Abstract
Background:
There is only little information about the effects of GABA receptors agonist and antagonist on morphine self-administration. Present study was designed to assess role of GABAB receptors in the regulation of morphine-reinforced self-administration.
Methods:
This study was performed in four groups of rats: (1) Saline group, which received saline in the self-administration session. (2) Morphine group, which received morphine in saline solution in the self-administration session. (3) Baclofen + Morphine group, which received both baclofen 20 min before self- administration test and morphine in the self-administration session. (4) Phaclofen + Morphine group, which received both phaclofen 20 min before self- administration test and morphine in the self-administration session. The number of lever pressing and self-infusion were recorded.
Results:
Morphine significantly increased the number of active lever pressing dose dependently in self-administration session in comparative with saline group. Administration of baclofen, 20 min before morphine self-administration produced significant decrease in the initiation of morphine self-administration during all session. Conversely, pre-treatment of phaclofen increased the number of active lever pressing and self-infusion in this test.
Conclusion:
Our results indicated a short-term treatment by baclofen, reduced morphine-maintenance response in a dose-dependent manner, suggesting that GABAB receptor agonists could be useful for reversing the neuroadaptations related to opiates.
Keywords: Baclofen, morphine addiction, phaclofen, self-administration
INTRODUCTION
Although drug addictive mechanisms in humans are considerably complex, animal models of addictive behavior have been useful in predicting the abuse liability of new compounds, in assessing the medication for the treatment of drug addiction, investigating the underlying neuropharmacological and molecular mechanisms. Self-administration is one of most powerful tools for investigating the manner in which neurochemical and neuropharmacological process influence behaviors related to drug reinforcement.[1]
It is well established reinforcing effect of drug abuse to the mesolimbic dopamine (DA) system. The mesolimbic dopamine (DA) system seems to be primarily responsible for the reinforcing effect of morphine.[2]
GABA is the primary inhibitory neurotransmitter in the brain and spinal cord.[3–6] The GABAergic system is closely relevated to the mesolimbic dopaminergic system, and is suppose involve in the modulation of reinforcing effects of several addictive drugs.[7,8] Importantly GABAergic receptors modulation has been reported to participate in drug-induced reinstatement of cocaine[9] and alcohol-seeking[10] as well as in the cue-induced reinstatement of nicotine-seeking behavior in rats.[11] There are also evidences that GABAergic system is involve to behavioral sensitization to morphine.[12–16] It has been shown that learning and memory play an important role in the development of opioids tolerance and dependence.[17–19] In the other hand, the GABAergic system has effect on the learning and memory retention.[20–23] Previous studies administration of GABAergic receptors agonists impairs memory and their antagonists facilitate retrieval it[24] and also memory acquisition and extinction can be altering by GABAergic system.[5]
Thus, the identification of new pharmacological anti-craving treatments for use in prevent of opioids relapse is a priority issue. There have been relatively few studies examine the role of GABAB receptor pathways in the extinction of opioids reward.[25] Manipulation of GABAB receptors transmission may facilitate or impede extinction learning depending on the time point of drug administration. Hence, it is interesting to evaluate role GABAB receptors in morphine self-administration test. Thus present study was performed to further investigate the role of GABAB receptors in morphine self-administration.
METHODS
Animals
48 male wistar rats with weighing 270-300 gram at the beginning of the experiment were used and housed in the cages with food and water made available ad libitum. Rats were maintained in a climate-controlled colony room at 23 ± 1 on a 12 h light-dark cycle. All experiments were conducted during the dark phase. The Ethic Committee for Animal Experiments at Isfahan University of Medical Sciences approved the study plan. All animals care and experimental procedures were in accord with protocols approved by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Drugs
Morphine sulfate and GABAB receptors agonist and antagonist's dosages, time course of effects and route of administration all selected based on previous studies. Morphine sulfate (Temad, Iran), phaclofen and buclofen (Sigma-Aldrich) was dissolved in sterile %0.9 saline just before the experiment.
Surgery
Rats were anesthetized with chloral hydrate (350 mg/kg i.p)[26] and followed by, if necessary maintenance dose. A cannula was inserted the jugular vein (for details see.[27] Cannula was guided subcutaneously up to the skull where it was fixed to a curved metal tube and secured onto the skull with small screws, fixed with dental acrylic cement and a styled cap was inserted into the distal end of the catheter to prevent its clogging and provide a closed system. Then a cannula was implanted in the CV region in order to injection GABAB receptors agonist and antagonist, as described below. Animals were placed in a stereotaxic frame in a flat-skull position and fixed. A midline incision was made the skin and underlying periostum retracted and the skull cleaned. Stainless steel, 23-gauge guide cannula was implanted 1 mm above the lateral cerebral ventricle. Stereotaxic was coordinated according to the rat brain atlas of Paxinos and Watson (CV: AP = 0.8 relative to bregma, L = 1.5 relative to midline, V = 4.2 relative to skull surface).[28] Cannula was fixed with dental acrylic cement and anchored by two screws placed in the skull. A stylet (26-gauge stainless steel) was placed into the guide cannula to allow it to maintain patency. After surgery, rats were given 300,000 units of procaine penicillin G (i.p) to prevent infection. The animals were allowed 5-7 days of recovery before beginning of the experiment
Experimental design
Male rats (N = 48) were randomly selected and divided into four groups (n = 12):
Saline group, which received saline 5 μL icv, 20 min before each session and saline in the self-administration session.
Morphine group, which received saline 5 μL icv, 20 min before each session and morphine in saline solution in the self-administration session.
Baclofen + morphine group, (BAC + M), which received both GABAB receptor agonist (baclofen 100 nmol in volume: 5 μL icv)[29] 20 min before self- administration test and morphine in the self-administration session.
Phaclofen + morphine group (PHA + M), which received both GABAB receptor antagonist (phaclofen 100 nmol in volume: 5 μL icv)[30] 20 min before self- administration test and morphine in the self-administration session.
Saline, baclofen and phaclofen were injected by microinjection and via cannula that before implanted in the CV region and one end that attached to a 50 μL Hamilton micro syringe by a polyethylene tube. Solution was infused slowly over a period of 5 min in per rat. Cannula was kept in place for an additional 3 min to minimize the drug backflow into the injection track.
Procedure
Briefly, to aid in acquisition of drug self-administration, rats were initially trained to press a lever using food as reinforcement before being surgically implanted with a chronic intravenous (i.e. chronic iv) jugular catheter. Training and testing were done in standard operant conditioning cages (21 × 21 × 28 cm) placed in a sound-attenuated room, ventilated with fans, based on the method used previously by others[1,31] with minor modifications. The apparatus was equipped with reinforcement lever (RL) and non- reinforcement lever (NRL), 2 cm above the floor, with a red light located 4 cm above the RL. The iv cannula was connected to an infusion pump via a swivel, allowing the animal to move relatively freely. Pressing of the reinforcement lever (RL), marked by a red light and resulted in 10 sec infusion of 0.2 ml fluid through an infusion pump. The fluid was saline in the control group and sulfate morphine in saline solution (5 mg/ml) in other groups.
Seven days after the recovery, and following 24 hrs. of food restriction, rats were placed in the operant chambers where a lever filled with food pellets was available. Animals were placed in the self-administration apparatus for 2 hrs. each day on an FR-1 schedule for 10 days.[32] The number of the RL and NRL were recorded by computer. In this study, the number of active lever pressing and number of infusion were regarded as a measure of the reinforcing action of the drug.[32,33]
By pressing the reinforcement lever, the rats received 0.2 ml fluid and small pellets in the first 5 days and fluid without pellets in the final 5 days of the experiment. Pressing the NRL did not deliver fluid or food. In the first 5 day of self-infusion period, the availability of food was restricted in order to reduce body weight by 15% which has been shown to facilitate the initiation of intravenous self-administration.[34] On the next 5 days, the animals had free access to their ad libitum food. Catheters were flushed daily with 0.2 mL saline containing heparin sulfate (50 IU/ml) during the recovery period as well as before and after the self-administration sessions. All operant sessions were conducted during the animal's dark cycle. Cannula potency was tested by the injection of sodium pentobarbital solution into the catheter and observation of animal behavior. Animals with patent catheters exhibit prominent signs of anesthesia (loss of muscle tone) a few seconds after the administration.[33]
Statistical analysis
The data obtained during self-administration sessions, the numbers of active and passive lever pressing and the numbers of infusion were analyzed by using one-way analysis of variance (ANOVA) and student's t-test. All results have been expressed as means ± S.E.M. different with P < 0.05 was considered significant.
RESULTS
In this study, the effects of GABAB receptors agonist and antagonist (baclofen and phaclofen) on morphine self- administration were studied in rats. For this purpose, saline (control and morphine groups) baclofen (baclofen + morphine group) and phaclofen (phaclofen + morphine group) injected (icv), 20 min before each session and received saline (control group) and morphine (morphine, baclofen + morphine and phaclofen + morphine groups) in self- administration. The number of self infusion (SI), reinforcement lever (RL) and non- reinforcement lever (NRL) were compared between and within groups. In morphine group that received morphine during 10 days in comparison with control group that received saline in self-infusion there was no significant difference in the number of SI during first 4 days between 2 groups. But in the last 6 days number of SI increased significantly in the morphine group [Figure 1].
Figure 1.
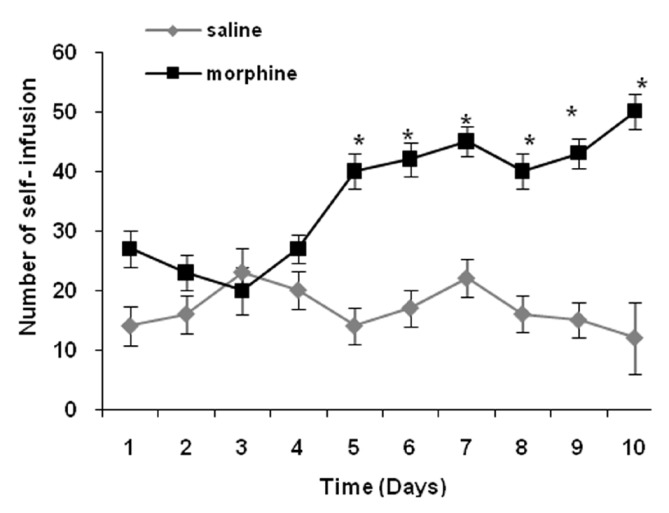
Comparison numbers of self-infusion (SI) between two groups of control and morphine. Rats received saline (control group) and morphine (morphine group) in self-administration apparatus. Saline 5 μL injected (icv) before each session and number of SI was compared among 2 groups using one-way ANOVA and student's t-test. Data are presented as mean ± SEM. There was significant difference between control and morphine groups. The number of SI in morphine group increased significantly except first 5 days (*P< 0.05)
The number of SI in baclofen + morphine group decreased and in phaclofen + morphine group increased significantly during 5-10 days in comparison with morphine group [Figure 2] (*P < 0.05) (+P < 0.05).
Figure 2.
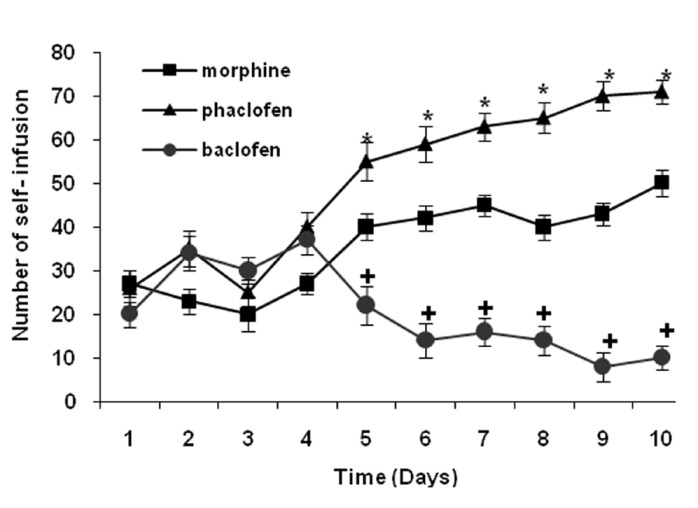
The number of self- infusion (SI) comparison between morphine, (BAC + M) and (PHA + M) groups. In three groups rats received morphine in self-administration apparatus and saline (morphine group), baclofen (BAC + M group) and phaclofen (PHA + M group) injected (icv) 20 min before each session and number of SI was compared among three groups using one-way ANOVA and student's t-test. Data are presented as mean ± SEM. This figure shows that pre-treatment with baclofen (GABAB receptors agonist) (100 nmol icv), decreased significantly SI in rats and pre-treatment with phaclofen (GABAB receptors antagonist) (100 nmol icv) increased significantly SI in comparative with morphine group in during 5-10 days (*P < 0.05) (+P < 0.05)
The numbers of RL and NRL in control and morphine groups were presented in [Figure 3]. The number of RL in morphine group increased significantly in comparison with control group except in the first 4 days in which the animals had free access to food (*P < 0.05). There was no significant difference in the NRL between 2 groups.
Figure 3.

Comparison number of reinforcement lever pressing and non-reinforcement lever pressing between saline and morphine groups during morphine self-administration. In the day testing rats received saline (saline and morphine groups) in self-administration apparatus and saline 5 μL injected (icv) 20 min before each session and number of RL and NRL was compared among 2 groups using one-way ANOVA and student's t-test. Data are presented as mean ± SEM. This figure shows that number of RL increased significantly in morphine group during 5-10 days. No significantly difference in the NRL was observed between two groups (*P< 0.05)
Numbers of RL and NRL between morphine, baclofen + morphine and phaclofen + morphine groups were presented in [Figure 4]. Numbers of RL in baclofen + morphine group decreased and in phaclofen + morphine group increased significantly during 5-10 days. No significantly difference in the NRL was observed between three groups (*P < 0.05), (+P < 0.05).
Figure 4.

Comparison number of reinforcement lever pressing and non-reinforcement lever pressing between morphine, (BAC + M) and (PHA + M) groups. In three groups rats received morphine in self-administration apparatus. And saline (morphine group), baclofen (BAC + M) groups and phaclofen (PHA + M) groups injected (icv), 20 min before each session. The number of RL and NRL was compared among three groups using one-way ANOVA and student's t-test. Data are presented as mean ± SEM. This figure shows that number of RL in (BAC + M) group decreased and in (PHA + M) group increased significantly during 5-10 days in comparative with morphine group (*P< 0.05), (+P< 0.05). No significantly difference in the NRL was observed between three groups
DISCUSSION
Results obtained in the present study demonstrate that morphine treatment in a manner of dose-dependent significantly enhanced morphine self-administration. In agreement with previous studies, which showed that morphine increased number of self-infusion and number of active lever pressing in self-administration session.[35] Soleki et al., (2005) found that the enhancement of GABAergic transmission by GABA uptake inhibitors potently reduces heroin discriminative stimulus.[36] Xi and Stein (2000) also found that increasing GABA concentrations in the VTA or VP blocks heroin self-administration in a GABAB receptor dependent manner.[37] Our results indicated that pre-treatment with GABAB receptor agonist (baclofen) significantly decreased morphine self-infusion and GABAB receptor antagonist (phaclofen) increased morphine self-infusion [Figure 2]. These findings are in agreement with the previous studies, which have shown that stimulation of GABAB receptors reduce self-infusion of the most common drug abuse, including nicotine, methamphetamine, heroine and cocaine.[38] Baclofen also prevents the increase NAc shell dopamine release after acute administration of morphine, nicotine and cocaine.[7,37] This regulatory effect of GABAB receptors agonist on drug reinforcement is also supported by earlier studies demonstrating that baclofen antagonized morphine-induced increases in synaptic neostriatal and nucleus accumbens DA levels.[1,39] Moreover similar conclusions obtained in other studies, which baclofen reduced heroine-induced DA release in the NAc and inhibited heroine self-administration behavior in a dose dependent manner. Microinjection baclofen into the VTA inhibited morphine-induced CCP in rats[7] and suppressed heroin self-administration behavior and heroin-induced DA release.[18] Others reported that this drug can modulate self-administration of cocaine and attenuate cocaine-maintenance responding and reinstatement of cocaine-reinforced responding.[40] Moreover baclofen, when administrated directly into the VTA in rats, decreases somatodendritic dopamine release in this area,[41] extracellular dopamine levels in VTA,[36] inhibit morphine-induced place preference in rats.[18] Results obtained in the present study demonstrate that selective GABAB receptor antagonist (phaclofen) reverse baclofen inhibition of morphine-maintenance response [Figure 2]. Similar results were obtained by other investigations which have been shown morphine induce place preference in a dose-dependent fashion[39] which this effect is blocked by intra-VTA microinjection of the GABAB receptor antagonist (2-hydroxy saclofen), administration of the GABAB receptor agonist (baclofen) into the VTA reduced heroin-induced DA release and this inhibitory effect of baclofen was abolished by intra-VTA microinjection of the GABAB receptor antagonist (2-hydroxy saclofen).[42]
Previous studies suggested that change in brain DA level are involved in the reward effects of opiates drug-seeking and drug maintenance behaviors.[35] The mesolimbic dopamine system seems to be primary response for reinforcing affects of morphine (7) so that; morphine increases the mesocorticolimbic dopaminergic system by inhibitory activity of GABAergic interneuron.[12] It is possible that morphine participates by stimulation of mu-opioid receptors situated on inhibitory GABAergic interneuron. Activation of these mu-opioid receptors hyperpolarizes GABAergic interneurons in turn increase firing rate and release of DA in the reward pathway. The present results show that injection baclofen intra cranial ventricle can have substantial effects on morphine self-administration, also that the anti-craving effect of GABAB receptor agonists may depend on their ability to stimulate GABAergic transmission by reducing the firing rate of DA neurons. However our findings suggest GABAergic system plays a predominant role in the attenuating the reinforcing effects of opiates via modulation of DA transmission. Taken together, these studies demonstrated a possibility that GABAB receptor stimulation is sufficient to suppress craving for morphine through modulation of the activity of the mesocorticolimbic DA system.
In conclusion, results indicate that a short-term treatment by baclofen reduced morphine-maintenance response and suggest that GABAB receptor agonists could be useful for reversing the neuroadaptations related to opiates.
Footnotes
Source of Support: This study was conducted as a thesis funded by Isfahan University of Medical Sciences
Conflict of Interest: None declared
REFERENCES
- 1.Yoon SS, Lee BH, Kim HS, Choi KH, Yun J, Jang EY, et al. Potential roles of GABA receptors in morphine self-administration in rats. Neurosci Lett. 2007;428:33–7. doi: 10.1016/j.neulet.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki T, Nurrochmad A, Qzaki M, Khotib J, Nakamura A, Imai S, et al. Effect of the GABAB receptor agonist baclophen on the μ-opiod receptor agonist-induced antinociceptive, emetic and rewarding effects. Neuropharmacology. 2005;49:1121–31. doi: 10.1016/j.neuropharm.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Dunbar SA, Karamian I, Yeatman A, Zhang J. Effect of reccurent withdrawal on spinal GABA release during chronic morphine infusion in the rat. Eur J Pharmacol. 2006;535:152–6. doi: 10.1016/j.ejphar.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Apergis-Schoute J, Pinto A, Pare D. Ulterastructural organization of medial prefronta inputs to the rhinal cortices. Eur J Neurosci. 2006;24:135–44. doi: 10.1111/j.1460-9568.2006.04894.x. [DOI] [PubMed] [Google Scholar]
- 5.Rae C, Nasrallah FA, Griffin JL, Balcar VJ. Now I know my ABC. A systems neurochemistry and functional metabolomic approach to understanding the GABAergic system. J Neurochem. 2009;109:109–16. doi: 10.1111/j.1471-4159.2009.05803.x. [DOI] [PubMed] [Google Scholar]
- 6.Wise R, Bozarth M. Action of drugs of abuse on brain reward systems: An update with specific attention to opiates. Pharmacol Biochem Behav. 1982;17:239–43. doi: 10.1016/0091-3057(82)90076-4. [DOI] [PubMed] [Google Scholar]
- 7.Fadda P, Scherma M, Fresu A, Collu M, Fratta W. Baclofen antagonizes nicotine, cocaineâ, and morphineâ induced dopamine release in the nucleus accumbens of rat. Synapse. 2003;50:1–6. doi: 10.1002/syn.10238. [DOI] [PubMed] [Google Scholar]
- 8.Campbell UC, Lac ST, Carroll ME. Effects of baclofen on maintenance and reinstatement of intravenous cocaine self-administration in rats. Psychopharmacology. 1999;143:209–14. doi: 10.1007/s002130050937. [DOI] [PubMed] [Google Scholar]
- 9.Colombo G, Serra S, Brunetti G, Vacca G, Carai MA, Gessa GL. Suppression by baclofen of alcohol deprivation effect in Sardinian alcohol-preferring (sP) rats. Drug Alcohol Depend. 2003;70:105–8. doi: 10.1016/s0376-8716(02)00333-2. [DOI] [PubMed] [Google Scholar]
- 10.Paterson NE, Froestl W, Markou A. Repiated administration of the GABAB receptor agonist CGP444532 decreasecue-inducd reinstatement of niccotine-seeking in rats. Neuropsychopharmacology. 2005;30:119–28. doi: 10.1038/sj.npp.1300524. [DOI] [PubMed] [Google Scholar]
- 11.Taber MT, Das S, Fibiger HC. Cortical regulation of subcortical dopamine release: Mediation via the ventral tegmental area. J Neurochem. 1995;65:1407–10. doi: 10.1046/j.1471-4159.1995.65031407.x. [DOI] [PubMed] [Google Scholar]
- 12.Zarrindast MR, Hoghooghi V, Rezayof A. Inhibition of morphine-induced amnesia in morphine-sensitized mice: Involvement of dorsal hippocampal GABAergic receptors. Neuropharmacology. 2008;54:569–76. doi: 10.1016/j.neuropharm.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Bartoletti M, Ricci F, Gaiardi M. A GABA B agonist reverses the behavioral sensitization to morphine in rats. Psychopharmacology. 2007;192:79–85. doi: 10.1007/s00213-006-0693-8. [DOI] [PubMed] [Google Scholar]
- 14.Jinno SH. Structural organization of long-rang GABAergic projection system of the hippocampus. Front Neuroanat. 2009;3:1–8. doi: 10.3389/neuro.05.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridderinkhof KR, Nieuwenhuis S, Braver TS. Medial frontal cortex function: An introduction and overview. Cogn Affect Behav Neurosci. 2007;7:261–5. doi: 10.3758/cabn.7.4.261. [DOI] [PubMed] [Google Scholar]
- 16.Xu NJ, Wang LZ, Wu CF, Pei G. Spatial learning and morphine-rewarded place preference negatively correlates in mice. Pharmacol Biochem Behav. 2007;68:389–94. doi: 10.1016/s0091-3057(00)00479-2. [DOI] [PubMed] [Google Scholar]
- 17.Leite-Morris KA, Fukudome EY, Shoeb MH, Kaplan GB. GABAB receptor activation in the ventral tegmental area inhibits the acquisition and expression of opiate-induced motor sensitization. J Pharmacol Exp Ther. 2004;308:667–78. doi: 10.1124/jpet.103.058412. [DOI] [PubMed] [Google Scholar]
- 18.Fan GH, Wang LZ, Qiu HC, Ma L, Pei G. Inhibition of calcium/calmodulin-dependent protein kinase II in rat hippocampus attenuates morphine tolerance and dependence. Mol Pharmacol. 1999;56:39–45. doi: 10.1124/mol.56.1.39. [DOI] [PubMed] [Google Scholar]
- 19.Zarrindast MR, Heidari-Darvishani A, Rezayof A, Fathi-Azarbaijani F, Jafari-Sabet M, Hajizadeh-Moghaddam A. Morphine-induced sensitization in mice: Changes in locomotor activity by prior scheduled exposure to GABAA receptor agents. Behav Pharmacol. 2007;18:303–10. doi: 10.1097/FBP.0b013e3282186baa. [DOI] [PubMed] [Google Scholar]
- 20.Brioni JD, Decker MW, Gamboa LP, Izquierdo I, Mcgaugh JL. Muscimol injections in the medial septum impair spatial learning. Brain Res. 1990;522:227–34. doi: 10.1016/0006-8993(90)91465-s. [DOI] [PubMed] [Google Scholar]
- 21.Lu L, Zeng S, Liu D, Ceng X. Inhibition of the amygdala and hippocampal calcium/calmodulin-dependent protein kinase II attenuates the dependence and relapse to morphine differently in rats. Neurosci Lett. 2000;291:191–5. doi: 10.1016/s0304-3940(00)01352-5. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa Y, Sasaki A, Takashima T. The GABAB receptor antagonist CGP36742 improves learned helplessness in rats. Eur J Pharmacol. 1999;381:1–7. doi: 10.1016/s0014-2999(99)00567-1. [DOI] [PubMed] [Google Scholar]
- 23.Castellano C, Brioni JD, Nagahara AH, Mcgaugh JL. Post-training systemic and intra-amygdala administration of the GABA-B agonist baclofen impairs retention1. Behav Neural Biol. 1989;52:170–9. doi: 10.1016/s0163-1047(89)90285-9. [DOI] [PubMed] [Google Scholar]
- 24.Heinrichs S, Leite-Morris K, Carey R, Kaplan G. Baclofen enhances extinction of opiate conditioned place preference. Behav Brain Res. 2009;59:353–9. doi: 10.1016/j.bbr.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Hao Y, Yang J, Sun J, Qi J, Dong Y, Wu CF. Lesions of the medial prefrontal cortex prevent the acquisition but not reinstatement of morphine-induced conditioned place preference in mice. Neurosci Lett. 2008;433:48–53. doi: 10.1016/j.neulet.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 26.Alaei H, Esmaeili M, Nasimi A, Pourshanazari A. Ascorbic acid decreases morphine self-administration and withdrawal symptoms in rats. Pathophysiology. 2005;12:103–7. doi: 10.1016/j.pathophys.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Mierzejewski P, Koros E, Goldberg SR, Kostowski W, Stefanski R. Intravenous self-administration of morphine and cocaine: A comparative study. Pol J Pharmacol. 2003;55:713–26. [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed. 2005. pp. 28–31. [DOI] [PubMed] [Google Scholar]
- 29.Ebenezer I, Baldwin B. Effect of intracerebroventricular administration of the GABAB-receptor agonist baclofen on operant feeding in satiated pigs. Br J Pharmacol. 1990;101:559–62. doi: 10.1111/j.1476-5381.1990.tb14120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giuliani S, Lecci A, Santicioli P, Del Bianco E, Maggi C. Effect of the GABAB antagonist, phaclofen, on baclofen-induced inhibition of micturition reflex in urethane-anesthetized rats. Neuroscience. 1992;48:217–23. doi: 10.1016/0306-4522(92)90350-b. [DOI] [PubMed] [Google Scholar]
- 31.Fattore L, Spano S, Cossu G, Deiana S, Fadda P, Fratta W. Cannabinoid CB1 antagonist SR 141716 A attenuates reinstatement of heroin self-administration in heroin-abstinent rats. Neuropharmacology. 2005;48:1097–104. doi: 10.1016/j.neuropharm.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 32.Alaei H, Pourshanazari A, Rafati A. Electrical stimulation of nucleus raphe dorsalis changes morphine self-administration and withdrawal symptoms in rats. Pathophysiology. 2002;9:1–5. doi: 10.1016/s0928-4680(02)00050-0. [DOI] [PubMed] [Google Scholar]
- 33.Sahraei H, Poorheidari G, Foadaddini M, Khoshbaten A, Asgari A, Noroozzadeh A, et al. Effects of nitric oxide on morphine self-administration in rat. Pharmacol Biochem Behav. 2004;77:111–6. doi: 10.1016/j.pbb.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Sahraei H, Motamedi F, Khoshbaten A, Zarrindast MR. Adenosine A (2) receptors inhibit morphine self-administration in rats. Eur J Pharmacol. 1999;383:107–13. doi: 10.1016/s0014-2999(99)00613-5. [DOI] [PubMed] [Google Scholar]
- 35.Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Rev. 2001;36:129–38. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- 36.Bartoletti M, Colantoni A, De Luca V, Gaiardi M. Single and repeated baclofen treatment attenuates the discriminative stimulus effects of morphine in rats. Pharmacol Biochem Behav. 2010;97:279–83. doi: 10.1016/j.pbb.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 37.Torregrossa MM, Kalivas PW. Microdialysis and the neurochemistry of addiction. Pharmacol Biochem Behav. 2008;90:261–72. doi: 10.1016/j.pbb.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spano MS, Fattore L, Fratta W, Fadda P. The GABAB receptor agonist baclofen prevents heroin-induced reinstatement of heroin-seeking behavior in rats. Neuropharmacology. 2007;52:1555–62. doi: 10.1016/j.neuropharm.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 39.Sahraei H, Amiri YA, Haeri-Rohani A, Sepehri H, Salimi SH, Pourmotabbed A, et al. Different effects of GABAergic receptors located in the ventral tegmental area on the expression of morphine-induced conditioned place preference in rat. Eur J Pharmacol. 2005;524:95–101. doi: 10.1016/j.ejphar.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Roberts D. Preclinical evidence for GABAB agonists as a pharmacotherapy for cocaine addiction. Physiol Behav. 2005;86:18–20. doi: 10.1016/j.physbeh.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 41.Yoon SS, Kim H, Choi KH, Lee BH, Lee YK, Lim SC, et al. Acupuncture suppresses morphine self-administration through the GABA receptors. Brain Res Bull. 2010;81:625–30. doi: 10.1016/j.brainresbull.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Tsuji M, Nakagawa Y, Ishibashi Y, Yoshii T, Takashima T, Shimada M, et al. Activation of ventral tegmental GABAB receptors inhibits morphine-induced place preference in rats. Eur J Pharmacol. 1996;313:169–73. doi: 10.1016/0014-2999(96)00642-5. [DOI] [PubMed] [Google Scholar]


