Abstract
Spermatozoa from azoospermic males can be retrieved from either the epididymis or the testis, depending on the type of azoospermia, using different surgical methods such as percutaneous epididymal sperm aspiration (PESA), testicular sperm aspiration (TESA), testicular sperm extraction (TESE), and microsurgical testicular sperm extraction (micro- TESE). After collecting the epididymal fluid or testicular tissue, laboratory techniques are used to remove contaminants, cellular debris, noxious microorganisms, and red blood cells. Processed spermatozoa may be used for intracytoplasmic sperm injection or eventually be cryopreserved. However, spermatozoa collected from either the epididymis or the testis are often compromised and more fragile than ejaculated ones. Therefore, sperm processing techniques should be used with great caution to avoid jeopardizing the sperm fertilizing potential in treatment cycles. In this review, we describe the current methods for processing surgically-retrieved specimens, either fresh or frozen- thawed, and provide the tips and pitfalls for facilitating the handling of such specimens. In addition, we present the available laboratory tools to aid in the identification of viable immotile spermatozoa to be used in conjunction with assisted reproductive techniques. Review of the literature was carried out using PubMed and Science Direct search engines.
KEY WORDS: Assisted reproductive techniques, azoospermia, epididymis, intracytoplasmic sperm injection, male infertility, review, sperm processing techniques, sperm retrieval techniques, spermatozoa, testis
INTRODUCTION
Two major breakthroughs occurred in the area of male infertility only 2 to 3 years apart.[1–3] The first was the development of intracytoplasmic sperm injection (ICSI) for the treatment of male factor infertility due to severely abnormal semen quality.[1] The second was the extension of ICSI to azoospermic males and the demonstration that spermatozoa derived from either the epididymis or the testis were capable of normal fertilization and pregnancy.[2,3] In the case of azoospermia, two totally different clinical situations exist, i.e., obstructive and non-obstructive azoospermia. In obstructive azoospermia (OA), spermatogenesis is normal, but a mechanical blockage exists in the genital tract, somewhere between the epididymis and the ejaculatory duct, or the epididymis and vas deferens are totally or partially absent. Causes of OA may be acquired or congenital. Acquired OA may be due to vasectomy, failure of vasectomy reversal, post-infectious diseases, surgical procedures in the scrotal, inguinal, pelvic or abdominal regions, and trauma. Congenital causes of OA include cystic fibrosis, congenital absence of the vas deferens (CAVD), ejaculatory duct or prostatic cysts, and Young's syndrome.[4] Non-obstructive azoospermia (NOA) comprises a spectrum of testicular histopathology resulting from various causes that include environmental toxins, medications, genetic and congenital abnormalities, varicocele, trauma, endocrinologic disorders, and idiopathic. Sperm can be easily obtained from men with OA for ICSI, whereas individuals exhibiting NOA have historically been the infertile men most difficult to treat.[4,5]
Several sperm retrieval methods have been developed to collect epididymal and testicular sperm for ICSI in azoospermic men. It is out of our scope to discuss which technique is best to surgically retrieve sperm. A comprehensive review of sperm retrieval techniques can be found elsewhere.[6] As a general rule, either percutaneous (PESA)[7] or microsurgical epididymal sperm aspiration (MESA)[2] can be successfully used to retrieve sperm from the epididymis in men with obstructive azoospermia. Testicular sperm aspiration (TESA) can be used to retrieve sperm from the testes either in men with OA who fail PESA as well as in those with NOA.[7,8] Testicular sperm extraction (TESE) using single or multiple open biopsies,[8–10] and more recently using microsurgery (micro-TESE), are indicated for men with NOA.[11–13]
Processing of surgically retrieved-spermatozoa differs from the commonly used methods for processing ejaculates. Sperm processing should not only ease the selection of the best quality spermatozoa for ICSI but also optimize their fertilizing ability, whenever possible. The laboratory has a crucial role in the management of these often compromised specimens, particularly in the cases of NOA and after the freeze-thawing process.[14] In order to achieve their goals, laboratory personnel should: (i) receive the best quality surgically-retrieved specimen possible, with minimal or no contaminants such as red blood cells and noxious microorganisms, (ii) minimize the iatrogenic cellular damage during sperm processing by mastering technical skills and controlling several factors, including centrifugation force and duration, exposure to ultraviolet light and temperature variation, laboratory air quality conditions, dilution and washing steps, quality of reagents, culture media, and disposable materials, and (iii) improve the sperm fertilizing potential, if possible, by using stimulants or selecting viable sperm for ICSI when only immotile spermatozoa is available. In this review, we provide laboratory description of the commonly used methods for epididymal and testicular sperm processing and present the clinical results of ICSI using testicular and epididymal sperm in azoospermic men. Finally, we critically discuss the tips and pitfalls of sperm processing techniques for surgically-retrieved specimens.
An overview of epididymal and testicular sperm retrieval techniques
Percutaneous sperm retrieval methods
Percutaneous sperm retrieval can be either diagnostic or therapeutic. In the former, it is used to confirm the presence of viable spermatozoa prior to ICSI. In the latter, it is carried out at the same day of oocyte retrieval or at the day before.
Percutaneous epididymal sperm aspiration
For PESA, a fine needle attached to a 1-mL tuberculin syringe is inserted through the scrotal skin into the epididymis.[6,7] Negative pressure is created by pulling the syringe plunger while the tip of the needle is gently moved in and out inside the epididymis until fluid is seen coming into the syringe. The amount of fluid aspirated is often minimal (~0.1 mL), except in cases of CAVD, in which 0.3-1.0 mL may be obtained. The aspirate is flushed into a tube containing warm sperm medium. The tube containing the epididymal aspirate is taken to the laboratory for immediate microscopic examination. PESA is repeated at a different site (from cauda to caput epididymis) until adequate number of motile sperm is retrieved. If PESA fails to retrieve motile sperm, testicular sperm retrieval can be attempted at the same operative time.
Testicular sperm aspiration
Despite of minor technical variations, the common principle of all methods described for TESA involves the needle insertion through the scrotal skin into the testis.[6,9] Then, testicular parenchyma is percutaneously aspirated using fine or large diameter needles. The needle is usually inserted at the anteromedial or anterolateral portion of the superior testicular pole, in an oblique angle towards the medium and lower poles. These areas are least likely to contain major branches of the testicular artery running superficially underneath the albuginea. Loupe-magnification may be used to avoid small vessels seen through the skin. Negative pressure is created by pulling the syringe plunger while the tip of the needle is moved in and out the testis in an oblique plane to disrupt the seminiferous tubules and sample different areas. The specimen is flushed into a tube containing warm sperm medium and is immediately transferred to the laboratory for microscopic examination. TESA or TESE may be performed at the contralateral testis if insufficient or no sperm are obtained.
Microsurgical sperm retrieval techniques
Microsurgical sperm retrieval can be performed under either local anesthesia in association with intravenous sedation or epidural anesthesia. Operating microscope and microsurgery technique are used throughout the procedures.
Microsurgical epididymal sperm aspiration
The surgical technique involves the exteriorization of the testis through a 2-3 cm transverse scrotal incision. The epididymal tunica is incised, and an enlarged tubule is then dissected and opened with sharp microsurgical scissors. Fluid exuding from the tubule is aspirated with the aid of a silicone tube or blunted needle attached to a tuberculin syringe. The aspirate is flushed into a tube containing warm sperm medium and is transferred to the laboratory for examination. MESA is repeated at a different site of the same epididymis (from cauda to caput) and/or at the contralateral one until adequate number of motile sperm is retrieved. If MESA fails to retrieve motile sperm, TESA or TESE can be performed at the same operative time.[2,6]
Microsurgical testicular sperm extraction
In micro-TESE, the delivery of the testis is carried out as described for MESA. Then, a single, large, mid-portion incision is made in an avascular area of the tunica albuginea under 6-8× magnification and the testicular parenchyma is widely exposed.[6,10–13] Dissection of the testicular parenchyma is undertaken at 16-25× magnification searching for enlarged islets of seminiferous tubules (more likely to contain germ cells and eventually normal sperm production). The superficial and deep testicular regions may be examined, if needed, and microsurgical-guided testicular biopsies are performed by carefully removing enlarged tubules using microsurgical forceps. If enlarged tubules are not seen, then any tubule different from the remaining ones in size is excised. If all tubules are identical in appearance, random micro-biopsies are performed at each testicular pole. The excised testicular tissue specimens are placed into the outer-well Petri dish containing sperm medium. Specimens are washed grossly to remove blood clots and are sent to the laboratory for processing and search for sperm. Albuginea and scrotal layers are closed using non-absorbable and absorbable sutures, respectively.
Conventional testicular sperm extraction (single or multiple open-biopsy TESE)
For conventional TESE, a standard open surgical biopsy technique is used to retrieve sperm without the aid of optical magnification.[6,8,9] TESE can be performed under either local anesthesia with or without intravenous sedation or under epidural anesthesia, and it is often carried out using the ‘window’ technique. Briefly, a transverse 2-cm incision is made through the anterior scrotal skin, dartos, and tunica vaginalis. A small self-retaining eyelid retractor is placed to improve exposure of the tunica albuginea since the testis is not exteriorized. The albuginea is incised for approximately 1-cm. Gentle pressure is made onto the testis to extrude testicular parenchyma. A small fragment (approximately 5 × 5 mm) is excised with sharp scissors and placed promptly in sperm culture medium. A single or multiple specimens can be extracted from the same incision. Alternatively, individual albuginea incisions can be made onto the upper, middle, and lower testicular poles to extract multiple biopsy specimens. Testicular specimens are sent to the laboratory for processing and immediate microscopic examination. Albuginea is closed using non-absorbable sutures.
Laboratory processing of epididymal and testicular specimens
Laboratory setup
All laboratory steps are performed using sterile techniques, and handling of the male gametes should be carried out under laminar flow. Ideally, sperm media should be protein-supplemented (5% HSA) and kept at 37°C.
In cases of epididymal (PESA and MESA) and testicular sperm aspirations (TESA), a tube containing sperm medium is sent to the operating room (sperm media is used to flush the aspirating system before aspiration and to incubate epididymal aspirates or testicular specimens upon collection). In cases involving testicular retrievals, several center-well dishes are prepared by transferring 0.5 mL and 1.0 mL sperm medium-aliquots to the inner and outer-dish wells, respectively (for TESA/TESE only). Some of them are kept onto a warm surface (37°C) inside the workstation, and others are sent to the operating room.
Tuberculin syringes connected with 26-gauge needles are prepared to be used as tools for mincing and squeezing seminiferous tubules in TESA/TESE processing A list of materials, equipment and reagents commonly used for laboratory handling of epididymal and testicular sperm is provided in Table 1.
Table 1.
Laboratory materials, equipment, and reagents commonly used for processing epididymal and testicular sperm

Laboratory processing of epididymal specimens
After epididymal retrievals, the tube containing the epididymal aspirate is transferred to the laboratory for processing [Figure 1]. Epididymal aspirates are diluted with sperm medium to avoid sperm agglutination (epididymal spermatozoa tend to agglutinate fast). A 10-20 μL aliquot of the mixture is placed and spread onto a Petri dish, and then examined under the inverted microscope to confirm the presence of motile sperm [Figure 2]. The surgeon is informed if an adequate number of motile sperm for ICSI is available. This step should take no more than 2-3 minutes because the patient is kept under anesthesia until a decision of continuing or finishing the surgical retrieval is made. If more epididymal specimens are taken, samples of similar quality are pooled together for processing.[14]
Figure 1.

A tube containing the epididymal aspirate obtained by percutaneous epididymal sperm aspiration
Figure 2.
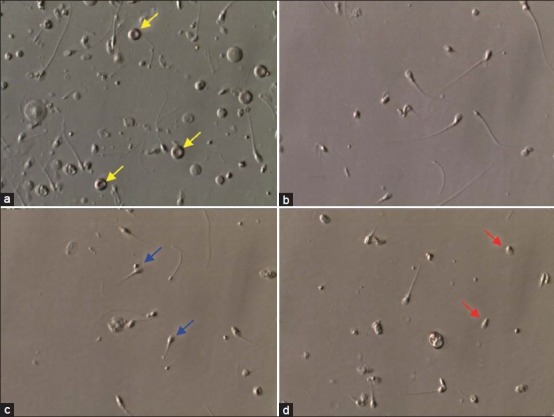
Photomicrograph showing the overall pattern of epididymal specimens obtained by percutaneous epididymal sperm aspiration (PESA). (a) Raw specimen showing contamination with debris and red blood cells (yellow arrows). (b) Processed specimen after dilution and washing with erythrocyte lysing buffer solution. (c) and (d) Raw specimens obtained from the epididymis cauda. Aspirates form the cauda are often rich in senescent spermatozoa (blue and red arrows). Photographs obtained from the inverted microscope equipped with Hoffman modulation phase contrast (Nikon Diaphot 300) under ×400 magnification
Upon completing epididymal retrievals, aspirate-containing tube(s) should be identified according to the epididymis side and site of aspiration (caput, corpus, or cauda), as well as to the presence of motile sperm. A decision is made upon the processing method to be used, i.e., simple washing or two-layer discontinuous mini-gradient centrifugation, based on a gross estimate of sperm density and motility. Gradient centrifugation is preferred when the specimen contains a high density of motile sperm, particularly if contaminated with red blood cells, cellular debris, and immotile sperm. Otherwise, simple washing is used.
For density gradient centrifugation, an aliquot of the epididymal aspirate up to 0.5 mL is layered over 0.3 mL-gradients of 45% and 90%, respectively, and centrifuged at ×300 g for 10 minutes. The pellet is re-suspended in fresh sperm medium, and washed by centrifugation at ×300g for 5 minutes. The supernatant is carefully removed, leaving about 0.2 mL of medium above the pellet. Then, the pellet is re-suspended and kept at 37°C until used for sperm injection or may be eventually cryopreserved.[14] When gradient centrifugation is chosen for epididymal sperm processing, it is advisable to spare and process part of the sample by simple washing. The reason is the unpredictability of gradient centrifugation to recover motile sperm in such cases. If recovery is less than desired, one can rely on the washed sample to select motile sperm for ICSI. Due to the relatively low sperm yields in percutaneous testicular and epididymal aspirations, it is recommended to use low volumes of media during sperm processing and wash the sample only once.
For simple washing, the epididymal aspirate is diluted with fresh sperm medium to a final volume of 1.5-2.0- mL. The mixture is centrifuged at ×300 g for 10 minutes, and the supernatant is discharged. The pellet is then re-suspended in approximately 0.2 mL of sperm medium. When a processed epididymal specimen is still contaminated with an excessive number of red blood cells, dilution and centrifugation with 2-mL erythrocyte lysing buffer may be required (Appendix). Centrifugation force and time should be carefully controlled to avoid jeopardizing the often compromised sperm motility.[15]
Laboratory processing of testicular specimens
Fragments of testicular parenchyma are placed into the outer Petri dish wells containing sperm medium. Under stereomicroscopy, the seminiferous tubules are identified and blood clots are removed using the needled-tuberculin syringes [Figure 3].
Figure 3.
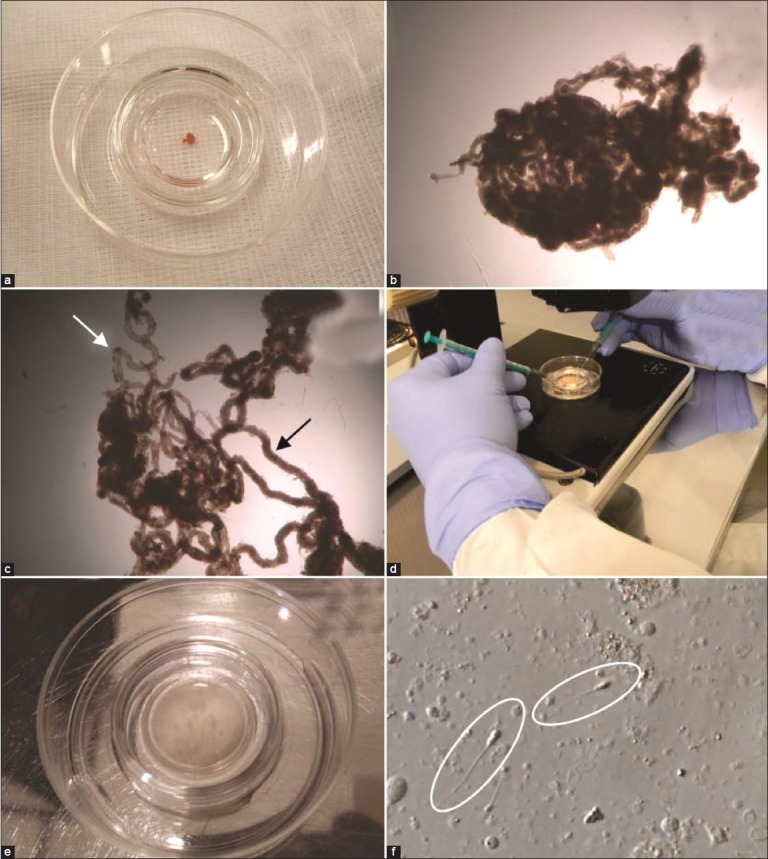
Laboratory processing of testicular specimens. (a) A fragment of testicular parenchyma obtained by microdissection testicular sperm extraction. (b) Testicular parenchyma fragment examined under stereomicroscopy before processing using ×40 magnification. (c) Seminiferous tubules examined under steromicroscopy using ×40 magnification. Observe the difference in diameter and appearance of the loosen tubules (arrows). (d) Mechanical mincing of the seminiferous tubules using needled-tuberculin syringes. (e) Aspect of the cell suspension after mechanical mincing of the tubules. No intact seminiferous tubule is seen. (f) Processed specimen examined under the inverted microscope using ×400 magnification. Testicular sperm were identified as shown
After initial washing, the seminiferous tubules are transferred to the inner-dish wells containing fresh sperm medium, and mechanical dispersion of the tubules is carried out by mincing repeatedly using needled-tuberculin syringes (one is used to hold tubules in place at the bottom of the dish and the other to squeeze and open them). This step is repeated until no intact tubules are seen [Figure 3].[14,16–18]
The homogenates are examined to confirm the presence of sperm under the inverted microscope. The surgeon is informed if an adequate number of sperm for ICSI is available. This step should take no more than 10 minutes because the patient is kept under anesthesia until a decision of continuing or finishing the surgical retrieval is made. If other testicular specimens are taken, the initial processing steps described above are carried out. Individual specimens should be identified according to the testis side, as well as to the presence of motile sperm.
Seminiferous tubules can be mechanically minced and shredded or enzymatically digested. Different laboratories opt for their own standard methods of processing. At Androfert, cell suspensions are transferred to centrifuge tubes containing fresh sperm medium upon the initial mincing of the seminiferous tubules is completed. The mixtures are subsequently centrifuged at ×300 g for approximately 7 minutes. The supernatants are discarded, and the pellets re-suspended in 0.2 mL of sperm medium.[14] When a processed testicular specimen is still contaminated with an excessive number of red blood cells, dilution and centrifugation with erythrocyte lysing buffer may be required (Appendix). At Montreal Reproductive Center, the finely minced tubules with cells are transferred to a centrifuge tube (suspended in 4-5 mL of media) and a quick spin (few seconds in a centrifuge machine) is done to make the large tissues to settle down. The supernatant is aspirated and transferred to a fresh tube and again centrifuged at a high speed (approximately ×350 g) for 12 minutes. The resulting pellet is washed once again with HEPES-buffered media and charged in the TESA dish under the oil. Usually, testicular aspirations are done one day prior to the actual oocyte pickup (OPU), and the processed sperm is kept under the oil and at room temperature overnight so that spermatozoa gain some motility [Figure 4].
Figure 4.
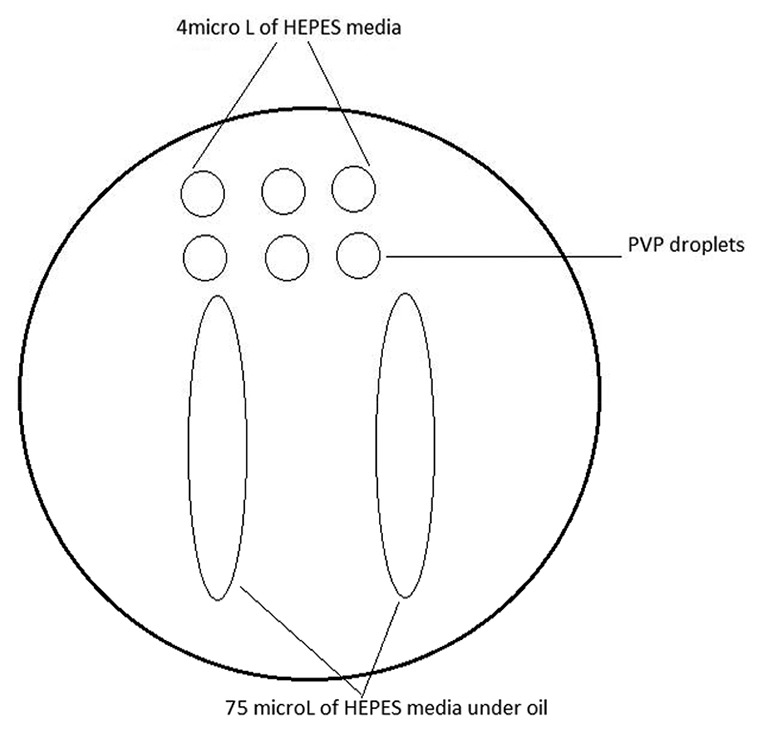
ICSI dish preparation for testicular sperm processed specimen one day before OPU. The processed cell suspension is loaded into the river-like HEPES medium. The small droplets are used for oocyte loading or can be removed and loaded with PVP droplets on the day of ICSI
The mechanical preparation has the advantage of being fast, requiring about 15-30 minutes, while enzymatic digestion is more time-consuming, requiring at least 4 hours for sperm preparation.[16] Studies comparing both techniques show conflicting results, but large series were unable to confirm the superiority of one technique over the other for processing fresh and frozen testicular sperm for ICSI.[16–19] Moreover, it has been shown that collagenase treatment of testicular tissue can initiate enhanced production of cytokines by macrophages, which can have a negative impact on spermatozoa.[20]
Laboratory processing of cryo-thawed epididymal and testicular specimens
The concept of cryopreservation may be used in association with sperm retrieval procedures. Some centers prefer to retrieve and intentionally cryopreserve sperm for future use. This strategy offers the advantage of avoiding ovarian stimulation when no sperm is obtained from testicular specimens. Epididymal and testicular spermatozoa may be cryopreserved using protocols routinely used for ejaculated sperm.[21,22] If sperm is found and frozen, thawing can be done at any time, thus obviating the need to organize two operations (oocyte and sperm retrieval) at the same day. Also, cryopreservation may be an interesting tool to spare left-over specimens that would be discharged after ICSI, especially if the treatment cycle does not result in a pregnancy. Future ICSI attempts may be carried out without repeated surgical retrievals. In most cases of epididymal retrievals, motile sperm will be available after thawing, and ICSI outcomes using motile fresh or frozen epididymal sperm seems not to differ.[23] Cryopreservation of testicular sperm is also advisable, especially for men with NOA who often require multiple ICSI attempts to conceive but may not have an adequate number of sperm available for repeated retrieval attempts.
After thawing, removal of cryoprotectant is carried out by simple washing. However, post-thawing, testicular sperm are often immotile or exhibit only a twitching motility. ICSI outcomes, including pregnancy rates, using immotile testicular sperm are lower than using motile testicular sperm.[23–26] Although methods for selecting immotile viable sperm for ICSI are available, results are either limited or not validated for cryopreserved specimens.[27–35] The simple in vitro incubation of fresh or frozen retrieved sperm may aid to obtain a more viable and functionally normal sperm population that obviates the risks of using immotile unselected sperm for ICSI. Sperm culture media have the components to support normal metabolism of immotile mature retrieved spermatozoa that may become motile by incubation.[36] However, in vitro incubation should be limited to a maximum of 48 h, since contamination by bacteria that normally come from the scrotal skin is almost a rule after a period of 36-48 h, even when strict sterile conditions are used in the laboratory. According to each group's results, different strategies can be developed. If freezing of surgically-retrieved specimens provides results similar to those with the use of fresh sperm, then the use of freezing specimens would be preferable. If not, fresh specimens are preferable. To date, our cryopreservation technique for surgically retrieval sperm is the standard liquid nitrogen vapor method using TEST-yolk buffer and glycerol as cryoprotectants.[21,22] Epididymal specimens are concentrated by washing before freezing, and testicular sperm are freed from the testicular parenchyma, i.e., testicular homogenates are frozen. Cryopreservation of few sperm into an empty zona pellucida and the use of stimulants before freezing may optimize results.[21,37,38]
Some laboratories freeze the whole tubules once they confirm the presence of spermatozoa. The freezing is done using cryovials and upon thawing one day prior to OPU; the tissue is minced properly and processed by density-gradient centrifugation (DGC) to make sure that junk tissues are removed before DGC by a quick spin). Usually, 80/40 gradient, 1 mL each is employed. Recently, it has been shown that human spermatozoa can be successfully vitrified, and this strategy may be of interest for preserving small quantities of surgically-retrieved gametes.[39]
Handling epididymal and testicular sperm for sperm injections
A Petri dish containing a series of microdrops under mineral oil is prepared for sperm pick-up from a processed epididymal or testicular cell suspension [Figure 5]. An aliquot of approximately 1-μL sperm suspension is loaded at the center of the polyvinylpyrrolidone (PVP) if the suspension contains motile sperm with progressive motility. After an incubation period of 10-20 minutes, morphologically normal motile spermatozoa are identified and picked up for ICSI using the injection micropipette at the edge of the PVP droplet. If progressive motility is low or absent and/or the sample is contaminated with cellular debris, a sperm suspension aliquot of approximately 1-4 microliters is loaded in a microdroplet containing buffered sperm culture medium to facilitate search and selection of motile sperm [Figure 4].[14] Testicular specimens can be incubated up to 48 hours before ICSI at room temperature in an attempt to improve testicular sperm motility.
Figure 5.
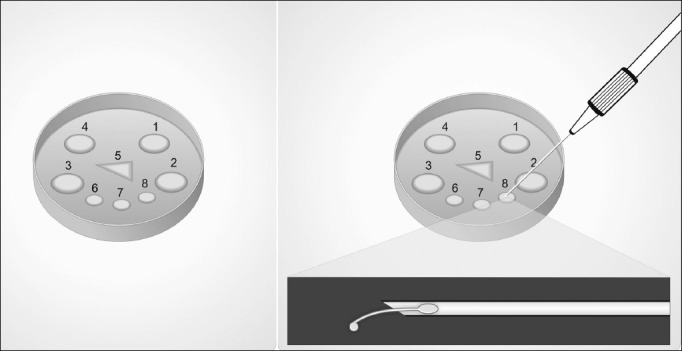
Preparation of Microdroplets. A 50 × 09 mm Petri dish containing several microdroplets of culture medium under mineral oil is prepared for sperm pick-up from a processed epididymal or testicular cell suspension. Microdroplets are prepared as follows: Four 10-μL sperm medium at dish periphery to load specimens (numbered 1 to 4), one 4-μL polyvinylpyrrolidone (PVP) at dish center in a triangle shape to pick up selected sperm for ICSI (number 5), and two to three 10-μL sperm medium at dish center below the PVP triangle for washing (numbered 6 to 8). Alternatively, one of the sperm medium-containing microdroplets (e.g., number 8) or the peripheric ones (1-4) may be replaced with the hyposmotic or motility-stimulant solutions, respectively (left). The hyposmotic swelling test is illustrated (right). The sperm tail is partially withdrawn from the injection micropipette into the HOS droplet. A swelling at the level of the tail tip may be seen under the inverted microscope with contrast at ×400 magnification
A small quantity of PVP is aspirated into the injection micropipette to improve control during sperm pick-up and to avoid blowing air bubbles during ejection of selected sperm into the PVP droplet. The injection micropipette should be washed free of any debris by repeated aspiration and release of PVP upon completion of picking up epididymal or testicular sperm. A slightly large diameter ICSI micro-injection pipette (e.g., Cook K-MPIP-3330 with an internal diameter of 5.5 micrometer) can help to avoid clogging of the needle while searching for the sperm. Just prior to sperm injections into the oocyte, a final morphologic sperm assessment is carried out (using conventional or high magnification) in the group of pre-selected gametes. Then, spermatozoon is immobilized, aspirated into the micropipette, and injected into the cytoplasm of metaphase-II oocytes.
In cases of non-obstructive azoospermia, testicular tissue removal is often 50 to 70-fold less when microsurgical testicular sperm retrieval is used compared to standard TESE.[12,40] The small amount of tissue extracted facilitates sperm processing. Selection of spermatozoa from a smaller population of contaminating testicular cells allows more ease and greater speed for sperm pick-up and injection process, as well as alleviates contamination and blockage of the injection needle with cells and debris. It is far less technically demanding and labor-intensive to extract spermatozoa from small volume specimens than large pieces of testicular tissue that must be dissected, red-blood cells lysed, and the rare spermatozoa searched for in a tedious fashion under an inverted microscope. TESE sperm processing may be incredibly labor-intensive, and the searching process may miss the rare spermatozoa within a sea of seminiferous tubules and other cells.[14] TESE/ micro-TESE may be scheduled either for the day of oocyte collection and ICSI or the day before. In the latter, processed specimens are incubated in a closed HEPES-buffered culture system (microdrops under mineral oil) at room temperature, inside a laminar flow cabinet or in a clean room, to avoid bacterial contamination. Culture of specimens at 37°C inside the incubator should be avoided since contamination with scrotum skin derived-bacteria is often seen. From our data, optimal fertilization by ICSI using surgically-retrieved sperm is obtained when the time frame from hCG administration to microinjection does not exceed 44 h.[14] Testicular tissue sperm processing, searching, and selection of viable spermatozoa for ICSI may take several hours in NOA cases. It has been our observation that it takes approximately 12 minutes to handle an individual testicular spermatozoon from processing to microinjection in cases of NOA, but only 5 minutes in OA. In other words, the average time required to perform ICSI in a standard NOA treatment cycle involving 8-12 metaphase-II oocytes is approximately 2 hours. As such, it is recommended that testicular retrievals are carried out the day before oocyte collection when a busy next day IVF laboratory workload is anticipated. Ideally, two laboratory technicians should work together during the initial steps of processing testicular specimens of patients with NOA (one mincing the tubules and the other searching for spermatozoa) to speed up the searching process and to allow a faster feedback to the surgeon who may decide to end the procedure if sperm is found or to continue extracting additional seminiferous tubules.
It has been suggested that testicular spermatozoa of men with severely impaired spermatogenesis have decreased fertilization potential, and may have a higher tendency to carry deficiencies such as the ones related to the centrioles and genetic material, which ultimately affect the capability of the male gamete to activate the egg and trigger the formation and development of a normal zygote and a viable embryo.[41] In fact, the clinical outcomes of ICSI using surgically-extracted testicular sperm in NOA seems to be lower than those obtained with either ejaculated or epididymal/testicular sperm from men with OA.[41]
Methods for selecting viable immotile sperm for ICSI
It has been observed that conventional seminal parameters have little or no influence in ICSI outcomes, except when only immotile spermatozoa are available.[23–25] In some cases after epididymal and testicular sperm retrieval, only immotile spermatozoa are obtained after processing of fresh or cryo-thawed epididymal and testicular specimens. Different strategies may be used to differentiate live immotile spermatozoa from dead ones, thus aiding in the selection of viable gametes for ICSI, as described below.
Hyposmotic swelling (HOS) test
The method for preparation of the hyposmotic solution is provided in the appendix. First, a single morphologically normal immotile spermatozoon is picked up from the sperm medium aspirated head-first into the pipette. Then, the pipette is immersed into the HOS microdroplet [Figure 5],[27,28] and only the sperm tail is moved out of the pipette tip into the hyposmotic solution. The tail is kept for 5-10 seconds into the solution, and it is observed if a tail tip swelling occurs. Sperm tail swelling is often minimal and is a marker of viability in fresh specimens. However, tail swelling is may not be suitable for testing cryopreserved sperm.[29]
If tail swelling is seen, the spermatozoon is aspirated back into the pipette and released it in a drop of fresh medium to allow osmotic re-equilibration (tail swelling often disappears in 5-20 seconds). If tail swelling is not seen, spermatozoon is discharged into the HOS solution, and the process is repeated with another sperm. The selected sperm populations are kept in the PVP droplet until an adequate number of viable sperm is available for ICSI.
The application of a single laser shot to the far end of the sperm tail has been shown to cause a curling of the tail only in viable sperm, similar to the reaction observed with the HOST, but this method has not been validated in cryo-thawed specimens.[42]
Sperm tail flexibility test
First, morphologically normal immotile spermatozoa are picked up from the sperm medium using the microinjection pipette and transferred to the PVP microdroplet. Then, sperm tail is touched with the tip of the microinjection pipette to move the tail up and down. The sperm tail is considered flexible when it moves independently of the sperm head. Sperm tail flexibility is considered a marker of sperm viability.[30,31] If the tail remains rigid upon touching and sperm head and tail move together as a block, spermatozoon is then considered non-viable for ICSI.
The procedure is repeated until an adequate number of viable sperm is selected for ICSI.
Motility stimulant sperm challenge using pentoxifylline
The method for preparation of the pentoxifylline solution is provided in the appendix. A 4-μL-aliquot of fresh or cryopreserved epididymal/testicular sperm suspension is loaded into the motility stimulant solution microdroplet and is incubated for approximately 20 minutes [Figure 4]. The specimen is examined microscopically to search for moving sperm. In cases of a positive test, a slight noticeable tail twitching is seen, and in rare occasions, vigorous twisting may be observed.[32,33,34] Motile sperm is picked up using the microinjection pipette and transferred to a fresh microdroplet of sperm medium. This step is repeated several times to wash out any residual pentoxifylline (PF) since it has been suggested in animal studies that PF is embryotoxic.[35] Motile spermatozoa are kept in culture or placed into the PVP droplet for sperm selection and intracytoplasmic injection.
CONCLUSIONS
The primary goal of epididymal and testicular sperm processing is the recovery of a clean sample containing motile sperm. Such specimens are more fragile, and often compromised in motility, as compared to the ones obtained from ejaculates. Laboratory techniques should be carried out with great caution not to jeopardize the sperm fertilizing potential. The whole process starts with the surgical collection of the best quality specimen as possible. During laboratory steps, minimal iatrogenic cellular damage may be achieved by strict control of centrifugation force and duration, exposure to ultraviolet light and temperature variation, laboratory air quality conditions, as well as the use of high quality reagents, materials, and equipments.
Epididymal sperm processing may be performed either by mini-gradient centrifugation or by simple washing using low volumes of culture media. Testicular specimens may be processed either by mechanical mincing of seminiferous tubules or by enzymatic digestion, with similar results, and homogenates are simply washed. The mechanical preparation is significantly faster than enzymatic digestion. The use of unselected immotile epididymal/testicular sperm for ICSI negatively impact clinical outcomes. Methods for selecting viable immotile sperm for ICSI are available.
The concept of cryopreservation may be useful for intentionally cryopreserve retrieved-sperm for future use or spare left-over specimens that would be discharged after ICSI. Different strategies can be developed according to each group's results. If freezing of surgically-retrieved specimens provides results similar to those with the use of fresh sperm, then the use of freezing specimens would be preferable. If not, fresh specimens are preferable.
APPENDIX
Erythrocyte lysing buffer solution (ELBS)
155 mM NH4Cl +10 mM KHCO3 + 2 mM EDTA dissolved in sterile water. Adjust the pH to 7.2, if necessary. Upon finishing the first dilution and centrifugation step, re- suspend the pellet with 2.0 mL of ELBS and keep the mixture at room temperature for 10 minutes. Then, centrifuge the sample at ×300 g for 5 minutes, discharge the supernatant, and re-suspend the pellet in 0.2 mL of fresh HEPES-buffered protein-supplemented sperm medium.[16]
Hyposmotic solution
Prepare a 150 mOsm/kg HOS solution by dissolving 7.35 mg sodium citrate and 13.51 mg fructose in 1 mL sterile reagent water.[29] Alternatively, a 139 mOsm/kg HOS solution can be prepared by mixing 1 mL sperm medium to 1 mL sterile reagent water.[27,28]
Pentoxifylline solution
Prepare a 5 mM solution of PF by dissolving 1.391 mg pentoxifylline (Sigma cat. No. P-1784) in 1 mL of HEPES-buffered culture medium.[32]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Palermo G, Joris H, Devroey P, van Steirteghem AC. Pregnancies after intracytoplasmic sperm injection of single spermatozoan into an oocyte. Lancet. 1992;340:17–8. doi: 10.1016/0140-6736(92)92425-f. [DOI] [PubMed] [Google Scholar]
- 2.Silber S, Nagy ZP, Liu J, Godoy H, Devroey P, Van Steirteghem AC. Conventional in-vitro fertilization versus intracytoplasmic sperm injection for patients requiring microsurgical sperm aspiration. Hum Reprod. 1994;9:1705–9. doi: 10.1093/oxfordjournals.humrep.a138778. [DOI] [PubMed] [Google Scholar]
- 3.Devroey P, Liu J, Nagy ZP, Goossens A, Tournaye H, Camus M, et al. Pregnancies after testicular extraction (TESE) and intracytoplasmic sperm injection (ICSI) in non-obstructive azoospermia. Hum Reprod. 1995;10:1457–60. doi: 10.1093/humrep/10.6.1457. [DOI] [PubMed] [Google Scholar]
- 4.Esteves SC, Miyaoka R, Agarwal A. An update on the clinical assessment of the infertile male. Clinics (Sao Paulo) 2011;66:691–700. doi: 10.1590/S1807-59322011000400026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esteves SC, Miyaoka R, Agarwal A. Surgical treatment of male infertility in the era of intracytoplasmic sperm injection-new insights. Clinics (Sao Paulo) 2011;66:1463–78. doi: 10.1590/S1807-59322011000800026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esteves SC, Miyaoka R, Agarwal A. Sperm retrieval techniques for assisted reproduction. Int Braz J Urol. 2011;37:570–83. doi: 10.1590/s1677-55382011000500002. [DOI] [PubMed] [Google Scholar]
- 7.Craft I, Tsirigotis M, Bennett V, Taranissi M, Khalifa Y, Hogewind G, et al. Percutaneous epididymal sperm aspiration and intracytoplasmic sperm injection in the management of infertility due to obstructive azoospermia. Fertil Steril. 1995;63:1038–42. doi: 10.1016/s0015-0282(16)57544-x. [DOI] [PubMed] [Google Scholar]
- 8.Craft I, Tsirigotis M. Simplified recovery, preparation and cryopreservation of testicular spermatozoa. Hum Reprod. 1995;10:1623–7. doi: 10.1093/oxfordjournals.humrep.a136142. [DOI] [PubMed] [Google Scholar]
- 9.Raviv G, Levron J, Menashe Y, Bider D, Dor J, Ramon J, et al. Sonographic evidence of minimal and short-term testicular damage after testicular sperm aspiration procedures. Fertil Steril. 2004;82:442–4. doi: 10.1016/j.fertnstert.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 10.Okada H, Dobashi M, Yamazaki T, Hara I, Fujisawa M, Arakawa S, et al. Conventional versus microdissection testicular sperm extraction for nonobstructive azoospermia. J Urol. 2002;168:1063–7. doi: 10.1016/S0022-5347(05)64575-2. [DOI] [PubMed] [Google Scholar]
- 11.Tsujimura A, Matsumiya K, Miyagawa Y, Tohda A, Miura H, Nishimura K, et al. Conventional multiple or microdissection testicular sperm extraction: A comparative study. Hum Reprod. 2002;17:2924–9. doi: 10.1093/humrep/17.11.2924. [DOI] [PubMed] [Google Scholar]
- 12.Schlegel PN. Testicular sperm extraction: Microdissection improves sperm yield with minimal tissue excision. Hum Reprod. 1999;14:131–5. doi: 10.1093/humrep/14.1.131. [DOI] [PubMed] [Google Scholar]
- 13.Ramasamy R, Lin K, Gosden LV, Rosenwaks Z, Palermo GD, Schlegel PN. High serum FSH levels in men with nonobstructive azoospermia does not affect success of microdissection testicular sperm extraction. Fertil Steril. 2009;92:590–3. doi: 10.1016/j.fertnstert.2008.07.1703. [DOI] [PubMed] [Google Scholar]
- 14.Schneider DT, Esteves SC. Male infertility and assisted reproductive technology: Lessons from the IVF. Open Reprod Sci J. 2011;3:138–53. [Google Scholar]
- 15.Esteves SC, Sharma RK, Thomas AJ, Agarwal A. Improvement in motion characteristics and acrosome status in cryopreserved spermatozoa by swim-up processing before freezing. Hum Reprod. 2000;15:2173–9. doi: 10.1093/humrep/15.10.2173. [DOI] [PubMed] [Google Scholar]
- 16.Verheyan G, De Croo I, Tournaye H, Pletincx I, Devroey P, van Steirteghem AC. Comparison of four mechanical methods to retrieve spermatozoa from testicular tissue. Hum Reprod. 1995;10:2956–9. doi: 10.1093/oxfordjournals.humrep.a135828. [DOI] [PubMed] [Google Scholar]
- 17.Salzbrunn A, Benson DM, Holstein AF, Shulze W. A new concept for the extraction of testicular spermatozoa as a tool for assisted fertilization (ICSI) Hum Reprod. 1996;11:752–5. doi: 10.1093/oxfordjournals.humrep.a019248. [DOI] [PubMed] [Google Scholar]
- 18.Nagy ZP, Verheyen G, Tournaye H, Devroey P, van Steirteghem AC. An improved treatment procedure for testicular biopsy specimens offers more efficent sperm recovery: Case series. Fertil Steril. 1997;68:376–9. doi: 10.1016/s0015-0282(97)81534-8. [DOI] [PubMed] [Google Scholar]
- 19.Baukloh V. Retrospective multicentre study on mechanic and enzymatic preparation of fresh and cryopreserved testicular biopsies. Hum Reprod. 2002;17:1788–94. doi: 10.1093/humrep/17.7.1788. [DOI] [PubMed] [Google Scholar]
- 20.Bryniarski K, Szczepanik M, Ptak M, Ptak W. Modulation of testicular macrophage activity by collagenase. Folia Histochem Cytobiol. 2005;43:37–41. [PubMed] [Google Scholar]
- 21.São Paulo, BrazilEsteves SC, Spaine DM, Cedenho AP Andrology and Human Reproduction Clinic. Effects of pentoxifylline treatment before freezing on motility, viability and acrosome status of poor quality human spermatozoa cryopreserved by the liquid nitrogen vapor method. Braz J Med Biol Res. 2007;40:985–92. doi: 10.1590/s0100-879x2006005000118. [DOI] [PubMed] [Google Scholar]
- 22.Verza S, Jr, Feijo CM, Esteves SC. Resistance of human spermatozoa to cryoinjury in repeated cycles of thaw-refreezing. Int Braz J Urol. 2009;35:581–91. doi: 10.1590/s1677-55382009000500010. [DOI] [PubMed] [Google Scholar]
- 23.Nagy ZP, Liu J, Joris H, Verheyen G, Tournaye H, Camus M, et al. The result of intracytoplasmic sperm injection is not related to any of the three basic sperm parameters. Hum Reprod. 1995;10:1123–9. doi: 10.1093/oxfordjournals.humrep.a136104. [DOI] [PubMed] [Google Scholar]
- 24.Fisher R, Baukloh V, Naether OG, Shulze W, Salzbrunn A, Benson DM. Pregnancy after intracytoplasmic sperm injection of spermatozoa from frozen-thawed testicular biopsy. Hum Reprod. 1996;11:2197–9. doi: 10.1093/oxfordjournals.humrep.a019075. [DOI] [PubMed] [Google Scholar]
- 25.Dafopoulos K, Griesinger G, Schultze-Mosgau A, Orief Y, Schöpper B, Nikolettos N, et al. Factors affecting outcome after ICSI with spermatozoa retrieved from cryopreserved testicular tissue in non-obstructive azoospermia. Reprod Biomed Online. 2005;10:455–60. doi: 10.1016/s1472-6483(10)60820-6. [DOI] [PubMed] [Google Scholar]
- 26.Konc J, Kanya K, Cseh S. The effect of condition/state of testicular spermatozoa injected to the outcome of TESE-ICSI-ET cycles. Eur J Obstet Gynecol Reprod Biol. 2008;141:39–43. doi: 10.1016/j.ejogrb.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Tsai YL, Katz E, Compton G, Garcia JE, Baramki TA. High fertilization rate obtained after intracytoplasmic sperm injection with 100% no motile spermatozoa selected by using a simple modified hypo-osmotic swelling test. Fertil Steril. 1997;68:373–5. doi: 10.1016/s0015-0282(97)81533-6. [DOI] [PubMed] [Google Scholar]
- 28.Sallam HN, Farrag A, Agameya AF, El-Garem Y, Ezzeldin F. The use of the modified hypo-osmotic swelling test for the selection of immotile testicular spermatozoa in patients treated with ICSI: A randomized controlled study. Hum Reprod. 2005;20:3435–40. doi: 10.1093/humrep/dei249. [DOI] [PubMed] [Google Scholar]
- 29.Esteves SC, Sharma RK, Thomas AJ, Jr, Agarwal A. Suitability of the hypo-osmotic swelling test for assessing the viability of cryopreserved sperm. Fertil Steril. 1996;66:798–804. [PubMed] [Google Scholar]
- 30.Soares JB, Glina S, Antunes N, Jr, Wonchockier R, Galuppo AG, Mizrahi FE. Sperm tail flexibility test: A simple test for selecting viable spermatozoa for intracytoplasmic sperm injection from semen samples without motile spermatozoa. Rev Hosp Clin Fac Med Sao Paulo. 2003;58:250–3. doi: 10.1590/s0041-87812003000500003. [DOI] [PubMed] [Google Scholar]
- 31.Oliveira NM, Sanchez RV, Fiesta SR, Salgado TL, Rodriguez R, Bethencourt JC, et al. Pregnancy with frozen-thawed and fresh testicular biopsy after motile and immotile sperm microinjection, using the mechanical touch technique to assess viability. Hum Reprod. 2004;19:262–5. doi: 10.1093/humrep/deh083. [DOI] [PubMed] [Google Scholar]
- 32.Esteves SC, Sharma RK, Thomas AJ, Jr, Agarwal A. Cryopreservation of human spermatozoa with pentoxifylline improves the post-thaw agonist-induced acrosome reaction rate. Hum Reprod. 1998;13:3384–9. doi: 10.1093/humrep/13.12.3384. [DOI] [PubMed] [Google Scholar]
- 33.Terriou P, Hans E, Giorgetti C, Spach JL, Salzmann J, Urrutia V, et al. Pentoxifylline initiates motility in spontaneously immotile epididymal and testicular spermatozoa and allows normal fertilization, pregnancy, and birth after intracytoplasmic sperm injection. J Assist Reprod Genet. 2000;17:194–9. doi: 10.1023/A:1009435732258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kovacic B, Vlaisavljevic V, Reljic M. Clinical use of pentoxifylline for activation of immotile testicular sperm before ICSI in patients with azoospermia. J Androl. 2006;27:45–52. doi: 10.2164/jandrol.05079. [DOI] [PubMed] [Google Scholar]
- 35.Yovich JL. Pentoxifylline: Actions and applications in assisted reproduction. Hum Reprod. 1993;8:1786–91. doi: 10.1093/oxfordjournals.humrep.a137935. [DOI] [PubMed] [Google Scholar]
- 36.Zhu J, Tsirigotis M, Pelekanos M, Craft I. In vitro maturation of testicular spermatozoa. Hum Reprod. 1996;11:231–2. doi: 10.1093/oxfordjournals.humrep.a019030. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Zheng XZ, Baramki TA, Compton G, Yazigi RA, Katzet E. Cryopreservation of a small number of fresh human testicular spermatozoa and testicular spermatozoa cultured in vitro for 3 days in an empty zona pellucida. J Androl. 2000;21:409–13. [PubMed] [Google Scholar]
- 38.Levi-Setti PE, Albani E, Negri L, Cesana A, Novara P, Bianchi S. Cryopreservation of a small number of spermatozoa in yolk-filled human zonae pellucidae. Arch Ital Urol Androl. 2003;75:195–8. [PubMed] [Google Scholar]
- 39.Isachenko E, Isachenko V, Weiss JM, Kreienberg R, Katkov II, Schulz M, et al. Acrosomal status and mitochondrial activity of human spermatozoa vitrified with sucrose. Reproduction. 2008;136:167–73. doi: 10.1530/REP-07-0463. [DOI] [PubMed] [Google Scholar]
- 40.Schlegel PN, Su LM. Physiologic consequences of testicular sperm extraction. Hum Reprod. 1997;12:1688–92. doi: 10.1093/humrep/12.8.1688. [DOI] [PubMed] [Google Scholar]
- 41.Verza S, Jr, Esteves SC. Sperm defect severity rather than sperm source is associated with lower fertilization rates after intracytoplasmic sperm injection. Int Braz J Urol. 2008;34:49–56. doi: 10.1590/s1677-55382008000100008. [DOI] [PubMed] [Google Scholar]
- 42.Aktan TM, Montag M, Duman S, Gorkemli H, Rink K, Yurdakul T. Use of laser to detect viable but immotile spermatozoa. Andrologia. 2004;36:366–9. doi: 10.1111/j.1439-0272.2004.00636.x. [DOI] [PubMed] [Google Scholar]


