Abstract
Background:
Massive ischemic cerebellar infarct (MICI) is a main source of stroke, which can lead to severe morbidity and mortality. There is no consensus in medical literature for the management of MICI. The choice is made between placing an external ventricular drainage, suboccipital decompressive craniectomy, and removal of necrotic tissue or conservative treatment. There are not many prospective studies, done on this subject.
Methods:
We retrospectively analyzed the clinical features, and imaging studies of 53 patients with MICI who had been treated by surgery or conservative treatment between January 2000 and December 2008 at the Department of Neurosurgery of the general hospital of Fort de France in Martinique. A total of 25 patients underwent surgery and 28 were treated medically.
Results:
The results show significantly better outcomes in the operated patients compared with the patients treated medically; Operated comatose patients demonstrated significant improvement in their Glasgow coma score (GCS) score with only two deaths. Whereas, nonoperated comatose patients lost points in their GCS with four deaths.
Conclusion:
The results of our study suggest that surgery may be an effective procedure and quite helpful for MICI in majority of cases.
Keywords: Cerebellar infarct, external ventricular derivation, neurovascular, suboccipital craniotomy
INTRODUCTION
Cerebellar infarction was described at the beginning of the 20th century but the surgical treatment was introduced much more later. It constitutes between 1% and 9-10.5% of cases in the series of patients with cerebral infarction.[1–7] It manifests mainly with headache, vertigo, nausea, vomiting, ataxia, gait disturbance, drowsiness, and dysarthria. The diagnosis is suspected when the signs and symptoms are present and it is confirmed by the imaging results (scanner and magnetic resonance imaging [MRI]). However, there is no consensus in medical literature about the management of massive ischemic cerebellar infarction (MICI). Thus the type and timing of the surgery and the criteria of selection are widely disputed. The choice can be made between ventriculostomy (external ventricular drainage; EVD), suboccipital decompressive craniectomy (SODC) and conservative treatment. The surgical treatment is decided when there are threats of herniation or brain stem compression. The purpose of our study is to verify the efficiency of surgery in short and medium term in MICI.
MATERIALS AND METHODS
The study was performed at the Department of Neurosurgery of the general hospital of Fort de France in Martinique (French West Indies). This regional unit provides neurosurgical service for a population of 600,000 (400,000 in Martinique and 200,000 in French Guyana). We retrospectively reviewed the medical charts of patients treated in our department during the 8-year period between January 2000 and December 2008 and included the patients who had been treated for a cerebellar infarct. We excluded the patients with the infarct involving the brain stem and secondary lesions due to tumor, trauma, or other major diseases. In each patient, case notes were reviewed to record history and clinical examinations and radiological findings. Statistical analysis was performed by EPI/INFO (version 6.04d ‘Epi Info) and MS Excel.
Baseline charactereistics of patients
A total of 53 patients were identified (32 male and 21 female). They had an average age of 58.73 years. Neurological signs and symptoms were: Gait disturbances, vertigo, nausea, vomiting, headache, drowsiness, dysarthria, neck pain, sensory disturbances, double vision, lower cranial involvement, 6th palsy, hemiparesis, gaze palsy, and extensor plantar response [Table 1 and Figure 1].
Table 1.
Signs and symptoms of patients
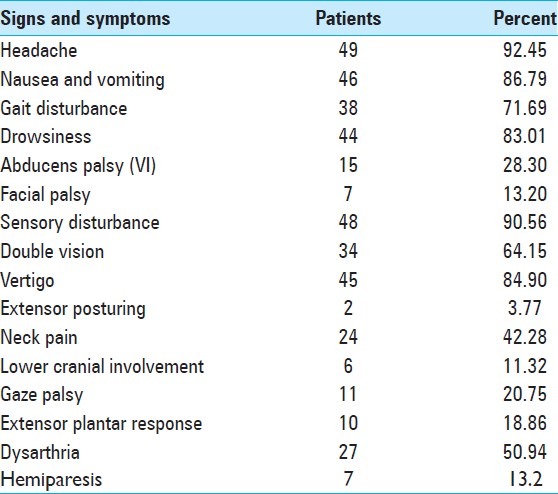
Figure 1.
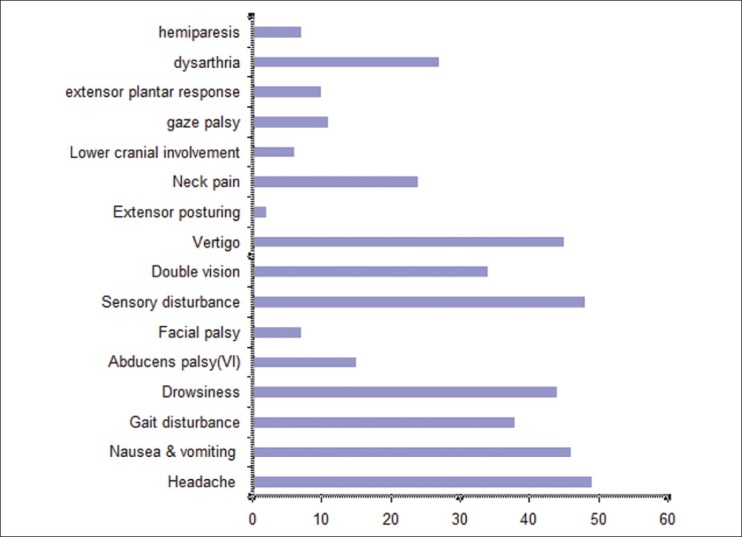
Signs and symptoms
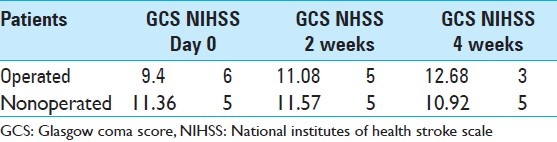
We reviewed the medical charts of patients during the 8-year period from January 2000 to December 2008. According to the protocol of our department, all the patients benefited from neurological supervision every 4 hours. We chose the Glasgow coma score (GCS) and the clinical examination and NIHSS as criteria of comparison for our study. Thus we compared these criteria at admission, at the 2-week mark, and again after 4 weeks [Table 2].
Table 2.
Glasgow coma score score and NIHSS (median) at admission, 2 and 4 weeks later
Radiological finding
All patients had an imaging evaluation. The computed tomography (CT) scan was performed in 49 cases. The MRI was performed in 25 cases. All patients had, after the surgery, a CT scan control at the 2-days mark, then again 1 week after. They also underwent MR imaging 1 month later. CT scan and MRI of patients were reviewed by the principal author to assess the size of lesion or presence of acute hydrocephalus and brain stem compression. We defined MICI as ischemic volume above 5 cm3 or/and when there was hydrocephalus or brain stem compression. Ischemic volume was measured by the workstation software used in department of Radiology at our facilities. Therapeutic decisions were taken by on-call neurosurgeon or in a clinical meeting of our department for each individual patient. A total of 28 patients were treated medically. A total of 25 patients underwent surgery, in which 22 patients underwent surgery immediately and 3 patients underwent surgery a second time after clinical deterioration. The surgical treatment consisted of a posterior fossa craniotomy (SODC) and/or a ventriculostomy (EVD). The medical treatment consisted of parenteral nutrition, symptomatic treatment including high blood pressure treatment, antiemetic treatment and if necessary the corticosteroid therapy, Mannitol therapy to preserve good hemodynamic and ventilation outcome.
RESULTS
A total of 15 patients were admitted with a GCS ≤7. Three criteria's were employed for reference to surgery: 1 – consciousness deterioration at admission (GCS) 2 – secondary deterioration of consciousness during hospitalization. 3 – infarct volume. A total of 22 patients underwent surgery as a matter of urgency. Two patients were operated on the day after admission because they had neurological deterioration and altered consciousness; and one patient underwent surgery within 2 days after admission for persistence of altered awareness and of neurological symptoms and ischemic infarct volume. The average age of operated patients was 59.67 years and for medically treated patients was 62.44 years [Table 3].
Table 3.
Average age of patients

The average age of operated patients is 2.77 years less than the patients who were medically treated. Tables 4 and 5 show number and proportion of patients according to age brackets. The majority of patients were aged between 50-60 and 60-70 years. These age groups constituted 62. 25% of all patients.
Table 4.
Number of patients according to age range
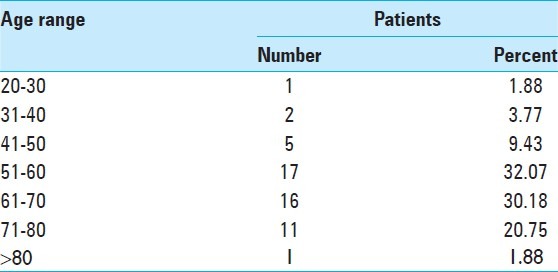
Table 5.
Proportion of operated and nonoperated patients according to age range
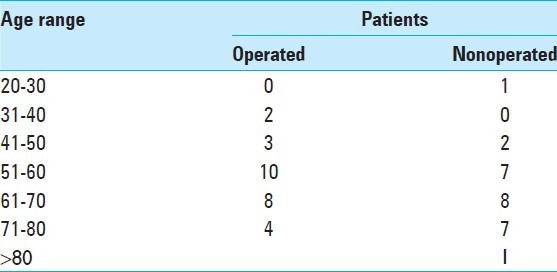
The patients with MICI less than 20 years and more than 80 years were extremely rare. The mean GCS in operated cases at admission was 9.4 and in medically treated patients was 11.36 [Table 2]. GCS and NIHSS of patients at admission and again at 2 and 4 weeks later were compared. This comparison shows a better result for the operated patients [Table 2]. We found an improvement of GCS and NIHSS after surgery. Operated patients obtained a mean increase of 1.58 points in GCS. Medically treated patients obtained a mean increase of 0.21 two weeks after. A great improvement was observed for the operated patients (3.28 points) after 4 weeks, whereas for medically treated patients, we found clinical deterioration (on average, patients lost 0.44 points in 4 weeks). However, they had obtained a small mean increase of 0.21 points 2 week after admission [Table 2]. Table 6 shows operated patients with their different types of surgery, their GCS on admission, and 4 weeks later.
Table 6.
Types of surgery and Glasgow coma score score in admission and 4 weeks later
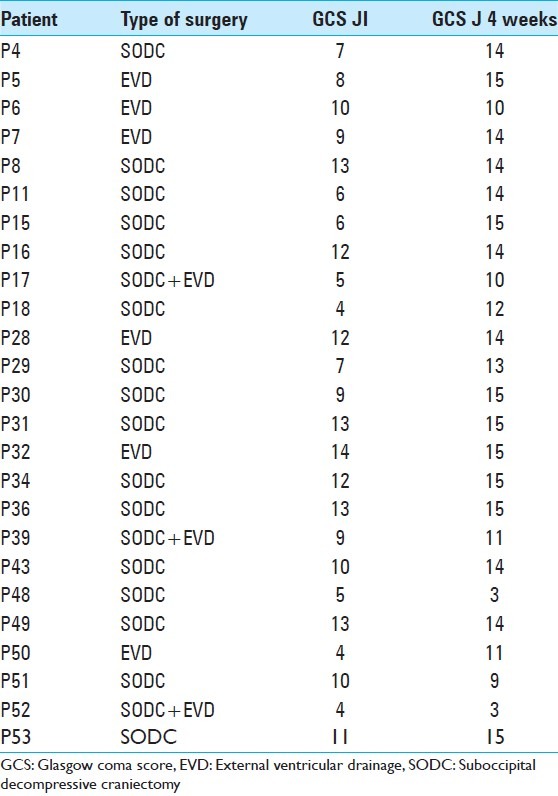
A total of 16 patients underwent posterior fossa craniectomy (SODC). Only six patients were operated by EVD and three patients underwent SODC and EVD at the same time. Table 8 shows the operated patients with their average GCS score at admission and 4 weeks after surgery.
Table 8.
Average GCS score of comatose patients at admission and in the 4th weeks
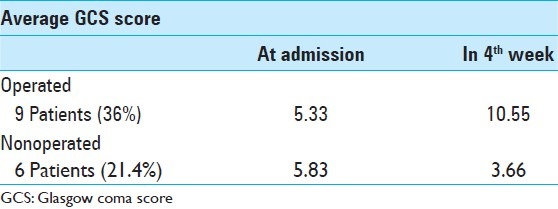
Study results show that nine patients were in a coma (GCS ≤7) for the operated group versus six patients for the medically treated group. Among the six medically treated patients, four died and two did not have improvement in GCS at the end of 4 weeks. Whereas for nine operated patients, we found two deaths and seven odemonstrated improvement in GCS score to ≥10. Therefore we think that early surgery is a good choice for MICI. However, considering the retrospective nature of study and the small number of patients, results must be interpreted cautiously [Table 7]. In view of the results, there was no significant difference between SODC and EVD [Tables 8 and 9].
Table 7.
Average Glasgow coma score score in operated patients at admission and 4 weeks later
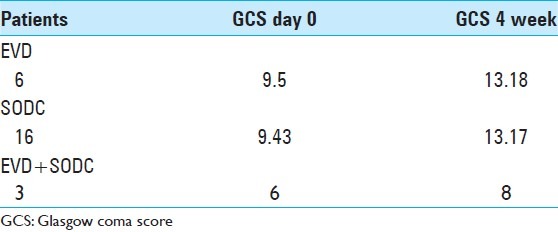
Table 9.
Average Glasgow coma score score of noncomatose patients at admission and in the 4th week
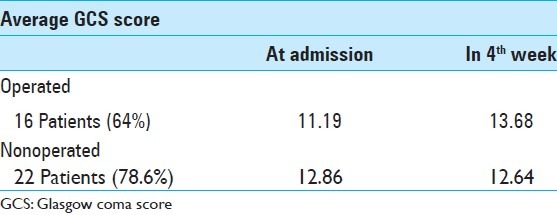
In spite of many studies a low GCS score was not a pejorative factor for outcome.[8,12–20] The 16 operated patients (64%) did not have any disturbance of consciousness in their 4th week (GCS 14 or 15). Whereas 13 nonoperated patients had a GCS score of 14-15 (46.42%) [Table 2]. Operated patients with GCS below 8 showed significant improvement in their GCS score (5 points on average). We had only two deaths. Whereas nonoperated comatose patients lost 2 point in their GCS with four deaths.
DISCUSSION
Besides direct signs of cerebellar infarct, cerebellum tissue affected by ischemic necrosis usually undergoes oedematous swelling. If it is voluminous enough, it provokes a mass effect on brain stem, fourth ventricle, cerebral aqueduct, and cisterna magna. This mass effect can potentially generate fatal complications including obstructive hydrocephalous and brain stem compression. In order to avoid complications, a rapid diagnosis and treatment planning is necessary. There is no specific data resulting from randomized trials in treatment of cerebellar infarct. Literature review gives different and at times contradictory results. In a series of 56 patients, Jüttler, et al.[9] reported that there were no significant differences in survival between space-occupying cerebellar infarct treated by EVD and SODC. Whereas in a series of 25 patients subdivided in two groups with EVD and with SODC, Kudo, et al.[10] demonstrate a significantly better prognosis in group of patients treated by SODC.
One of the largest studies of cerebellar infarct is the German–Austrian infarction study,[7] which includes 84 patients. In this series with 34 craniotomies, 14 ventriculostomies and 36 patients were medically treated, surgical treatment for MICI is not found to be superior to medical treatment in awake/drowsy or somnolent/stupor patients. In the other grand series with 71 patients with an infarct in posterior fossa, Mathew, et al.[11] found out that most patients were managed successfully conservatively. It is necessary to note that in this series that there is no difference between MICI and other minor types of cerebellar infarct. In their series, Ito, et al.[7] recommend SODC in comatose patients only in cases where ICP cannot be controlled by EVD. In the series of 11 patients, Chen, et al.[4] found neurological improvement in 7 patients after SODC. The reason of this controversy in literature is lack of group control and absence of well-defined criterion as well as lack of randomized trials. According to our result, surgery shows a good result in MICI. In our patients postoperative GCS scores (4 weeks after) were higher in surgically treated comatose patients. While nonoperated comatose patients lost 2 points in their GCS. Operated noncomatose patients gained 2.5 points in the postoperative period. However, no significant difference was found in GCS scoring in the preoperative and postoperative period in nonoperated noncomatose patients. In majority of cases, surgical decisions were made as a matter of urgency. A low GCS score was not a pejorative factor for outcome and therefore we believe that early surgery is a good choice for MICI. Surgery seems to be more effective in younger patients especially because they have shorter recovery period. It should be noted that given the retrospective nature of our study and the small number of patients, results must be interpreted cautiously.
Surgical treatment (SODC or EVD) should be considered based on timely identification of patients who will benefit from this intervention.
CONCLUSION
This study performed in our department with 53 surgically versus medically treated patients. The results corroborate that surgery can be quite helpful in majority of cases in MICI. Surgery seems to be more effective in younger patients. They actually had shorter recovery period. In spite of certain studies to have a GCS score lower than 7, it was not a pejorative factor for the outcome. We think that an early surgery is necessary to avoid a neurological deterioration in the great majority of cases.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2013/4/1/28/107906
REFERENCES
- 1.Amarenco P, Lévy C, Cohen A, Touboul PJ, Roullet E, Bousser MG. Causes and mechanism or territorial and nonterritorial cerebellar infarcts in 115 consecutive patients. Stroke. 1994;25:105–12. doi: 10.1161/01.str.25.1.105. [DOI] [PubMed] [Google Scholar]
- 2.Auer LM, Auer T, Sayama I. Indication for surgical treatment of cerebellar haemorrhage and infarction. Acta Neurochir (Wien) 1986;79:74–9. doi: 10.1007/BF01407448. [DOI] [PubMed] [Google Scholar]
- 3.Caplan LR. Size and flow matter in the posterior circulation. J Neurol Neurosurg Psychiatry. 2009;80:1057. doi: 10.1136/jnnp.2009.174482. [DOI] [PubMed] [Google Scholar]
- 4.Chen HJ, Lee TC, Wei CP. Treatment of cerebellar infarction by decompression suboccipital craniectomy. Stroke. 1992;23:957–61. doi: 10.1161/01.str.23.7.957. [DOI] [PubMed] [Google Scholar]
- 5.Coltamai L, Maeder P, Croquelois A. Atypical blood supply to the cerebellar hemispheres by isolated superior cerebellar arteries. Eur Neurol. 2010;63:127–8. doi: 10.1159/000277612. [DOI] [PubMed] [Google Scholar]
- 6.Eldow JA, Newman-Toker DE, Savits SI. Diagnosis and initial management of cerebellar infarction. Lancet Neurol. 2008;7:951–64. doi: 10.1016/S1474-4422(08)70216-3. [DOI] [PubMed] [Google Scholar]
- 7.Ito M, Sonokawa T, Mishina H, Hishii M, Sato K. Surgical management of comatose patients with cerebellar infarction. J Clin Neursci. 1994;1:251–6. doi: 10.1016/0967-5868(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 8.Ivamoto HS, Numoto M, Donaghy RM. Surgical decompression for cerebral and cerebellar infarcts. Stroke. 1974;5:365–70. doi: 10.1161/01.str.5.3.365. [DOI] [PubMed] [Google Scholar]
- 9.Jauss M, Krieger D, Hornig C, Schramm J, Busse O. Surgical and medical management of patients with massive cerebellar infarction: Results of the German-Austrian cerebellar infarction study. J Neurol. 1999;246:257–64. doi: 10.1007/s004150050344. [DOI] [PubMed] [Google Scholar]
- 10.Jensen MB, St Louis EK. Management of acute cerebellar stroke. Arch Neurol. 2005;62:537–544. doi: 10.1001/archneur.62.4.537. [DOI] [PubMed] [Google Scholar]
- 11.Jüttler E, Schweickert S, Ringleb PA, Huttner HB, Köhrmann M, Aschoff A, et al. Long-term outcome after surgical treatment for space-occupying cerebellar infarction: Experience in 56 patients. Stroke. 2009;40:30606–6. doi: 10.1161/STROKEAHA.109.550913. [DOI] [PubMed] [Google Scholar]
- 12.Kudo H, Kawaguchi T, Minami H, Kuwamura K, Miyata M, Kohmura E. Controvery of surgical treatment for severe cerebellar infarction. J Stroke Cerebrovasc Dis. 2007;16:259–62. doi: 10.1016/j.jstrokecerebrovasdis.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Mathew P, Teasdale B, Oluoch-olunya D. Neurological management of cerebellar haematoma and infarct. J Neuorol Neurosurg Psychiatry. 1995;59:287–92. doi: 10.1136/jnnp.59.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathew S, Pandian JD. Stroke in patients with dengue. J Stroke Cerebrovasc Dis. 2010;19:253–6. doi: 10.1016/j.jstrokecerebrovasdis.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Kahn M, Polyzoldis KS. Massive cerebellar infarction. Stroke. 1983;14:745–51. doi: 10.1161/01.str.14.5.745. [DOI] [PubMed] [Google Scholar]
- 16.Pfefferkorn T, Eppinger U, Linn J, Birnbaum T, Herzog J, Straube A, et al. Long term outcome after suboccipital decompressive craniectomy for malignant cerebellar infarction. Stroke. 2009;40:3045–50. doi: 10.1161/STROKEAHA.109.550871. [DOI] [PubMed] [Google Scholar]
- 17.Rieke K, Krieger D, Adams HP, Aschoff A, Meyding-Lamadé U, Hacke W. Therapeutic strategies in space occupying cerebellar infarction based on clinical, neuroradiological and neurophysiological data. Cerebrovasc Dis. 1991;3:45–55. [Google Scholar]
- 18.Schmahmann JD, Macmore J, Vangel M. Cerebellar stroke without motor deficit: Clinical evidence for motor and non-motor domains within the human cerebellum. Neuroscience. 2009;162:852–61. doi: 10.1016/j.neuroscience.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsitsopoulos PP, Tobieson L, Enblad P, Marklund N. Clinical outcome following surgical treatment for bilateral cerebellar infarction. Acta Neurol Scand. 2011;123:345–51. doi: 10.1111/j.1600-0404.2010.01404.x. [DOI] [PubMed] [Google Scholar]
- 20.Weisberg LA. Acute cerebellar hemorrhage and CT evidence of tight posterior fossa. Neurology. 1986;36:858–60. doi: 10.1212/wnl.36.6.858. [DOI] [PubMed] [Google Scholar]


