression of origin assembly in metaphase depends on inhibition of RLF-B/Cdt1 by geminin. Nature Cell Biol. 2001;3:107–113. doi: 10.1038/35055000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodgson B, Li A, Tada S, Blow JJ. Geminin becomes activated as an inhibitor of Cdt1/RLF-B following nuclear import. Curr Biol. 2002;12:678–683. doi: 10.1016/s0960-9822(02)00778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGarry TJ, Kirschner MW. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell. 1998;93:1043–1053. doi: 10.1016/s0092-8674(00)81209-x. [DOI] [PubMed] [Google Scholar]
- 8.Wohlschlegel JA, Dwyer BT, Dhar SK, Cvetic C, Walter JC, Dutta A. Inhibition of eukaryotic replication by geminin binding to Cdt1. Science. 2000;290:2309–2312. doi: 10.1126/science.290.5500.2309. [DOI] [PubMed] [Google Scholar]
- 9.Li A, Blow JJ. Non-proteolytic inactivation of geminin requires CDK-dependent ubiquitination. Nature Cell Biol. 2004 doi: 10.1038/ncb1100. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blow JJ. Preventing re-replication of DNA in a single cell cycle: evidence for a replication licensing factor. J Cell Biol. 1993;122:993–1002. doi: 10.1083/jcb.122.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubota Y, Takisawa H. Determination of initiation of DNA replication before and after nuclear formation in Xenopus egg cell free extracts. J Cell Biol. 1993;123:1321–1331. doi: 10.1083/jcb.123.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blow JJ, Nurse P. A cdc2-like protein is involved in the initiation of DNA replication in Xenopus egg extracts. Cell. 1990;62:855–862. doi: 10.1016/0092-8674(90)90261-c. [DOI] [PubMed] [Google Scholar]
- 13.Kraft C, Herzog F, Gieffers C, Mechtler K, Hagting A, Pines J, et al. Mitotic regulation of the human anaphase-promoting complex by phosphorylation. EMBO J. 2003;22:6598–6609. doi: 10.1093/emboj/cdg627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zachariae W, Nasmyth K. Whose end is destruction: cell division and the anaphase-promoting complex. Genes Dev. 1999;13:2039–2058. doi: 10.1101/gad.13.16.2039. [DOI] [PubMed] [Google Scholar]
- 15.Peters JM. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol Cell. 2002;9:931–943. doi: 10.1016/s1097-2765(02)00540-3. [DOI] [PubMed] [Google Scholar]
- 16.Nishiyama A, Tachibana K, Igarashi Y, Yasuda H, Tanahashi N, Tanaka K, et al. A nonproteolytic function of the proteasome is required for the dissociation of Cdc2 and cyclin B at the end of M phase. Genes Dev. 2000;14:2344–2357. doi: 10.1101/gad.823200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahaffey DT, Gorbea C, Rechsteiner M. Evidence that DNA replication is not regulated by ubiquitin-dependent proteolysis in Xenopus egg extract. Exp Cell Res. 2003;288:225–234. doi: 10.1016/s0014-4827(03)00088-0. [DOI] [PubMed] [Google Scholar]
- 18.Bach I, Ostendorff HP. Orchestrating nuclear functions: ubiquitin sets the rhythm. Trends Biochem Sci. 2003;28:189–195. doi: 10.1016/S0968-0004(03)00055-0. [DOI] [PubMed] [Google Scholar]
- 19.Schnell JD, Hicke L. Non-traditional functions of ubiquitin and ubiquitin-binding proteins. J Biol Chem. 2003;278:35857–35860. doi: 10.1074/jbc.R300018200. [DOI] [PubMed] [Google Scholar]
- 20.Blow JJ. Control of chromosomal DNA replication in the early Xenopus embryo. EMBO J. 2001;20:3293–3297. doi: 10.1093/emboj/20.13.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coverley D, Downes CS, Romanowski P, Laskey RA. Reversible effects of nuclear membrane permeabilization on DNA replication: evidence for a positive licensing factor. J Cell Biol. 1993;122:985–992. doi: 10.1083/jcb.122.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]