Abstract
BACKGROUND:
Oocyte donation is an invaluable therapy for couples with impending or complete ovarian failure. In addition, oocyte donation affords a scientific opportunity to study the unique biologic participation of the uterus in the process of human embryo implantation.
AIM:
To identify the recipient variables that may have a significant impact on pregnancy outcome in order to optimize results of an oocyte donation program.
DESIGN AND SETTINGS:
A prospective study conducted from March 1, 2010 to March 31, 2011 at a private tertiary care IVF Clinic. Materials and methods A total of 270 recipients resulting in embryo transfer as a result of oocyte donation were enrolled. Clinical and Ongoing pregnancy rates, Implantation rates were calculated according to different age groups, Endometrial thickness, Indication, Day and number of embryos transferred. Data was evaluated as chi square analyses with comparative significance determined at P <.05.
RESULTS:
In recipients less than 40 years of age, higher ongoing pregnancy and implantation rates (41.9% and 24.6%) were seen as compared to recipients above 40 years (21.8% and 13.18%) respectively. Pregnancy and implantation rates increased with increasing endometrial thickness but the difference was not statistically significant. A higher ongoing pregnancy rate (40.9% vs.28.8%) and implantation rate (23% vs.19.6%) was demonstrated with Day 3 embryo transfer compared to Day 2 transfer.
CONCLUSION:
A declining endometrial receptivity may result in lower implantation and pregnancy rates in recipients above the age of 40 years, more pronounced after the age of 45 years. An endometrial thickness of >8 mm is considered ideal before transfer. Transfer of two selected embryos on day 3 yields a favorable pregnancy outcome with reduced multiple pregnancy rates. Recipient's age above 45 years has negative impact on pregnancy outcome whereas embryo transfers on Day 3 yields better pregnancy
KEY WORDS: Aging uterus, embryo implantation, endometrial thickness, oocyte donation, uterine receptivity, uterine senescence
INTRODUCTION
According to the last report from the NARI 2006 (National ART Registry of India), recipient cycles were performed in approximately 12% of all ART cycles. In 50% of recipient cycles, a dedicated donor was stimulated and rest of 50% recipients had egg sharing with a patient. Pregnancy rate was 42.1% and 36.1%, respectively. The success of oocyte donation may be influenced by various factors including age of the oocyte donor, quality, and number of embryos transferred, age and endometrial receptivity of the recipient. Implantation rate is considered the most sensitive and accurate variable and should serve as the basis of assessment and comparison for studies of uterine receptivity.[1] The current study aims to identify recipient-related variables that may predict the pregnancy outcome in order to optimize results of an oocyte donation program.
MATERIALS AND METHODS
A prospective study of 270 recipients, resulting in embryo transfer as a result of oocyte donation, occurring from March 1, 2010 to March 31, 2011 at a private tertiary care IVF clinic. All regulatory policies regarding oocyte donation were observed including the signing of appropriate donor and recipient consent forms required by The Indian Council of Medical Research (ICMR) guidelines for oocyte donation were obtained. In all anonymous egg donation cycles volunteer donor aged between 21 to 31 years were recruited. A donor's oocytes were shared between two recipients provided that each recipient received a minimum 7 oocytes. A complete medical and past reproductive history of all donors was reviewed. Ovarian reserve was assessed by baseline ultrasound for antral follicle count and ovarian volume measurement. All recipients, their partners and all donors underwent serum testing for ABO Rh, human immunodeficiency virus, syphilis, and Hepatitis B and C, and were subjected to a psychological evaluation. Recipients above 40 years were referred to a physician for medical fitness before starting treatment. Donors were stimulated with recombinant FSH (Gonal-F, Merck Serono, Switzerland or Recagon, Organon, Ireland) from day 2 of their menstrual cycle with a dose of ranging from 200-225 IU. The GnRH antagonist Ganerelix (orgalutran, Serono) was introduced according to a multiple- dose protocol (0.25 mg/day) when a leading follicle of 14 mm was present. Triggering was performed when at least three follicles of 18 mm were present, with 250 mcg recombinant HCG (Ovitrelle, Serono, Italy). The oocyte retrieval was scheduled 35 hours after triggering.
In all recipients, a mock cycle with estrogen priming for mid-cycle endometrial thickness was studied and hysteroscopy was done only if indicated. The recipient's age ranged from 23 to 55 years (mean age 37.5). An average of 2.8 embryos were transferred per recipient. Synchronization was achieved between donors and recipients cycle as shown in Figure 1. Cycling recipients were put on oral contraceptive pills and daily administration of 0.5 mg sc of leuprolide acetate starting on the 17/18th day (when 4-5 pill are left) of the previous cycle. The hormone replacement therapy was started on the 2nd day of the cycle if patient was down regulated (confirmed by SerumE2 levels, if less than 30 pg/ml) and increasing doses of oestradiol valerate orally (Progynova, Schering AG Berlin, Zydus Healthcare India), which was given as follows: 2 mg twice a day for the first 4 days of the cycle, followed by 2 mg thrice a day for the next 4 days. Dose was increased to 4 mg bid, i.e, 8 mg /day only if endometrium thickness was less than 8 mm on 10th/11th day. Same dose was continued until the oocyte donation was available. Non-cycling patients were started with hormone therapy without any down regulation. After a minimum of 11-12 days of priming with estrogen if endometrium thickness was appropriate, recipients were advised administration of intravaginal progesterone puregest (Sun Pharma) at the dose of 200 mg, three times a day beginning from the day of donors pick up. Embryos were replaced on the 2nd or 3rd day of progesterone supplementation.
Figure 1.
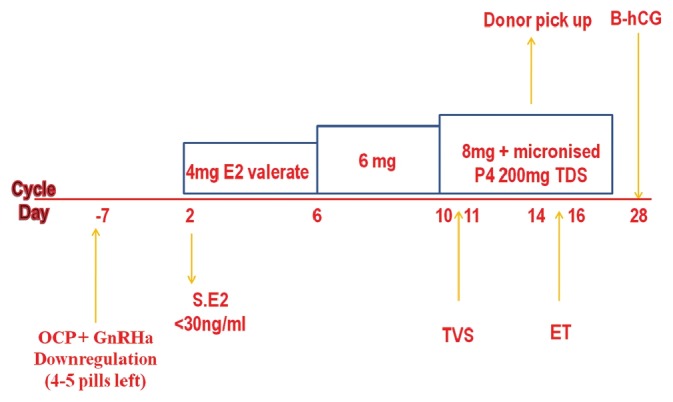
Endometrium preparation protocol for recipients
Each anonymous donor was matched phenotypically with one or two potential recipients so that each recipient received minimum of seven oocytes. The match of a specific donor with a specific recipient was based on chronology of completion of requirements and similarities of physical features. There were no program requirements that contributed to the matching process. Therefore, it is unlikely that any bias existed in the matching process that might account for any of the differences detected between recipient groups. Mature oocytes were classified as metaphase II at the time of aspiration. Intracytoplasmic Sperm Injection (ICSI) was performed for all. The resultant embryos were rated in the laboratory according to their morphologic characteristics and blastomere number on day 2/3 after oocyte retrieval. In the current study only grade 1 embryos were transferred. At 14 days after the embryo transfer, a blood test for ß-hCG assessment was performed and if positive, transvaginal ultrasonography was performed one week later to confirm the presence of a gestational sac. Estrogen and progesterone supplementation was continued until a negative pregnancy test or, if a pregnancy had resulted, through 12 weeks of the pregnancy.
Data were evaluated as chi square analyses with comparative significance determined at P<0.05.
RESULTS
The clinical pregnancy and embryo implantation rates of the oocyte recipient group were 95 of 270 (35.18%) and 150 of 780 (19.23%), respectively, resulting in 48 (50.52%) singleton, 39 (41.05%) twin, and 8 (8.42%) triplet gestations. Of the 95 pregnancies, 85 eventuated in ongoing pregnancies (31.48%).
Clinical pregnancy was defined as the presence of at least one intrauterine gestational sac on ultrasound in the first trimester. The implantation rate was calculated by dividing the maximum number of gestational sacs seen on ultrasound in the first trimester by the number of embryos transferred in the corresponding cycles. Ongoing pregnancy was a pregnancy continued beyond 20 weeks. Miscarriage rate was the difference between the clinical pregnancy and ongoing rates divided by the clinical pregnancy rate.
Recipient age
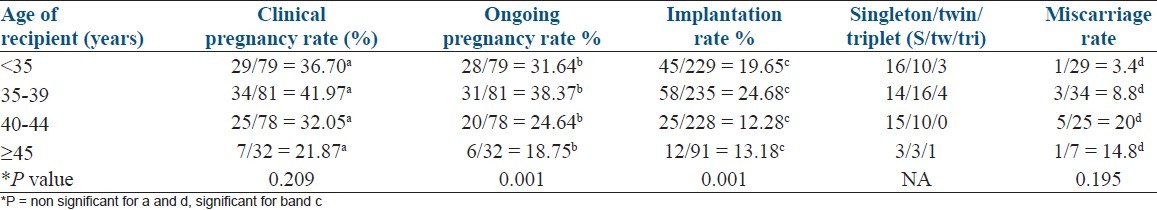
While evaluating the relationship between recipient's age and pregnancy rate in four age groups, a significant decline in the ongoing pregnancy and implantation rate was noted in recipients above 40 years of age (P = 0.001). Clinical pregnancy rate also decreased above 40 but was not found to be statistically significant. Miscarriage rate increased in recipients above 40 years [Table 1].
Table 1.
Clinical pregnancy, implantation rate and ongoing pregnancy rates relative to the age of recipients
Endometrial thickness
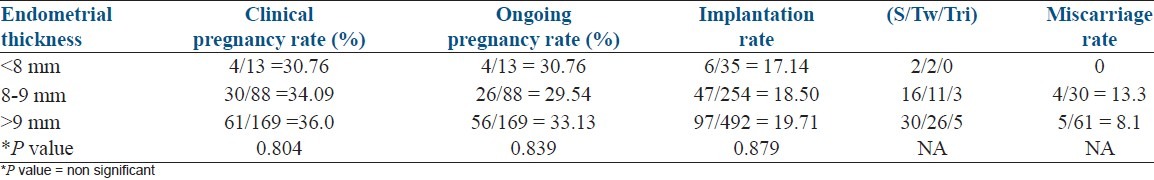
Pregnancy rates of all recipients who underwent embryo transfer were calculated at each measure of endometrial thickness. However, none was statistically significant as shown in Table 2. Embryo transfer was not done in current study if endometrial thickness was less than 6 mm.
Table 2.
Clinical pregnancy, implantation rate, and ongoing pregnancy rates relative to endometrial thickness of recipient
Day of embryo transfer
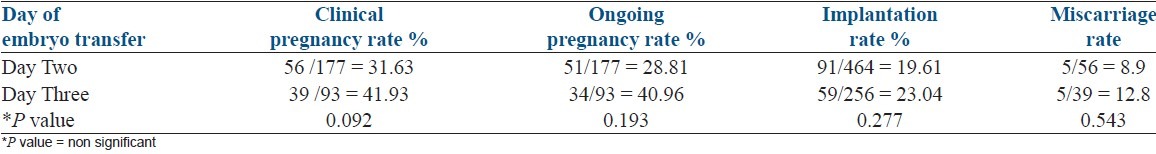
An increase in clinical pregnancy, ongoing pregnancy, and implantation rate with Day 3 embryo transfer was demonstrated, although it was not statistically significant as shown in Table 3.
Table 3.
Clinical pregnancy, implantation rate, ongoing pregnancy rates relative to the day of embryo transfer
Although number of cases with two embryo transfer was less in present study (30 out of 270 cases), transfer of two embryos resulted in lower multiple pregnancy rates with no triplet pregnancies. However, clinical and ongoing pregnancy and implantation rates were not adversely affected [Table 4a, Graph 1].
Table 4a.
Clinical pregnancy, implantation rate, ongoing pregnancy rates relative to the number of embryos transferred

Graph 1.
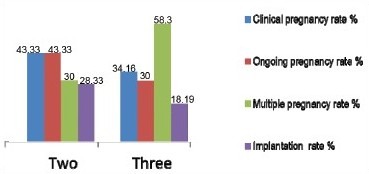
Clinical pregnancy, implantation rate, multiple pregnancy and ongoing pregnancy rates relative to the number of rmbryos transferred
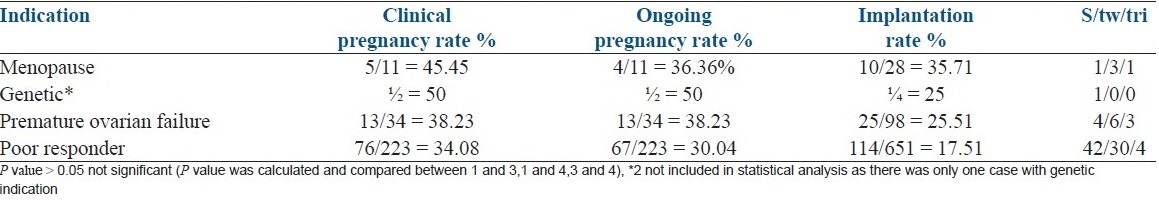
Clinical outcomes according to recipient diagnosis
The variables of clinical pregnancy rate, ongoing pregnancy and implantation rate showed no statistically significant difference between the recipients in the different diagnostic subgroups as shown in Table 4. Poor responders group included patients with advanced maternal age ≥ 40 years, a previous poor ovarian response (one or two episodes of POR after maximal stimulation), low AMH (<1 ng/ml), low AFC (<4-5) and small ovarian volume on baseline sonography. Usually presence minimal two criteria were considered essential before deciding for oocyte donation.
Table 4.
Clinical pregnancy, implantation rate, ongoing pregnancy rates relative to the indication for oocyte donation
Past history
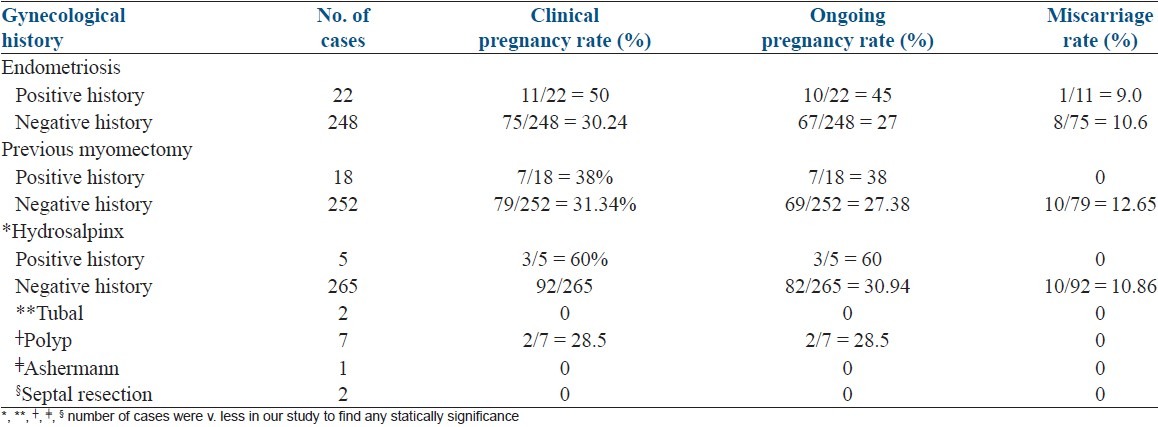
There was no adverse pregnancy outcome in recipients with a history of endometriosis, previous myomectomy. Table 5 shows the impact of various gynecological factors.
Table 5.
Clinical pregnancy, ongoing pregnancy and miscarriage rates relative to past history of gynecological diseases
DISCUSSION
In the current study recipient's age above 40 year, had a negative impact on embryo implantation efficiency and ongoing pregnancy. However, this effect of uterine senescence did not affect the clinical pregnancy rate. Miscarriage rate (MR) was higher in recipients above 40 years. The mean donor age for each of the recipient subgroups was similar (26 years). Average endometrial thickness was same in recipients less than 40 years and above 40 years (9.64 and 9.8, respectively). The Average no. of embryos transferred (<40 years = 2.88, >40 years = 2.89) and their quality was also same in each subgroup. The inverse relation between recipient age and pregnancy outcome seen in current study has been reported by many other investigators.[2–6] A large study of 17,339 recipient cycles aggregated by SART (Society for Assisted Reproductive Technology) and the CDC (Centers for Disease Control and Prevention) derived from donated eggs showed that Pregnancy Rate (PR) and Implantation Rate (IR) did not vary until patient age reached the ‘′late forties′’ and decreased significantly from this time on (even more clearly from 50 years onward). In addition, the increase in MR was close to significant. The obvious disadvantages associated with analyzing data collected from a large number of centers (a lack of control over the protocols and documentation of the information used).[2] A recent large single center study of 3089 cycles from Valencia concluded that the pregnancy and implantation rate were significantly lower from 45 years onwards and even worse after 50 years. In this series, the miscarriage rate increase was also significant.[3] A significant decline in the implantation rate associated with increasing recipient's age has also been reported by other authors.[1,5] Diminished uterine receptivity is one attractive possibility for these results. This might be due to reduced uterine blood flow with increased age.[6,7] or a decreased sensitivity to progesterone effects.[8] Histologic, ultrastructural, and biochemical changes like subepithelial extracellular matrix deposition, stromal angiosclerosis, which become more common with age.[2,6] The implantation rate, which is a far more sensitive and accurate dependent variable, was not analyzed in studies that denied an age related effect of the uterus.[9–13] However, ongoing surveillance is needed to ascertain any effect of recipient age on other clinical outcomes.
Endometrial thickness
In the current study, pregnancy rate was maximum in cycles where the endometrial thickness measured ≥9 mm and least when it was less than 8 mm but it was not statistically significant. No pregnancies occurred in cycles where endometrial thickness was less than 7 mm. Multiple pregnancy rates and miscarriage rate was also not significantly different among groups. In a study of 343 recipient cycles studied over a period of 3 years, cycles where endometrium measured ≥9 had greatest pregnancy rate, significantly higher than in those cycles where ET was ≥8 mm. A thin endometrium of less than 6 mm resulted in poor pregnancy rate.[12] In a study of 134 recipient cycles with discordant outcome, comparison between pregnant and nonpregnant subgroups showed that none of the pregnant recipients had endometrium less than 8 mm, whereas lining <8 mm was found in six failed cycles (14.5%).[14] However, in a large study of 3089 recipient cycles, no specific endometrial thickness significantly determined cycle prognosis in terms of PR,IR, or MR. Even a thin endometrium(<6 mm) had good PR and IR, without an increased MR.[3] These findings confirm data previously published by others.[15] Pregnancies and live birth have been reported to occur even at 4 mm endometrium.[12,15] So the exact limit below which implantation is unlikely to occur has been hard to define. No significant association was found between endometrial thickness and implantation rate but pregnancy outcome was improved in patients showing an endometrial lining thickness >8 mm.[16] Endometrial pattern in artificially prepared cycles does not influence pregnancy prognosis.[12]
Indication
Oocyte donation is an invaluable therapeutic option for a growing list of clinical indications, yielding excellent results. In the current study, most common indication for oocyte donation was poor responders. Pregnancy outcome does not depend on the indication for oocyte donation.[13,17,18]
Day and number of embryo transfer
In the current study, day three embryo transfers showed an increase in clinical pregnancy and ongoing pregnancy rate as compared to day two transfer, but the difference is not statistically significant. Average numbers of embryos transferred in day three vs. day two group were 2.7 and 2.9, respectively. Embryo quality was constant. Mean age of the recipients in both groups was also same (37.5 and 37.6). The Cochrane review 2009 on day 3 vs. day 2 embryo transfer following IVF/ICSI has not shown any increase in live birth, although increase in clinical pregnancy rate was found.[19]
Increasing the number of embryos transferred was associated with an overall significantly enhanced multiple pregnancy rates. Twin pregnancies were significantly higher when three embryos were transferred. There were no triplets when two embryos were transferred.[16]
Previous history of myomectomy and endometriosis in the recipient has not affected the pregnancy outcome in our study. Endometriosis may be relevant in the context of a natural cycle, but its possible negative impact seems to be overcome by standard endometrial priming protocols used in OD cycles. The same may be true for adenomyosis.[20,21]
Other recipient-related variables with adverse affect on pregnancy outcome in OD programme, reported in literature include chemotherapy and radiotherapy,[13] Asherman's syndrome,[1] and hydrosalpinx.[1,17] Endometrial patterns, serum oetradiol levels, transfer difficulty are not studied in the current study, but none of these have been reported to be useful in predicting the success oocyte donation cycles.[12,14,15] The relevance of a smoking habit and the exact influence of a high BMI is still understudy.[6]
Limitation of current study is that pregnancies were followed only till 20 wk, which does not guarantee live birth. Also, pregnancy associated complications were not studied.
CONCLUSION
Our study shows that lower implantation and pregnancy rates in recipients begin above the age of 40 years and become pronounced after the age of 45 years. A leading possibility is declining endometrial receptivity. Endometrial thickness is not a useful predictor of success in oocyte donation cycles however ideal endometrial thickness before transfer is found to be ≥8 mm. Transfer of two selected embryos reduces the occurrence of multiple pregnancies. Embryo transfer on day two or three does not influence the pregnancy outcome. Indication for oocyte donation, previous history of myomectomy and endometriosis in recipients do not affect the pregnancy outcome.
ACKNOWLEDGMENTS
I thank Dr. Manish Banker and Dr. Pravin Patel, Medical Directors of The Pulse women's Hospital, Ahmedabad and Nova Pulse IVF Clinics, for their guidance and support. I thank Dr. Bharat Joshi, Chief of Embryology of The Pulse women's Hospital, Ahmedabad and Nova Pulse IVF Clinics, for the constant guidance, encouragement and supervision in completing this study. I thank Dr. Sangeeta Gulati, Lecturer in SPM Department at Dayanand Medical College Ludhiana, for her great help in calculating statistics. I also thank all the patients for their willingness and support for this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Moomjy M, Cholst I, Mangieri R, Rosenwaks Z. Oocyte donation: Insights into implantation. Fertil Steril. 1999;71:15–21. doi: 10.1016/s0015-0282(98)00420-8. [DOI] [PubMed] [Google Scholar]
- 2.Toner JP, Grainger DA, Frazier LM. Clinical outcomes among recipients of donated eggs: An analysis of the U.S. national experience, 1996–1998. Fertil Steril. 2002;78:1038–45. doi: 10.1016/s0015-0282(02)03371-x. [DOI] [PubMed] [Google Scholar]
- 3.Reis Soares S, Troncoso C, Bosch E. Age and uterine receptiveness: Predicting the outcome of Oocyte donation cycles. J Clin Endocrinol Metab. 2005;90:4399–404. doi: 10.1210/jc.2004-2252. [DOI] [PubMed] [Google Scholar]
- 4.Yaron Y, Ochshorn Y, Amit A, Kogosowski A, Yovel I, Lessing JB. Oocyte donation in Israel: A study of 1001 initiated treatment cycles. Hum Reprod. 1998;13:1819–24. doi: 10.1093/humrep/13.7.1819. [DOI] [PubMed] [Google Scholar]
- 5.Borini A, Bianchi L, Violini F, Maccolini A, Cattoli M, Flamigni C. Oocyte donation program (pregnancy and implantation rates in women of different ages sharing oocytes from single donor) Fertil Steril. 1996;65:94–7. doi: 10.1016/s0015-0282(16)58033-9. [DOI] [PubMed] [Google Scholar]
- 6.Soares SR, Velasco JA, Fernandez M, Bosch E, Remoh J, Pellicer A, et al. Clinical factors affecting endometrial receptiveness in oocyte donation cycles. Fertil Steril. 2008;89:491–501. doi: 10.1016/j.fertnstert.2008.01.080. [DOI] [PubMed] [Google Scholar]
- 7.Meldrum DR. Female reproductive aging—ovarian and uterine factors. Fertil Steril. 1993;59:1–5. doi: 10.1016/s0015-0282(16)55608-8. [DOI] [PubMed] [Google Scholar]
- 8.Steer CV, Tan SL, Mason BA, Campbell S. Midluteal-phase vaginal color Doppler assessment of uterine artery impedance in a subfertile population. Fertil Steril. 1994;61:53–8. doi: 10.1016/s0015-0282(16)56452-8. [DOI] [PubMed] [Google Scholar]
- 9.Paulson R, Hatch I, Lobo R, Sauer M. Cumulative conception and live birth rates after oocyte donation (implications regarding endometrial receptivity) Hum Reprod. 1997;12:835–9. doi: 10.1093/humrep/12.4.835. [DOI] [PubMed] [Google Scholar]
- 10.Abdalla H, Wren M, Thomas A, Korea L. Age of the uterus does not affect pregnancy or implantation rates; a study of egg donation in women of different ages sharing oocytes from the same donor. Hum Reprod. 1997;12:827–9. doi: 10.1093/humrep/12.4.827. [DOI] [PubMed] [Google Scholar]
- 11.Remohi J, Gartner B, Gallardo E, Yalil S, Simon C, Pellicer A. Pregnancy and birth rates after oocyte donation. Fertil Steril. 1997;67:717–23. doi: 10.1016/s0015-0282(97)81372-6. [DOI] [PubMed] [Google Scholar]
- 12.Noyes N, Hampton BS, Berkeley A, Licciardi F, Grifo J, Krey L. Factors useful in predicting the success of oocyte donation: A 3-year retrospective analysis. Fertil Steril. 2001;76:9–27. doi: 10.1016/s0015-0282(01)01823-4. [DOI] [PubMed] [Google Scholar]
- 13.Sauer MV, Paulson RJ, Ary BA, Lobo RA. Three hundred cycles of oocyte donation at the University of Southern California: Assessing the effect of age and infertility diagnosis on pregnancy and implantation rates. J Assist Reprod Genet. 1994;11:92–6. doi: 10.1007/BF02215994. [DOI] [PubMed] [Google Scholar]
- 14.Zenke U, Chetkowski RJ. Transfer and uterine factors are the major recipient-related determinants of success with donor eggs. Fertil Steril. 2004;82:850–6. doi: 10.1016/j.fertnstert.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 15.Remohí J, Ardiles G, García-Velasco J, Gaitán P, Simón C, Pellicer A. Endometrial thickness and serum oestradiol concentrations as predictors of outcome in oocyte donation. Hum Reprod. 1997;12:2271–6. doi: 10.1093/humrep/12.10.2271. [DOI] [PubMed] [Google Scholar]
- 16.Mirkin S, Gimeno TG, Bovea C, Stadtmauer L, Gibbons WE, Oehninger S. Factors associated with an optimal pregnancy outcome in an oocyte donation program. J Assist Reprod Genet. 2003;20:400–8. doi: 10.1023/A:1026236726568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen MA, Lindheim SR, Sauer MV. Hydrosalpinges adversely affect implantation in donor oocyte cycles. Hum Reprod. 1999;14:1087–9. doi: 10.1093/humrep/14.4.1087. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Velasco JA, Isaza V, Caligara C, Pellicer A, Remohi J, Simon C. Factors that determine discordant outcome from shared oocytes. Fertil Steril. 2003;80:54–60. doi: 10.1016/s0015-0282(03)00545-4. [DOI] [PubMed] [Google Scholar]
- 19.Oatway C C, Gunby J, Daya S. Day three versus day two embryo transfer following in vitro fertilization or intracytoplasmic sperm injection. Cochrane Database Syst Rev. 2004;(2):CD004378. doi: 10.1002/14651858.CD004378.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Diaz I, Navarro J, Blasco L, Simon C, Pellicer A, Remohi J. Impact of stage III-IV endometriosis on recipients of sibling oocytes: Matched case-control study. Fertil Steril. 2000;74:31–4. doi: 10.1016/s0015-0282(00)00570-7. [DOI] [PubMed] [Google Scholar]
- 21.Sung L, Mukherjee T, Takeshige T, Bustillo M, Copperman A. Endometriosis is not detrimental to embryo implantation in oocyte recipients. J Assist Reprod Genet. 1997;14:152–6. doi: 10.1007/BF02766132. [DOI] [PMC free article] [PubMed] [Google Scholar]


