Abstract
Objective The aim of this study was to determine whether young boys with fragile X syndrome (FXS) exhibit abnormal physiological or behavioral responses to a moderately intense auditory stimulus, as heightened sensory reactivity is believed to contribute to problem behaviors in this population. Methods We examined the physiological basis, via heart activity, of auditory startle in young boys with FXS (n = 22) compared with typically developing controls (n = 27). Associations with mental age, behavioral reactivity, and chronological age were examined. Results Results suggest that older boys with FXS display increased cardiac reactivity to auditory input than younger boys with FXS that distinguishes them from typically developing controls. Higher mental age was associated with decreased latency to react. Conclusions Results contribute to increased understanding of the pathology in sensory processing in boys with FXS, which can inform refinement of the phenotype in young children with FXS and aid in the development of efficacious psychopharmacological and/or behavioral interventions.
Keywords: auditory startle, development, fragile X syndrome, sensory reactivity
Introduction
Fragile X syndrome (FXS) is the leading known genetic cause of intellectual disability, with an estimated prevalence of 1 in 3,600 individuals (Hagerman et al., 2009). Hypermethylation of the promoter region of the FMR1 gene results in a reduction or absence of fragile X mental retardation protein (FMRP) that is associated with the clinical features of the syndrome. Physical features are mild and include a long narrow face, protruding ears, and macroorchidism in postpubertal boys (Hagerman, 1999). At present, there is no cure for FXS, and treatment efforts focus on early identification and remediation of developmental deficits.
Although the majority of males with FXS have intellectual disabilities, problem behaviors most concern parents and clinicians. Individuals with FXS exhibit hyperactivity, inattentiveness, anxiety, perseverative language, social avoidance, self-injury, and aggression (Hatton et al., 2002; Roberts, Boccia, Bailey, Hatton, & Skinner, 2001; Sullivan et al., 2006; Symons, Clark, Hatton, Skinner, & Bailey, 2003). These problem behaviors interfere with learning and social outcomes (Hatton et al., 2002, 2006; Roberts, Mazzocco, Murphy, & Hoehn-Saric, 2008) and can negatively affect their family’s emotional well-being (Roberts et al., 2008) and quality of life (Wheeler, Skinner, & Bailey, 2008). One of the most common comorbid conditions in FXS is autism, with 25–52% of children with FXS meeting DSM-based diagnostic criteria (Clifford et al., 2007; Garon et al., 2009; Hall, Lightbody, & Reiss, 2008), and up to 90% displaying at least one autistic symptom (Hagerman, 2002). Understanding the underlying features of FXS is imperative for treatment initiatives and for understanding fundamental early developmental considerations.
Although the underlying mechanisms associated with problem behaviors in FXS are not yet fully understood, heightened sensory reactivity secondary to hyperarousal is believed to contribute to the emergence and intensity of problem behaviors observed in boys with FXS (Cohen, 1995; Miller et al., 1999; Roberts et al., 2001). Sensory processing deficits as measured by both parent report and observational measures affect up to 90% of young boys with FXS and reflect both hyporesponsiveness, characterized as diminished reactivity associated with delayed latency of response or lack of orienting, and hyperresponsiveness, characterized as excessive or exaggerated reactivity associated with aversion or avoidance of stimuli (Baranek et al., 2008). Sensory processing deficits are problematic and have been linked to lower levels of school participation, self-care, and play in school-aged boys with FXS (Baranek et al., 2002). Although sensory processing deficits are reported in a number of clinical groups (autism and developmental delay) and are not specific to FXS, their prevalence and pattern appear distinct in FXS. In one of two studies to conduct group comparisons of sensory processing responses of children with FXS (mean age: 31 months), children with FXS and children with autism had more parent-reported sensory symptoms than children with developmental delays of mixed etiology and mental age-matched typical controls; however, children with FXS displayed less energy and were weaker in their motor responses than all three groups (Rogers, Hepburn, & Wehner, 2003). In a study using retrospective video analysis of sensory-motor features, findings reflect that infants with FXS (9–12 months of age) displayed increased posturing, leg stereotypies, and less mature use of objects compared with groups of infants with autism, generalized developmental delay, and chronological age-matched typically developing infants (Baranek et al., 2005).
The underlying mechanisms for sensory processing deficits in FXS are not well understood. Some evidence suggests that low levels of FMRP predict sensory processing abnormalities (Miller et al., 1999); however, this finding has not been replicated in other studies (Baranek et al., 2008). There are also mixed findings for an association between sensory processing variables and mental age or IQ (Baranek et al., 2005, 2008; Rogers et al., 2003). Longitudinal studies of sensory processing changes as a function of chronological age are limited, with one study finding a shift from a predominant hyporesponsive pattern in early infancy to an increasing hyperresponsive pattern in later preschool years (Baranek et al., 2008).
Recently, research has examined the physiological basis for sensory processing deficits in FXS. In general, this work suggests that excessive reactivity, not baseline (Baranek et al., 2008; Hagerman et al., 2002), measures of arousal are related to abnormal sensory responses in FXS. Using auditory event-related brain potentials, elevated N1 amplitudes in the auditory brainstem of four adults with FXS have been reported (Rojas et al., 2001). Similarly, elevated N1 amplitudes, larger N2 global field power, and no habituation of N1 with limited sensitization of N2 for repeated tones have been reported in four children with FXS (Castren, Paakkonen, Tarkka, Ryynanen, & Partanen, 2003). This is consistent with exaggerated electrodermal responses and poor habituation to a series of auditory stimuli reported in 15 individuals with FXS (Miller et al., 1999). Additionally, studies have found that males with FXS have deficits in prepulse inhibition, a marker of sensorimotor gating, and that this deficit was more pronounced in individuals with FXS with and without autism than in individuals with idiopathic autism (Frankland et al., 2004; Yuhas et al., 2011).
Although existing studies provide critical information to better understand the physiological basis of sensory processing difficulties in FXS, this work is limited in several ways. First, the samples in these studies are small and age restricted. Second, these physiological studies failed to examine predictors of physiological responses, including FMRP and developmental variables such as chronological age and mental age, as has been done in behavioral work (Baranek et al., 2008). Our study was designed to investigate the physiological basis of auditory startle in young boys with FXS using heart activity. We selected heart activity as the primary biomarker of interest given that it reflects both sympathetic and parasympathetic nervous system contributions, captures responses in real time, and is tolerable to most young children with FXS. The focus on the auditory system was because of evidence that boys with FXS exhibit abnormal responsivity in this system (Miller et al., 1999) and to design constraints that precluded a comprehensive sensory battery, as sensory responsivity was a secondary aim of the primary study. Two research questions guided this work. First, do young boys with FXS exhibit an abnormal physiological response to a moderately intense auditory stimulus? Second, is chronological age, mental age, behavioral reactivity, or FMRP associated with the physiological response to auditory stimuli in young boys with FXS? We hypothesize that boys with FXS will exhibit an abnormal physiological response to the auditory stimulus compared with typically developing controls, and that this response will relate to chronological age, mental age, behavioral reactivity, and FMRP.
Methods
This study used a cross-sectional case control design. Participants were drawn from a larger study examining early development and family adaptation to FXS (see Bailey et al., 1998). Study procedures were approved by the Institutional Review Board at the University of North Carolina at Chapel Hill.
Participants
Two gender and chronological age-matched groups of boys (ages: ∼1–10.5 years) participated in this study. The target group consisted of 23 boys with FXS (M = 4.91 years, standard deviation [SD] ± 2.54) as verified by genetic report, and the control group included 27 typically developing boys (M = 4.62 years, SD ± 2.27). Only boys participated in this study because boys are more severely affected with less heterogeneity (Hagerman, 1997). Boys with FXS were diagnosed with the full mutation through cytogenetic testing and DNA analysis. Typical development was defined as the product of a full-term gestation, no documented or suspected disability, and normal hearing and vision per parental report. No participants were taking psychoactive medication or had current cardiovascular disease, and no participants appeared to have trouble hearing the instructions of the examiner. Participants were recruited through several sources, including a national listserv for parents of children with FXS, a database of participants, and web-based advertisements posted on FXS organization web sites. Typically developing boys were recruited from local child care programs and schools, and all lived in a southeastern community where the study took place. Additional details regarding the participants’ characteristics are displayed in Table I.
Table I.
Participant Characteristics
| Demographics | n | M (SD) | Minimum | Maximum |
|---|---|---|---|---|
| Chronological age (years) | ||||
| Fragile X syndrome | 22 | 4.91 (2.54) | 1.30 | 10.30 |
| Typical | 27 | 4.62 (2.27) | 1.11 | 10.60 |
| Mental agea | 22 | 2.83 (1.30) | 1.08 | 5.83 |
| FMRPa | 20 | 7.90 (7.85) | 2.50 | 39.00 |
Note. FMRP = fragile X mental retardation protein; SD = standard deviation.
aFragile X syndrome sample only.
Measures
Heart Activity
Heart activity data were collected at a 1 ms resolution (500 Hz) using the Mini-Logger 2000 (1994) system, a radio telemetry system using Polar chest belts for R-wave detection to identify that interbeat interval (IBI), which is the time interval between heart beats. Heart activity data were edited by hand by research staff who had undergone extensive training (∼40 hr) in recognizing and editing heart activity via the training module of the MxEdit program (1989). Editing files consisted of scanning the data for outliers relative to adjacent data and modifying those points by summing or dividing them to be consistent with the surrounding data. One participant was dropped because of a poor signal. Data were analyzed during baseline and reactivity. Baseline was calculated as the mean IBI during a 5-min phase immediately before the stimulus onset. Participants were seated and watching a video during this time. Reactivity was measured in two ways. First, a change score was calculated as the shortest IBI value within the stimulus phase minus the mean prestimulus baseline value. Second, the latency to the shortest IBI poststimulus was calculated in seconds. Latency to IBI was selected to reflect the efficiency or maturation of the autonomic nervous system, as shorter latency is generally associated with optimal orientation and attention. The shortest IBI was selected to reflect the upper range of responsivity that could be masked by using mean levels. To our knowledge, this is a novel indicator of heart activity; thus, results should be interpreted with caution. We selected heart activity over other measures (such as galvanic skin response) to allow obtainment of both a generalized index of arousal and an index of parasympathetic tone via heart activity.
Leiter-R
The Leiter-R, a nonverbal measure used to assess cognitive function in children and adolescents aged 2–20 years (Roid & Miller, 1997), was individually administered to each child with FXS as part of a larger study. This assessment took place on the same day as the auditory startle experiment. The Leiter-R was not administered to the control group, as the research questions did not address the relationship of mental age to physiological response in this group. The Brief IQ (M = 100, SD ± 15) has reliabilities ranging from .88 to .90 and is highly correlated to the Wechsler Intelligence Scale for Children, 3rd edition (r = .85). The majority (>75%) of participants were at or near the floor for this measure; therefore, the range of standard scores was severely restricted, which significantly impacted our ability to detect relationships among mental age and heart activity in this sample. Thus, a metal age score was calculated as the age equivalent based on raw scores.
Fragile X Mental Retardation Protein
Parental consent was required to draw blood from participants to analyze for percentage of FMRP-positive lymphocytes using immunocytochemistry techniques (Bailey, Hatton, Tassone, Skinner, & Taylor, 2001). Although attempted with all FXS participants, we were successful at obtaining FMRP for 90% (n = 20) of our sample. Because of the lack of variability and small sample size, FMRP was not included in our analyses; however, these values are provided descriptively (Table I).
Behavioral Reactivity and Recovery
Behavioral reactivity and recovery were examined by reviewing the videotaped sessions. Data were collected at 100ths of a second. We examined behavioral reactivity in two ways. First, we rated behavioral reactivity using a rating scale of (1) no behavioral reaction, (2) mild behavior reactivity, (3) moderate behavioral reactivity, and (4) severe behavioral reactivity. See Table II for a specific description of the behavioral variables. Second, we measured behavioral latency to react, which was defined as the time it took for the child to begin to look at the alarm clock or display a startle response. We then examined behavioral recovery by measuring the latency to return to task following the stimulus. Return to task was measured by reviewing the child’s behavior during the minute before the alarm. A child was considered to have returned to task if, for at least 15 s, he watched the video or engaged in a behavior that was observed during the minute before the alarm. A child who did not recover behaviorally within 60 s was given the maximum recovery time score of 60 s. If the examiner did not give the child at least 30 s to recover, he was dropped from the recovery analyses.
Table II.
Descriptions of Behavioral Codes
| Code | Description | Value |
|---|---|---|
| No behavioral reaction | No behavioral reaction | 1 |
| Mild behavioral reaction | The child looks at the alarm or examiner, showing no sign of distress or aversion; this could include a social reference | 2 |
| Moderate behavioral reaction | Child looks at the alarm and may reach out to touch it and/or make a “neutral” comment about it (e.g., “What was that?” “The alarm rang.”). This could also include a disruption in behavioral stream. | 3 |
| Severe behavioral reaction | This includes a display of negative affect or a reaction such as a facial expression that looks “fearful” (e.g., a scowl of the face or eyebrows), and/or a “negative” comment about the alarm (e.g., “That is loud,”), and/or a negative behavioral reaction (e.g., child pushes clock away, covers ears, and backs away), and/or a startle response (e.g., child shows a sudden shift in body position, e.g., a muscle twitch). | 4 |
| Behavioral latency to react | The amount of time it takes the child to respond to the alarm. This can be observed as the child looking at the alarm and/or touching and turning toward it. The time was calculated from the beginning of the alarm to the child’s response. | 0–300 s |
| Behavioral latency to recover | Behavioral observations of orientation back to watching the video or of doing what he was doing before the alarm for a minimum of 15 s. Back on task was observed as not looking or talking about the alarm but looking at the video, playing with a toy, or talking about something other than the alarm. | 0–60 s |
Experimental Procedures
Parents provided written informed consent for their child’s participation in the study. Participants were assessed between 8:00 a.m. and 10:00 a.m. to account for circadian rhythms (Fallen & Kamath, 1995). Sessions were videotaped to synchronize the heart activity with the session phases. The prestimulus baseline phase involved the child watching a nonviolent segment of a child-engaging video (i.e., The Lion King) for 5 min. Both groups watched the same 5-min video. At the end of 5 min, a child-friendly (i.e., Mickey Mouse) alarm clock rang for 5 s, serving as the auditory stimulus. This was an unexpected stimulus. The clock was placed ∼24 inches from the participants. The peak sound pressure level was 79 decibels at 24 inches. Octave band measurements revealed that the greatest spectral concentration was in the high frequency area (8,000 Hz). The video continued playing throughout the auditory stimulus and for 2 min after the stimulus ended. Physiological and behavioral responses to the auditory stimulus were designated on review of the videotaped assessments, which were synchronized with event marks inserted into the heart activity data files.
Results
Descriptive Statistics
Descriptive statistics were calculated for all variables to determine the distribution of values to test those distributions against the assumptions of used analyses and to provide an overview of the primary variables (see Table III).
Table III.
Descriptive Statistics of Physiological Variables
| Physiology | n | M (SD) | Minimum | Maximum |
|---|---|---|---|---|
| IBI baseline | ||||
| Fragile X syndrome | 22 | 537 (86) | 376 | 735 |
| Typically developing | 27 | 574 (72) | 442 | 751 |
| IBI shortest | ||||
| Fragile X syndrome | 22 | 445 (62) | 350 | 577 |
| Typically developing | 27 | 484 (51) | 391 | 574 |
| Latency to shortest IBI (ms) | ||||
| Fragile X syndrome | 22 | 46,638 (33,905) | 4,830 | 103,460 |
| Typically developing | 27 | 56,356 (41,648) | 520 | 178,498 |
Note. IBI = interbeat interval; SD = standard deviation.
Correlates of Physiological Response in FXS
Mental Age
Partial correlation coefficients were computed among mental age and change in IBI, and mental age and latency to shortest IBI while holding chronological age constant. The partial correlations revealed no significant relationship between mental age and change in IBI in boys with FXS (r = .21, p = .36); however, there was a moderate relationship between mental age and latency to shortest IBI in boys with FXS (r = .51, p = .02) indicating that as mental age increased the latency to react increased.
Group Differences in Physiological Response
Linear regression models were conducted to investigate the presence of group differences in physiological response to an auditory stimulus. First, differences were examined in the magnitude of change between mean baseline IBI and shortest IBI for boys with FXS versus typically developing boys. Second, differences were examined in the latency to shortest IBI from stimulus onset. In addition to the grouping variable, child chronological age and an age by group interaction were included in each regression model to assess whether the association between group and either outcome variable (change in IBI and latency to shortest IBI) was dependent on child chronological age.
Change in IBI
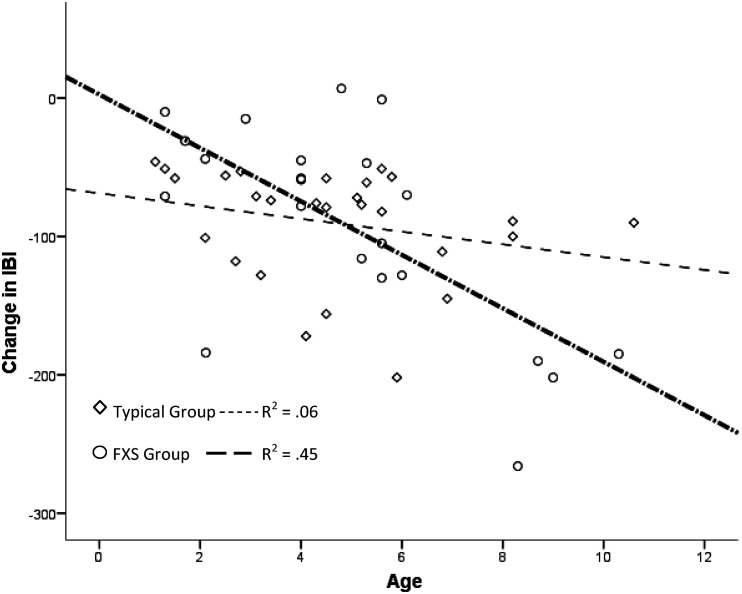
No significant difference between boys with FXS and typically developing boys was revealed for change in IBI [F(1, 47) = .015, p = .90]; however, a significant interaction of chronological age by group on change in IBI was found [F(3, 45) = 7.55, p < .01]. An adjusted R-squared indicated that ∼29% of the variability in the change in IBI value is accounted for by the interaction between chronological age and group membership. An examination of the effect of group on age revealed that only the FXS group showed an increased cardiac response (greater change/reactivity) to the auditory startle as age increased accounting for a large amount of variability for the FXS group (R2 = .43). The typically developing children showed a pattern of dampening cardiac response; however, that effect was not significant and accounted for little of the variance (R2 = .06). These analyses suggest group differences with typically developing boys displaying little change in cardiac responsivity across ages, whereas boys with FXS display increased cardiac reactivity across age. See Figure 1.
Figure 1.
Interaction of chronological age by group membership on change in IBI. IBI = interbeat interval.
Latency to Shortest IBI
No significant difference between boys with FXS and typically developing boys was revealed in the latency to the shortest IBI value from stimulus onset [F(1, 47) = .777, p = .38, R2 = .016]. In addition, no significant interaction of chronological age by group on latency to shortest IBI was found [F(3, 45) = .768, p = .52, R2 = .049].
Group Difference in Behavioral Response
The behavioral reactivity scales of the two groups were compared using a Wilcoxon Signed-ranks test. No significant differences between groups were found on behavioral reactivity (T = −0.25, p > .05). Paired samples t-tests were used to compare groups on latency to react and latency to recover. No significant differences were found for the latency to react [t(25) = −2.98, p > .05] or latency to recover [t(21) = −0.10, p > .05].
Behavior
Pearson correlations were computed among the three behavioral variables (behavioral reactivity, latency to reactivity, and latency to recover) and change in IBI and latency to shortest IBI. The Pearson correlations revealed no significant relationship between any of the behavior variables and the physiological variables for either group. See Table IV for a summary of these results.
Table IV.
Correlations Between Behavioral and Physiological Measures
| Typical |
FXS |
Combined |
||||
|---|---|---|---|---|---|---|
| Behavioral Measure | IBIa | Change IBI | IBIa | Change IBI | IBIa | Change IBI |
| Reactivity scale | ||||||
| r | −.08 | .02 | −.08 | .19 | −.11 | .14 |
| p | .68 | .92 | .72 | .38 | .47 | .36 |
| n | 27 | 27 | 23 | 23 | 49 | 49 |
| Latency to react | ||||||
| r | .14 | −.19 | .01 | −.30 | .06 | −.26 |
| p | .49 | .35 | .96 | .16 | .67 | .08 |
| n | 26 | 26 | 23 | 23 | 49 | 49 |
| Latency to recover | ||||||
| r | −.35 | .25 | .05 | .19 | −.09 | .18 |
| p | .10 | .25 | .84 | .40 | .57 | .23 |
| n | 24 | 24 | 22 | 22 | 46 | 46 |
Note. FXS = fragile X syndrome; IBI = interbeat interval.
aLatency to shortest IBI.
Discussion
Although individuals with FXS are noted to have problem behaviors associated with sensory processing deficits, few studies have examined the underlying physiological and behavioral mechanisms in young children. In the present study, we investigated cardiac and behavioral responsivity to auditory input in young boys with FXS and a chronological age-matched typically developing control group. Age-related changes have long been documented in FXS but have primarily focused on the well-studied decline in IQ and linear effects (e.g., increase in social anxiety with age), with few studies adopting a developmental perspective looking at how age effects may differ cross-sectionally across ages (Bailey et al., 1998; Roberts, Weisenfeld, Hatton, Heath, & Kaufmann, 2007). Recent studies have emerged suggesting a nonlinear age effect on the sensory responses and negative affect in young boys with FXS using behavioral measures (Baranek et al., 2008; Shanahan et al., 2008). More recently, Heilman, Harden, Zageris, Berry-Kravis, and Porges (2011) confirmed that males with FXS display an atypical autonomic profile exhibiting a faster baseline heart rate and decreased respiratory sinus arrhythmia compared with typically developing males.
The primary finding of the current study is that young boys with FXS exhibit unique cardiac reactivity profiles across age that differentiate them from typically developing boys. Boys with FXS display increased cardiac reactivity associated with increasing age, whereas typically developing boys display little change in cardiac responsivity across age. These results suggest a developmental shift in physiological arousal that parallels recent findings from our group. Recently, we reported an age-related relationship between cardiac activity and the severity of autistic behavior in 31 boys with FXS, aged 8–40 months (Roberts, Tonnsen, Robinson & Shinkareva, 2012). Cross-sectional analyses indicated increased autistic behavior was associated with lower heart rate at younger ages but with elevated heart rate at older ages. Results from these studies suggest a relationship between physiological arousal and core phenotypic features in FXS that emerge and shift within the first years of life that are consistent with findings from behavioral studies (Baranek et al., 2008; Shanahan et al., 2008). This work lends evidence that observed behavioral characteristics may be rooted in abnormal physiological regulation associated with abnormal brain development secondary to FMR1 dysfunction.
Understanding the developmental trajectory of early phenotypic features of FXS is imperative, as research supports the notion that phenotypic expression may change over time (Baranek et al., 2008; Roberts et al., 2012), and that the phenotype in infancy is likely not a simple downward extension of that observed in early childhood. Research on early development is particularly crucial to further define the infant–toddler phenotype, and to help determine whether and at what age the phenotypic expression may “shift.” Understanding this phenomenon is of importance when considering treatment implications, as validated treatments of older children and adults may not be suitable for infants and young children. Improved understanding of the pathology in processing sensory stimuli in children with behavior problems, like FXS, may be helpful in the development of efficacious psychopharmacological and/or behavioral interventions that have promise to improve the developmental trajectory of these children and prevent primary deficits from emerging into additional secondary risk factors.
Our findings contribute to the refinement of the complex relationship between arousal and behavior in FXS, which has only recently begun to emerge. Specifically, we report an association between mental age and reactivity (i.e., latency to shortest IBI) controlling for chronological age. This indicates that as intellectual abilities increase, boys with FXS show less sensory reactivity. Also, consistent with our previous work (Baranek et al., 2008), we report that heart activity is not related to behavioral sensory reactivity. The lack of relationship between behavioral indicators and physiological measures in the current study is important, as it suggests that sensory reactivity may be occurring in FXS independent of behavioral indicators. However, the association of physiological arousal and sensory reactivity is likely complex, as others have reported a relationship between electrodermal activity and sensory behaviors in FXS (Miller et al., 1999). The discrepancies between our work and others suggest that the association of sensory behaviors to arousal may be more closely linked to the sympathetic system given that electrodermal activity is primarily regulated by the sympathetic system, whereas heart activity is regulated by both sympathetic and parasympathetic components of the autonomic nervous system.
Although this study contributes to our understanding of the physiological mechanisms associated with sensory processing deficits in young boys with FXS, there are a number of limitations and areas for future research. First, we did not include a mental age or clinical comparison group (i.e., autism and idiopathic developmental disability), and we relied on standard screening procedures of parental report to document typical development and the absence of hearing or vision deficits rather than in-depth evaluation of these variables. Another limitation of our study was inclusion of a single stimulus (alarm) in only one sensory modality (auditory) rather than a comprehensive inclusion of multiple stimuli across modalities. We also used a novel indicator of heart activity (shortest IBI) that appears useful, but replication is needed before its validity can be established with confidence. Additionally, because of low levels of parental consent for blood draws, our sample size was insufficient to conduct in-depth analyses examining the relationship of behavior and physiology to FMRP that could further elucidate heterogeneity in this sample. Finally, we used a cross-sectional design that limits the inferences that can be drawn until our findings are replicated by a larger scale longitudinal study. Future work should address these limitations to include relevant clinical groups (e.g., idiopathic autism) in a longitudinal design with multiple sensory modalities examined.
This study has implications for clinicians, particularly with regard to increasing awareness of underlying indicators of physiological arousal at different ages that impact adaptive responses in boys with FXS, even when behavioral indicators of auditory reactivity are not as obvious. This study has implications for clinicians in that a lack of behavioral reactivity does not preclude the presence of sensory processing deficits, as evidenced by our physiological data. Also, our findings have implications for more specifically targeting psychopharmacological and environmental interventions if our finding that sensory reactivity is closely associated with sympathetic functioning. For example, medications known to attenuate sympathetic activation and environmental modifications associated with decreasing arousal activation (e.g., relaxation techniques and reducing stimuli) could be particularly beneficial in reducing these problem behaviors. Finally, our results indicate that lower functioning boys (e.g., lower mental age) with FXS may be at heightened risk for physiological dysregulation associated with sensory reactivity; therefore, this within-group variability is important in the detection and treatment to optimize outcomes.
Funding
This research was supported in part by grants from the Office of Special Education Programs, US Department of Education (H023B70035) (PI: Bailey) and the National Institute of Mental Health (R01MH0901194-01A1) (PI: Roberts).
Conflicts of interest: None declared.
References
- Bailey D B, Jr, Hatton D D, Tassone F, Skinner M, Taylor A K. Variability in FMRP and early development in males with FXS. American Journal on Mental Retardation. 2001;1:16–27. doi: 10.1352/0895-8017(2001)106<0016:VIFAED>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bailey D B, Jr, Mesibov G B, Hatton D D, Clark R D, Roberts J E, Mayhew L. Autistic behavior in young boys with fragile X syndrome. Journal of Autism and Developmental Disorders. 1998;28:499–508. doi: 10.1023/a:1026048027397. [DOI] [PubMed] [Google Scholar]
- Baranek G T, Chin Y, Hess L, Yankee J, Hatton D, Hooper S. Sensory processing correlates of occupational performance in children with fragile X syndrome: Preliminary findings. American Journal of Occupational Therapy. 2002;56:538–546. doi: 10.5014/ajot.56.5.538. [DOI] [PubMed] [Google Scholar]
- Baranek G T, Danko C D, Skinner M L, Bailey D B, Jr, Hatton D D, Roberts J E, Mirrett P L. Video analysis of sensory-motor features in infants with fragile X syndrome at 9-12 months of age. Journal of Autism and Developmental Disorders. 2005;35:645–656. doi: 10.1007/s10803-005-0008-7. [DOI] [PubMed] [Google Scholar]
- Baranek G T, Roberts J E, David F J, Sideris J, Mirrett P L, Hatton D D, Bailey D B., Jr Developmental trajectories and correlates of sensory processing in young boys with fragile X syndrome. Physical and Occupational Therapy in Pediatrics. 2008;28:79–98. doi: 10.1300/j006v28n01_06. [DOI] [PubMed] [Google Scholar]
- Castren M, Paakkonen A, Tarkka I M, Ryynanen M, Partanen J. Augmentation of auditory N1 in children with fragile X syndrome. Brain Topography. 2003;15:165–171. doi: 10.1023/a:1022606200636. [DOI] [PubMed] [Google Scholar]
- Clifford S, Dissanayake C, Bui Q M, Huggins R, Taylor A K, Loesch D Z. Autism spectrum phenotype in males and females with fragile X full mutation and premutation. Journal of Autism and Developmental Disorders. 2007;37:738–747. doi: 10.1007/s10803-006-0205-z. [DOI] [PubMed] [Google Scholar]
- Cohen I L. A theoretical analysis of the role of hyperarousal in the learning and behavior of fragile X males. Mental Retardation and Developmental Disabilities Research Reviews. 1995;1:286–291. [Google Scholar]
- Fallen E L, Kamath M V. Circadian rhythms of heart rate variability. In: Malik M, Camm A J, editors. Heart rate variability. New York: Futura; 1995. pp. 293–309. [Google Scholar]
- Frankland P W, Wang Y, Rosner B, Shimizu T, Balleine B W, Dykens E M, Ornitz E M, Silva A J. Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr-1 knockout mice. Molecular Psychiatry. 2004;9:417–425. doi: 10.1038/sj.mp.4001432. [DOI] [PubMed] [Google Scholar]
- Garon N, Bryson S E, Zwaigenbaum L, Smith I M, Brian J, Roberts W, Szatmari P. Temperament and its relationship to autistic symptoms in a high-risk infant sib cohort. Journal of Abnormal Child Psychology. 2009;37:59–78. doi: 10.1007/s10802-008-9258-0. [DOI] [PubMed] [Google Scholar]
- Hagerman R J. Fragile X Syndrome: Meeting the challenges of diagnosis and care. Contemporary Pediatrics. 1997;14:31–59. [Google Scholar]
- Hagerman R J. Fragile X syndrome. In: Hagerman R J, editor. Neurodevelopmental disorders. Oxford: Oxford University Press; 1999. pp. 61–132. [Google Scholar]
- Hagerman R J. Physical and behavioral phenotype. In: Hagerman R J, Hagerman P J, editors. Fragile X syndrome: Diagnosis, treatment, and research. 3rd ed. Baltimore, MD: Johns Hopkins University Press; 2002. pp. 3–109. [Google Scholar]
- Hagerman R J, Berry-Kravis E, Kaufmann W E, Ono M Y, Tartaglia N, Lachiewicz A, Kronk R, Delahunty C, Hessl D, Visootsak J, Picker J, Gane L, Tranfaglia M. Advances in the treatment of fragile X syndrome. Pediatrics. 2009;123:378–390. doi: 10.1542/peds.2008-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman R J, Miller L J, McGrath-Clarke J, Riley K, Goldson E, Harris S W, McIntosh D N. Influence of stimulants on electrodermal studies in fragile X syndrome. Microscopy Research and Technique. 2002;57:168–173. doi: 10.1002/jemt.10067. [DOI] [PubMed] [Google Scholar]
- Hall S S, Lightbody A A, Reiss A L. Compulsive, self-injurious, and autistic behavior in children and adolescents with FXS. American Journal on Mental Retardation. 2008;113:44–53. doi: 10.1352/0895-8017(2008)113[44:CSAABI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hatton D D, Hooper S R, Bailey D B, Jr, Skinner J L, Sullivan K M, Wheeler A. Problem behavior in boys with fragile X syndrome. American Journal of Medical Genetics. 2002;108:105–116. doi: 10.1002/ajmg.10216. [DOI] [PubMed] [Google Scholar]
- Hatton D D, Sideris J, Skinner M, Mankowski J, Bailey D B, Jr, Roberts J E, Mirrett P L. Autistic behavior in children with fragile X syndrome: Prevalence, stability, and the impact of FMRP. American Journal of Medical Genetics Part A. 2006;140A:1804–1813. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- Heilman K J, Harden E R, Zageris D M, Berry-Kravis E, Porges S V. Autonomic regulation in fragile X syndrome. Developmental Psychobiology. 2011;53:785–795. doi: 10.1002/dev.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L J, McIntosh D N, McGrath J, Shyu V, Lampe M, Taylor AK, Tassone F, Neitzel K, Stackhouse T, Hagerman R J. Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: A preliminary report. American Journal of Medical Genetics. 1999;83:268–279. [PubMed] [Google Scholar]
- Mini-Logger 2000. Sunriver, OR: Mini Mitter Co., Inc; 1994. Computer software. [Google Scholar]
- MxEdit . Bethesda, MD: Delta-Biometrics, Inc; 1989. Computer software. [Google Scholar]
- Roberts J E, Boccia M L, Bailey D B, Jr, Hatton D D, Skinner M. Cardiovascular indices of physiological arousal in boys with fragile X syndrome. Developmental Psychobiology. 2001;29:107–123. doi: 10.1002/dev.1035. [DOI] [PubMed] [Google Scholar]
- Roberts J E, Mazzocco M M, Murphy M M, Hoehn-Saric R. Arousal modulation and cognitive task performance in females with fragile X or turner syndrome. Journal of Autism and Developmental Disorders. 2008;38:20–27. doi: 10.1007/s10803-007-0356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J E, Tonnsen B L, Robinson A, Shinkareva S. Heart activity and autistic behavior in infants and toddlers with fragile X syndrome. American Journal on Intellectual and Developmental Disabilities. 2012;117:90–102. doi: 10.1352/1944-7558-117.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J E, Weisenfeld L A, Hatton D D, Heath M, Kaufmann W E. Social approach and autistic behavior in children with fragile X syndrome. Journal of Autism and Developmental Disorders. 2007;37:1748–1760. doi: 10.1007/s10803-006-0305-9. [DOI] [PubMed] [Google Scholar]
- Rogers S J, Hepburn S, Wehner E. Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. Journal of Autism and Developmental Disorders. 2003;33:631–642. doi: 10.1023/b:jadd.0000006000.38991.a7. [DOI] [PubMed] [Google Scholar]
- Roid G H, Miller L J. Leiter International Performance Scale- Revised. Wood Dale, IL: Stoelting Company; 1997. [Google Scholar]
- Rojas D C, Benkers T L, Rogers S J, Teale P D, Reite M L, Hagerman R J. Auditory evoked magnetic fields in adults with fragile X syndrome. Neuroreport. 2001;12:2573–2576. doi: 10.1097/00001756-200108080-00056. [DOI] [PubMed] [Google Scholar]
- Shanahan M, Roberts J, Hatton D, Reznik J, Goldsmith H. Early temperament and. negative reactivity in boys with FXS. Journal of Intellectual Disability Research. 2008;52:842–854. doi: 10.1111/j.1365-2788.2008.01074.x. [DOI] [PubMed] [Google Scholar]
- Sullivan K, Hatton D D, Hammer J, Sideris J, Hooper S, Ornstein P, Bailey D B., Jr AD/HD symptoms in children with FXS. American Journal of Medical Genetics Part A. 2006;140A:2275–2288. doi: 10.1002/ajmg.a.31388. [DOI] [PubMed] [Google Scholar]
- Symons F J, Clark R D, Hatton D D, Skinner M, Bailey D B., Jr Self-injurious behavior in young boys with FXS. American Journal of Medical Genetics. 2003;118A:115–121. doi: 10.1002/ajmg.a.10078. [DOI] [PubMed] [Google Scholar]
- Wheeler A C, Skinner D G, Bailey D B., Jr Perceived quality of life in mothers of Children with fragile X syndrome. American Journal on Mental Retardation. 2008;113:159–177. doi: 10.1352/0895-8017(2008)113[159:PQOLIM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Yuhas J, Cordeiro L, Tassone F, Ballinger E, Schneider A, Long J M, Ornitz E M, Hessl D. Brief report: Sensorimotor gating in idiopathic autism and autism associated with fragile X syndrome. Journal of Autism and Developmental Disorders. 2011;41:248–253. doi: 10.1007/s10803-010-1040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]