Abstract
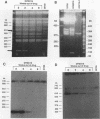
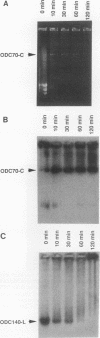
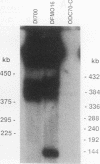
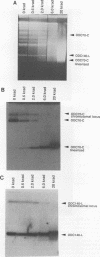
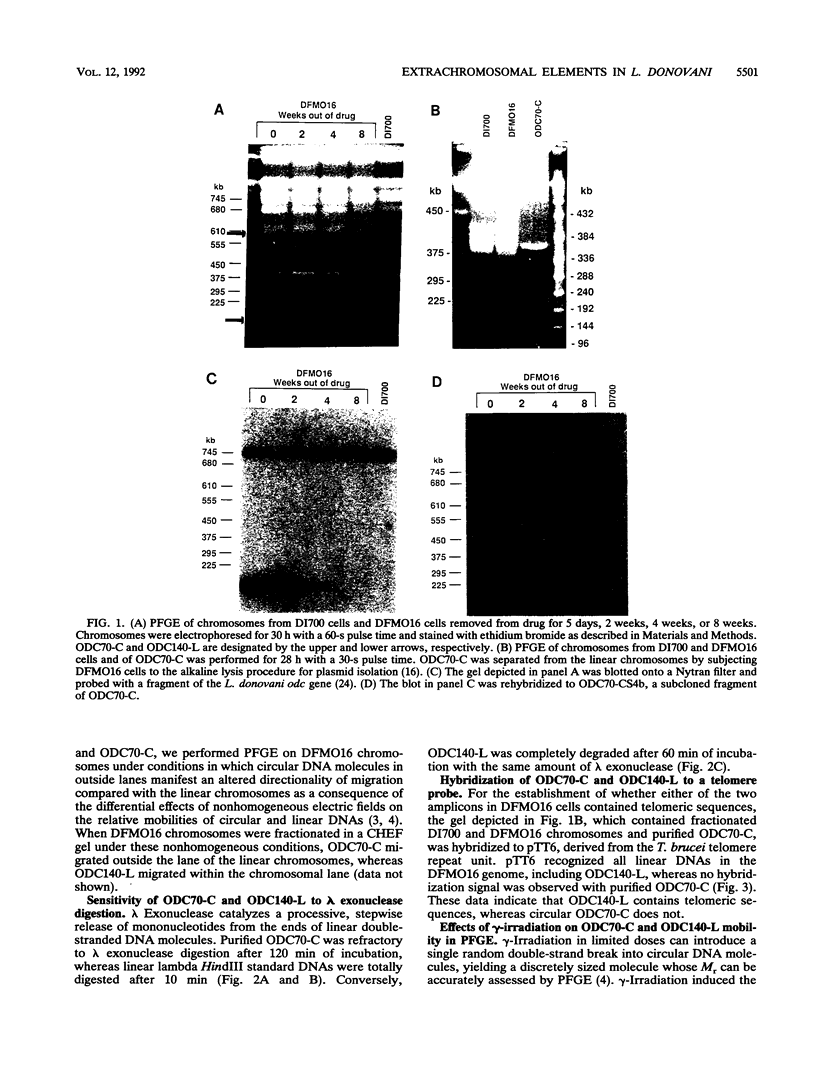
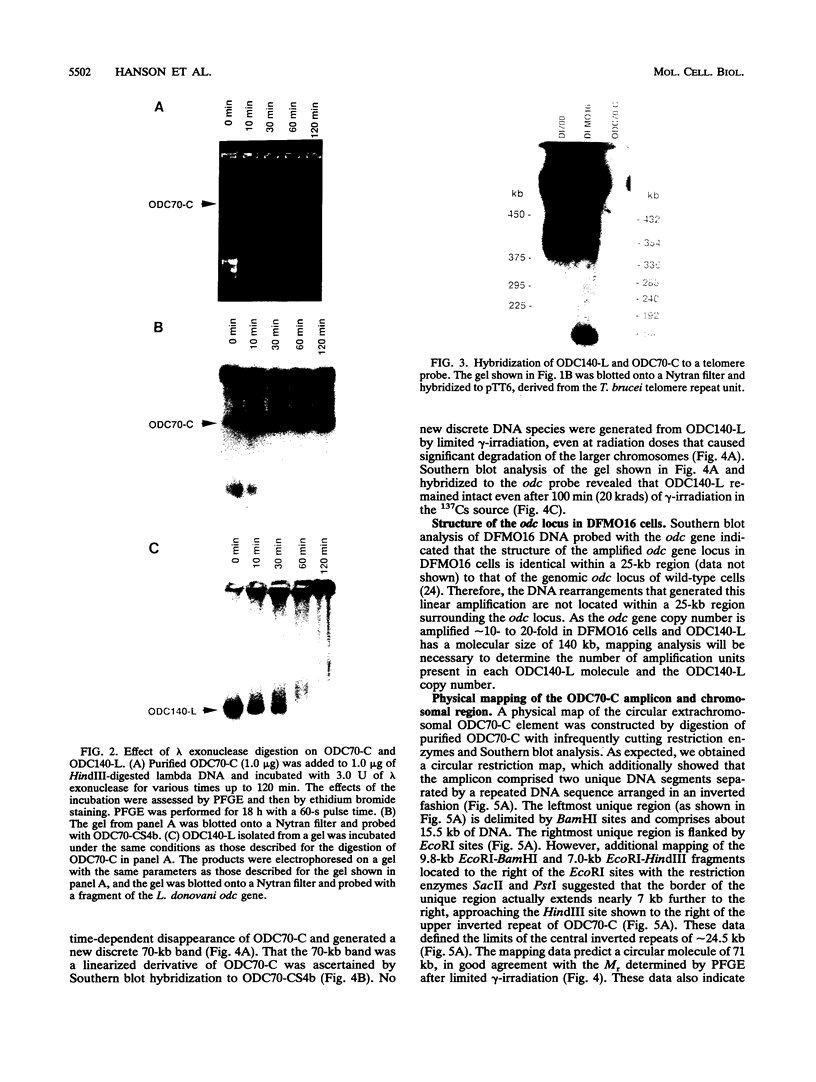
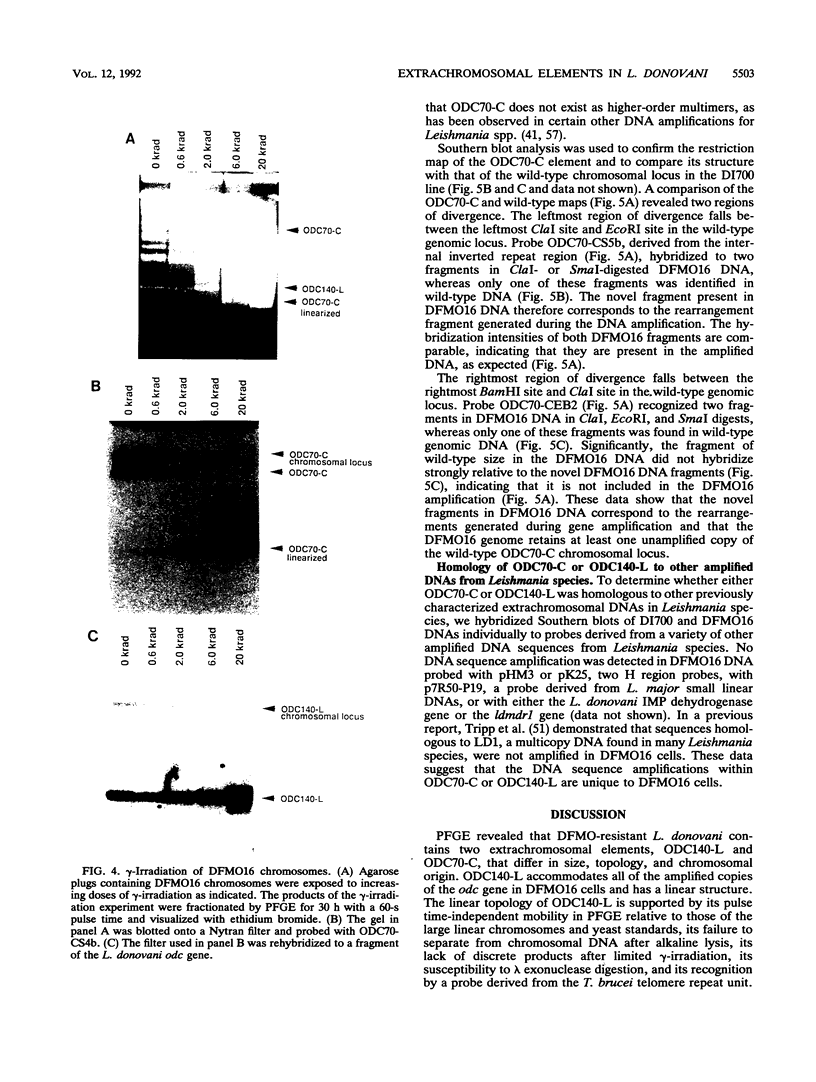
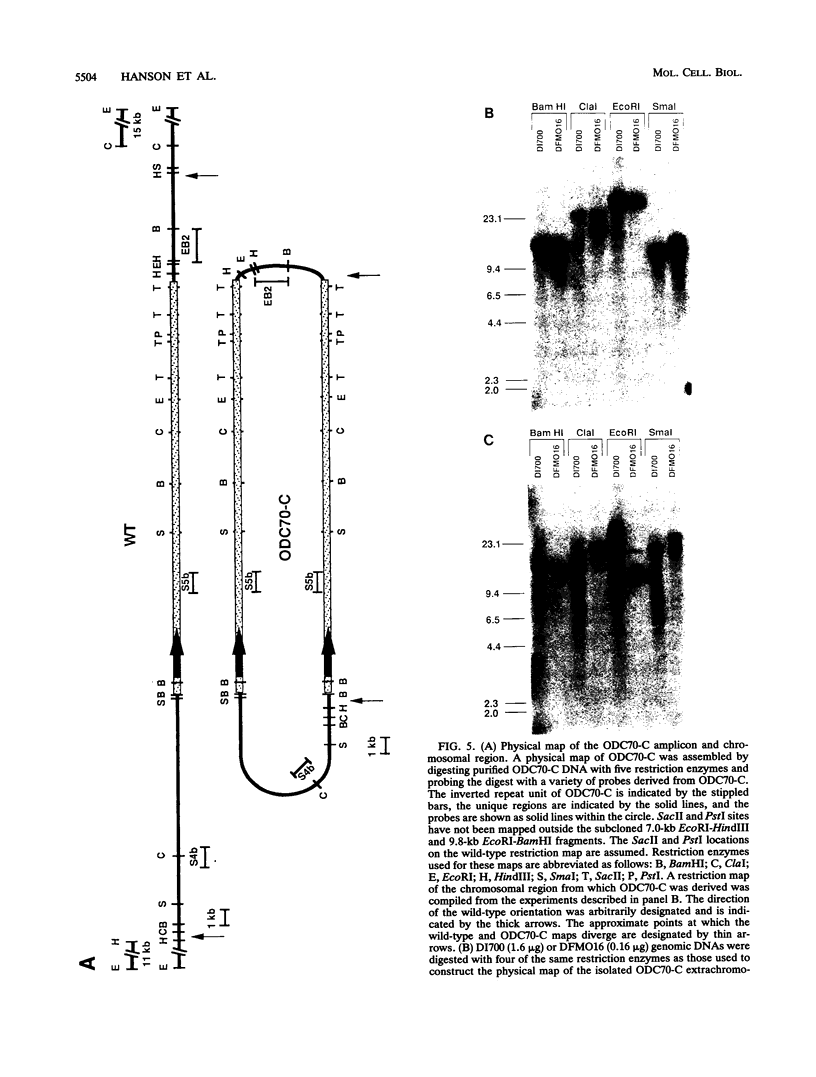
We describe the first example of unstable gene amplification consisting of linear extrachromosomal DNAs in drug-resistant eukaryotic cells. alpha-Difluoromethylornithine (DFMO)-resistant Leishmania donovani with an amplified ornithine decarboxylase (ODC) gene copy number contained two new extrachromosomal DNAs, both present in 10 to 20 copies. One of these was a 140-kb linear DNA (ODC140-L) on which all of the amplified copies of the odc gene were located. The second was a 70-kb circular DNA (ODC70-C) containing an inverted repeat but lacking the odc gene. Both ODC140-L and ODC70-C were derived from a preexisting wild-type chromosome, probably by a conservative amplification mechanism. Both elements were unstable in the absence of DFMO, and their disappearance coincided with a decrease in ODC activity and an increase in DFMO growth sensitivity. These results suggest the possibility that ODC70-C may play a role in DFMO resistance. These data expand the diversity of known amplification mechanisms in eukaryotes to include the simultaneous unstable amplification of both linear and circular DNAs. Further characterization of these molecules will provide insights into the molecular mechanisms underlying gene amplification, including the ability of linear amplified DNAs to acquire telomeres and the determinants of chromosomal stability.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacchi C. J., Nathan H. C., Hutner S. H., McCann P. P., Sjoerdsma A. Polyamine metabolism: a potential therapeutic target in trypanosomes. Science. 1980 Oct 17;210(4467):332–334. doi: 10.1126/science.6775372. [DOI] [PubMed] [Google Scholar]
- Beverley S. M. Characterization of the 'unusual' mobility of large circular DNAs in pulsed field-gradient electrophoresis. Nucleic Acids Res. 1988 Feb 11;16(3):925–939. doi: 10.1093/nar/16.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley S. M., Coburn C. M. Recurrent de novo appearance of small linear DNAs in Leishmania major and relationship to extra-chromosomal DNAs in other species. Mol Biochem Parasitol. 1990 Aug;42(1):133–141. doi: 10.1016/0166-6851(90)90121-2. [DOI] [PubMed] [Google Scholar]
- Beverley S. M., Coderre J. A., Santi D. V., Schimke R. T. Unstable DNA amplifications in methotrexate-resistant Leishmania consist of extrachromosomal circles which relocalize during stabilization. Cell. 1984 Sep;38(2):431–439. doi: 10.1016/0092-8674(84)90498-7. [DOI] [PubMed] [Google Scholar]
- Beverley S. M. Estimation of circular DNA size using gamma-irradiation and pulsed-field gel electrophoresis. Anal Biochem. 1989 Feb 15;177(1):110–114. doi: 10.1016/0003-2697(89)90023-7. [DOI] [PubMed] [Google Scholar]
- Beverley S. M. Gene amplification in Leishmania. Annu Rev Microbiol. 1991;45:417–444. doi: 10.1146/annurev.mi.45.100191.002221. [DOI] [PubMed] [Google Scholar]
- Biessmann H., Mason J. M., Ferry K., d'Hulst M., Valgeirsdottir K., Traverse K. L., Pardue M. L. Addition of telomere-associated HeT DNA sequences "heals" broken chromosome ends in Drosophila. Cell. 1990 May 18;61(4):663–673. doi: 10.1016/0092-8674(90)90478-w. [DOI] [PubMed] [Google Scholar]
- Bishop R. P. Extensive homologies between Leishmania donovani chromosomes of markedly different size. Mol Biochem Parasitol. 1990 Jan 1;38(1):1–11. doi: 10.1016/0166-6851(90)90198-u. [DOI] [PubMed] [Google Scholar]
- Bishop R. P., Miles M. A. Chromosome size polymorphisms of Leishmania donovani. Mol Biochem Parasitol. 1987 Jul;24(3):263–272. doi: 10.1016/0166-6851(87)90158-7. [DOI] [PubMed] [Google Scholar]
- Callahan H. L., Beverley S. M. Heavy metal resistance: a new role for P-glycoproteins in Leishmania. J Biol Chem. 1991 Oct 5;266(28):18427–18430. [PubMed] [Google Scholar]
- Coons T., Hanson S., Bitonti A. J., McCann P. P., Ullman B. Alpha-difluoromethylornithine resistance in Leishmania donovani is associated with increased ornithine decarboxylase activity. Mol Biochem Parasitol. 1990 Feb;39(1):77–89. doi: 10.1016/0166-6851(90)90010-j. [DOI] [PubMed] [Google Scholar]
- Cruz A., Beverley S. M. Gene replacement in parasitic protozoa. Nature. 1990 Nov 8;348(6297):171–173. doi: 10.1038/348171a0. [DOI] [PubMed] [Google Scholar]
- Detke S., Chaudhuri G., Kink J. A., Chang K. P. DNA amplification in tunicamycin-resistant Leishmania mexicana. Multicopies of a single 63-kilobase supercoiled molecule and their expression. J Biol Chem. 1988 Mar 5;263(7):3418–3424. [PubMed] [Google Scholar]
- Doberstyn E. B. Resistance of Plasmodium falciparum. Experientia. 1984 Dec 15;40(12):1311–1317. doi: 10.1007/BF01951884. [DOI] [PubMed] [Google Scholar]
- Dunn B., Szauter P., Pardue M. L., Szostak J. W. Transfer of yeast telomeres to linear plasmids by recombination. Cell. 1984 Nov;39(1):191–201. doi: 10.1016/0092-8674(84)90205-8. [DOI] [PubMed] [Google Scholar]
- Ellenberger T. E., Beverley S. M. Multiple drug resistance and conservative amplification of the H region in Leishmania major. J Biol Chem. 1989 Sep 5;264(25):15094–15103. [PubMed] [Google Scholar]
- Garvey E. P., Coderre J. A., Santi D. V. Selection and properties of Leishmania tropica resistant to 10-propargyl-5,8-dideazafolate, an inhibitor of thymidylate synthetase. Mol Biochem Parasitol. 1985 Oct;17(1):79–91. doi: 10.1016/0166-6851(85)90129-x. [DOI] [PubMed] [Google Scholar]
- Hahn P. J., Nevaldine B., Longo J. A. Molecular structure and evolution of double-minute chromosomes in methotrexate-resistant cultured mouse cells. Mol Cell Biol. 1992 Jul;12(7):2911–2918. doi: 10.1128/mcb.12.7.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamkalo B. A., Farnham P. J., Johnston R., Schimke R. T. Ultrastructural features of minute chromosomes in a methotrexate-resistant mouse 3T3 cell line. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1126–1130. doi: 10.1073/pnas.82.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson S., Adelman J., Ullman B. Amplification and molecular cloning of the ornithine decarboxylase gene of Leishmania donovani. J Biol Chem. 1992 Feb 5;267(4):2350–2359. [PubMed] [Google Scholar]
- Henderson D. M., Sifri C. D., Rodgers M., Wirth D. F., Hendrickson N., Ullman B. Multidrug resistance in Leishmania donovani is conferred by amplification of a gene homologous to the mammalian mdr1 gene. Mol Cell Biol. 1992 Jun;12(6):2855–2865. doi: 10.1128/mcb.12.6.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower R. C., Ruiz-Perez L. M., Wong M. L., Santi D. V. Extrachromosomal elements in the lower eukaryote Leishmania. J Biol Chem. 1988 Nov 15;263(32):16970–16976. [PubMed] [Google Scholar]
- Iovannisci D. M., Beverley S. M. Structural alterations of chromosome 2 in Leishmania major as evidence for diploidy, including spontaneous amplification of the mini-exon array. Mol Biochem Parasitol. 1989 May 1;34(2):177–188. doi: 10.1016/0166-6851(89)90009-1. [DOI] [PubMed] [Google Scholar]
- Iovannisci D. M., Ullman B. High efficiency plating method for Leishmania promastigotes in semidefined or completely-defined medium. J Parasitol. 1983 Aug;69(4):633–636. [PubMed] [Google Scholar]
- Katakura K., Peng Y., Pithawalla R., Detke S., Chang K. P. Tunicamycin-resistant variants from five species of Leishmania contain amplified DNA in extrachromosomal circles of different sizes with a transcriptionally active homologous region. Mol Biochem Parasitol. 1991 Feb;44(2):233–243. doi: 10.1016/0166-6851(91)90009-u. [DOI] [PubMed] [Google Scholar]
- Kink J. A., Chang K. P. Tunicamycin-resistant Leishmania mexicana amazonensis: expression of virulence associated with an increased activity of N-acetylglucosaminyltransferase and amplification of its presumptive gene. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1253–1257. doi: 10.1073/pnas.84.5.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laban A., Tobin J. F., Curotto de Lafaille M. A., Wirth D. F. Stable expression of the bacterial neor gene in Leishmania enriettii. Nature. 1990 Feb 8;343(6258):572–574. doi: 10.1038/343572a0. [DOI] [PubMed] [Google Scholar]
- Ma C., Looney J. E., Leu T. H., Hamlin J. L. Organization and genesis of dihydrofolate reductase amplicons in the genome of a methotrexate-resistant Chinese hamster ovary cell line. Mol Cell Biol. 1988 Jun;8(6):2316–2327. doi: 10.1128/mcb.8.6.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann C., Davis R. W. Structure and sequence of the centromeric DNA of chromosome 4 in Saccharomyces cerevisiae. Mol Cell Biol. 1986 Jan;6(1):241–245. doi: 10.1128/mcb.6.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunberg J. H., Kaufman R. J., Schimke R. T., Urlaub G., Chasin L. A. Amplified dihydrofolate reductase genes are localized to a homogeneously staining region of a single chromosome in a methotrexate-resistant Chinese hamster ovary cell line. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5553–5556. doi: 10.1073/pnas.75.11.5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette M., Fase-Fowler F., Borst P. The amplified H circle of methotrexate-resistant leishmania tarentolae contains a novel P-glycoprotein gene. EMBO J. 1990 Apr;9(4):1027–1033. doi: 10.1002/j.1460-2075.1990.tb08206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauletti G., Lai E., Attardi G. Early appearance and long-term persistence of the submicroscopic extrachromosomal elements (amplisomes) containing the amplified DHFR genes in human cell lines. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2955–2959. doi: 10.1073/pnas.87.8.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters W. The problem of drug resistance in malaria. Parasitology. 1985 Apr;90(Pt 4):705–715. doi: 10.1017/s003118200005232x. [DOI] [PubMed] [Google Scholar]
- Petrillo-Peixoto M. L., Beverley S. M. Amplified DNAs in laboratory stocks of Leishmania tarentolae: extrachromosomal circles structurally and functionally similar to the inverted-H-region amplification of methotrexate-resistant Leishmania major. Mol Cell Biol. 1988 Dec;8(12):5188–5199. doi: 10.1128/mcb.8.12.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pologe L. G., de Bruin D., Ravetch J. V. A and T homopolymeric stretches mediate a DNA inversion in Plasmodium falciparum which results in loss of gene expression. Mol Cell Biol. 1990 Jun;10(6):3243–3246. doi: 10.1128/mcb.10.6.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz J. C., Choi K. H., von Hoff D. D., Roninson I. B., Wahl G. M. Autonomously replicating episomes contain mdr1 genes in a multidrug-resistant human cell line. Mol Cell Biol. 1989 Jan;9(1):109–115. doi: 10.1128/mcb.9.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz J. C., Wahl G. M. Formation of an inverted duplication can be an initial step in gene amplification. Mol Cell Biol. 1988 Oct;8(10):4302–4313. doi: 10.1128/mcb.8.10.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured cells. J Biol Chem. 1988 May 5;263(13):5989–5992. [PubMed] [Google Scholar]
- Shea C., Glass D. J., Parangi S., Van der Ploeg L. H. Variant surface glycoprotein gene expression site switches in Trypanosoma brucei. J Biol Chem. 1986 May 5;261(13):6056–6063. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Debatisse M., Giulotto E., Wahl G. M. Recent progress in understanding mechanisms of mammalian DNA amplification. Cell. 1989 Jun 16;57(6):901–908. doi: 10.1016/0092-8674(89)90328-0. [DOI] [PubMed] [Google Scholar]
- Tripp C. A., Myler P. J., Stuart K. A DNA sequence (LD1) which occurs in several genomic organizations in Leishmania. Mol Biochem Parasitol. 1991 Aug;47(2):151–156. doi: 10.1016/0166-6851(91)90174-5. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Cornelissen A. W., Michels P. A., Borst P. Chromosome rearrangements in Trypanosoma brucei. Cell. 1984 Nov;39(1):213–221. doi: 10.1016/0092-8674(84)90207-1. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Liu A. Y., Borst P. Structure of the growing telomeres of Trypanosomes. Cell. 1984 Feb;36(2):459–468. doi: 10.1016/0092-8674(84)90239-3. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Schwartz D. C., Cantor C. R., Borst P. Antigenic variation in Trypanosoma brucei analyzed by electrophoretic separation of chromosome-sized DNA molecules. Cell. 1984 May;37(1):77–84. doi: 10.1016/0092-8674(84)90302-7. [DOI] [PubMed] [Google Scholar]
- Wahl G. M. The importance of circular DNA in mammalian gene amplification. Cancer Res. 1989 Mar 15;49(6):1333–1340. [PubMed] [Google Scholar]
- White T. C., Fase-Fowler F., van Luenen H., Calafat J., Borst P. The H circles of Leishmania tarentolae are a unique amplifiable system of oligomeric DNAs associated with drug resistance. J Biol Chem. 1988 Nov 15;263(32):16977–16983. [PubMed] [Google Scholar]
- Wilkie A. O., Lamb J., Harris P. C., Finney R. D., Higgs D. R. A truncated human chromosome 16 associated with alpha thalassaemia is stabilized by addition of telomeric repeat (TTAGGG)n. Nature. 1990 Aug 30;346(6287):868–871. doi: 10.1038/346868a0. [DOI] [PubMed] [Google Scholar]
- Wilson K., Collart F. R., Huberman E., Stringer J. R., Ullman B. Amplification and molecular cloning of the IMP dehydrogenase gene of Leishmania donovani. J Biol Chem. 1991 Jan 25;266(3):1665–1671. [PubMed] [Google Scholar]