Abstract
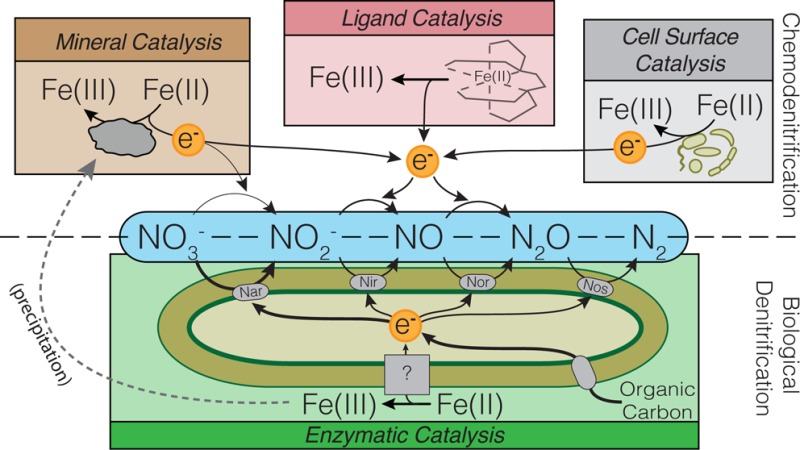
This study introduces a newly isolated, genetically tractable bacterium (Pseudogulbenkiania sp. strain MAI-1) and explores the extent to which its nitrate-dependent iron-oxidation activity is directly biologically catalyzed. Specifically, we focused on the role of iron chelating ligands in promoting chemical oxidation of Fe(II) by nitrite under anoxic conditions. Strong organic ligands such as nitrilotriacetate and citrate can substantially enhance chemical oxidation of Fe(II) by nitrite at circumneutral pH. We show that strain MAI-1 exhibits unambiguous biological Fe(II) oxidation despite a significant contribution (∼30–35%) from ligand-enhanced chemical oxidation. Our work with the model denitrifying strain Paracoccus denitrificans further shows that ligand-enhanced chemical oxidation of Fe(II) by microbially produced nitrite can be an important general side effect of biological denitrification. Our assessment of reaction rates derived from literature reports of anaerobic Fe(II) oxidation, both chemical and biological, highlights the potential competition and likely co-occurrence of chemical Fe(II) oxidation (mediated by microbial production of nitrite) and truly biological Fe(II) oxidation.
Introduction
Fe(II)/Fe(III) is an important redox couple in natural environments.1 In anoxic systems, iron oxidation can be mediated by several biological agents, such as anoxygenic phototrophs2,3 and nitrate-dependent chemotrophs.4,5 While the enzymatic machinery for Fe(II) oxidation has been identified and characterized for two anoxygenic phototrophs,3,6,7 comparable catalysts have not yet been identified for nitrate-dependent chemotrophs. Toward this end, we isolated a fast growing Fe(II) oxidizing, nitrate-dependent chemotroph from the iron-rich tropical Lake Matano,8 with the intention of developing it into a model genetic system. However, work with the isolate highlighted a second, often overlooked aspect of Fe(II) oxidation in anoxic environments: direct chemical interaction with nitrite (a form of chemodenitrification9). Being able to distinguish the mechanisms and turnover rates of direct biological versus abiotic components of anaerobic Fe(II) oxidation is necessary to gain a complete understanding of the biogeochemical coupling of the N and Fe redox cycles. Here, we expand our understanding of chemodenitrification by experimental elucidation of how organic ligands promote abiotic Fe(II) oxidation by nitrite, and discuss its relevance to assessing the potential co-occurrence of chemical and biological Fe(II) oxidation.
The isolation and characterization of an increasing number of microorganisms capable of nitrate-dependent anaerobic Fe(II) oxidation in recent years4,5,10−16 has revealed the potential for chemotrophic recycling of Fe(II) in anoxic systems. However, deconvolving the chemical and biological aspects of this process remains challenging in many environmental settings17,18 and even laboratory studies.19,20 The complication arises whenever denitrifying organisms reduce nitrate in iron-rich anoxic systems, where the metabolic intermediate nitrite can oxidize Fe(II).21−26 This was recently highlighted in a review by Picardal,27 which underscored that while biologically induced (through the production of nitrite during biological denitrification), Fe(II) oxidation can be abiotically catalyzed and proceed by chemodenitrification. Because Fe(II) oxidation may also be directly catalyzed by (potentially the same) denitrifying organisms, two competing pathways exist whose precise mechanisms and relative importance in nature are poorly understood. While the physiology of nitrate-dependent Fe(II)-oxidizing bacteria has been the subject of a growing number of studies,16,24,28−30 the chemical aspect of anaerobic Fe(II) oxidation by nitrite has received less attention,27,31 despite its relevance to constraining the extent of its microbial counterpart.
Rapid oxidation of Fe(II) by nitrite in strongly acidic conditions was described as early as 1936,32 with high reaction rates linked to the generation and subsequent degradation of nitrous acid (pKa = 3.4). At circumneutral pH, nitrite is stable and anaerobic Fe(II) oxidation requires a catalyst or suitable Fe(II)-containing mineral to proceed at appreciable rates. Acceleration of this process has been reported with a number of specific Fe(II) mineral phases and catalysts, such as Cu2+,33 iron oxides and hydroxides,31,34−37 green rust,25,38 as well as siderite39 and vivianite,23 and even microbial surfaces,22 providing possible reaction mechanisms for Fe(II)-oxidizing chemodenitrification. The same is true for nitrate, which is generally less reactive toward Fe(II) than nitrite at circumneutral pH,40 but can similarly benefit from metal and mineral catalysis.41,42 However, metals and surfaces are not the only agents for chemical catalysis. While the kinetic effects of ligands (including EDTA, NTA, and citrate) on iron redox processes in oxic environments have been explored before43−46 and often lead to acceleration of Fe(II) oxidation, much less is known about their effects in the absence of molecular oxygen. Several studies have investigated the effect of ligands on iron redox processes in acidic conditions and solvents,47,48 but with the notable exception of studies on microbial Fe(II) oxidation in the presence of EDTA,13,49 little is known about the impact of ligands at circumneutral pH.
Here, we investigate the effect of several Fe(II)-chelating ligands on iron-oxidizing chemodenitrification to (1) assess true biological Fe(II) oxidation in the newly isolated β-proteobacterium Pseudogulbenkiania sp. strain MAI-1 and (2) elucidate the role ligands could play more generally in abiotic Fe(II) oxidation in laboratory and environmental settings. We use Paracoccus denitrificans as a model strain to show how Fe(II) oxidation can appear to be directly biologically catalyzed when, in fact, much of this activity may only be indirectly biologically mediated. We describe the kinetics and potential reaction mechanism of the chemical oxidation of Fe(II) by nitrite observed in these experiments and discuss their relevance for the interpretation of laboratory and environmental studies. We place our findings in the context of chemical and biological oxidation rates reported in the literature to evaluate their relative importance in anaerobic Fe(II) oxidation.
Materials and Methods
Media
All reagent solutions were autoclaved or filter-sterilized prior to use. The basal medium for all experiments was a freshwater medium containing 500 mg/L MgSO4·7H2O, 300 mg/L NH4Cl, 100 mg/L CaCl2·2H2O, and 5.4 mg/L KH2PO4·H2O2. For microbial cultures, the medium was amended with a 1000× vitamin mix (final concentrations in the medium: 40 μg/L 4-aminobenzoic acid, 10 μg/L D-biotin, 100 μg/L nicotinic acid, 50 μg/L Ca pantothenate, 100 μg/L pyridoxamine·2HCl, 100 μg/L thiamine·2Cl) and a 1000× trace element solution (final concentrations in the medium: 1.1 mg/L FeSO4·7H2O, 42 μg/L ZnCl2, 50 μg/L MnCl2·4H2O, 190 μg/L CoCl2·6H2O, 2 μg/L CuCl2· 2H2O, 24 μg/L NiCl2·6H2O, 18 μg/L Na2MoO4·2H2O, 300 μg/L H3BO3).50 For aerobic cultures, the medium was buffered to pH 7.2 with 20 mM phosphate. For anoxic experiments, the medium was pH buffered with 22 mM NaHCO3 and adjusted to pH 7 with 1 M HCl under an oxygen free atmosphere containing 15% CO2. Phosphate addition was minimal (but not microbially growth inhibiting) to avoid precipitation of vivianite (Fe3(PO4)2·8H2O) at high Fe(II) concentrations. The final ionic strength was ∼0.04 M. Anoxic solutions were prepared using O2-free deionized water and stored anoxically for at least three days prior to use. Reactant solutions containing nitrite were always prepared fresh from an anoxic stock solution kept at pH 11 to avoid degradation through self-decomposition. All glassware and plastics were autoclaved and stored anoxically for at least three days prior to use.
Bacterial Strains
Paracoccus denitrificans strain ATCC 19367 was obtained from the United States Department of Agriculture culture collection and was grown routinely in anoxic freshwater medium under denitrifying conditions with succinate as the growth substrate. Pseudogulbenkiania sp. strain MAI-1 is a newly isolated β-proteobacterium that was routinely grown in anoxic freshwater medium under denitrifying conditions with acetate as the growth substrate.
Isolation
Cultures of anaerobic Fe(II) oxidizing chemotrophs were enriched by inoculating freshwater medium supplemented with 10 mM FeCl2, 10 mM Na3NTA, 2 mM Na acetate, and 5 mM NaNO3 with samples from a microbial mat in the litterol zone of iron-rich tropical Lake Matano, Sulawesi Island, Indonesia.8 Enrichments were incubated at 30 °C in the dark. After a few days, some enrichments developed the characteristic dark green color of Fe(III)-NTA, indicating Fe(II) oxidation. Cultures exhibiting fast Fe(II) oxidation were transferred successively to fresh Fe(II)-containing medium. After four transfers, serial dilutions of enrichments were plated on YP agar pates (0.3% yeast extract, 0.3% Difco Bacto Peptone, 1.2% agarose) and incubated aerobically at 30 °C in the dark to identify strains potentially suitable for genetic manipulation. Colonies were picked and subcultured in the Fe(II) enrichment medium. Fast Fe(II) oxidizers were plated again, and the purity was assessed by phase-contrast microscopy. The 1497-bp 16S rRNA gene sequence of strain MAI-1 was deposited in the GenBank database under the accession number HQ714499. The pure strain was deposited with the American Type Culture Collection under the ATCC number BAA-2177.
Analytical Techniques
The concentration of Fe(II) was determined colorimetrically at 562 nm using the ferrozine [3-(2-pyridyl)-5,6 bis(4-phenylsulfonic acid)-1,2,4-triazine, monosodium salt] assay51 without prior acidification of analyte. Sample acidification in the presence of nitrite led to underestimation of Fe(II) concentrations31 and was therefore avoided (see Supporting Information Figure S4). The assay was calibrated using ferrous ammonium sulfate hexahydrate of known concentration. Nitrite was determined colorimetrically at 520 nm using sulfanilamide and N-1-napthylethylenediamine dihydrochloride.52 The chelator EDTA is incompatible with this assay,53 but none of the ligands used in this study interfere with nitrite determination (Supporting Information Figure S5). The assay was calibrated using a commercial nitrite standard (Fluka Analytical TraceCERT). Samples for Fe(II) and nitrite determination in microbial cultures were obtained with a sterile disposable syringe flushed for 30 s with 20%CO2/80%N2. The evolution of N2O in abiotic reactions was assessed qualitatively by gas-chromatography using a Hewlett-Packard 5890 Series II Plus Gas Chromatograph equipped with a Thermal Conductivity Detector. Samples were injected onto a HP-MOLSIV column (30m, 0.32 mm inner diameter (ID), 12 μm film) and eluted with helium at a flow rate of 10 mL/min using a temperature gradient from 35 to 240 °C (4 min at 35 °C, 35 °C/min up to 140 °C, 25 °C/min up to 240 °C). Formation of the nitrosyliron-NTA complex (Fe(II)-NTA-NO) was assessed qualitatively by monitoring its characteristic absorption peaks (440 nm and 600 nm)54,55 spectroscopically. Growth of microbial cultures was followed by optical density at 600 nm (OD600) in cultures without iron and at 700 nm (OD700) in cultures with iron. This wavelength was used to decrease distortion by Fe(III)-NTA, which absorbs strongly at 600 nm. OD700 underestimates optical density as compared to OD600.
Experimental Procedure
Kinetic Fe(II) oxidation experiments were conducted inside an anaerobic chamber (Coy Laboratory Products, Inc.) equipped with palladium catalysts for O2 removal. The chamber contained ∼3%H2/15% CO2/82% N2 and experiments were performed at 25 °C using a digital heat block. Samples were taken at varying time points and analyzed immediately for Fe(II) and nitrite concentrations using a BioTek Synergy 4 Microplate Reader housed inside the chamber. Oxidation experiments were conducted in sterile basal freshwater medium containing 2 mM Fe(II) and 2 mM NO2– and were amended alternatively with 2 mM nitrilotriacetate (NTA), 300 mg/L Pahokee Peat Humic Acid (PPHA, International Humic Substances Society), 0.1, 0.5, or 2 mM citrate, 300 mg/L PPHA + 2 mM citrate. PPHA was selected as the humic acid of choice due to its high solubility and low capacity for storing redox equivalents that could rereduce Fe(III) and interfere with the experiment.56 Control experiments included incubations of Fe(II) with or without NTA in the absence of nitrite or in the presence of 2 mM nitrate. pH was measured at the beginning and conclusion of each experiment.
Pseudogulbenkiania sp. strain MAI was grown in triplicate at 30 °C in the dark in freshwater medium amended with 0.5 mM acetate, 4 mM Fe(II), and 8 mM NTA, and a headspace of ∼3%H2/15% CO2/82% N2. Cultures were sampled regularly for nitrite accumulation and Fe(II) oxidation.
Paracoccus denitrificans was grown in triplicate at 30 °C in the dark in freshwater medium amended with 10 mM succinate and 20 mM nitrate and sampled regularly for nitrite accumulation. Upon reaching a nitrite concentration of ∼5 mM, 5 mL of each culture was withdrawn and processed anaerobically as follows: each withdrawn sample was divided in four. Two aliquots were left unchanged while the other two were filter sterilized using a 0.2 μm syringe filter. All aliquots were spiked with ∼5 mM Fe(II) and one of each set (one unfiltered P. denitrificans and one filter-sterilized aliquot) was further amended with 10 mM citrate (all from 1 M stock solutions to avoid sample dilution). No citrate was present in cultures prior to spiking. Aliquots were incubated at 25 °C for 4 h and sampled at regular intervals as described in the kinetic Fe(II) oxidation experiments. The remaining cultures were reincubated at 30 °C for continued monitoring of growth and nitrite accumulation.
Computation
Nonlinear least-squares model fits and parameter estimates for kinetic data were computed using the statistical model analysis functionality provided by Wolfram Mathematica (v. 8.0). Fe(II) speciation in solution was estimated using the Visual MINTEQ equilibrium speciation model (v. 3.0) with stability constants provided by King57 (Fe(II)-carbonate complexes) and the MINTEQ database58 (all other Fe(II) species) and precomputed humic substance properties based on the NICA-Donnan model.59 Chemical oxidation of Fe(II) with nitrite produced by MAI-1 was modeled using Euler’s method to calculate stepwise solutions of eq 5. Nitrite concentrations at each time step were calculated by linear interpolation between closest measurement time points. Chemical oxidation with concomitant biological NO consumption was modeled by assuming complete NO removal and subsequent lack of Fe(II)-NTA-NO complex formation.
Results
The enrichment of fast growing anaerobic Fe(II) oxidizing chemotrophs lead to the successful isolation of Pseudogulbenkiania sp. strain MAI-1, a novel β-proteobacterium closely related to the lithoautotrophic Fe(II) oxidizer Pseudogulbenkiania sp. 200216,28 (96.9% 16S rRNA gene sequence similarity, 97.3% to the type strain Pseudogulbenkiania subflava BP-560). MAI-1 has several key characteristics necessary for routine genetic manipulation: the strain forms colonies on plates (aerobically within 24 h), grows rapidly both aerobically and anaerobically (overnight at 30 °C), is sensitive to antibiotics, and cryopreserves well. Most importantly, it displays the desired phenotype: rapid nitrate dependent Fe(II) oxidation (10 mM in less than 24 h, Supporting Information Figure S1) in the presence of a chelator, nitrilotriacetate (NTA), that prevents the formation of mineral precipitates (which could obscure cells in automated assays) but does not serve as a growth substrate for the organism (Supporting Information Figure S2). When first isolated, MAI-1 appeared to be an ideal candidate for elucidating the genes required for nitrate dependent Fe(II) oxidation. However, although Fe(II)-NTA is highly stable in abiotic controls in the presence of nitrate (Figure 1; Supporting Information Figure S1), adding Fe(II)-NTA to filter-sterilized spent MAI-1 growth medium that had accumulated substantial amounts of nitrite lead to rapid Fe(II) oxidation with concomitant nitrite reduction (Supporting Information Figure S3). The strain’s ability to use a wide range of chelators as a carbon substrate (e.g., citrate, humic acids, DTPA) and its inability to grow and oxidize free Fe2+ (Supporting Information Figure S1) precluded avoiding NTA. Additionally, MAI-1 cannot use alternate electron acceptors (e.g., DMSO, TMAO, fumarate), requiring the use of nitrate (and consequentially risking the production of nitrite) for anaerobic culturing.
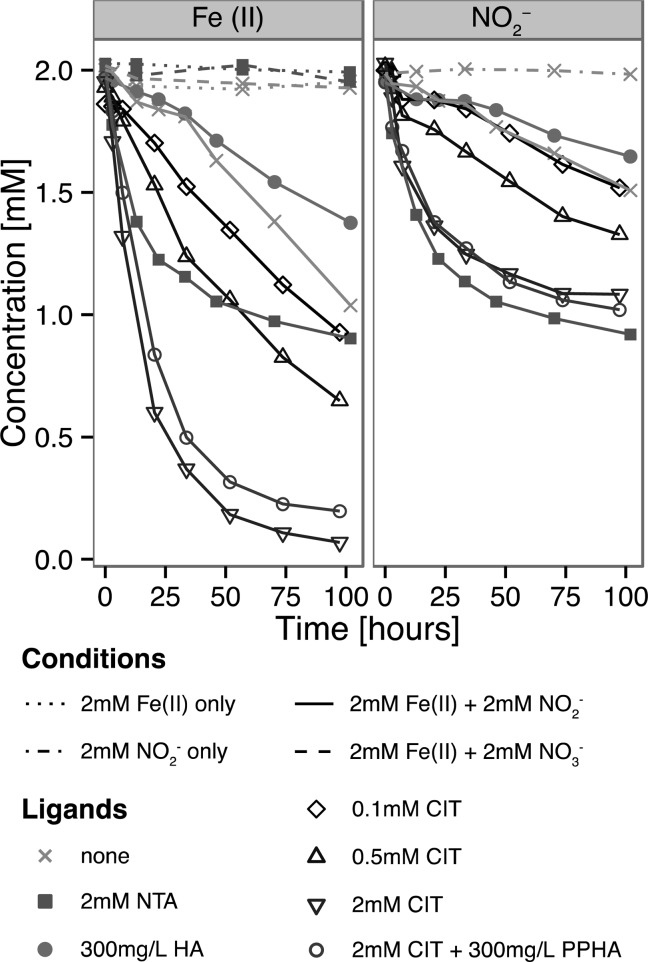
Figure 1.
Ligands affect the abiotic oxidation of Fe(II) by NO2–. Error bars omitted for clarity (relative standard deviation of Fe(II) and NO2– quantitation from all seven experiments estimated at 3% and 2%, respectively).
To quantitatively assess the effect of Fe(II) chelation on chemical oxidation by nitrite at circumneutral pH, we conducted kinetic experiments with NTA as well as two environmentally relevant Fe(II)-chelating ligands (citrate, CIT, and Pahokee Peat Humic Acid, PPHA). Attempts to investigate the effect of Fe(II) chelation with the siderophore desferoxamine (DFO) and the organic pollutant ethylenediaminetetraacetate (EDTA) proved unsuccessful because of interference with the ferrozine assay and the nitrite assay, respectively (Supporting Information Figure S5). They were not pursued further. Figure 1 shows the oxidation of Fe(II) and concomitant reduction of NO2– over the course of ∼100 h (4.2 days) for each condition. Nitrite-free controls without any oxidant or amended with nitrate show little Fe(II) oxidation (a maximum of 2% without oxidant, 5% with nitrate; see Table 1) over the course of the experiment. This provided confidence that O2 contamination is not a significant source of error in our experimental setup and suggested that nitrate is relatively unreactive toward Fe(II) even in the presence of ligands (see abiotic control in Supporting Information Figure S1). Nitrite in the absence of iron shows high stability, confirming the expected absence of nitrite self-decomposition that occurs at acidic pH.61 In the absence of any chelating moieties, less than 9% of Fe(II) is oxidized by nitrite within the first 22 h. Similar control experiments in previous reports have yielded Fe(II) oxidation rates at ∼8% Fe(II) within 10 h,36 ∼9% within 20 h,39 and ∼1% within 24 h.37 Complexation by both citrate and NTA, however, leads to rapid depletion of Fe(II) and nitrite, indicating that these organic ligands can accelerate Fe(II) oxidation by nitrite (Figure 1).
Table 1. Summary of Kinetic Fe(II) Oxidation Experiments by Nitritea.
| reactant
changes within ∼100 h |
Fe(II)
oxidation |
NO2– reduction |
|||||
|---|---|---|---|---|---|---|---|
| ΔFe(II) | ΔNO2– | ΔFe(II)/ΔNO2– | model | kapp (LCI;UCId) | model | kapp (LCI;UCId) | |
| [μM] (%b) | [μM] (%b) | (±1σ)c | R2 | [10–3 M–1 s–1] | R2 | [10–3 M–1 s–1] | |
| Controls | |||||||
| 2 mM NO2– only | –3 (0%) | ||||||
| 2 mM Fe(II) only | –3 (0%) | ||||||
| +2 mM NTA | –35 (2%) | ||||||
| +2 mM NO3– | –91 (5%) | ||||||
| +2 mM NTA + 2 mM NO3– | –64 (3%) | ||||||
| Kinetically Unresolved | |||||||
| 2 mM Fe(II) + 2 mM NO2– | –963 (48%) | –478 (24%) | 2.0 ± 0.3 | ||||
| +0.1 mM citrate | –933 (50%) | –480 (24%) | 1.9 ± 0.2 | ||||
| +300 mg/L PPHA | –592 (30%) | –303 (16%) | 2.0 ± 0.4 | ||||
| Second-Order Kinetics | |||||||
| +0.5 mM citrate | –1281 (66%) | –686 (34%) | 1.9 ± 0.2 | 0.9995 | 0.98 (0.92;1.04) | 0.9995 | 1.04 (0.88;1.19) |
| +2 mM citrate | –1883 (96%) | –945 (47%) | 2.0 ± 0.1 | 0.9979 | 4.67 (4.18;5.17) | 0.9992 | 4.31 (3.57;5.06) |
| +2 mM citrate +300 mg/L PPHA | –1773 (90%) | –931 (48%) | 1.9 ± 0.1 | 0.9963 | 3.31 (2.85;3.78) | 0.9997 | 3.59 (3.24;3.93) |
| +2 mM NTA | –1119 (55%) | –1065 (54%) | 1.1 ± 0.1 | 0.9987 | 6.66 (5.19;8.13) | 0.9993 | 6.11 (5.15;7.07) |
The rate constant kapp is reported for reactions that are described well by second-order kinetics. The experiments were conducted at 25°C, pH 6.9 to 7.1. The p-values for the model parameter kapp are <0.001 for all conditions. R2 is the adjusted regression coefficient for the least-squares fit.
Percentage change of [Fe(II)] and [NO2–] relative to starting concentrations.
Derived by error propagation from measurement errors (relative standard deviation of Fe(II) and NO2– quantitation during experiments estimated at 3% and 2% respectively).
Lower (LCI) and upper (UCI) 95% confidence interval of parameter derived from model fit.
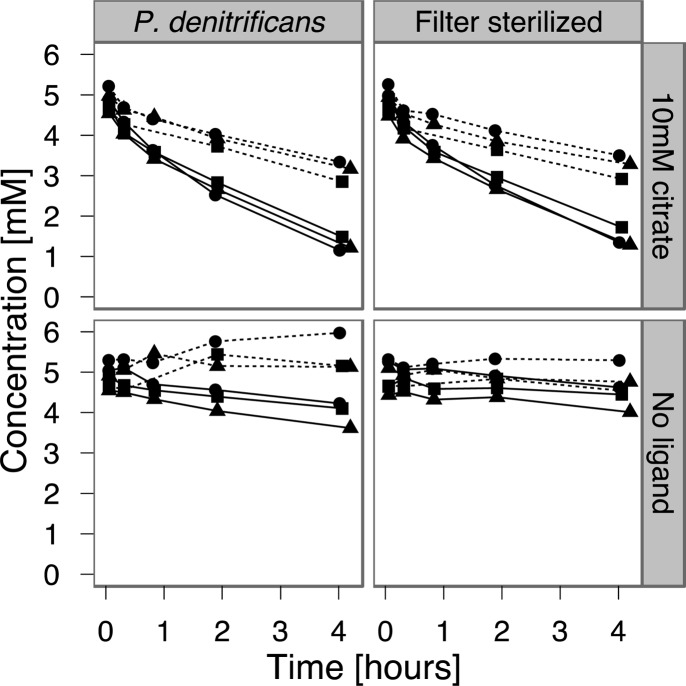
Equipped with an estimate for the extent of chemical Fe(II) oxidation by nitrite in the presence of NTA, we grew MAI-1 in the presence of Fe(II)–NTA while closely monitoring the accumulation of nitrite (Figure 2) to model the maximal abiotic Fe(II) oxidation resulting from an abiotic reaction with nitrite. Given the strong effect of citrate on the chemical oxidation of Fe(II) by nitrite, we also tested the hypothesis that abiotic Fe(II) oxidation could be mediated by the biological production of nitrite during denitrification in general. For this purpose, P. denitrificans, a model denitrifying microorganism, was grown anaerobically on succinate and nitrate, such that substantial quantities of nitrite accumulated during early exponential growth (Supporting Information Figure S6). After accumulation of ∼5 mM nitrite, filter sterilized culture medium as well as active cultures of P. denitrificans were amended with ∼5 mM Fe(II) with or without 10 mM citrate. Figure 3 illustrates the resulting oxidation of Fe(II) over the course of 4 h. Moderate oxidation occurred in the absence of chelation both with P. denitrificans cultures as well as in spent medium (up to 21% and 12%, respectively). Higher oxidation rates for cultures are likely a consequence of continued denitrification by P. denitrificans, increasing the measured pool of nitrite by up to 13%. However, the most striking feature is the rapid depletion of Fe(II) and nitrite (up to 76% Fe(II), 38% NO2–) observed with the addition of 10 mM citrate, regardless of the presence of P. denitrificans (Table 2, Figure 3).
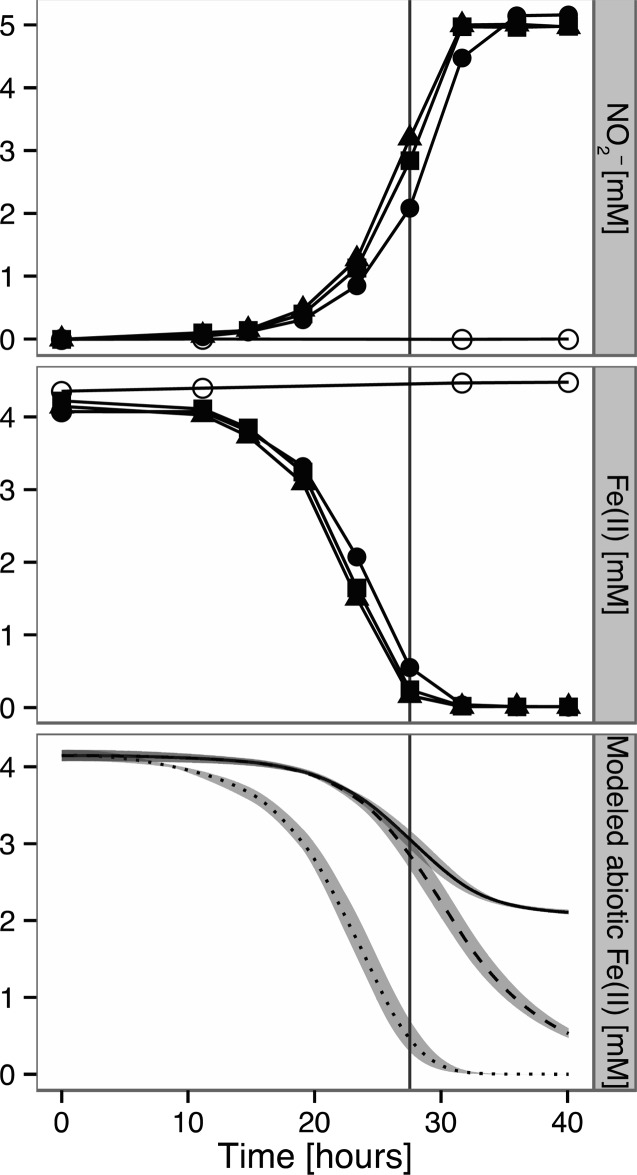
Figure 2.
Fe(II) oxidation by Pseudogulbenkiania sp. strain MAI-1 during anaerobic growth with nitrate. Nitrite accumulation during growth depicted in top panel, concomitant Fe(II) oxidation in middle panel, modeled abiotic Fe(II) oxidation in bottom panel (see Materials and Methods for details on computation). Solid and dashed lines indicate Fe(II) oxidation without/with biological NO consumption, respectively. Dotted line indicates Fe(II) oxidation with 6× higher rate constant and NO consumption. Model range for three biological replicates shaded in gray. Vertical line indicates time point addressed in text. Experiment conducted in biological triplicates (solid markers) and with abiotic control (empty circles, ○). All data are shown.
Figure 3.
Fe(II) oxidation in P. denitrificans cultures and filter-sterilized spent medium. Fe(II) concentrations shown as solid lines, NO2– concentrations as dashed lines. Samples are drawn from triplicate cultures (Supporting Information Figure S6) after accumulation of ∼5 mM NO2– and spiked with Fe(II) ± citrate at 0 h. All data are shown.
Table 2. Summary of Kinetic Fe(II) Oxidation Experiments by Nitrite in P. denitrificans Cultures and Spent Mediuma.
| reactant
changes within ∼4 h |
Fe(II)
oxidation |
NO2– reduction |
||||
|---|---|---|---|---|---|---|
| ΔFe(II) | ΔNO2– | model | kapp (LCI;UCIc) | model | kapp (LCI;UCIc) | |
| [mM] (%b) | [mM] (%b) | R2 | [103 M–1 s–1] | R2 | [103 M–1 s–1] | |
| P. denitrificans | ||||||
| #1 | –3.7 (76%) | –1.9 (36%) | 0.9991 | 12 (11;14) | 0.9991 | 11 (8;15) |
| #2 | –3.3 (73%) | –1.8 (36%) | 0.9984 | 11 (9;13) | 0.9996 | 10 (8;12) |
| #3 | –3.2 (69%) | –1.8 (38%) | 0.9977 | 10 (7;13) | 0.9985 | 11 (6;17) |
| Filter Sterilized | ||||||
| #1 | –3.6 (73%) | –1.8 (33%) | 0.9990 | 11 (9;12) | 0.9981 | 10 (6;15) |
| #2 | –3.2 (71%) | –1.7 (34%) | 0.9985 | 11 (9;13) | 0.9995 | 10 (8;12) |
| #3 | –3.2 (65%) | –1.7 (37%) | 0.9983 | 9 (7;11) | 0.9988 | 12 (7;17) |
The experiment was conducted at 25 °C. P-values for the model parameter k2 are <0.01. R2 is the adjusted regression coefficient for the least-squares fit.
Percentage change of [Fe(II)] and [NO2–] relative to starting concentrations.
Lower (LCI) and upper (UCI) 95% confidence interval of parameter derived from model fit.
Discussion
Reaction Mechanism and Kinetics
Understanding the kinetics of Fe(II) oxidation in the presence of ligands provides the tools for predicting the potential effects of ligand-enhanced Fe(II) oxidation in microbial systems. The total consumption of Fe(II) and nitrite (Table 1) suggests that Fe(II) oxidation by nitrite proceeds with 2:1 Fe(II)/NO2– stoichiometry regardless of complexation (no ligand, PPHA, citrate), with the notable exception of NTA, which appears to deplete Fe(II) and NO2– in a 1:1 ratio. The 2:1 stoichiometry is in agreement with literature reports that the predominant product of nitrite reduction at pH regimes between 6 and 8 is N2O,22,33,36,37,62 according to the following representative net reaction:
| 1 |
where Fe2+ can be unbound Fe2+ or a ligand-bound Fe(II)-L species, and Fe3+ can be ligand-bound Fe(III)-L or contained within an (oxy)hydroxide mineral (e.g., FeOOH). This net reaction likely comprises a number of elementary reaction steps; we consider the following three to contextualize our observations:
| 2 |
| 3 |
| 4 |
Equations 3(63) and 4(64) proceed rapidly at circumneutral pH, with eq 2 being the rate limiting step (k1≈ k2). Accordingly, the reaction consumes 2 Fe(II) for every NO2–, except in the case of NTA. Both citrate and NTA complexes with ferrous iron can bind nitric oxide such that the following reactions can occur in competition with eq 3:
| 5 |
| [6] |
However, Fe(II)-NTA forms a considerably stronger complex with NO (k6≈ 2.1 × 107 M–1 s–1, Keq = 106.26)54,65,66 than Fe(II)-citrate (k5 ≈ 4.4 × 105 M–1 s–1, Keq = 102.83)66 or Fe2+ alone (k3≈ 6.2 × 105 M–1 s–1, Keq = 102.65),63 potentially preventing eq 4 from proceeding. For example, if 100 μM Fe(II) reacted with 100 μM NO2– to form NO in the presence of 2 mM NTA, more than 99.98% of the produced NO would form the highly stable Fe(II)-NTA-NO complex. The 1:1 stoichiometry of Fe(II) oxidation by nitrite observed in the presence of NTA is likely a consequence of this stable Fe(II)–NTA–NO complex formation. As expected, we confirmed evolution of N2O during Fe(II) oxidation by nitrite by gas chromatography in the presence of citrate, but no N2O formed in the presence of NTA (Supporting Information Figure S8); the formation of the Fe(II)–NTA–NO complex could be observed instead (Supporting Information Figure S9).
Based on the rate-limiting, Fe(II) and NO2– dependent first reaction step (eq 2), a plausible scheme for the overall reaction kinetics is a second-order rate expression with overall rate constant kapp in analogy with oxidation of Fe(II) and Mn(II) by O257,67
| 7 |
| 8 |
where Fe(II) comprises the total pool of ferrous iron (free Fe2+ as well as all complexed Fe(II)). Given the equimolarity of initial total Fe(II) and NO2– in our experimental setup, we integrate eqs 7 and 8 to yield the following decay equations (see the Supporting Information for details):
| 9 |
| 10 |
Least-squares fits of eqs 9 and 10 to our experimental results for Fe(II) and NO2– depletion provide two separate estimates of the overall rate constant kapp for each condition (Tables 1 and 2). Reactions without a ligand and with low citrate or PPHA are better described by a linear least-squares fit (apparent zero-order kinetics) and are therefore considered kinetically unresolved (no kapp determined). Elementary reaction steps and kinetic constraints for these conditions cannot be deduced from our observations, and it remains unclear why the reactions appear to be zero-order. Oxidation in these conditions likely proceeds as a consequence of ferric (oxy)hydroxide precipitation (observed visually) and subsequent heterogeneous autocatalysis as reported by Tai and Dempsey (2009).37 Apparent zero-order kinetics could reflect the complex balance between the generation of catalytic mineral surfaces and depletion of dissolved Fe(II) and nitrite.
At higher concentrations of citrate and NTA, the reactions remained homogeneous and are in agreement with a second-order kinetic interpretation of our data (Tables 1 and 2 and Supporting Information Figure S7). Rate constants derived from Fe(II) oxidation and nitrite reduction agree well within their 95% confidence intervals, lending further credence to the model. The pH remained close to 7.0 in all conditions, with an average change of 0.1 by the end of the experiment (Supporting Information Table S1), suggesting that the presence of the ligands, rather than fluctuations in pH are responsible for the observed differences in reaction kinetics. The reaction progression observed in the presence of PPHA suggests that chelation of Fe(II) by the humic acid moieties (10% of the initial Fe(II) pool is organically complexed) has little to no effect on the kinetics of iron oxidation (see Figure 1, PPHA and CIT + PPHA). Rather than accelerating Fe(II) oxidation, PPHA appears to have a slight retarding effect. In contrast to experiments without a ligand, PPHA is likely to impede iron oxide formation and autocatalysis as a result of its high affinity for Fe(III). In combination with citrate, PPHA leads to diminished formation of the Fe(II)-citrate complex (Supporting Information Table S2), which appears to reduce the overall reaction rate (Table 1).
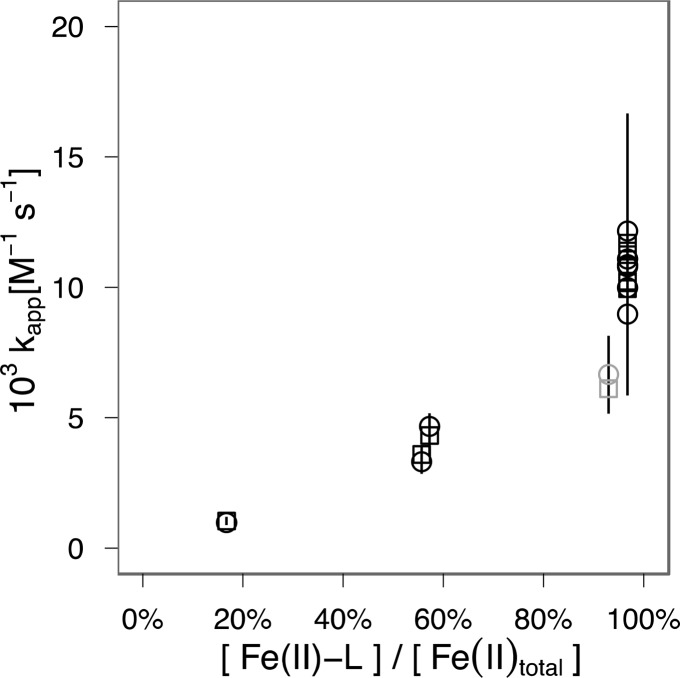
Additional information for predicting the contribution of chemical Fe(II) oxidation, especially in well-defined laboratory systems, can be gained from identifying the reactive species. In analogy to Fe(II) and Mn(II) oxidation by O2, the overall rate constant kapp observed in our experiments can likely be explained in terms of the weighted sum of the oxidation rates of individual Fe(II) species57,67kapp = ∑ kiαi where αi is the fraction of each Fe(II) species in solution and ki the species-specific second-order rate constant for oxidation by nitrite. A comparison of kapp with the extent of Fe(II) complexation for each experimental condition (Figure 4; Supporting Information Table S2) suggests that the Fe(II)-L complex is involved in accelerating Fe(II) oxidation, although the effect is ligand-specific (no effect for PPHA, variable magnitude for citrate and NTA). The observed reaction rates at low species fractions of Fe(II)-L (<20%) suggest the existence of other Fe(II) species with appreciable nitrite-dependent oxidation rates. We speculate that the carbonate species Fe(II)–CO3–OH– and Fe(II)–(CO3)22– (Supporting Information Table S2) could provide such reactive species in analogy to their role in Fe(II) oxidation by molecular oxygen.57 However, the precise mechanism and species-specific reaction rates ki for the observed oxidation of Fe(II) by nitrite are beyond the scope of this report and await further study. Due to the uncertainty surrounding the reactive species involved, we recommend caution in applying the rate constants derived in Tables 1 and 2 to aqueous environments with widely differing Fe(II) complexation, pH, or ionic strength.
Figure 4.
Rate constants increase with increasing degree of Fe(II) complexation. Second-order rate constants for oxidation experiments in the presence of citrate (black symbols) and NTA (gray symbols) are plotted against the degree of Fe(II) complexation by citrate/NTA. Rate constants derived from [Fe(II)] depicted as circles (○), constants derived from [NO2–] as squares (□). Error bars indicate 95% confidence intervals (Tables 1 and 2). Details on speciation can be found in Supporting Information Table S1. Larger confidence intervals for data reported in Table 2 are a consequence of reduced temporal resolution and greater deviation from the assumption that initial Fe(II) and NO2– concentrations are equimolar.
Biological Fe(II) Oxidation by Pseudobulkeniania sp. Strain MAI-1
Using the kinetic rate constants derived for the oxidation of Fe(II) by nitrite in the presence of NTA with the nitrite accumulation measured in culture of MAI-1 (Figure 2), we modeled the purely abiotic Fe(II) oxidation that would result from the interaction of Fe(II) with the accumulated nitrite (Figure 2, bottom), assuming the presence of cell surfaces22 to have negligible effects on purely chemical oxidation. Even if we conservatively assume the upper 95% confidence interval for the rate constant (8.13 M–1 s–1; see Table 1) and that produced NO is biologically consumed (thus leaving more Fe(II) free to react by preventing formation of the highly stable Fe(II)-NTA-NO complex), abiotic oxidation would maximally account for ∼30%/35% (solid vs dashed curve) of the observed Fe(II) oxidation after 28 h (time point indicated by vertical line in Figure 2). In fact, a 6× higher rate constant (combined with biological consumption of any produced NO) would be required to attribute observed Fe(II) oxidation to purely chemical processes (Figure 2, dotted model). Based on the kinetic quantification of chemical oxidation of Fe(II), it thus becomes evident that Pseudogulbenkiania sp. MAI-1 can directly oxidize Fe(II), establishing the organism as a novel neutrophilic nitrate-dependent chemotroph with unambiguous biological Fe(II)-oxidizing activity. The potential to easily genetically manipulate this strain makes it a good candidate for elucidating the machinery involved in biological Fe(II) oxidation. Whether the biological component of Fe(II) oxidation in MAI-1 occurs via a dedicated enzyme system or via nonspecific reactions with redox active components of the cell, such as periplasmic thiols or components of the electron transport chain,25,26 is a question that could be addressed in the future.
Chemical vs Biological Fe(II) Oxidation in Laboratory and Environmental Studies
Given the aforementioned difficulty in discriminating between chemical and biological contributions to anaerobic Fe(II) oxidation in many systems, it can be informative to compare Fe(II) oxidation rates observed in a variety of environmental and laboratory settings. Table 3 provides an overview of the maximal Fe(II) oxidation rates reported in a number of publications on chemical and biological Fe(II) oxidation in nitrite/nitrate rich anoxic environments at circumneutral pH. Several observations are particularly noteworthy:
-
(i)
The majority of observed maximal rates of chemical and biological Fe(II) oxidation fall within a similar range of values (∼10–100 μM/h), highlighting the likely competition and co-occurrence of chemical and biological processes involved in the coupled biogeochemical cycling of iron and nitrogen. Moreover, because nitrite is produced and often accumulates during the microbial denitrification process, they are intrinsically coupled. This biologically induced chemical oxidation of iron (via the microbial production of nitrite) in organic rich environments such as soils and wetlands is likely to contribute significantly to the cycling of iron and immobilization of metal contaminants and organic pollutants on iron (oxy)hydroxides. High oxidation rates reported for environmental samples with mixed contributions from biological and chemical catalysis20 illustrate the interplay of these processes and call for caution in interpreting an observed effect to stem from solely one or the other mechanism.
-
(ii)
In the case of mineral accelerated Fe(II) oxidation, the presence of amorphous hydrous ferric oxide (HFO/ferrihydrite)9,31,37 and green rust42 appears to cause the most significant acceleration of Fe(II) oxidation (see Table S3 for additional detail on rate constants derived for mineral catalysis). This effect is likely to be highly relevant in natural settings where poorly crystalline iron oxides are ubiquitous. However, it is also important to consider this effect in laboratory studies where iron oxides precipitate over the course of an experiment and can provide catalytic surfaces for chemodenitrification as suggested previously.23−25
-
(iii)
In the case of ligand-enhanced Fe(II) oxidation by nitrite, the absence of a major effect of the humic acid representative PPHA and low environmental abundance of the anthropogenic ligand NTA (maximal levels of 10–100 nM in aqueous systems),1 suggests that citrate (detected in soil solutions in appreciable quantities, ∼100 μM range)68 is likely to be the only ligand investigated in this study that could be relevant in natural systems. In laboratory studies of iron oxidizing microorganisms in the presence of citrate or NTA, the ligands’ effect on oxidation kinetics is a crucial aspect of Fe(II) depletion that cannot be disregarded. This is particularly clear from the experiment reported in Figure 3 that confirms ligand-enhanced chemical oxidation of Fe(II) by nitrite can be an important side effect of microbial denitrification. Here, chemical Fe(II) oxidation could be mistaken for direct biological catalysis by P. denitrificans; while direct catalysis may indeed be at play, it would simply be challenging to unambiguously identify without appropriate controls. In conclusion, this study serves as a reminder of the complex interplay between direct and indirect biological effects involving metal transformations. In the case of denitrifying microorganisms, the extent to which these different processes catalyze Fe(II) oxidation likely depends on the precise culturing conditions and must be evaluated on a case-by-case basis.
Table 3. Maximal Rates of Fe(II) Oxidation Reported for Various Anaerobic Processes at Circumneutral pH (25–30 °C, Except Where Otherwise Indicated).
| experimental
conditions |
max. rates | ||||||
|---|---|---|---|---|---|---|---|
| pH | buffer | Fe(II) | nitrite | nitrate | ΔFe(II) [μM/h] | reference | |
| Chemical (Abiotic) | |||||||
| +30 mg/L lepidocrocite (γ-FeOOH) | 7.5 | autotitration | 0.2 mM | 0.2 mM | –7 | (36), Figure 5 | |
| +30 mg/L lepidocrocite (γ-FeOOH) | 8.5 | autotitration | 0.2 mM | 0.2 mM | –40 | (36), Figure 5 | |
| Fe(II) as siderite (10 g/L ∼ 80 mM) | 6 | MES/PIPES/HEPES | 10 g/L | 4.6 mM | –265 | (39), Figure 5 | |
| Fe(II) as siderite (10 g/L ∼ 80 mM) | 6.5 | MES/PIPES/HEPES | 10 g/L | 4.6 mM | –169 | (39), Figure 5 | |
| Fe(II) as siderite (10 g/L ∼ 80 mM) | 7.9 | MES/PIPES/HEPES | 10 g/L | 4.6 mM | –140 | (39), Figure 5 | |
| +2.5 mM Fe(II) as HFO, 64 μM average solid-bound Fe(II) | 6.8 | PIPES | 0.38 mM | 0.38 mM | –158 | (37), Table 1, #6 | |
| +17.5 mM Fe(III) as HFO, 188 μM average solid-bound Fe(II) | 6.8 | PIPES | 0.34 mM | 0.32 mM | –301 | (37), Table 1, #11 | |
| F(II) as green rust | 8.25 | autotitration | 10.81 mM | 14.2 mM | –139 | (42), Table 1 | |
| +2 mM NTA | 7 | bicarbonate | 2 mM | 2 mM | –192 | this study, Table 1 | |
| +2 mM CIT | 7 | bicarbonate | 2 mM | 2 mM | –134 | this study, Table 1 | |
| +10 mM CIT, P. denitrificans spent medium | 7 | bicarbonate | 5 mM | 5 mM | –1695 | this study, Table 2 | |
| +10 mM CIT, P. denitrificans culture | 7 | bicarbonate | 5 mM | 5 mM | –1910 | this study, Table 2 | |
| Mixed (Chemical + Biological) | |||||||
| D. frappieri strain G, Fe(II) complexed by 10 mM NTA | ∼7 | bicarbonate | 4.8 mM | 1.4 mM | 2.5 mM | –294 | (20), Figure 5 |
| D. frappieri strain G, Fe(II) as smectite | ∼7 | bicarbonate | 3 mM | 1.4 mM | 5 mM | –175 | (20), Figure 6 |
| Pseudogulbenkiania sp. MAI-1, Fe(II)-NTA | 7 | bicarbonate | 4 mM | 5 mM | 10 mM | –360 | this study, Figure 2 |
| Chemotrophic | |||||||
| enrichment culture, +1 mM acetate | 7 | bicarbonate | 10 mM | ? | 3 mM | –106 | (4), Figure 1 |
| enrichment culture containing Sideroxydans species | 6.8 | bicarbonate | 10 mM | ? | 4 mM | –156 | (29), Figure 1a |
| Pseudogulbenkiania strain 2002 | 6.8 | bicarbonate | 10 mM | ? | 2.2 mM | –74 | (16), Figure 4 |
| strain HidR2, +1 mM acetate | 6.7 | bicarbonate | 6 mM | <30 μM | 5 mM | –66 | (14), Figure 2 |
| Ferroglobus placidus, 85C | 7 | bicarbonate | 2 mM | up to 550 μM | 0.64 mM | –173 | (5), Figure 4 |
| cell suspension of D. suillum, grown on acetate + nitrate | 6.8 | bicarbonate | 10 mM | ? | 10 mM | –4700 | (12), Figure 3a |
| Paracoccus ferrooxidans, +25 mM EDTA, +1 mM ethanol | 7 | bicarbonate | 25 mM | ? | 5 mM | –1600 | (13), Figure 3a |
| Acidovorax sp. strain BoFeN1, +2 mM acetate | 6.8 | bicarbonate | 2.5 mM | <1 mM | 5 mM | –48 | (15), Figure 2 |
| Acidovorax sp. strain BoFeN1, +5 mM acetate | 7 | bicarbonate | 10 mM | 0 mM | 10 mM | –240 | (30), Figure 1a |
| Acidovorax sp. strain 2AN, +1.6 mM acetate | 6.85 | bicarbonate | 8.3 mM | up to 1 mM | 5 mM | –158 | (24), Figure 2a |
| Acidovorax sp. strain 2AN, + 4 mM EDTA, +1.2 mM ethanol | 7 | PIPES | 4 mM | ? | 5 mM | –970 | (49), Figure 3c |
| Dechloromonas sp. UWNR4, + 4 mM EDTA, +1.2 mM ethanol | 7 | PIPES | 4 mM | ? | 5 mM | –950 | (49), Figure 3d |
| lake sediment slurry | ∼7 | bicarbonate | 1.4 mM | 0.01 mM | 1 mM | –6 | (69), Figure 3 |
| Phototrophic | |||||||
| Rhodopseudomonas palustris strain TIE-1, + 0.2 mM citrate | 7 | bicarbonate | 4.5 mM | –21 | (3), Figure 2 | ||
| Rhodobacter capsulatus strain SB1003, +0.2 mM citrate | 7 | bicarbonate | 0.1 mM | –34 | (3), Figure 4 | ||
| Rhodobacter capsulatus strain SB1003, +1 mg/L HA | 7 | bicarbonate | 0.1 mM | –50 | (70), Figure 4 | ||
| Rhodobacter capsulatus strain SB1003, +0.2 mM NTA | 7 | bicarbonate | 0.1 mM | –112 | (70), Figure 4 | ||
Acknowledgments
We thank Jim Morgan for many insightful conversations and inspiring S.H.K. to pursue this project, Sean Crowe, CarriAyne Jones, Arne Sturm, Sulung Nomosatryo, David Fowle, and Don Canfield for sample acquisition and fieldwork in Indonesia, Nathan Dalleska and the Caltech Environmental Analysis Center for instrumentation that benefited this project, Andreas Kappler, Nicole Klüglein, and Jay Labinger for helpful discussions, members of the Newman Lab and the anonymous reviewers for constructive criticism that improved the manuscript. This work was supported by grants to D.K.N from the Dreyfus Foundation and the Howard Hughes Medical Institute (HHMI). D.K.N. is an HHMI Investigator. S.H.K. is an HHMI International Student Research Fellow.
Supporting Information Available
Derivation of reaction equations and additional tables and figures as described in the text. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Stumm W.; Morgan J.. Redox conditions in natural waters. In Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed.; John Wiley & Sons: New York, 1996; Chapter 8.5, p 464. [Google Scholar]
- Ehrenreich A.; Widdel F. Anaerobic oxidation of ferrous iron by purple bacteria, a new-type of phototrophic metabolism. Appl. Environ. Microbiol. 1994, 60, 4517–4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y.; Kappler A.; Croal L.; Newman D. Isolation and characterization of a genetically tractable photo autotrophic Fe(II)-oxidizing bacterium, Rhodopseudomonas palustris strain TIE-1. Appl. Environ. Microbiol. 2005, 71, 4487–4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub K.; Benz M.; Schink B.; Widdel F. Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Appl. Environ. Microbiol. 1996, 62, 1458–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafenbradl D.; Keller M.; Stetter K. Ferroglobus placidus gen. nov., sp. nov., a novel hyperthermophilic archaeum that oxidizes Fe2+ at neutral pH under anoxic conditions. Arch. Microbiol. 1996, 166, 308–314. [DOI] [PubMed] [Google Scholar]
- Croal L. R.; Jiao Y.; Newman D. K. The fox operon from Rhodobacter strain SW2 promotes phototrophic Fe(II) oxidation in Rhodobacter capsulatus SB1003. J. Bacteriol. 2007, 189, 1774–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva I. H. I.; Newman D. K. D.; Louro R. O. R. Functional characterization of the FoxE iron oxidoreductase from the photoferrotroph Rhodobacter ferrooxidans SW2. J. Biol. Chem. 2012, 287, 25541–25548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe S. A.; O’Neill A. H.; Katsev S.; Hehanussa P.; Haffner G. D.; Sundby B.; Mucci A.; Fowle D. A. The biogeochemistry of tropical lakes: A case study from Lake Matano, Indonesia. Limnol. Oceanogr. 2008, 53, 319–331. [Google Scholar]
- Tiedje J. M.Ecology of denitrification and dissimilatory nitrate reduction to ammonium. Biology of Anaerobic Microorganisms; John Wiley & Sons: New York, 1988; pp 179–244. [Google Scholar]
- Edwards K. J.; Rogers D. R.; Wirsen C. O.; McCollom T. M. Isolation and characterization of novel psychrophilic, neutrophilic, Fe-oxidizing, chemolithoautotrophic α- and γ-proteobacteria from the deep sea. Appl. Environ. Microbiol. 2003, 69, 2906–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub K.; Schonhuber W.; Buchholz-Cleven B.; Schink B. Diversity of ferrous iron-oxidizing, nitrate-reducing bacteria and their involvement in oxygen-independent iron cycling. Geomicrobiol. J. 2004, 21, 371–378. [Google Scholar]
- Lack J. G.; Chaudhuri S. K.; Chakraborty R.; Achenbach L. A.; Coates J. D. Anaerobic biooxidation of Fe(II) by Dechlorosoma suillum. Microbial Ecol. 2002, 43, 424–431. [DOI] [PubMed] [Google Scholar]
- Kumaraswamy R. R.; Sjollema K. K.; Kuenen G. G.; van Loosdrecht M. M.; Muyzer G. G. Nitrate-dependent [Fe(II)EDTA]2- oxidation by Paracoccus ferrooxidans sp. nov., isolated from a denitrifying bioreactor. Systematic Appl. Microbiol. 2006, 29, 276–286. [DOI] [PubMed] [Google Scholar]
- Benz M.; Brune A.; Schink B. Anaerobic and aerobic oxidation of ferrous iron at neutral pH by chemoheterotrophic nitrate-reducing bacteria. Arch. Microbiol. 1998, 169, 159–165. [DOI] [PubMed] [Google Scholar]
- Kappler A.; Schink B.; Newman D. K. Fe(III) mineral formation and cell encrustation by the nitrate-dependent Fe(II)-oxidizer strain BoFeN1. Geobiology 2005, 3, 235–245. [Google Scholar]
- Weber K.; Pollock J.; Cole K.; O’Connor S.; Achenbach L.; Coates J. Anaerobic nitrate-dependent iron(II) bio-oxidation by a novel lithoautotrophic betaproteobacterium, strain 2002. Appl. Environ. Microbiol. 2006, 72, 686–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu Y.; Takagi M.; Yamaguchi M. Participation of iron in denitrification in waterlogged soil. Soil Biol. Biochem. 1978, 10, 21–26. [Google Scholar]
- Matocha C. J.; Coyne M. S. Short-term response of soil iron to nitrate addition. Soil Sci. Soc. Am. J. 2007, 71, 108–117. [Google Scholar]
- Brons H. J.; Hagen W. R.; Zehnder A. J. B. Ferrous iron dependent nitric-oxide production in nitrate reducing cultures of Escherichia coli. Arch. Microbiol. 1991, 155, 341–347. [DOI] [PubMed] [Google Scholar]
- Shelobolina E. S.; VanPraagh C. G.; Lovley D. R. Use of ferric and ferrous iron containing minerals for respiration by Desulfitobacterium frappieri. Geomicrobiol. J. 2003, 20, 143–156. [Google Scholar]
- Cooper D. C. D.; Picardal F. W. F.; Coby A. J. A. Chemical and biological interactions during nitrate and goethite reduction by Shewanella putrefaciens 200. Appl. Environ. Microbiol. 2003, 69, 3517–3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coby A. J.; Picardal F. W. Inhibition of NO3– and NO2– reduction by microbial Fe(III) reduction: Evidence of a reaction between NO2– and cell surface-bound Fe2+. Appl. Environ. Microbiol. 2005, 71, 5267–5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miot J.; Benzerara K.; Morin G.; Bernard S.; Beyssac O.; Larquet E.; Kappler A.; Guyot F. Transformation of vivianite by anaerobic nitrate-reducing iron-oxidizing bacteria. Geobiology 2009, 7, 373–384. [DOI] [PubMed] [Google Scholar]
- Chakraborty A.; Roden E. E.; Schieber J.; Picardal F. Enhanced growth of Acidovorax sp. strain 2AN during nitrate-dependent Fe(II) oxidation in batch and continuous-flow systems. Appl. Environ. Microbiol. 2011, 77, 8548–8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantke C.; Obst M.; Benzerara K.; Morin G.; Ona-Nguema G.; Dippon U.; Kappler A. Green rust formation during Fe(II) oxidation by the nitrate-reducing Acidovorax sp. strain BoFeN1. Environ. Sci. Technol. 2012, 46, 1439–1446. [DOI] [PubMed] [Google Scholar]
- Carlson H. K.; Clark I. C.; Melnyk R. A.; Coates J. D. Toward a mechanistic understanding of anaerobic nitrate-dependent iron oxidation: Balancing electron uptake and detoxification. Frontiers Microbiol. 2012, 3, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picardal F. F. Abiotic and microbial interactions during anaerobic transformations of Fe(II) and NOx–. Frontiers Microbiol. 2012, 3, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K. A.; Hedrick D. B.; Peacock A. D.; Thrash J. C.; White D. C.; Achenbach L. A.; Coates J. D. Physiological and taxonomic description of the novel autotrophic, metal oxidizing bacterium, Pseudogulbenkiania sp. strain 2002. Appl. Microbial Biotechnol. 2009, 83, 555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blöthe M.; Roden E. E. Composition and activity of an autotrophic Fe(II)-oxidizing, nitrate-reducing enrichment culture. Appl. Environ. Microbiol. 2009, 75, 6937–6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehe E. M.; Gerhardt S.; Schink B.; Kappler A. Ecophysiology and the energetic benefit of mixotrophic Fe(II) oxidation by various strains of nitrate-reducing bacteria. Fems Microbiol. Ecol. 2009, 70, 335–343. [DOI] [PubMed] [Google Scholar]
- Weber K. A.; Picardal F. W.; Roden E. E. Microbially catalyzed nitrate-dependent oxidation of biogenic solid-phase Fe(II) compounds. Environ. Sci. Technol. 2001, 35, 1644–1650. [DOI] [PubMed] [Google Scholar]
- Abel E.; Schmid H.; Pollak F.. Kinetik der oxydation von ferro-ion durch salpetrige Säure. Chem. Monthly, 1936. [Google Scholar]
- Moraghan J. T.; Buresh R. J. Chemical reduction of nitrite and nitrous-oxide by ferrous iron. Soil Sci.Soc. Am. J. 1977, 41, 47–50. [Google Scholar]
- Vancleemput O.; Baert L. Nitrite stability influenced by iron compounds. Soil Biol. Biochem. 1983, 15, 137–140. [Google Scholar]
- Vancleemput O.; Samater A. Nitrite in soils: Accumulation and role in the formation of gaseous N compounds. Fertilizer Res. 1996, 45, 81–89. [Google Scholar]
- Sørensen J.; Thorling L. Stimulation by lepidocrocite (γ-FeOOH) of Fe(II)-dependent nitrite reduction. Geochim. Cosmochim. Acta 1991, 55(5), 1289–1294. [Google Scholar]
- Tai Y.-L.; Dempsey B. A. Nitrite reduction with hydrous ferric oxide and Fe(II): Stoichiometry, rate, and mechanism. Water Res. 2009, 43, 546–552. [DOI] [PubMed] [Google Scholar]
- Hansen H. C. B.; Kragholm Borggaard O.; Sørensen J. Evaluation of the free energy of formation of Fe(II)–Fe(III) hydroxide-sulphate (green rust) and its reduction of nitrite. Geochim. Cosmochim. Acta 1994, 58, 2599–2608. [Google Scholar]
- Rakshit S.; Matocha C. J.; Coyne M. S. Nitrite reduction by siderite. Soil Sci. Soc. Am. J. 2008, 72, 1070. [Google Scholar]
- Buresh R. J.; Moraghan J. T. Chemical reduction of nitrate by ferrous iron. J. Environ. Quality 1976, 5, 320–325. [Google Scholar]
- Ottley C.; Davison W.; Edmunds W. Chemical catalysis of nitrate reduction by iron(II). Geochim. Cosmochim. Acta 1997, 61, 1819–1828. [Google Scholar]
- Hansen H. C. B.; Koch C. B.; Nancke-Krogh H.; Borggaard O. K.; Sorensen J. Abiotic nitrate reduction to ammonium: Key role of green rust. Environ. Sci. Technol. 1996, 30, 2053–2056. [Google Scholar]
- Theis T. L.; Singer P. C. Complexation of iron(II) by organic-matter and its effect on iron(II) oxygenation. Environ. Sci. Technol. 1974, 8, 569–573. [Google Scholar]
- Pham A. N.; Waite T. D. Modeling the kinetics of Fe(II) oxidation in the presence of citrate and salicylate in aqueous solutions at pH 6.0–8.0 and 25 °C. J. Phys. Chem. 2008, 112, 5395–5405. [DOI] [PubMed] [Google Scholar]
- Demmink J.; Beenackers A. Oxidation of ferrous nitrilotriacetic acid with oxygen: A model for oxygen mass transfer parallel to reaction kinetics. Ind. Eng. Chem. Res. 1997, 36, 1989–2005. [Google Scholar]
- Zang V.; van Eldik R. Kinetics and mechanism of the autoxidation of iron(II) induced through chelation by ethylenediaminetetraacetate and related ligands. Inorg. Chem. 1990, 29, 1705–1711. [Google Scholar]
- Zang V.; Kotowski M.; van Eldik R. Kinetics and mechanism of the formation of FeII(EDTA)NO in the system FeII(EDTA)/NO/HONO/NO2– in aqueous solutions. Inorg. Chem. 1988, 27, 3279–3283. [Google Scholar]
- Fanning J. C. The interaction of iron complexes with small nitrogen-containing molecules and ions. Coord. Chem. Rev. 1991, 110, 235–273. [Google Scholar]
- Chakraborty A.; Picardal F. Induction of nitrate-dependent Fe(II) oxidation by Fe(II) in Dechloromonas sp. strain UWNR4 and Acidovorax sp. strain 2AN. Appl. Environ. Microbiol. 2013, 79, 748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croal L.; Johnson C.; Beard B.; Newman D. Iron isotope fractionation by Fe(II)-oxidizing photoautotrophic bacteria. Geochim. Cosmochim. Acta 2004, 68, 1227–1242. [Google Scholar]
- Stookey L. L. Ferrozine—A new spectrophotometric reagent for iron. Anal. Chem. 1970, 42, 779–781. [Google Scholar]
- Griess Reagent System Technical Bulletin, TB229; Promega: Madison, WI, 2009; pp 1–8.
- Colman B. P. Understanding and eliminating iron interference in colorimetric nitrate and nitrite analysis. Environ. Monit. Assess 2009, 165, 633–641. [DOI] [PubMed] [Google Scholar]
- Lin N.; Littlejohn D.; Chang S. G. Thermodynamics and kinetics of the coordination of NO to Fe(II) NTA in aqueous solutions. Ind. Eng. Chem. Proc. 1982, 21, 725–728. [Google Scholar]
- Schneppensieper T.; Wanat A.; Stochel G.; van Eldik R. Mechanistic information on the reversible binding of NO to selected iron(II) chelates from activation parameters. Inorg. Chem. 2002, 41, 2565–2573. [DOI] [PubMed] [Google Scholar]
- Bauer I.; Kappler A. Rates and extent of reduction of Fe(III) compounds and O2 by humic substances. Environ. Sci. Technol. 2009, 1–7. [DOI] [PubMed] [Google Scholar]
- King D. Role of carbonate speciation on the oxidation rate of Fe(II) in aquatic systems. Environ. Sci. Technol. 1998, 32, 2997–3003. [Google Scholar]
- Smith R.; Martell A. NIST standard reference database 46. NIST Critically Selected Stability Constants of Metal Complexes; NIST: Gaithersburg, MD, 1998.
- Kinniburgh D.; Milne C.; Benedetti M.; Pinheiro J.; Filius J.; Koopal L.; Van Riemsdijk W. Metal ion binding by humic acid: Application of the NICA-Donnan model. Environ. Sci. Technol. 1996, 30, 1687–1698. [Google Scholar]
- Lin M.-C.; Chou J.-H.; Arun A. B.; Young C.-C.; Chen W.-M. Pseudogulbenkiania subflava gen. nov., sp. nov., isolated from a cold spring. Int. J. Syst. Evol. Micr. 2008, 58, 2384–2388. [DOI] [PubMed] [Google Scholar]
- Van Cleemput O.; Baert L. Theoretical considerations on nitrite self-decomposition reactions in soils. Soil Sci. Soc. Am. J. 1976, 40, 322–324. [Google Scholar]
- Bonner F. T.; Pearsall K. A. Aqueous nitrosyliron (II) chemistry. 1. Reduction of nitrite and nitric oxide by iron (II) and (trioxodinitrato) iron (II) in acetate buffer. Intermediacy of nitrosyl hydride. Inorg. Chem. 1982, 21, 1973–1978. [Google Scholar]
- Kustin K.; Taub I. A.; Weinstoc E. A kinetic study of formation of ferrous-nitric oxide complex. Inorg. Chem. 1966, 5, 1079–1082. [Google Scholar]
- Pearsall K. A.; Bonner F. T. Aqueous nitrosyliron(II) chemistry. 2. Kinetics and mechanism of nitric-oxide reduction—The dinitrosyl complex. Inorg. Chem. 1982, 21, 1978–1985. [Google Scholar]
- Demmink J.; van Gils I.; Beenackers A. Absorption of nitric oxide into aqueous solutions of ferrous chelates accompanied by instantaneous reaction. Ind. Eng. Chem. Res. 1997, 36, 4914–4927. [Google Scholar]
- Schneppensieper T.; Wanat A.; Stochel G.; Goldstein S.; Meyerstein D.; van Eldik R. Ligand effects on the kinetics of the reversible binding of NO to selected aminocarboxylato complexes of iron(II) in aqueous solution. Eur. J. Inorg. Chem. 2001, 2317–2325. [Google Scholar]
- Morgan J. J. Kinetics of reaction between O2 and Mn(II) species in aqueous solutions. Geochim. Cosmochim. Acta 2005, 69, 35–48. [Google Scholar]
- Jones D. Organic acids in the rhizosphere—A critical review. Plant Soil 1998, 205, 25–44. [Google Scholar]
- Senn D. B. Nitrate controls on iron and arsenic in an urban lake. Science 2002, 296, 2373–2376. [DOI] [PubMed] [Google Scholar]
- Poulain A. J.; Newman D. K. Rhodobacter capsulatus catalyzes light-dependent Fe(II) oxidation under anaerobic conditions as a potential detoxification mechanism. Appl. Environ. Microbiol. 2009, 75, 6639–6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Smith R.; Martell A. NIST standard reference database 46. NIST Critically Selected Stability Constants of Metal Complexes; NIST: Gaithersburg, MD, 1998.