Abstract
OBJECTIVE:
To compare Letrozole (5 mg) and clomiphene citrate (100 mg) as first line ovulation induction drug in infertile PCOS women.
STUDY DESIGN:
Prospective Randomised trial.
SETTING:
A Tertiary level infertility centre. Patients: 103 infertile PCOS women
INTERVENTION(S):
Treatment naïve infertile PCOS women were randomised to treatment with 5 mg letrozole (51 patients) or 100 mg clomiphene citrate (52 patients) daily starting day 2 to day 6 of menstrual cycle. Timed intercourse or Intra Uterine Insemination (IUI) was advised 24 to 36 hours after Human Chorionic Gonadotropin (HCG) injection.
MAIN OUTCOME MEASURES:
Ovulation rate, mono or multi follicular rate, days to ovulation, endometrial thickness, serum progesterone, serum estrogen, pregnancy rate, miscarriage rate.
RESULTS:
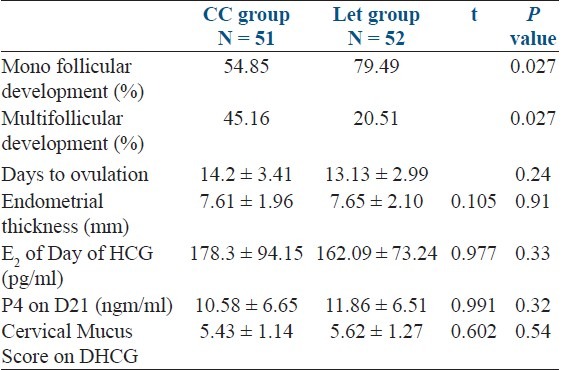
The mean age, Body Mass Index (BMI), duration of infertility in both Clomiphene Citrate (CC) and Letrozole groups were similar.Ovulation rate was 73.08% in letrozole group and 60.78% in CC, which was not statistically significant (P=0.398). There was no statistically significant difference between Endometrial thickness (CC 7.61 ±1.96, Let 7.65 ± 2.10), Sr E2 on day of HCG (CC 178.3 ± 94.15, Let 162.09 ± 73.24), Days to ovulation (CC 14.2 ± 3.41; Let 13.13 ± 2.99) and Sr P4 on D21 (CC 10.58 ± 6.65; Let 11.86 ± 6.51). Monofolliculo genesis (CC 54.84, Let 79.49 %, P=0.027) and Pregnancy rate (CC 7.84%, Let 21.56% P=0.0125) were statistically significantly higher in letrozole group.
CONCLUSION:
Our study shows that letrozole has excellent pregnancy rates compared to clomiphene citrate. Letrozole should be considered at par with clomiphene citrate as first line drug for ovulation induction in infertile PCOS women.
KEY WORDS: Clomiphene citrate, letrozole, ovulation induction, PCOS
INTRODUCTION
Polycystic ovary syndrome (PCOS) is the commonest endocrinopathy resulting in anovulatory infertility in young women. Recent years have seen a significant rise in women presenting with PCOS and a concomitant spurt in scientific interest to understand the syndrome.
There are many clinical manifestations of the syndrome, and infertility due to chronic anovulation is one of the commonest.[1–3] Clomiphene citrate (CC) is a long-standing, standard drug for ovulation induction and is still considered as first-line option in PCOS women.[4,5] However, clomiphene has certain well-defined disadvantages. Treatment with CC is associated with discrepancy in ovulation and pregnancy rates (60-85%; 10-20%). Miscarriage rate is higher than general population,[6,7] and 20-25% PCOS women are resistant to clomiphene.[8,9] Anti-estrogenic effect of CC leads to prolonged depletion of estrogens receptors, adversely affecting endometrial growth and development as well as quantity and quality of cervical mucus.
Letrozole is an orally-active aromatase inhibitor, with good potential for ovulation induction. It has been in use for few years now, and numbers of researchers have studied this molecule as an option for ovulation induction.[8,10] Letrozole acts by reducing estrogen production by blocking androgens to estrogens conversion. Additionally, it has no adverse effect on endometrium and cervical mucus. In India, letrozole was approved for ovulation induction from 2006 to 2011 by the Drug controller general of india (DCGI). Letrozole has been shown to have good ovulation rate in CC-resistant PCOS women.[11] Indian PCOS women have high prevalence of insulin resistance[12] and thus are likely to have high CC resistance. Letrozole could prove to be a good alternative for ovulation induction in such women.
This prospective randomized trial was carried out to compare the effects of 5 mg of letrozole with 100 mg CC as first-line ovulation induction drug in treatment-naive infertile PCOS women.
MATERIALS AND METHODS
This study was conducted at a private hospital with a large gynecological practice. The prospective randomized trial was conducted between July 2010 and July 2011. Study protocol was approved by the institutional ethics committee.
103 infertile PCOS women were recruited from outpatient department. A single consultant (author) was responsible for diagnosis and recruitment of the patients. PCOS was diagnosed according to Rotterdam criteria. All women were treatment-naοve i.e. had not undergone any significant treatment for infertility/ovulation induction earlier. Patients with hyper prolactinemia, thyroid disorder, male factor, suspected tubal factor, endometriosis, unexplained infertility were not included in the study. Patients were randomized by lottery to receive either 100 mg of CC or 5 mg of letrozole daily for 5 days starting Day2 of menstrual cycle. Follicular monitoring was done by transvaginal sonography starting day 8 of menstrual cycle till a follicle attained 17-18 mm diameter. A single injection of HCG 10,000 IU was given if at least one follicle attained 17-18 mm. Cervical mucus was scored on day of HCG according to a cervical mucus scoring criteria [Table 1]. Timed intercourse or IUI was advised as per the patient requirement after 24-36 hrs of HCG. A final scan after 48 hrs was done for all patients to confirm rupture of follicle. If not ruptured, a repeat scan was done after 72 hrs to diagnose luteinized unruptured follicle. Ovulation was confirmed by sonographic finding and day 21 progesterone. Serum E2 on day of HCG and serum P4 on day 21 was done for all patients. Luteal support (duphastone 10 mg daily) was given to all patients. Urine Serum beta human chorionic gonadotropin (βHCG) was done after one week of missed periods. Ongoing pregnancy diagnosed following visualization of cardiac activity by TVS. Women who failed to ovulate with 100 mg cc or 5 mg letrozole were dropped from the study.
Table 1.
Cervical mucus scoring criteria
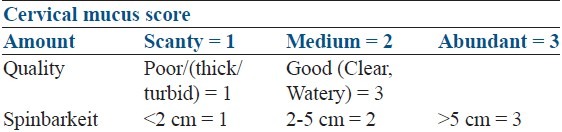
Primary outcome measures were ovulation rate, endometrial thickness, mono Vs. multifollicular rate, and days to ovulation. Secondary outcome measures were pregnancy and miscarriage rate. Chi-square and student's ‘t’ test was carried out for statistical analysis.
Results were expressed as mean and standard deviation of the mean. A P value of < 0.05 was considered significant.
RESULTS
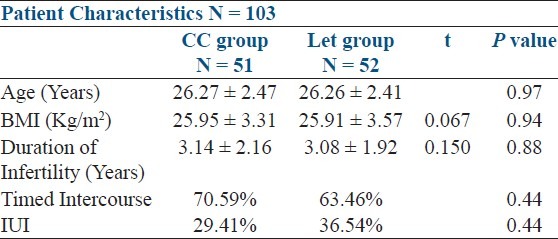
The study comprised of 103 patients; 51 patients in the CC group and 52 patients in the letrozole group. Age, duration of infertility, BMI, presenting signs and symptoms were similar in both groups [Table 2].
Table 2.
Patient characteristics of both CC and Let groups
The rate of multiple follicular development was statistically significantly, greater in the CC group (CC 45.16%, Let 20.51%, P = 0.027).
The ovulation rate was 60.78% in CC group and 73.08% in Let group (P = 0.39). There was no statistically significant difference between the two groups in endometrial thickness on the day of HCG (CC 7.61 ± 1.96 mm, Let 7.65 ± 2.10 mm P = 0.91). Similarly, there was no statistically significant difference between the two groups in serum E2 on day of HCG, cervical mucus score, days to ovulation, and serum P4 on D21 [Table 3].
Table 3.
Outcome of ovarian stimulation
Pregnancy occurred in 4 out of 51 (7.84%) in CC group and in 11 out of 52 (21.56%) in the Let group, the difference was highly statistically significant (P = 0.015) [Table 4].
Table 4.
Outcome of treatment
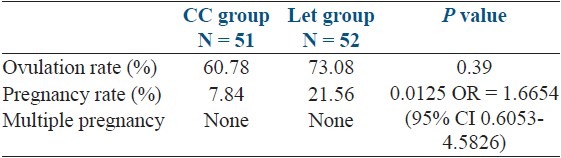
There were no twin pregnancies in either category. There was 1 second trimester pregnancy loss in CC group. Two patients were lost to follow-up in the letrozole group.
DISCUSSION
Clomiphene citrate (CC) has been used for ovulation induction since 1960s. It is still considered first-line drug for anovulatory PCOS women. However, clomiphene resistance (15-20%), endometrial thinning, and poor cervical mucus (15-50% of cases) makes it ineffective in many situations.[13–17] Letrozole, which is an aromatase inhibitor, has been explored as a good alternative by many researchers, but the evidence about its efficacy as compared to clomiphene is conflicting.
Letrozole creates an estrogen-deficient environment by blocking conversion of androgens to estrogens. This releases the pituitary from negative feedback of estrogens and releases FSH. Also, an added positive effect is increased follicular sensitivity to FSH through amplification of FSH receptor gene expression.[18–21] Thus, ovulation induction by letrozole should be better than by CC in terms of follicular growth and endometrial development.
Letrozole has also been shown to be effective in ovulation induction in CC-resistant PCOS women.[11] Hyper-insulinemia, which is closely associated with PCOS, is thought to be one of the causative factors for CC resistance. The prevalence of insulin resistance in PCOS is close to 50%.[22] This could be one more reason for letrozole to be a better first-line drug compared to clomiphene citrate.
In our study, ovulation rate was 60.78% with CC and 73.08% with letrozole, which was not statistically significant (P = 0.398). Others reported similarly, Badawy et al.[23] (CC 70.9%, Let 67.5%), Bayer et al.[24] (CC 74.7%, Let 65.7%), and M. Zeinalzadeh et al.[25] (CC 72%, Let 86%). In majority of the studies, no statistically significant difference is found between CC and letrozole in ovulation rate. Multi- follicular development was statistically significantly higher in outstudy (CC 45.16%, Let 20.51%, P = 0.027). This is expected and corroborated by number of studies.[23–25] Letrozole resulted in mono-folliculogenesis in 79.49% of cases, which is optimal for ovulation induction in PCOS women. However, where multiple follicular development is needed, letrozole may be inadequate.
The mean endometrial thickness was slightly higher in letrozole group, 7.65 ± 2.1 compared to CC 7.61 ± 1.96. Badawy et al.[23] in their study of 438 patients with 1063 cycles, one of the largest studies comparing CC and letrozole, reported statistically significantly higher endometrial thickness in CC group (9.2 ± 0.7) vs. letrozole (8.1 ± 0.2, P = 0.021). They attributed this effect to greater number of mature follicles and higher serum E2 levels. Mitwally and Casper[8] found letrozole associated with greater endometrial thickness. Cortinez et al.[26] found normal morphologic features of endometrium and full expression of pinopodes during implantation window when letrozole was used. Few studies have shown no significant difference between the two groups with regard to effect on endometrium.[27,28] In a recent study by Banerjee et al.,[29] 147 Indian women with PCOS were compared between letrozole (2.5 mg) Vs. clomiphene (100 mg). Mean endometrial development was 8.72 ± 11.41 mm in letrozole and 8.78 ± 1.16 mm in CC group (P = 0.004).
Pregnancy rate per cycle was astonishingly high with letrozole in our study (21.56%) Vs. (7.84%) (P = 0.015) Badawy et al.,[23] , with 438 women (1063 cycles), reported slightly better pregnancy rate in CC group (15.1%) letrozole and 17.9 % in CC group). Bayer et al.,[24] with 74 women, Zeinalzaden et al.,[25] with 107 women, both reported slightly better pregnancy rates with letrozole; however, no statistically significant difference between the two groups.
In a meta-analysis by He and Jiang,[30] the clinical efficacy and safety of letrozole was compared with clomiphene for ovulation induction in PCOS women. This is one of the largest meta-analysis of the subject published. Six RCTs involving 841 patients were analyzed. There were no significant differences in pregnancy rate, abortion rate, and multiple pregnancy rate between the two groups. The evidence from ovulation rate was not enough to support either of the drugs.
The high pregnancy rate in our study could be because of high prevalence of insulin resistance and central visceral obesity in Indian PCOS women, which predisposes to clomiphene resistance. Ganesh et al.[11] published the largest series of 1387 Indian PCOS women with clomiphene resistance. They were randomized to receive letrozole, CC + FSH or only FSH. Letrozole group had an ovulation rate of 79.30% and pregnancy rate of 23.39%. PR in the letrozole only group was highest. A similar study by Begum et al.[31] from Bangladesh, on 64 PCOS women who had failed to ovulate with CC 100 mg, showed a high PR of 40.63% with letrozole 7.5 mg compared to 15% with CC 150 mg.
CONCLUSION
Our study showed statistically significantly higher mono-follicular development and pregnancy rates when used as first-line ovulation induction drug in infertile PCOS women. This enhanced response to letrozole could be related to ethnic differences in PCOS women. More Indian studies are needed to find the correlation, if any, between hyper-insulinemia, central obesity, and CC resistance in PCOS women.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Kovacs G, Wood C. The current status of polycystic ovary syndrome. Aust N Z J Obstet Gynaecol. 2001;41:65–8. doi: 10.1111/j.1479-828x.2001.tb01296.x. [DOI] [PubMed] [Google Scholar]
- 2.Guzick D. Polycystic ovary syndrome, symptolatology, pathophysiology and epidemiology. Am J Obstet Gynecol. 1998;179:89–93. doi: 10.1016/s0002-9378(98)70238-8. [DOI] [PubMed] [Google Scholar]
- 3.Slowey MJ. Polycystic ovary syndrome: New perspective on an old problem. South Med J. 2001;94:190–5. [PubMed] [Google Scholar]
- 4.Casper RF. Letrozole versus clomiphene citrate: Which is better for ovulation induction? Fertil Steril. 2009;92:858–9. doi: 10.1016/j.fertnstert.2007.03.094. [DOI] [PubMed] [Google Scholar]
- 5.Amsterdam ESHRE/ASRM – Sponsored 3rd PCOS consensus workshop Group. Consensus on women's health aspects of polycystic ovary syndrome (PCOS) Hum Reprod. 2012;27:14–24. [Google Scholar]
- 6.Franks S, Adams J, Mason H, Polson D. Ovulatory disorders in women with polycystic ovary syndrome. Clin Obstet Gynecol. 1985;12:605–32. [PubMed] [Google Scholar]
- 7.Kistner RW. Induction of ovulation with clomiphene citrate (clomid) Obstet Gynecol Surv. 1965;20:873–900. doi: 10.1097/00006254-196512000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Mitwally MF, Casper RF. Use of an aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil Steril. 2001;75:305–9. doi: 10.1016/s0015-0282(00)01705-2. [DOI] [PubMed] [Google Scholar]
- 9.Wessman A, McArdle CR, Achiron R. Ultrasound in infertility. In: Machelle M, Seibel MD, editors. Infertility-a comprehensive text. 2nd ed. Stamford CT: Appleton and Lange; 1996. pp. 447–92. [Google Scholar]
- 10.Sammour A, Biljan MM, Tan SL, Tulandi T. Prospective randomized trial comparing the effects of letrozole and clomiphene citrate (CC) on follicular development, endometrial thickness and pregnancy rate in patients undergoing superovulation prior to intrauterine insemination. 57th Annual Meeting of the American Society for Reproductive Medicine. Orlando, FL: 2001. (Abstract O-291) Fertil Steril. 2001;76(Suppl 1):S110. [Google Scholar]
- 11.Ganesh A, Goswami SK, Chattopadhyay R, Choudhury K, Chakravarty B N. Comparison of letroz with continuous gonadotropins and clomiphene gonadotropin combination for ovulation induction. In 1387 PCOS women after clomiphene citrate failure: A randomized prospective clinical trial. J Assist Reprod Genet. 2009;26:19–24. doi: 10.1007/s10815-008-9284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandit K, Goswami S, Ghosh S, Mukhopadhyay P, Chowdhury S. Insulin resistance in metabolic syndrome in Indian PCOS South Asians. Indian J Endocrinal Metab. 2012;16:44–55. doi: 10.4103/2230-8210.91187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonen Y, Casper RF. Sonographic determination of a possible adverse effect of clomiphene citrate on endometrial growth. Hum Reprod. 1990;5:670–4. doi: 10.1093/oxfordjournals.humrep.a137165. [DOI] [PubMed] [Google Scholar]
- 14.Yagel S, Ben-Chetrit A, Anteby E, Zacut D, Hochner-Celnikier D, Ron M. The effect of ethinyl estradiol on endometrial thickness and uterine volume during ovulation induction by clomiphene citrate. Fertil Steril. 1992;57:33–6. doi: 10.1016/s0015-0282(16)54772-4. [DOI] [PubMed] [Google Scholar]
- 15.Dickey RP, Olar TT, Taylor SN, Curole DN, Matulich EM. Relationship of endometrial thickness and pattern to fecundity in ovulation induction cycles: Effect of clomiphene citrate alone and with human menopausal gonadotropin. Fertil Steril. 1993;59:756–60. doi: 10.1016/s0015-0282(16)55855-5. [DOI] [PubMed] [Google Scholar]
- 16.Opsahl MS, Robins ED, O’Connor DM, Scott RT, Fritz MA. Characteristics of gonadotropin response, follicular development, and endometrial growth and maturation across consecutive cycles of clomiphene citrate treatment. Fertil Steril. 1996;66:533–9. [PubMed] [Google Scholar]
- 17.Eden JA, Place J, Carter GD, Jones J, Alaghband-Zadeh J, Pawson ME. The effect of clomiphene citrate on follicular phase increase in endometrial thickness and uterine volume. Obstet Gynecol. 1989;73:187–90. [PubMed] [Google Scholar]
- 18.Weil SJ, Vendola K, Zhou J, Adesanya OO, Wang J, Okafor J, et al. Aandrogen receptor controlled ovarian hyperstimulation in the primate ovary: Cellular localization, regulation, and functional correlation. J Clin Endocrinol Metab. 1998;83:2479–85. doi: 10.1210/jcem.83.7.4917. [DOI] [PubMed] [Google Scholar]
- 19.Weil SJ, Vendola K, Zhou J, Bondy CA. Androgen and follicle stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab. 1999;84:2951–6. doi: 10.1210/jcem.84.8.5929. [DOI] [PubMed] [Google Scholar]
- 20.Vendola K, Zhou J, Wang J, Famuyiwa OA, Bienre M, Bondy CA. Androgens promote oocyte insulin-like growth factor I expression and initiation of follicle development in the primate ovary. Biol Reprod. 1999;61:353–7. doi: 10.1095/biolreprod61.2.353. [DOI] [PubMed] [Google Scholar]
- 21.Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgen stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;101:2622–9. doi: 10.1172/JCI2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glintborg D, Menriksen JE, Andresen M, Hagen C, Hangaard J, Rasmussen PE, et al. Prevalence of endocrine diseases and abnormal glucose tolerance tests in 340 Caucasian premenopausal women with hirsutism as referral diagnosis. Fertil steril. 2004;84:1570–9. doi: 10.1016/j.fertnstert.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 23.Badawg A, Aal IA, Abulalta M. Climiphene Citrate or Letrozole for ovulation induction in women with polycystic ovarian syndrome: A prospective randomized trial. Fertil Steril. 2009;92:849–52. doi: 10.1016/j.fertnstert.2007.02.062. [DOI] [PubMed] [Google Scholar]
- 24.Bayar U, Tanriverdi HA, Aykut B, Ayoglu F, Ozcan O, Erdal K. Letrozole vs clomiphene citrate in patients with ovulatory infertility. Fertil Steril. 2006;85:1045–8. doi: 10.1016/j.fertnstert.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 25.Zeinalzadeh M, Basirat Z, Esmalpour M. Efficacy of letrozole in ovulation indication compared to that of clomiphene citrate in patients with polycystic ovarian syndrome. J Reprod Med. 2010;55:36–40. [PubMed] [Google Scholar]
- 26.Cortinez I, De Carvaiho D, Vantman F, Gabler G, Iniguez R, Vega M. Hormonal profile and endometrial morphology in letrozole-controlled ovarian hyperstimulation in ovulatory infertile patients. Fertil Steril. 2005;83:110–5. doi: 10.1016/j.fertnstert.2004.05.099. [DOI] [PubMed] [Google Scholar]
- 27.Fisher SA, Reid RL, Van Vugt DA, Casper RF. A randomized double-blind comparison of the effects of clomiphene citrate and the aromatase inhibitor on ovulatory function in normal women. Fertil Steril. 2002;78:280–5. doi: 10.1016/s0015-0282(02)03241-7. [DOI] [PubMed] [Google Scholar]
- 28.Kilic-Okman T, Kucuk M, Altaner S. Comparison of the effects of letrozole and clomiphene citrate on ovarian follicles, endometrium, and hormone levels in the rat. Fertil Steril. 2003;80:1330–2. doi: 10.1016/j.fertnstert.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Banerjee Ray P, Ray A, Chakroborti PS. Comparison of efficacy of letrozole and clomiphene citrate in ovulation induction in Indian women with polycystic ovarian syndrome. Arch Gynecol Obstet. 2012;285:873–7. doi: 10.1007/s00404-011-2091-7. [DOI] [PubMed] [Google Scholar]
- 30.He D, Jiang F. Meta analysis of letrozole versus clomiphene citrate in polycystic ovary syndrome. Reprod Biomed Online. 2011;23:91–6. doi: 10.1016/j.rbmo.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 31.Begum MR, Ferdous J. Comparison of efficacy of aromatase inhibitor and clomiphene citrate in induction of ovulation in polycystic ovarian syndrome. Fertil Steril. 2009;92:853–7. doi: 10.1016/j.fertnstert.2007.08.044. [DOI] [PubMed] [Google Scholar]


