Abstract
OBJECTIVES:
To evaluate whether three daily doses of GnRH agonist (Inj. Lupride 1 mg SC) administered 6 days after oocyte retrieval increases ongoing pregnancy rates following embryo transfer (ET) in cycles stimulated with the long GnRH agonist protocol.
SETTINGS AND DESIGN:
Prospective randomized controlled study in a tertiary care center.
MATERIALS AND METHODS:
Four hundred and twenty six women undergoing ET following controlled ovarian stimulation with a long GnRH agonist protocol were included. In addition to routine luteal-phase support (LPS) with progesterone, women were randomized to receive three 1 mg doses of Lupride 6 days after oocyte retrieval. Computer-generated randomization was done on the day of ET. Ongoing pregnancy rate beyond 20th week of gestation was the primary outcome measure. The trial was powered to detect a 13% absolute increase from an assumed 27% ongoing pregnancy rate in the control group, with an alpha error level of 0.05 and a beta error level of 0.2.
RESULTS:
There were 59 (27.69%) ongoing pregnancies in the GnRHa group, and 56 (26.29%) in the control group (P = 0.827). Implantation, clinical pregnancy and multiple pregnancy rates were likewise similar in the GnRHa and placebo groups.
CONCLUSIONS:
Three 1 mg doses of Lupride administration 6 days after oocyte retrieval in the long protocol cycles does not result in an increase in ongoing pregnancy rates.
KEY WORDS: GnRH agonist, implantation rate, luteal-phase adjuvant, ongoing pregnancy
INTRODUCTION
Luteal-phase deficiency occurs frequently in ovulation induction cycles using pituitary down-regulation with a GnRH agonist as well as in those using GnRH antagonists for ART.[1–4] To cope with this problem, different regimens of luteal-phase support have been suggested.[2] Most of these regimens involve use of two therapeutic agents, HCG and progesterone, used separately or together in different doses and routes of administration.[2] Adjuvants have been tried to further better the luteal support with either of these two agents used primarily for luteal support such as estradiol, aspirin, vitamin C etc., Some recent data, however, have suggested a beneficial effect of GnRH agonist administered in the luteal phase on ART outcomes.[5–10] The mechanism of the presumed beneficial effect of luteal-phase GnRH agonist administration is not clear and may be due to the drug action at multiple levels. It was hypothesized that GnRH agonist may support the corpus luteum by stimulating the secretion of LH by pituitary gonadotroph cells or by acting directly on the endometrium through the locally expressed GnRH receptors or by directly acting on the embryo itself.[6] The administration of a single dose of GnRH agonist in the luteal phase was also shown to increase pregnancy, implantation, delivery and birth rates in recipients of donated oocytes in whom ovulation was suppressed and the corpus luteum was thus absent, suggesting a direct effect of GnRH agonist on the endometrium or the embryo.[5] Despite stressing the importance of this finding, properly conducted trials to evaluate the reproducibility of these results were advised before widespread adoption of this simple yet seemingly effective strategy.[11]
In this report, we present a prospective randomized, controlled trial aimed to assess the effect of a three 1 mg doses of Inj. Lupride, administered 6 days after oocyte retrieval, on the probability of ongoing pregnancy rates following embryo transfer (ET) in cycles stimulated with a long GnRH agonist protocol.
MATERIALS AND METHODS
This prospectively randomized study was conducted from October 2010 to October 2011. Four hundred and twenty six infertile couples IVF/ICSI in a long agonist downregulation protocol and meeting the inclusion criteria were included. Patients were randomly assigned to two groups. In the study group, patients received three doses of GnRH agonist (Inj. Lupride 1 mg SC), starting from day 6 after oocyte retrieval, along with progesterone support in the luteal phase. Progesterone support was given as natural micronized progesterone vaginally in dose of 400 mg twice a day alternating with injectable natural micronized progesterone 100 mg intramuscularly starting from the day of oocyte pickup. Women in other group (control group) received only progesterone support in the luteal phase after IVF/ICSI. Approval for the study was obtained from the Hospital Ethics Committee.
Inclusion criteria
-
(i)
Couples undergoing ART with their own gametes.
-
(ii)
Couples having at least one good embryo available for transfer.
-
(iii)
All downregulated cycles.
Exclusion criteria
-
(i)
Age more than 38 years.
-
(ii)
Untreated hydrosalpinx.
-
(iii)
Intramural fibroids more than 4 cm or adenomyosis.
-
(iv)
Endometrium less than 6 mm on the day of hCG.
Randomization protocol and data management
Couples selected for an IVF/ICSI attempt using long protocol were randomized by computer-generated randomization table between study group and control group on the day of ET by a nurse who assigned participants to their groups. The data assessor and the biological team performing the ART were blinded to group assignment. Trial was not placebo controlled as the outcome measures were objective.
Interventions
Pituitary suppression was achieved with daily s.c. injections of Buserelin 0.5 mg (Suprefact) starting on the mid-luteal phase (21st day) of the preceding cycle. Buserelin dose was reduced to 0.25 mg/day from the day of stimulation following menstruation and continued at the same dose until recombinant hCG (Ovitrelle) injection. Controlled ovarian stimulation was started with recombinant human follicle stimulating hormone (rFSH) (Gonal-F, Serono, Bari, Italy) on the second or third day of menstrual bleeding after confirming downregulation. The daily rFSH dose ranged between 150 and 300 IU, depending on body mass index and age of the women, and the anticipated ovarian response. Dose adjustment was done according to follicular development and serum estradiol levels. Recombinant hCG 500 mcg was administered subcutaneously when there were at least two follicles of 18 mm with other follicles of >/=14 mm. Stimulation protocols and the indications for hCG injection or cycle cancellation did not change throughout the study period. Oocyte retrieval was undertaken 35 hours after the administration of hCG. Fertilization was achieved with IVF/ICSI depending on indication of IVF. Embryo transfer was done on day 2. Cleavage stage embryos were graded according to Hardarson et al. A maximum of three embryos were transferred under ultrasound guidance using the Cooks catheter. Women allocated to GnRH agonist group were injected 1 mg Lupride subcutaneously on 6th, 7th and 8th days after oocyte retrieval. All women were given progesterone support (Vaginal 400 mg twice daily alternating with injectable natural micronized progesterone 100 mg) starting from the day of oocyte pickup. Luteal-phase support was continued until the pregnancy test performed 14 days after ET. Women with a positive pregnancy test continued the vaginal progesterone until the 10th week of gestation.
Outcome measures
Pregnancy rate, implantation rate, clinical pregnancy rate, clinical pregnancy with fetal heart beat and ongoing pregnancy rate were the main outcome measures.
Pregnancy rate: Number of patients with serum beta hCG >20 mIU/ml on day 14 after OCR divided by the total number of patients.
Implantation rate: Number of gestational sacs observed divided by the number of embryos transferred.[12]
Clinical pregnancy: A pregnancy diagnosed by ultrasonographic visualization of one or more gestational sacs or definitive clinical signs of pregnancy. It includes ectopic pregnancy. Note: Multiple gestational sacs are counted as one clinical pregnancy.[12]
Ongoing pregnancy was defined as pregnancy proceeding beyond the 20th gestational week.
Statistical analysis
Sample size calculation
Ongoing pregnancy rate was the primary outcome measure. In a previous trial, it has been reported that a single dose of GnRH agonist injection in the mid-luteal phase of ICSI-ET cycles increased ongoing pregnancy rate per ET from 38% to 46.8%. More than 900 treatment cycles would be required to detect an absolute 8.8% increase from 27% in ongoing pregnancy rate with an alpha error level of 0.05 and beta error level of 0.2. This figure was not considered feasible for a single center trial, which was planned to be completed in a year, and therefore the trial was designed to include 426 patients, which would enable detection of an absolute increase in ongoing pregnancy rate by 13% from the assumed 27% in the control group with the same alpha and beta error levels.
The difference of 13% was arbitrarily defined in order to complete the trial in a year. However, 13% was compatible with the 95% confidence interval (CI) of the difference between ongoing pregnancy rates per ET (-2.6% to 20.27%) in the previous report.
Statistical tests
Efficacy analysis was done according to the intention-to-treat principle. Differences between the study groups were assessed with Chi-squared test with Yates’ correction or Fisher's exact test for categorical variables, and independent samples t-test for continuous variables. For continuous and binary variables, as well as for differences between outcome variables, 95% CIs were calculated.
RESULTS
The trial was completed as planned and the results are reported in accordance with the CONSORT statement. Patient enrolment was done on the day of ET. 556 women were assessed for eligibility to participate. One hundred and thirty-one women who did not meet the inclusion criteria or met the exclusion criteria were excluded. Finally, 426 women were enrolled and randomized [Figure 1].
Figure 1.
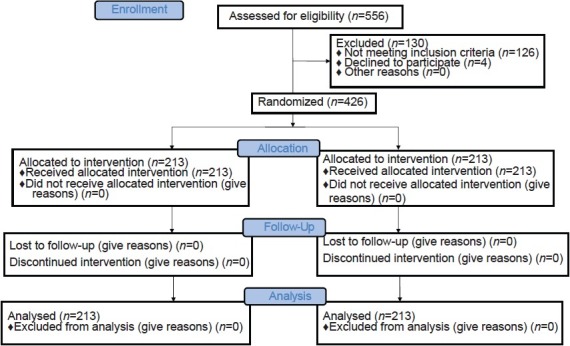
CONSORT 2010 Flow Diagram
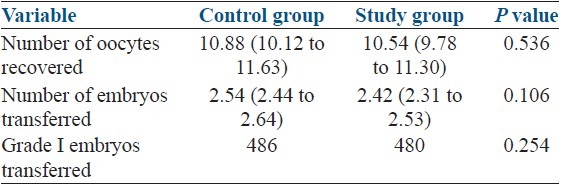
Two groups were comparable in terms of basic demographic characteristics and the features of ART cycles [Table 1]. Women in both groups received a similar number and quality of embryos [Table 1]. The groups were also comparable regarding etiology of infertility and indication of treatment [Table 2].
Table 1.
Patient and cycle characteristics
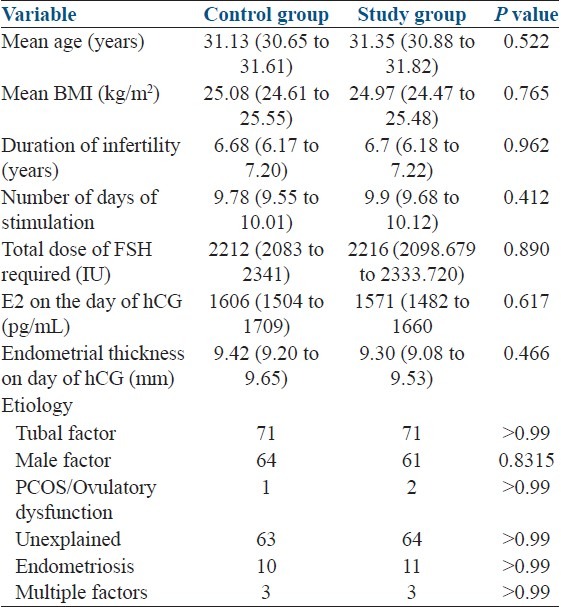
Table 2.
Embryological data
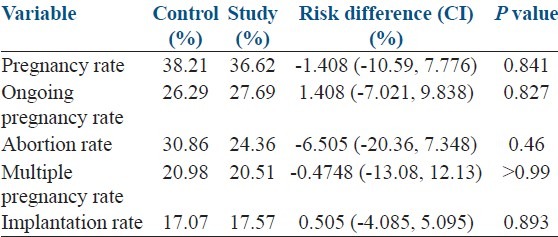
There were 59 (27.69%) ongoing pregnancies in the study group, and 56 (26.29%) in the control group. Multiple pregnancies constituted 20.51% of clinical pregnancies in the study group, whereas 20.98% of clinical pregnancies were multiples in the control group. Implantation rates were 17.57% and 17.07% in the GnRH agonist and control groups, respectively. Ongoing pregnancy, clinical pregnancy, implantation and multiple pregnancy rates were not significantly different between the groups [Table 3].
Table 3.
Results
DISCUSSION
The present prospective, randomized, case controlled study was performed to assess the effect of three doses of Lupride, administered 6 days after ICSI, on the probability of ongoing (1mg) pregnancy rate following ET in women stimulated with a long GnRH agonist protocol. Although ongoing pregnancy and implantation rates were higher in the GnRH agonist group, when compared with controls, 27.69% vs. 26.29%; 17.57% vs. 17.07% respectively observed differences were short of reaching statistical significance. However, the trial was powered to detect an absolute 13% increase in ongoing pregnancy rate from an assumed 27% in the control group. Hence, differences, <13% in absolute value cannot be excluded, since the present trial was not adequately powered to detect such differences. The 95% CI of the difference between ongoing pregnancy rates does not exclude differences between –7% and 9.8%, which means there's a probability of three doses GnRH agonist administration increasing the ongoing pregnancy rate by >5%, and this would be regarded as clinically significant. On the other hand, in the general population of women stimulated with the long protocol, a negative effect of the intervention tested cannot be refuted as well.
The use of GnRH analogs in the context of LPS has been investigated previously by various researchers. Whether the use of GnRH analogs alone could provide adequate luteal support in assisted reproduction cycles was investigated by Pirard et al., whereas Tesarik et al. analyzed the effects of GnRH analog administered as an adjunct to progesterone and estradiol valerate during the luteal phase on implantation and live birth rates.
Tesarik et al. investigated whether 0.1 mg triptorelin injection 6 days after ICSI to recipients in an oocyte donation program increased implantation rates. The authors reported significantly increased implantation rates with GnRH agonist administration when compared with placebo. However, the clinical pregnancy rate was not found to be significantly increased in the GnRH agonist group. The same group reported similar results in a subsequent trial that included women undergoing ICSI–ET with their own gametes. Subjects were randomly allocated to receive either 0.1 mg triptorelin or placebo 6 days after ICSI. Women in all groups received luteal support in the form of recombinant hCG (rhCG) injection, vaginal micronized progesterone and estradiol valerate tablets in addition to the study medication. Implantation and live birth rates per transferred embryo were significantly increased by GnRH agonist administration in women stimulated either with the long protocol or with a GnRH antagonist. Although the mechanism underlying the proposed favorable effect of GnRH agonist administration in the luteal phase was not clear, the authors speculated that it might have been due to effects on the embryo, endometrium and/or, unlike the donation model in the first trial, on the corpora lutea. Embryonic effects were thought to be more pronounced as implantation rates were increased more significantly than the pregnancy rates.
There are several points that raise concern in the analysis of the data presented in the previous studies by Tesarik et al. including those regarding sample size analyses, measuring implantation rate as the outcome, overoptimistic assumptions in the sample size analysis, definition of live birth rates, which have been very well discussed by Ata et al.
While addition of GnRH agonist as luteal-phase adjuvant was found to be beneficial by Tesarik et al., 2004, 2006;[5,7] Qublan et al., 2008[8] and Isik et al. 2009,[10] it was not found to have any significant effect by Ata et al., 2008 and Fouad AboHamila, 2011[13] [Table 4].
Table 4.
Review of literature
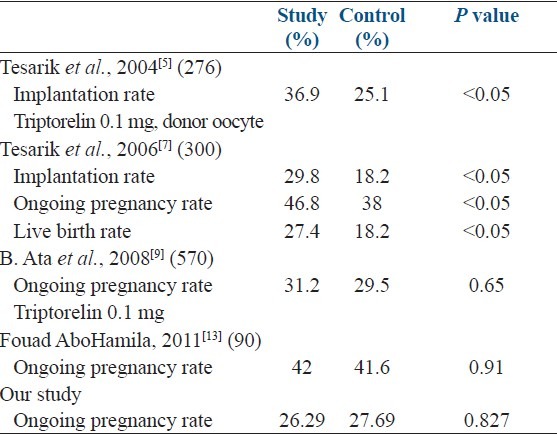
Recent metaanalysis done by Oliveira et al.[14] also failed to demonstrate any positive beneficial effect of luteal-phase-GnRHa administration on clinical pregnancy rate per transfer (P = 0.06) and ongoing pregnancy (P = 0.23) in IVF cycles stimulated with long protocol.
Additionaly, as most of previous studies demonstrating beneficial effect of administration of GnRH agonist as luteal-phase adjuvant had sample size ≤300, any small effect might have been exaggerated due to small sample size. As against those studies failing to demonstrate any beneficial effect had large sample size but still not adequate to refute any small beneficial effect on ongoing pregnancy rate.
There may be several explanations for the ineffectiveness of Lupride administered during the luteal phase, including the continuing downregulated state of the GnRH receptors in reproductive organs. The period between the last GnRH agonist injection in the long protocol and the subsequent injection in the luteal phase was 8 days in our study. In women stimulated with a long GnRH agonist protocol, downregulation of GnRH receptors may not be totally abated 8 days after cessation of GnRH agonist injections.[15–17] Pirard et al. investigated the effect of buserelin on the luteal phase in women stimulated with a GnRH antagonist protocol. Although recovery after the GnRH antagonist suppression is expected to occur rapidly, when compared with recovery after the GnRH agonist downregulation in a long protocol, even daily administration of 0.1 mg buserelin was not found to adequately support the luteal phase. Median serum progesterone levels in women receiving daily 0.1 mg buserelin injections did not reach 20 ng/ml at any point in time. Consequently, Pirard et al.'s findings do not suggest that even daily 0.1 mg GnRH agonist injections would not have a significant effect on corpus luteum function. GnRH receptors have been shown to be present in the human uterus.[18]
Although there are molecular studies suggesting a direct effect of GnRH agonists on endometrial function, research has failed to show any clinically relevant effect.[19,20] Additionally, prolonged GnRH agonist administration can be expected to cause downregulation of endometrial GnRH receptors similar to pituitary receptors, and render them, at least relatively, refractory to a subsequent administration of Inj. Lupride 1 week after downregulation.
There are several reports suggesting that GnRH agonists can enhance embryo development in vitro. GnRH agonist added to the culture medium was found to support in vitro development of porcine and murine embryos in a dose-dependent fashion, and the presence of GnRH and GnRH receptor mRNAs were demonstrated in preimplantation human embryos.[21–23] However, a direct effect on embryos of GnRH agonist administered subcutaneously to the mother is highly unlikely. Unlike in vitro culture conditions, the GnRH agonist either needs to pass to the endometrial cavity to directly affect embryos that have not attached to the decidua, or must be present in the maternal circulation when the early uteroplacental circulation is established. Establishment of uteroplacental circulation requires invasion of the endometrium, arterioles and spiral arteries by the syncytiotrophoblast, and formation of lacunae within the syncytiotrophoblast.
Formation of the lacunae is reported to occur 8 days after fertilization in a naturally occurring pregnancy.[24] This corresponds to the eighth day post-ICSI or insemination in an assisted reproduction cycle. Provided that the half-life of Lupride is 3 h, it is difficult to suggest any systemic effect at the time of establishment of uteroplacental circulation.[25] However, any indirect action of Lupride on embryos or on embryo–endometrium cross-talk cannot be completely refuted.
In conclusion, our results fail to demonstrate an unequivocal beneficial effect of a three doses of Lupride 1 mg, administered 6 days after oocyte retrieval, with regard to ongoing pregnancy rates in women stimulated with the long GnRH protocol. The observed 1.4% increase in ongoing pregnancy rates could have reached statistical significance had the sample size been larger, but from a clinical point of view it is questionable whether this would be regarded significant. The idea of significantly increasing pregnancy rates with such a simple and relatively inexpensive method is alluring, but to the best of our knowledge the available data in the literature, including the present trial, do not support this theory yet. In our opinion, this strategy should not be recommended as routine practice unless its efficacy and safety, especially with regard to developing embryos, are supported by further trials.
ACKNOWLEDGMENTS
The authors are thankful to Dr. M Kochar, Dr. Shweta Mittal, Dr. Neeti Tiwari and Dr. Ruma Satwik for providing subjects and inputs for the study. We express gratitude to the staff of IVF laboratory, Sir Ganga Ram Hospital and patients who participated in the study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Macklon NS, Fauser BC. Impact of ovarian hyperstimulation on the luteal phase. J Reprod Fertil. 2000;55:101–8. [PubMed] [Google Scholar]
- 2.Pritts EA, Atwood AK. Luteal phase support in infertility treatment: A meta-analysis of the randomized trials. Hum Reprod. 2002;17:2287–99. doi: 10.1093/humrep/17.9.2287. [DOI] [PubMed] [Google Scholar]
- 3.Beckers ND, Macklon NS, Eijkemans MJ, Ludwig M, Felberbaum RE, Diedrich K, et al. Nonsupplemented luteal phase characteristics after the administration of recombinant human chorionic gonadotropin, recombinant luteinizing hormone, or gonadotropin releasing hormone (GnRH) agonist to induce final oocyte maturation in in vitro fertilization patients after ovarian stimulation with recombinant follicle-stimulating hormone and GnRH antagonist co treatment. J Clin Endocrinol Metab. 2003;88:4186–92. doi: 10.1210/jc.2002-021953. [DOI] [PubMed] [Google Scholar]
- 4.Kolibianakis EM, Bourgain C, Platteau P, Albano C, Van Steirteghem AC, Devroey P. Abnormal endometrial development occurs during the luteal phase of nonsupplemented donor cycles treated with recombinant follicle-stimulating hormone and gonadotropin-releasing hormone antagonists. Fertil Steril. 2003;80:464–6. doi: 10.1016/s0015-0282(03)00663-0. [DOI] [PubMed] [Google Scholar]
- 5.Tesarik J, Hazout A, Mendoza C. Enhancement of embryo developmental potential by a single administration of GnRH agonist at the time of implantation. Hum Reprod. 2004;19:1176–80. doi: 10.1093/humrep/deh235. [DOI] [PubMed] [Google Scholar]
- 6.Pirard C, Donnez J, Loumaye E. GnRH agonist as novel luteal support: Results of a randomized, parallel group, feasibility study using intranasal administration of buserelin. Hum Reprod. 2005;20:1798–804. doi: 10.1093/humrep/deh830. [DOI] [PubMed] [Google Scholar]
- 7.Tesarik J, Hazout A, Mendoza-Tesarik R, Mendoza N, Mendoza C. Beneficial effect of luteal phase GnRH agonist administration on embryo implantation after ICSI in both GnRHa and antagonist treated ovarian stimulation cycles. Hum Reprod. 2006;21:2572–9. doi: 10.1093/humrep/del173. [DOI] [PubMed] [Google Scholar]
- 8.Qublan H, Amarin Z, Al-Qudah M, Diab F, Nawasreh M, Malkawi S, et al. Luteal phase support with GnRHa improves implantation and pregnancy rates in IVF cycles with endometrium of < or = 7mm on day of egg retrieval. Hum Fertil. 2008;11:43–7. doi: 10.1080/14647270701704768. [DOI] [PubMed] [Google Scholar]
- 9.Ata B, Yakin K, Balaban B, Urman B. GnRH agonist protocol administration in the luteal phase in ICSI–ET cycles stimulated with the long GnRH agonist protocol: A randomized, controlled double blind study. Hum Reprod. 2008;23:668–73. doi: 10.1093/humrep/dem421. [DOI] [PubMed] [Google Scholar]
- 10.Isik AZ, Caglar GS, Sozen E, Akarsu C, Tuncay G, Ozbıcer T, et al. Single dose GnRHa administration in luteal phase of GnRHa cycles: A prospective randomised study. Reprod Biomed Online. 2009;19:472–7. doi: 10.1016/j.rbmo.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Lambalk CB, Homburg R. GnRH agonist for luteal support in IVF.Setting the balance between enthusiasm and caution? Hum Reprod. 2006;21:2580–2. doi: 10.1093/humrep/del321. [DOI] [PubMed] [Google Scholar]
- 12.Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, et al. The International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) Revised Glossary on ART Terminology, 2009. Hum Reprod. 2009;24:2683–7. doi: 10.1093/humrep/dep343. [DOI] [PubMed] [Google Scholar]
- 13.AboHamila F, Maged A, AboulFoutouh A. Progesterone supplementation is just enough for luteal phase support in ICSI cycles. Kasr Al-Aini. J Obstet Gynecol. 2011;2:14–9. [Google Scholar]
- 14.Oliveira JB, Baruffi R, Petersen CG, Mauri AL, Cavagna M, Franco JG., Jr Administration of single-dose GnRH agonist in the luteal phase in ICSI cycles: A meta-analysis. Reprod Biol Endocrinol. 2010;8:107. doi: 10.1186/1477-7827-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smitz J, Devroey P, Camus M, Deschacht J, Khan I, Staessen C, et al. The luteal phase and early pregnancy after combined GnRH-agonist/HMG treatment for superovulation in IVF or GIFT. Hum Reprod. 1988;3:585–90. doi: 10.1093/oxfordjournals.humrep.a136750. [DOI] [PubMed] [Google Scholar]
- 16.Broekmans FJ, Bernardus RE, Berkhout G, Schoemaker J. Pituitary and ovarian suppression after early follicular and mid-luteal administration of a LHRH agonist in a depot formulation: Decapeptyl CR. Gynecol Endocrinol. 1992;6:153–61. doi: 10.3109/09513599209015549. [DOI] [PubMed] [Google Scholar]
- 17.DiLuigi AJ, Nulsen JC. Effects of gonadotropin-releasing hormone agonists and antagonists on luteal function. Curr Opin Obstet Gynecol. 2007;19:258–65. doi: 10.1097/GCO.0b013e3281338874. [DOI] [PubMed] [Google Scholar]
- 18.Reshef E, Lei ZM, Rao CV, Pridham DD, Chegini N, Luborsky JL. The presence of gonadotropin receptors in nonpregnant human uterus, huma placenta, fetal membranes, and decidua. J Clin Endocrinol Metab. 1990;70:421–30. doi: 10.1210/jcem-70-2-421. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi S, Futamura N, Minoura H, Toyoda N. Possible direct effect of gonadotropin releasing hormone on human endometrium and decidua. Life Sci. 1998;62:1187–94. doi: 10.1016/s0024-3205(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 20.Fauser BC, Devroey P. Reproductive biology and IVF: Ovarian stimulation and luteal phase consequences. Trends Endocrinol Metab. 2003;14:236–42. doi: 10.1016/s1043-2760(03)00075-4. [DOI] [PubMed] [Google Scholar]
- 21.Casan EM, Raga F, Polan ML. GnRH mRNA and protein expression in human preimplantation embryos. Mol Hum Reprod. 1999;5:234–9. doi: 10.1093/molehr/5.3.234. [DOI] [PubMed] [Google Scholar]
- 22.Raga F, Casan EM, Kruessel J, Wen Y, Bonilla-Musoles F, Polan ML. The role of gonadotropin-releasing hormone in murine preimplantation embryonic development. Endocrinology. 1999;140:3705–12. doi: 10.1210/endo.140.8.6899. [DOI] [PubMed] [Google Scholar]
- 23.Nam DH, Lee SH, Kim HS, Lee GS, Jeong YW, Kim S, et al. The role of gonadotropin-releasing hormone (GnRH) and its receptor in development of porcine preimplantation embryos derived from in vitro fertilization. Theriogenology. 2005;63:190–201. doi: 10.1016/j.theriogenology.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Moore K, Persaud T. Introduction to the developing human. In: Moore K, Persaud T, editors. The Developing Human Clinically Oriented Embryology. Philadelphia: Saunders; 2003. p. 4. [Google Scholar]
- 25.Shoham Z. Drugs used for controlled ovarian hyperstimulation: Clomiphene citrate, aromatase inhibitors, gonadotropins, and gonadotropin-releasing hormone analogs. In: Gardner D, Weissman A, Howles C, Shoham Z, editors. Textbook of Assisted Reproduction Techniques. London: Taylor and Francis; 2004. p. 532. [Google Scholar]


