Abstract
Huntington's disease (HD) is a dominantly inherited neurodegenerative disease best known for chorea. The disorder includes numerous other clinical features including mood disorder, eye movement abnormalities, cognitive disturbance, pendular knee reflexes, motor impersistence, and postural instability. We describe a mild case of HD early in the disease course with depression and subtle neurological manifestations. In addition, we review MRI and diffusion tensor imaging features in this patient. The bicaudate ratio, a measure of caudate atrophy, was increased. Fractional anisotropy values of the bilateral caudate and putamen were increased, signifying neurodegeneration of these structures in HD.
Key words: Huntington's disease, Diffusion tensor imaging, Fractional anisotropy
Introduction
Huntington's disease (HD) is a dominantly inherited neurodegenerative disease caused by mutations in the IT-15 gene on chromosome 4, which was subsequently renamed HTT. More specifically, the disease is linked to expansion of the CAG repeat tract of this gene, which codes for polyglutamine repeats in the huntingtin protein [1].
George Huntington provided the classic description of the disorder in 1872 from cases in the East Hampton area of Long Island, N.Y. The best known manifestation is chorea, which consists of random, fluid involuntary hyperkinetic movements. The term is derived from the Greek word χoρεíα, meaning a traditional circle dance [2]. It is important to note that, while movements in chorea may resemble a dance, they are not rhythmic or repetitive [2]. Chorea, likely due to HD, was also referred to as ‘magrums’ at the time of George Huntington's case description [3].
George Huntington discussed ‘tendency to insanity and suicide’ in his original paper [1]. Psychiatric manifestations may include irritability, anxiety, obsessive compulsive disorder, paranoid ideation, and psychosis. Depression is the most common psychiatric feature of HD and suicidal ideation occurs in 5–10% of HD patients [1]. Other neurologic manifestations include eye movement abnormalities, cognitive disturbance, pendular knee reflexes, motor impersistence [1], and postural instability [4]. Juvenile onset HD presents primarily with dystonia and rigidity [1].
We describe a mild case of adult onset HD early in the disease course along with MRI and diffusion tensor imaging (DTI) features. Increased fractional anisotropy (FA) of striatum has been linked to neurodegeneration in HD [5].
Case Presentation
The patient is a 50-year-old right-handed Caucasian male who was diagnosed with HD at age 47 by an outside movement disorders specialist. His father developed HD at age 60 and died at 73. His father had 41 CAG repeats of the HTT gene. The patient received a clinical diagnosis of HD given mild neurological findings in the context of genetically confirmed family history.
There is a history of depression since his diagnosis, which has become worse over the past week. He was admitted for psychiatric evaluation due to suicidal ideation with plans to overdose on sleeping pills. Stressors include his upcoming divorce. In addition, he complains of mild cognitive disturbance for at least one year, with difficulty processing information in distracting environments. He has a history of alcohol abuse and quit Alcoholics Anonymous about six months ago. There is no history of cigarette or drug use. Review of systems was significant for insomnia. He has never taken tetrabenazine. His medications include paroxetine 30 mg q.h.s., aripiprazole 5 mg q.a.m., naltrexone 50 mg q.a.m., memantine 10 mg b.i.d., and trazodone 200 mg q.h.s.
Upon examination, he was alert and oriented to person, place, and time. Speech was fluent. Luria three-step test was normal bilaterally. There was blunted affect and mild apathy. Mini mental state examination score was 29/30. Cranial nerve examination was significant for saccadic pursuit, slow saccade initiation and velocity in the horizontal and vertical directions, and motor impersistence exhibited by inability to fully protrude the tongue for five seconds. Reflexes were 2+/4 throughout except for pendular knee reflexes. There was no motor weakness and no sensory loss. There was only mild intermittent chorea in the fingertips bilaterally and rare lower facial chorea. Gait was mildly wide based. He could ambulate without assistance. There was profound instability with tandem gait and tandem stance. MRI imaging was performed due to the patient's perceived cognitive decline. He improved after one week of inpatient psychotherapy and was released for outpatient psychiatric and neurologic follow-up.
Materials and Methods
Images were obtained on a 3.0-Tesla Phillips Achieva MRI system. Bicaudate ratio (BCR), an imaging measure that correlates with caudate atrophy in HD, was obtained by measuring the shortest inter-caudate distance (seen as indentation of the frontal horns) on axial slice and dividing it by the distance between the outer tables of the skull along the same line, multiplied by 100 [6]. Phillips Fibertrak software was used to analyze DTI values on regions of interest which were defined as caudate head bilaterally and putamen bilaterally on axial slices.
Results
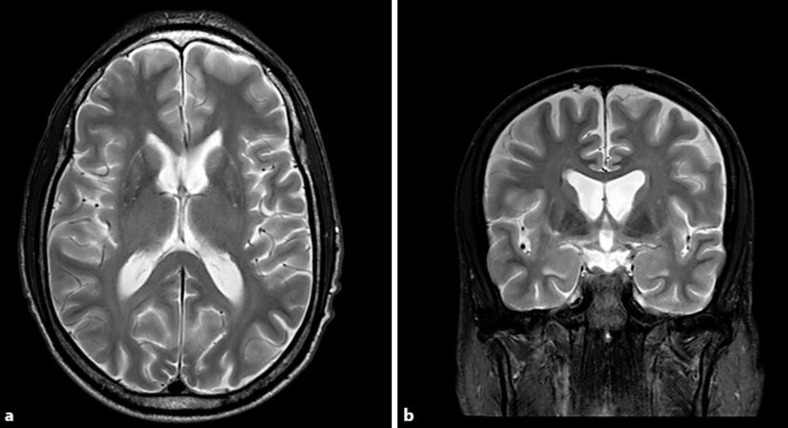
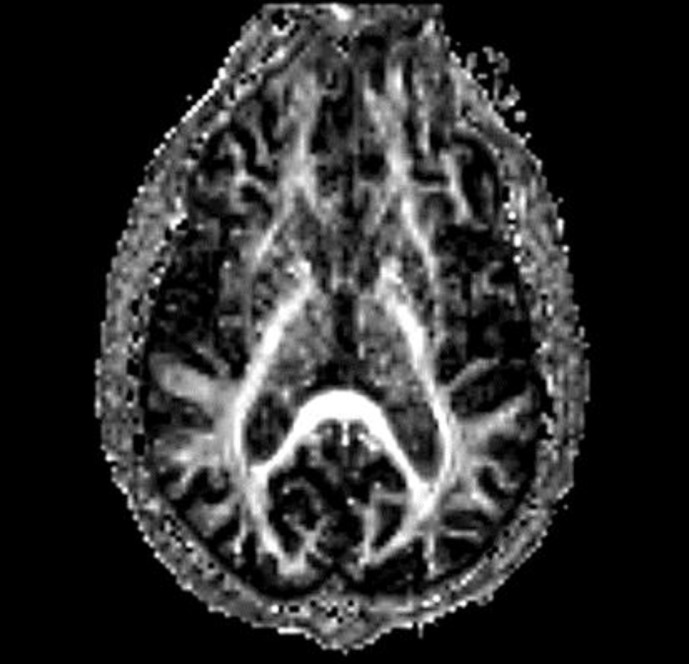
MRI brain showed caudate atrophy and ex vacuo dilatation of lateral ventricles (fig. 1). Axial BCR in the patient was increased compared to control at 16 versus 12, respectively. FA (fig. 2) was increased in the bilateral caudate and putamen versus control (table 1).
Fig. 1.
Caudate atrophy with ex vacuo dilatation of the frontal horns of lateral ventricles is seen on axial (a) and coronal (b) T2-weighted MRI images.
Fig. 2.
Axial fractional anisotropy map.
Table 1.
Fractional anisotropy values of bilateral caudate and putamen in the patient and control
| Fractional anisotropy |
||
|---|---|---|
| Patient | Control | |
| Left caudate head | 0.365 | 0.312 |
| Left putamen | 0.464 | 0.368 |
| Right caudate head | 0.383 | 0.378 |
| Right putamen | 0.418 | 0.351 |
Discussion
Many classical features of early HD are present in this case. Depression and suicidality were managed with inpatient psychiatry admission and psychotherapy, followed by outpatient therapy. Subtle neurologic manifestations included slowing of saccade imitation and velocity, saccadic pursuit, difficulty with tongue protrusion due to motor impersistence, pendular knee reflexes, and postural instability demonstrated by the inability to maintain tandem stance. Mild chorea did not require treatment in this case. Tetrabenazine is FDA approved for the treatment of HD chorea and its efficacy is supported by clinical evidence [7]. Other pharmacotherapy options for chorea include amantadine and levatiracetam [8].
On MRI imaging, caudate atrophy and ex vacuo dilatation of the frontal horns, which is typical for HD, was visualized. Increased BCR served as quantitative confirmation for this finding [6]. On DTI imaging, FA was increased in bilateral caudate and putamen. Increase in FA in white matter is linked to more coherent fiber orientation and normal structure, but in striatal grey matter increased FA is due to neurodegeneration. Douaud et al. [9] propose that increased FA in striatal grey matter structures is due to the loss of efferent fibers which in turn leads to increased coherence. For example, in HD there is preferential loss of putamino-pallidal connections that are oriented mediolaterally as relative preservation of anterior-posterior and dorsal-ventral cortical-putaminal fibers. Hence, loss of these mediolateral fibers may lead to more uniform fiber orientation and higher FA values.
In conclusion, we have presented a patient who is early in the course of Huntington's disease and who already exhibits basal ganglia neurodegeneration with atrophy and changes in FA.
Disclosure Statement
Dr. Fekete received honoraria from Medlink, Inc., and serves as a consultant for Teva Neuroscience, Inc., and Lundbeck, LLC.
References
- 1.Ha AD, Jankovic J. Huntington disease and other genetic choreas. In: Albanese A, Jankovic J, editors. Hyperkinetic Movement Disorders: Differential Diagnosis and Treatment. Oxford, UK: Wiley-Blackwell; 2012. [Google Scholar]
- 2.Walker RH. Acquired Choreas. In: Albanese A, Jankovic J, editors. Hyperkinetic Movement Disorders: Differential Diagnosis and Treatment. Oxford, UK: Wiley-Blackwell; 2012. [Google Scholar]
- 3.Goetz CG, Chmura TA, Lanska DJ. History of Chorea: Part 3 of the MDS-Sponsored History of Movement Disorders Exhibit, Barcelona, June 2000. Mov Disord. 2001;16:331–338. doi: 10.1002/mds.1066. [DOI] [PubMed] [Google Scholar]
- 4.Fekete R, Davidson A, Ondo WG, Cohen HS. Effect of tetrabenazine on computerized dynamic posturography in Huntington disease patients. Parkinsonism Relat Disord. 2012;18:896–898. doi: 10.1016/j.parkreldis.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohanna I, Georgiou-Karistianis N, Egan GF. Connectivity-based segmentation of the striatum in Huntington's disease: Vulnerability of motor pathways. Neurobiol Dis. 2011;42:475–481. doi: 10.1016/j.nbd.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Harris GJ, Pearlson GD, Peyser CE, Aylward EH, Roberts J, Barta PE, Chase GA, Folstein SE. Putamen volume reduction on magnetic resonance imaging exceeds caudate changes in mild Huntington's disease. Ann Neurol. 1992;31:69–75. doi: 10.1002/ana.410310113. [DOI] [PubMed] [Google Scholar]
- 7.Mestre T, Ferreira J, Coelho MM, Rosa M, Sampaio C. Therapeutic interventions for symptomatic treatment in Huntington's disease. Cochrane Database Syst Rev. doi: 10.1002/14651858.CD006456.pub2. DOI: 10.1002/14651858.CD006456.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Walker FO. Huntington's disease. Lancet. 2007;369:218–228. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- 9.Douaud G, Behrens TE, Poupon C, et al. In vivo evidence for the selective subcortical degeneration in Huntington's disease. Neuroimage. 2009;46:958–966. doi: 10.1016/j.neuroimage.2009.03.044. [DOI] [PubMed] [Google Scholar]