Abstract
Gaucher’s disease is a sphingolipidosis characterized by a specific deficiency in an acidic glucocerebrosidase, which results in aberrant accumulation of glucosylceramide primarily within the lysosome. Gaucher’s disease has been correlated with cases of myeloma, leukemia, glioblastoma, lung cancer, and hepatocellular carcinoma, although the reasons for the correlation are currently being debated. Some suggest that the effects of Gaucher’s disease may be linked to cancer, while others implicate the therapies used to treat Gaucher’s disease. This debate is not entirely surprising, as the speculations linking Gaucher’s disease with cancer fail to address the roles of ceramide and glucosylceramide in cancer biology. In this review, we will discuss, in the context of cancer biology, ceramide metabolism to glucosylceramide, the roles of glucosylceramide in multidrug-resistance, and the role of ceramide as an anticancer lipid. This review should reveal that it is most practical to associate elevated glucosylceramide, which accompanies Gaucher’s disease, with the progression of cancer. Furthermore, this review proposes that the therapies used to treat Gaucher’s disease, which augment ceramide accumulation, are likely not linked to correlations with cancer.
Keywords: Gaucher’s disease, cancer, glucosylceramide, ceramide, sphingolipid
I. SPHINGOLIPIDS
Sphingolipids are an extensive class of lipids with roles as components of cellular membranes and as cellular signaling intermediates.1–9 Sphingolipids are unique from other lipids due to the presence of nitrogen bound at the 2-carbon position of the sphingoid backbone.1,10,11 This feature arises from an amino acid, usually serine, and is critical to the biophysical nature of sphingolipids within membranes. Sphingolipids have been shown to play profound roles in normal cellular homeostasis as well as a diversity of pathological conditions.6 Owing to these critical roles, sphingolipid metabolism is tightly controlled by post-translational regulation of key enzymes as well as by subcellular restriction that limits the activities of enzymes to lipid species present within specific membranes.1 Deficiencies or aberrant regulation of sphingolipid-metabolizing enzymes can result in disease pathologies. These problems can often be reflected by global changes to the sphingolipidome, rather than changes in specific lipids, due to the highly integrated nature of sphingolipid metabolism.12
A. Basic Metabolism
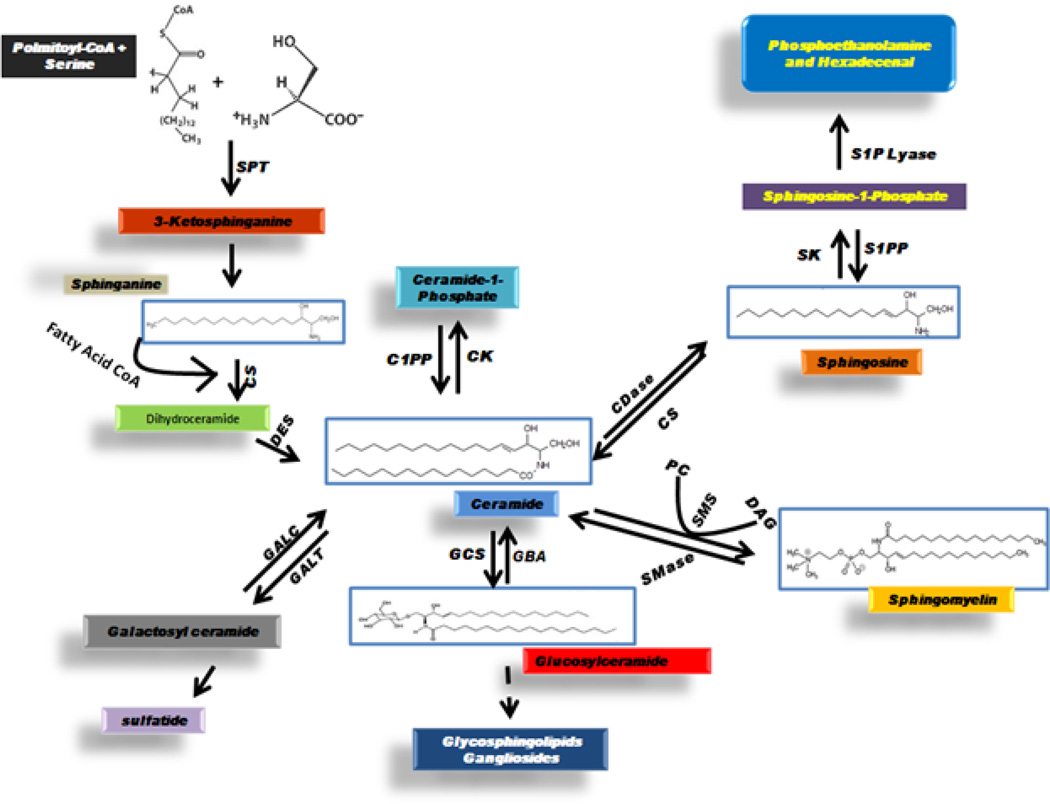
The de novo synthesis of sphingolipids begins with the typical condensation of serine and palmitoyl-CoA, a reaction catalyzed by the enzyme serine palmitoyl-transferase, to yield the product 3-ketosphinganine (3-ketodihydrosphingosine).1,10,11 Recent studies have shown that other amino acids, such as glycine and alanine, can substitute for serine and lead to the biosynthesis of a series of novel sphingolipids.1 Following the usual condensation of serine with palmitoyl-CoA, 3-ketosphinganine reductase reduces 3-ketosphinganine to sphinganine (dihydrosphingosine), and then sphinganine is acylated at the amide position by dihydroceramide synthase to yield dihydroceramide.1,10,11 Importantly, acylation can add a variety of fatty acid chains to the amide position, resulting in dihydroceramides with different chain lengths and varying degrees of unsaturation.1,6,10 Desaturation by dihydroceramide desaturase results in ceramide formation, with ceramide serving as the hypothetical center of sphingolipid metabolism (Fig. 1).1,3,5,6,10,11,13
FIGURE 1. Ceramide metabolism.
Ceramide serves as the hypothetical center of sphingolipid metabolism. Ceramide is generated de novo from the condensation of palmitoyl-CoA with serine, in a process producing intermediate metabolites, 3-ketosphinganine, sphinganine (dihydrosphingosine), and dihydroceramide. Ceramide can be phosphorylated to ceramide-1-phosphate, can be broken down to sphingosine and then phosphorylated to sphingosine-1-phosphate, or can be converted to sphingomyelin or glycosphingolipids via head group addition. Glucosylceramide synthase (GCS) catalyzes the conversion of ceramide to glucosylceramide while acidic glucocerebrosidase (GBA), the enzyme defective in Gaucher’s disease, removes glucose to regenerate ceramide. SPT: serine palmitoyltransferase; CS: ceramide synthase; DES: dihydroceramide desaturase; GALC: galactosylceramidase (galacto-cerebrosidase); GALT: ceramide galactosyltransferase; GCS: glucosylceramide synthase; SMase: sphingomyelinase; SMS: sphingomyelin synthase; PC: phosphatidylcholine; DAG: diacylglycerol; CDase: ceramidase; C1PP: ceramide-1-phosphate phosphatase; CK: ceramide kinase; S1PP: sphingosine-1-phosphate phosphatase; SK: sphingosine kinase.
Ceramide can serve as a precursor to many complex sphingolipids such as glucosylceramide, galactosylceramide, lactosylceramide, various gangliosides, and sphingomyelin.1,3,10,11 Specific enzymes add glucose, galactose, or phosphocholine to form glucosylceramide, galactosylceramide, or sphingomyelin, respectively.1,3,10,11 Lactosylceramide and gangliosides are then synthesized from glucosylceramide by the further addition of sugars, as well as sialic acid, in the case of gangliosides.1,10,11 Conversely, the degradation of sphingomyelin by sphingomyelinases, and glucosylceramide by cerebrosidases, liberates ceramide in mechanisms known commonly as the salvage pathway for ceramide generation.1,3,6,10,11 Catabolism of ceramide by ceramidases, enzymes that deacylate ceramide, results in the generation of sphingosine.1,6,10,11,14,15 Ceramide can then be regenerated from sphingosine by re-acylation.1,6 Lipid kinases are a key important feature of sphingolipid metabolism and mediate the formation of the most recognized bioactive sphingolipid mediators. Two sphingosine kinases and a ceramide kinase have been identified, phosphorylating sphingosine, sphinganine, and ceramide.1,3,6 Phosphatases have also been described that catabolically regulate sphingosine-1-phosphate (S1P), sphinganine-1-phosphate, and ceramide-1-phosphate. S1P can also be irreversibly degraded by a specific lyase.1,3,6
B. Subcellular Localization of Metabolism
The primary synthesis of sphingolipids begins in the membrane of the endoplasmic reticulum and continues to the membrane of the Golgi apparatus and then to the plasma membrane.1,10,11 The sphingolipid composition of the nuclear and mitochondrial membranes is also highly influenced by enzymatic activity identified and restricted within those specific membranes. The breakdown of sphingolipids occurs at the plasma membrane and even more so in the lysosome.1,10,11 Importantly, the sphingomyelinases and ceramidases have been identified and characterized by their pH optimums. For the most part, enzymes with neutral or alkaline pH optima exist and breakdown their substrates at the plasma membrane, while acidic enzymes, including the cerebrosidases, are localized to the lysosome, which serves as the primary subcellular location of sphingolipid catabolism.1,6,10,11,16 Several transport proteins have been identified that can actively move specific sphingolipids between membranes, including ABC (ATP-binding cassette) transporters such as P-glycoprotein,1,17 CERT (ceramide-transfer protein),18 and FAPP2.1,18 Sphingolipid transport proteins are important; they re-locate sphingolipids to different membranes, or different sides of membranes, to facilitate the continued synthesis of complex sphingolipids.
C. Ceramide/S1P Biostat
The most studied bioactive sphingolipids are ceramide and S1P. Ceramide is has been implicated primarily as a regulator of cell stress, including oxidative stress, as well as cell death.1,6 Ceramide has been shown to induce apoptosis through activation of caspases and altering mitochondrial membrane potential. Ceramide can also regulate signal transduction by specific interactions with kinases and phosphatases. One such example is activation of PKCζ by direct binding of ceramide, which results in antagonized pro-survival and mitogenic signaling by Akt/PKB.19 In contrast, S1P regulates development, cell growth and survival, motility, inflammation, thermotolerance, angiogenesis, and tumorgenesis.1,6,20–23 S1P regulates these processes by binding to specific G-protein coupled receptors known as S1P receptors 1–5.23 Recent studies have also uncovered novel intracellular targets for S1P that include the receptor-associated ubiquitin ligase TRAF2 as well as histone deacetylases.24,25 More specifically, S1P was found to a) stimulate NF-κB signaling via direct binding to TRAF2 and b) act as a direct inhibitor of histone deactetylases.24,25 Collectively, these studies have resulted in a ceramide/S1P ‘biostat’ model in which the relative ratio of ceramide to S1P can regulate cellular fate.1 In this model, accumulation of ceramide results in detrimental cellular processes, while clearance of ceramide and accumulation of S1P promotes cell survival and growth.1 In reality, this model can be extended to other aspects of ceramide metabolism whereby the conversion of ceramide to other metabolites, such as glucosylceramide, can also have a profound effect on cellular survival. A recent study has shown that histone deacetylase inhibitors can prevent the degradation and restore the activity of glucocerebrosidase in fibroblasts derived from patients with Gaucher’s disease.26 Furthermore, a separate study showed that glucocerebrosidase inhibition of mesenchymal stromal cells, mimicking Gaucher’s disease, resulted in a decrease in S1P.27 Perhaps these studies, in light of the new concept that S1P is a histone deacetylase inhibitor,25 provide insight on a novel sphingolipid-based feedback loop that may be dysregulated in some instances of Gaucher’s disease.
II. GAUCHER’S DISEASE
Several inheritable disorders of complex sphingolipid catabolism have been identified. These sphingolipidoses result in the aberrant accumulation of complex sphingolipids within subcellular compartments such as the lysosome. Gaucher’s disease is both a sphingolipidosis and a lysosomal storage disorder due to a specific deficiency in an acidic glucocerebrosidase (GBA, acid β-glucosidase) that results in accumulation of glucosylceramide primarily within the lysosome.28–32 A cytosolic glucocerebrosidase that has activity at neutral pH appears to play no role in Gaucher’s disease.33 The disease arises from recessive mutations in the GBA gene found on chromosome 1.30–32,34–36 It has been reported that nearly 1 in 100 people in the United States are carriers of a mutation that is responsible for the most common type of Gaucher’s disease (type I).30,34 Gaucher’s disease is subclassified into three clinical groups: a) type I or non-neuropathic, b) type II or acute neuropathic, and c) type III or chronic neuropathic (Table 1).30 Hepatomegaly and splenomegaly are associated with all types of Gaucher’s disease, and they can onset early in life or in adulthood with the most common form of Gaucher’s disease (type I).30,31,34,36 Neuropathic Gaucher’s disease (type II and III) can be associated with a range of neurological problems.30 Type II Gaucher’s disease has an onset very early in life and progressively becomes very severe.30 Most individuals with type II Gaucher’s disease die by age two.30 The symptoms of type III Gaucher’s disease can begin at any point in life and are characterized by less severe yet variably progressive neurological symptoms.30
TABLE 1.
Overview of Gaucher’s Disease
| Gaucher Disease | Type I | Type II | Type III |
|---|---|---|---|
| Prevalence | Most common, 99% of cases | Rare | Rare |
| Onset | Adulthood | Infancy | Adolescence or Early Adulthood |
| Disease Pattern | Chronic, non-neuropathic | Acute, neuropathic | Chronic, neuropathic |
| Severity | Good treatment outcome | Most severe; rapid disease progression | Intermediate; CNS involvement is progressive |
| Course | Normal life expectancy | Death at early age | Intermediate; Dependent on organ involvement |
| Organ Involvement | Spleen and bone | CNS, liver, spleen, lymph nodes, bone | CNS, spleen, liver, lymph nodes |
| Glucocerebrosidase Activity | Detectable but reduced level of activity | Virtually no detectable activity | Moderate to low level of activity |
A. Gaucher’s Disease Treatment
The treatment of Gaucher’s disease is currently accomplished by either enzyme replacement therapy (ERT) or substrate reduction therapy (SRT).28,30,36–39 These therapeutic options take different approaches that either directly or indirectly address the problem of defective glucocerebrosidase and therefore an over-accumulation of glucosylceramide. ERT is the most direct option in which several variations of glucocerebrosidase have been FDA-approved for patient use.28,36,37,39 These include alglucerase (placental derivative of glucocerebrosidase), imiglucerase (human recombinant imiglucerase), and velaglucerase alfa (human fibroblast-derived glucocerebrosidase).28–30,36,37,39–45 Alglucerase is no longer clinically available as it has been replaced by imiglucerase. Taliglucerase alfa, a recombinant glucocerebrosidase derived from plants, is currently being reviewed by the FDA.30,39 Alternatively, SRT has emerged as an indirect option that seeks to prevent glucosylceramide accumulation by limiting its production by glucosylceramdie synthase.28,36 Miglustat is an imino sugar analog of glucose that is an inhibitor of glucosylceramide synthase.28,38,42,46,47 Miglustat is currently the only FDA-approved SRT for Gaucher’s disease. Eliglustat tartrate is another SRT that is currently in phase III clinical trials after the completion of a successful phase II clinical trial.48,49 Eliglustat tartrate is structurally similar to D-threo-1-phenyl-2-decanoylamino-3-morpholino-propanol (PDMP), which is a ceramide-like inhibitor of glucosylceramide synthase.47,50,51 Other therapeutic options have been or are being considered for Gaucher’s disease including bone marrow transplantation (for non-neurological Gaucher’s disease), gene therapy, and pharmacological chaperone therapy.28,30,32,46,47,50–52 Isofagomine tartrate is a pharmacological chaperone that was under recent pre-clinical development and was designed to bind to and help stabilize the misfolded glucocerebrosidase that arises from the specific N370S mutation.52 However after initial clinical trials with minimal “clinically meaningful improvements,” further clinical development of isofagomine tartrate was discontinued (October 2, 2009 press release from Amicus Therapeutics). Other pharmacological chaperones are still being evaluated for the treatment of Gaucher’s disease.
B. Gaucher’s Disease and Cancer
Gaucher’s disease has been linked in some instances to certain cancers. Predominantly, cases of myeloma associate with Gaucher’s disease.29,31,42,53,54 However, leukemia, glioblastoma, lung cancer, and hepatocellular carcinoma have all been documented in patients with Gaucher’s disease.29,31,42,54 The reasons for the correlation are currently being debated. Some suggestions have been made that implicate the therapies used to treat Gaucher’s disease as being responsible for increased correlations with certain cancers.29,55 One hypothesis suggests that depleting glycosphingolipids can adversely affect potential anticancer immune responses.29,55 Namely, it has been suggested that invariant natural killer T (iNKT) cells recognize complex glycosphingolipids.56 Therefore, therapies that block the formation of these glycolipid antigens, such as SRT therapies used for Gaucher’s disease, may in fact block potential iNKT cell-mediated antitumor activity.56,57 Similarly, it has been speculated that glycosphingolipids may exert anti-inflammatory roles and that SRT treatments for Gaucher’s disease promote carcinogenic inflammation.29,55 This position has gained traction in part because SRT treatments augment the amount of ceramide, which has been previously linked as a mediator of pro-inflammatory pathways.1,6
In contrast, however, there appears to be little or no clinical evidence that the prevalence of cancer in patients with Gaucher’s disease has changed during the era of either ERT or SRT. In some instances, the prevalence of cancer in patients with Gaucher’s disease during the ERT era has remained unchanged or has dropped.31 Additionally, a recent study evaluating the increased risk for cancer in Gaucher’s patients with the specific N370S mutation of glucocerebrosidase found that 69.6% of the patients who developed cancers were diagnosed with cancer before ERT was initiated.42 These discrepancies with the hypothesis that ERT or SRT are linked to cancer are not entirely surprising, as the speculations linking Gaucher’s disease with cancer fail to address the roles of ceramide and glucosylceramide in cancer biology. Namely, the role of ceramide has been extended well beyond that of a pro-apoptotic lipid or mediator within pro-inflammatory pathways. Now it is recognized that a specific ceramide metabolite, ceramide-1-phosphate, is a potent pro-inflammatory mediator, while novel anti-inflammatory roles are being uncovered for ceramide.1,6,58–61 Moreover, ceramide-based therapeutics are now emerging as potent anticancer agents,1 which further suggests that ceramide-augmenting therapies like SRT and ERT are likely not responsible for any correlation with cancer in Gaucher’s patients. Furthermore, the role of glucosylceramide is more closely tied to the development of therapy resistance rather than carcinogenesis.1,62–66 We and others have shown in models of cancer that a) ceramide neutralization to glucosylceramide is a primary mechanism of therapy resistance,1,3,62,63,67 b) glucosylceramide can upregulate the expression of the multidrug efflux pump P-glycoprotein,63–65 and c) glucosylceramide can block chemotherapeutic-induced NADPH oxidase 2 assembly and subsequent oxidative cell death.66 Therefore, it is more practical to associate elevated glucosylceramide, which accompanies Gaucher’s disease, with the progression of cancer.
III. SPHINGOLIPIDS AND CANCER
Several pro- or anti-cancer effects are closely linked to sphingolipids, responses particularly due to the distinct roles of specific bioactive species.1,3 Most notably, the ceramide/S1P biostat theory indicates that ceramide regulates anti-cancer cellular fate while S1P is pro-oncogenic and pro-metastatic.1,3,6,21 Recent research has now implicated unique anti-cancer or pro-cancer effects for different ceramide species, having varying acyl chain lengths.1,68–70 These studies have benefited from advances in analytical methods for lipid detection and quantification, which most certainly will continue to reveal new insights between specific sphingolipid metabolites and cancer. In similar fashion to the ceramide/S1P biostat model, other ceramide metabolites, such as glucosylceramide, are also associated with cancer development and/or progression, either due to the depletion of ceramide or due to specific inherent oncogenic roles.1,3 As a reflection of the biostat models, most chemotherapeutics and radiation treatments have been shown to induce significant accumulations of ceramide, while drug-resistant cancers posses the ability to clear ceramide via metabolic routes.1,3 New chemotherapeutic approaches are therefore being designed to elevate ceramide levels by increasing ceramide synthase activity, increasing ceramide generation via salvage synthesis from complex sphingolipids, decreasing ceramide neutralizing/catabolic enzyme activity, or by increasing delivery of endogenous non-metabolizable analogues of ceramide.1
A. Glucosylceramide Cancer Biology I: Ceramide Neutralization
Enzymes that neutralize ceramide or degrade ceramide have been studied and shown to be upregulated in drug-resistant cancers.1,3 This includes upregulation of glucosylceramide synthase and drug transporters, such as P-glycoprotein, which are important in the conversion of ceramide to glucosylceramide.1,62,64 Intriguingly, the inclusion of inhibitors of glucosylceramide synthase or P-glycoprotein concurrently with common chemotherapeutics can restore sensitivity of resistant cancers to treatments.1,62,64 With particular regard to P-glycoprotein, an ABC transporter that acts as a drug efflux pump, our group uncovered a novel role of this protein in the metabolism of ceramide to glucosylceramide. Namely, we showed that common antagonists of P-glycoprotein block ceramide glycosylation and can thus be effectively utilized to increase intracellular levels of ceramide.71–74 This effect occurs because P-glycoprotein facilitates the movement of newly synthesized glucosylceramide from the cytosolic leaflet of the Golgi to the inner leaflet where further glycosylation can occur.63–65,75 This event specifically influences the initial glycosylation ceramide because glucosylceramide can block further glucosylceramide synthase activity by product inhibition. P-glycoprotein at the Golgi therefore potentiates the continued metabolism of ceramide to glycosylated derivatives.
An understanding of the relationship between glucosylceramide synthase and P-glycoprotein is fundamentally important when considering prospective treatments for multidrug-resistant cancers. The anticancer efficacy of inhibitors of ceramide glycosylation is likely related to whether they target glucosylceramide synthase, P-glycoprotein, or both (Fig. 2). The imino sugar analog of glucose, Miglustat, is a glucosylceramide synthase inhibitor that is effective and approved for the treatment of Gaucher’s disease, although at micromolar dosage, that likely is responsible for clinically-limiting side effects.38,46,47,76,77 Miglustat inhibits ceramide glycosylation by targeting glucosylceramide synthase but not P-glycoprotein.38,77 The anticancer efficacy of Miglustat has also been evaluated in cellular models of cancer with some success. However, in vivo data are lacking for Miglustat as an anticancer therapeutic and its specific efficacy within cancer-specific ceramide glycosylation pathways is questioned due to substantial off-target effects towards other glucosidases that have no impact on any glycosphingolipid metabolism.38,47,76 Moreover, these off-target effects of the SRT Miglustat are possibly responsible for aberrant immune-modulation that may influence potential antitumor immune surveillance. In contrast, analogs of glucosylceramide have been shown to exert significant anticancer efficacy in both in vitro and in vivo models of multidrug resistant cancer.1 These stereospecific glucosylceramide analogs, sometimes referred to as P-series compounds (PDMP, PPMP, PPPP), have been shown to block both glucosylceramide synthase and P-glycoprotein.1,28,47,62,63,78 Variations of these analogs are intended to improve selectivity and/or solubility. The efficacy of these glucosylceramide analogs in both animal models of cancer and clinical trials in Gaucher’s patients, in the case of Eliglustat tartrate, has been achieved at low dosage (nanomolar) and with minimal side effects.28,47–49,67 Recently, our laboratory has found success in formulating PDMP within nanoliposomes, with or without ceramide, and this has allowed for substantial in vitro and in vivo efficacy in models of glioblastoma, neuroblastoma, and pancreatic cancer.66,67 Finally, other inhibitors of P-glycoprotein, such as PSC833 and cyclosporin A, have also been shown to effectively block ceramide glycosylation in cancer models.1,62,64,71 These inhibitors were originally designed to target P-glycoprotein’s drug-effluxing activity, but they have not gained clinical traction or approval because efficacy has never been reached before dose-limiting toxicity.1 However, the investigation of these compounds at low doses in combination with ceramide-based therapeutics has provided new promise for these agents.62 Excitingly, it has also been revealed that the anti-breast cancer chemotherapeutic tamoxifen, also a potent inhibitor of P-glycoprotein and inhibitor of ceramide glycosylation, can act in concert with ceramide-based therapies to enhance ceramide-governed cell-death endpoints.1,62,64,79
FIGURE 2. Protein junctures for the action of glucosylceramide synthase inhibitors and P-glycoprotein antagonists.
P-glycoprotein (P-gp) directed transport of glucosylceramide into the Golgi. Upon conversion of ceramide to glucosylceramide by glucosylceramide synthase (GCS), glucosylceramide is transported into the Golgi lumen by P-gp for conversion to higher glycosphingolipids. Inhibitors of ceramide glycosylation exert effects at either GCS, P-gp, or both. LCS: lactosylceramide synthase.
B. Glucosylceramide Cancer Biology II: Regulation of P-glycoprotein
Recently, our research groups have explored novel links between glucosylceramide synthase activity and the regulation of P-glycoprotein expression. As mentioned, both glucosylceramide synthase and P-glycoprotein play pivotal roles in the generation of glycosphingolipids by facilitating glucosylceramide production and translocation into the Golgi. We initially showed that antisense oligonucleotides targeting glucosylceramide synthase in multidrug-resistant cell lines could down-regulate P-glycoprotein gene expression (MDR1).63,64 Moreover, this study also demonstrated that PPMP also diminished P-glycoprotein expression in multidrug-resistant cancer cells.63 In a later study, transient overexpression of glucosylceramide synthase was demonstrated to upregulate P-glycoprotein expression.65 These studies demonstrated a direct cellular pathway linking the initial enzyme in glycosphingolipid biosynthesis, glucosylceramide synthase, to the development of multidrug-resistance by genetic regulation of P-glycoprotein. More specific investigation revealed that this pathway was mediated by complex glycosphingolipid metabolites of glucosylceramide, specifically globo series glycosphingolipids, and that these regulated nuclear localization of the transcription regulator beta-catenin via upstream regulation of cSrc kinase.65 The elucidation of this signaling cascade may have profound consequences not only for the development of multidrug-resistance but also for links between cancer and Gaucher’s disease. Gaucher’s patients have elevated levels of many complex glycosphingolipids by virtue of not being able to breakdown glucosylceramide, which then causes a “back-up” of structurally associated lipids.
C. Glucosylceramide Cancer Biology III: Inhibition of NADPH Oxidase 2
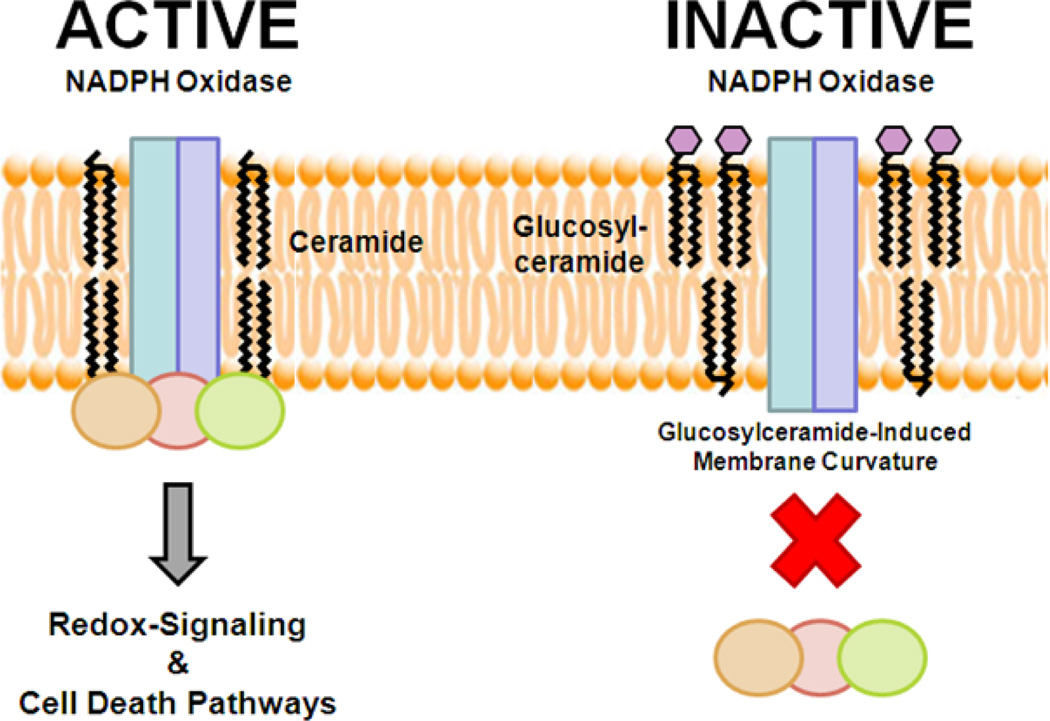
In addition to links between glucosylceramide synthase and the genetic regulation of P-glycoprotein, we have uncovered another novel regulatory role for glucosylceramide in the context of multidrug-resistant cancer. Specifically, we revealed that glucosylceramide can directly interfere with the assembly and function of NADPH oxidase 2 in cancer cells.66 NADPH oxidases are a large family of oxidoreductases that play important roles in redox-regulation of cellular signaling, mitogenesis, motility, proliferation, and cell death.80 The NADPH oxidase 2 is activated in response to stimuli, which allow cytosolic subunit phosphorylation and translocation to a complex with membrane-bound subunits.80 Not surprisingly, this activity is mediated by ceramide and can be stimulated by ceramide-based and ceramide-regulating therapeutics.66,81 This dependence on ceramide as a bioactive regulator also means that mechanisms of multidrug-resistance that clear ceramide can also prevent NADPH oxidase 2 activity. However, by utilizing exogenously delivered glucosylceramide, we were able to show that assembly of NADPH oxidase 2 in response to chemotherapeutics was blocked, demonstrating a specific mechanism of action for glucosylceramide (Fig. 3).66 This study has profound consequences for Gaucher’s patients, which have elevated circulating levels of glucosylceramide that may act to impede NADPH oxidase 2 in many tissues.66 It may therefore be of little surprise that some immune system abnormalities observed in Gaucher’s disease are similar to those observed in patients that suffer from chronic granulomatous disease, a genetic deficiency of the phagocytic NADPH oxidase.66 However, uncommonly and especially in the absence of a splenectomy, Gaucher’s disease patients rarely have the increased risk for pyogenic infections that are seen in patients with chronic granulomatous disease. Lastly, it should be noted that beta-catenin signaling is a target of NADPH oxidase 2-mediated redox-regulation,80 both directly and indirectly. This may provide another link within the signaling cascade revealed by our research associating glucosylceramide synthase activity and P-glycoprotein expression.
FIGURE 3. Glucosylceramide blocks the assembly of the NADPH oxidase.
The NADPH oxidase is composed of two membrane-bound subunits (rectangles: gp91phox, p22phox) and three cytosilic subunits (circles: p40phox, p47phox, p67phox). In response to stimulation by chemotherapeutics, or inflammatory mediators, ceramide stimulates assembly and activation of the NADPH oxidase. An active NADPH oxidase can mediate redox-sensitive signaling as well as cell death pathways. In contrast, glucosylceramide blocks assembly of the cytosolic subunits with the membrane subunits by promoting changes in membrane curvature. Therefore, glucosylceramide blocks NADPH oxidase-mediated redox-signaling and cell death pathways.
IV. SPHINGOLIPIDS AS A LINK BETWEEN GAUCHER’S DISEASE AND CANCER
Following a review of what is known regarding glucosylceramide biosynthesis and cancer, substantive links between Gaucher’s disease and cancer can begin to be made. The most important consideration is that glucosylceramide accumulation, or accumulation of complex glycosphingolipids due to glucocerebrosidase deficiency, is intimately linked to the expression of P-glycoprotein and the development of multidrug-resistance.1,62,64 This in itself is likely not carcinogenic but may exacerbate the progression and severity of malignancies once they occur. It is easy to understand the clinical significance of glucosylceramide accumulation in oncology, especially when considering regulation of NADPH oxidase 2-mediated redox-signaling and the redox-sensitive transcription factor beta-catenin.65,66 In theory, the therapies used for the treatment of Gaucher’s disease may be able to avert aggressive cancer progression, more so by synergizing with ceramide-based therapeutics. Importantly, development of inhibitors that target both glucosylceramide synthase and P-glycoprotein, such as the P-series drugs developed by Radin and Shayman, serve the best chance to treat Gaucher’s disease and links with cancer progression.82,83 In contrast, the current SRTs for Gaucher’s disease, like Miglustat, may not be able to effectively synergize with ceramide-based and ceramide-regulating therapeutics at concentrations that are non-toxic to normal cells and tissues.
In contrast to links between the glucosylceramide accumulation during Gaucher’s disease and cancer is the hypothesis that the therapies for Gaucher’s disease may promote oncogenesis. In the context of sphingolipid biology, this brings to light the question of the role of ceramide in cancer, because both SRT and ERT result in ceramide accumulation at the expense of glycosphingolipids. In our opinion, when considering the role of ceramide in cancer, it seems unlikely that the treatments for Gaucher’s disease would contribute to the development of malignancies. Not only is ceramide a pro-apoptotic sphingolipid, but it has been well documented as an important mediator of many anticancer strategies.1,3 Furthermore, ceramide-containing therapeutics have been shown to have nearly complete selectivity for cancer cells, at low doses.1,2,67,84 Moreover, the mechanisms of drug resistance are intimately tied to ceramide clearance.1,3,64 Therefore, it is unlikely that therapies that augment ceramide levels would be carcinogenic. The caveat would be the metabolism of ceramide to a pro-oncogenic sphingolipid like S1P.1,3,20
Lastly, the idea that Gaucher’s disease therapies promote oncogenesis suggests that depletion of complex glycosphingolipids, and thereby an accumulation of ceramide, promotes inflammation or reduces immune responses. Importantly though, this idea does not account for the role of ceramide as an anti-inflammatory sphingolipid. The most current research now suggests that ceramide has profound anti-inflammatory roles.1,58–60 The earlier indications of ceramide as a pro-inflammatory agent almost always failed to account for its conversion to ceramide-1-phosphate, which is now considered to be a potent pro-inflammatory mediator.61 Two representative studies argue for the role of ceramide as an anti-inflammatory lipid. Initially, our group demonstrated that ceramide nanoliposomes reduce corneal inflammation.60 These studies were more recently confirmed in elegant work by the Hannun/Obeid group, showing that glucocerebrosidase-derived ceramide, or exogenous ceramide, could prevent IL-6 production.58,59 Not only has this study confirmed a role for ceramide as an anti-inflammatory lipid, but it has done so in a model directly relevant to the questions posed in Gaucher’s disease, due to manipulations of the responsible enzyme. Altogether, the roles of ceramide as an anti-inflammatory lipid and as a potent anticancer lipid indicate that the occurrence of cancer in patients with Gaucher’s disease treated with SRTs or ERTs is perhaps coincidental. We therefore propose that the most relevant connection between Gaucher’s disease and cancer is the augmentation of cancer progression, not necessarily carcinogenesis, due to the pathological accumulation of glucosylceramide and complex glycosphingolipids.
ACKNOWLEDGMENT
This work was supported by the National Institutes of Health (Grant Nos. GM77391 and CA143755) awarded to Myles C. Cabot, John Wayne Cancer Institute.
ABBREVIATIONS
- ABC
ATP-binding cassette
- C1PP
ceramide-1-phosphate phosphatase
- CDase
ceramidase
- CERT
ceramide-transfer protein
- CK
ceramide kinase
- CS
ceramide synthase
- DAG
diacylglycerol
- DES
dihydroceramide desaturase
- ERT
enzyme replacement therapy
- GALC
galactosylceramidase
- GALT
ceramide galactosyltransferase
- GBA
glucocerebrosidase
- GCS
glucosylceramide synthase
- iNKT
invariant natural killer T
- LCS
lactosylceramide synthase
- PC
phosphatidylcholine
- PDMP
D-threo-1-phenyl-2-decanoylamino-3-morpholino-propanol
- P-gp
P-glycoprotein
- S1P
sphingosine-1-phosphate
- S1PP
sphingosine-1-phosphate phosphatase
- SK
sphingosine kinase
- SMase
sphingomyelinase
- SMS
sphingomyelin synthase
- SPT
serine palmitoyltransferase
Footnotes
A. Potential Conflicts of Interest
Penn State research foundation has licensed ceramide-based drug delivery systems to Keystone Nano, Inc. and Mark Kester is the Chief Medical officer of Keystone Nano.
REFERENCES
- 1.Barth BM, Cabot MC, Kester M. Ceramide-Based Therapeutics for the Treatment of Cancer. Anticancer Agents Med Chem. 2011;11(9):911–919. doi: 10.2174/187152011797655177. [DOI] [PubMed] [Google Scholar]
- 2.Fox TE, Finnegan CM, Blumenthal R, Kester M. The clinical potential of sphingolipid-based therapeutics. Cell Mol Life Sci. 2006;63(9):1017–1023. doi: 10.1007/s00018-005-5543-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryland LK, Fox TE, Liu X, Loughran TP, Kester M. Dysregulation of sphingolipid metabolism in cancer. Cancer Biol Ther. 2011;11(2):138–149. doi: 10.4161/cbt.11.2.14624. [DOI] [PubMed] [Google Scholar]
- 4.Eyster KM. The membrane and lipids as integral participants in signal transduction: lipid signal transduction for the non-lipid biochemist. Adv Physiol Educ. 2007;31(1):5–16. doi: 10.1152/advan.00088.2006. [DOI] [PubMed] [Google Scholar]
- 5.Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274(5294):1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 6.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9(2):139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 7.Kester M, Kolesnick R. Sphingolipids as therapeutics. Pharmacol Res. 2003;47(5):365–371. doi: 10.1016/s1043-6618(03)00048-3. [DOI] [PubMed] [Google Scholar]
- 8.Morales A, Lee H, Goni FM, Kolesnick R, Fernandez-Checa JC. Sphingolipids and cell death. Apoptosis. 2007;12(5):923–939. doi: 10.1007/s10495-007-0721-0. [DOI] [PubMed] [Google Scholar]
- 9.Saddoughi SA, Song P, Ogretmen B. Roles of bioactive sphingolipids in cancer biology and therapeutics. Subcell Biochem. 2008;49:413–440. doi: 10.1007/978-1-4020-8831-5_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Futerman AH, Riezman H. The ins and outs of sphingolipid synthesis. Trends Cell Biol. 2005;15(6):312–318. doi: 10.1016/j.tcb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Futerman AH, Hannun YA. The complex life of simple sphingolipids. EMBO Rep. 2004;5(8):777–782. doi: 10.1038/sj.embor.7400208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng W, Kollmeyer J, Symolon H, Momin A, Munter E, Wang E, Kelly S, Allegood JC, Liu Y, Peng Q, Ramaraju H, Sullards MC, Cabot M, Merrill AH., Jr Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta. 2006;1758(12):1864–1884. doi: 10.1016/j.bbamem.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Hannun YA, Luberto C. Ceramide in the eukaryotic stress response. Trends Cell Biol. 2000;10(2):73–80. doi: 10.1016/s0962-8924(99)01694-3. [DOI] [PubMed] [Google Scholar]
- 14.He X, Okino N, Dhami R, Dagan A, Gatt S, Schulze H, Sandhoff K, Schuchman EH. Purification and characterization of recombinant, human acid ceramidase. Catalytic reactions and interactions with acid sphingomyelinase. J Biol Chem. 2003;278(35):32978–32986. doi: 10.1074/jbc.M301936200. [DOI] [PubMed] [Google Scholar]
- 15.Okino N, He X, Gatt S, Sandhoff K, Ito M, Schuchman EH. The reverse activity of human acid ceramidase. J Biol Chem. 2003;278(32):29948–29953. doi: 10.1074/jbc.M303310200. [DOI] [PubMed] [Google Scholar]
- 16.Weinreb NJ, Brady RO, Tappel AL. The lysosomal localization of sphingolipid hydrolases. Biochim Biophys Acta. 1968;159(1):141–146. doi: 10.1016/0005-2744(68)90251-9. [DOI] [PubMed] [Google Scholar]
- 17.Gouaze-Andersson V, Cabot MC. Glycosphingolipids and drug resistance. Biochim Biophys Acta. 2006;1758(12):2096–2103. doi: 10.1016/j.bbamem.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Yamaji T, Kumagai K, Tomishige N, Hanada K. Two sphingolipid transfer proteins, CERT and FAPP2: their roles in sphingolipid metabolism. IUBMB Life. 2008;60(8):511–518. doi: 10.1002/iub.83. [DOI] [PubMed] [Google Scholar]
- 19.Fox TE, Houck KL, O’Neill SM, Nagarajan M, Stover TC, Pomianowski PT, Unal O, Yun JK, Naides SJ, Kester M. Ceramide recruits and activates protein kinase C zeta (PKC zeta) within structured membrane microdomains. J Biol Chem. 2007;282(17):12450–12457. doi: 10.1074/jbc.M700082200. [DOI] [PubMed] [Google Scholar]
- 20.Spiegel S, Milstien S. Sphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem. 2002;277(29):25851–25854. doi: 10.1074/jbc.R200007200. [DOI] [PubMed] [Google Scholar]
- 21.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4(8):604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 22.Payne SG, Milstien S, Spiegel S. Sphingosine-1-phosphate: dual messenger functions. FEBS Lett. 2002;531(1):54–57. doi: 10.1016/s0014-5793(02)03480-4. [DOI] [PubMed] [Google Scholar]
- 23.Pyne S, Pyne N. Sphingosine 1-phosphate signalling via the endothelial differentiation gene family of G-protein-coupled receptors. Pharmacol Ther. 2000;88(2):115–131. doi: 10.1016/s0163-7258(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 24.Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, Maceyka M, Jiang H, Luo C, Kordula T, Milstien S, Spiegel S. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465(7301):1084–1088. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, Spiegel S. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325(5945):1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu J, Yang C, Chen M, Ye DY, Lonser RR, Brady RO, Zhuang Z. Histone deacetylase inhibitors prevent the degradation and restore the activity of glucocerebrosidase in Gaucher disease. Proc Natl Acad Sci U S A. 2011;108(52):21200–21205. doi: 10.1073/pnas.1119181109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campeau PM, Rafei M, Boivin MN, Sun Y, Grabowski GA, Galipeau J. Characterization of Gaucher disease bone marrow mesenchymal stromal cells reveals an altered inflammatory secretome. Blood. 2009;114(15):3181–3190. doi: 10.1182/blood-2009-02-205708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abe A, Wild SR, Lee WL, Shayman JA. Agents for the treatment of glycosphingolipid storage disorders. Curr Drug Metab. 2001;2(3):331–338. doi: 10.2174/1389200013338414. [DOI] [PubMed] [Google Scholar]
- 29.Choy FY, Campbell TN. Gaucher disease and cancer: concept and controversy. Int J Cell Biol. 2011;2011:150450. doi: 10.1155/2011/150450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grabowski GA. Phenotype, diagnosis, and treatment of Gaucher’s disease. Lancet. 2008;372(9645):1263–1271. doi: 10.1016/S0140-6736(08)61522-6. [DOI] [PubMed] [Google Scholar]
- 31.Mistry PK, Weinreb NJ, Brady RO, Grabowski GA. Gaucher disease: resetting the clinical and scientific agenda. Am J Hematol. 2009;84(4):205–207. doi: 10.1002/ajh.21384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Motabar O, Huang W, Marugan JJ, Southall N, DeBernardi M, Zheng W, Sidransky E. Identification of modulators of the N370S mutant form of glucocerebrosidase as a potential therapy for Gaucher disease. In: Rusconi L, Kilstrup-Nielsen C, Landsberger N, editors. Probe reports from the NIH Molecular Libraries Program [Internet] Bethesda, MD: National Center for Biotechnology Information (US); 2010–2010. Mar 29, [updated 2011 Mar 3]. (2011). [Google Scholar]
- 33.Aerts JM, Donker-Koopman WE, van der Vliet MK, Jonsson LM, Ginns EI, Murray GJ, Barranger JA, Tager JM, Schram AW. The occurrence of two immunologically distinguishable beta-glucocerebrosidases in human spleen. Eur J Biochem. 1985;150(3):565–574. doi: 10.1111/j.1432-1033.1985.tb09058.x. [DOI] [PubMed] [Google Scholar]
- 34.Weinreb NJ, Andersson HC, Banikazemi M, Barranger J, Beutler E, Charrow J, Grabowski GA, Hollak CE, Kaplan P, Mankin H, Mistry PK, Rosenbloom BE, Vom Dahl S, Zimran A. Prevalence of type 1 Gaucher disease in the United States. Arch Intern Med. 2008;168(3):326–327. doi: 10.1001/archinternmed.2007.128. author reply 327–8. [DOI] [PubMed] [Google Scholar]
- 35.Weinreb NJ, Deegan P, Kacena KA, Mistry P, Pastores GM, Velentgas P, vom Dahl S. Life expectancy in Gaucher disease type 1. Am J Hematol. 2008;83(12):896–900. doi: 10.1002/ajh.21305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimran A. How I treat Gaucher disease. Blood. 2011;118(6):1463–1471. doi: 10.1182/blood-2011-04-308890. [DOI] [PubMed] [Google Scholar]
- 37.Beutler E, Kay A, Saven A, Garver P, Thurston D, Dawson A, Rosenbloom B. Enzyme replacement therapy for Gaucher disease. Blood. 1991;78(5):1183–1189. [PubMed] [Google Scholar]
- 38.Butters TD, Dwek RA, Platt FM. Imino sugar inhibitors for treating the lysosomal glycosphingolipidoses. Glycobiology. 2005;15(10):43R–52R. doi: 10.1093/glycob/cwi076. [DOI] [PubMed] [Google Scholar]
- 39.Lachmann RH. Enzyme replacement therapy for lysosomal storage diseases. Curr Opin Pediatr. 2011;23(6):588–593. doi: 10.1097/MOP.0b013e32834c20d9. [DOI] [PubMed] [Google Scholar]
- 40.Burrow TA, Grabowski GA. Velaglucerase alfa in the treatment of Gaucher disease type 1. Clin Investig (Lond) 2011;1(2):285–293. doi: 10.4155/cli.10.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He X, Galpin JD, Tropak MB, Mahuran D, Haselhorst T, von Itzstein M, Kolarich D, Packer NH, Miao Y, Jiang L, Grabowski GA, Clarke LA, Kermode AR. Production of active human glucocerebrosidase in seeds of Arabidopsis thaliana complex-glycan-deficient (cgl) plants. Glycobiology. 2011 doi: 10.1093/glycob/cwr157. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taddei TH, Kacena KA, Yang M, Yang R, Malhotra A, Boxer M, Aleck KA, Rennert G, Pastores GM, Mistry PK. The underrecognized progressive nature of N370S Gaucher disease and assessment of cancer risk in 403 patients. Am J Hematol. 2009;84(4):208–214. doi: 10.1002/ajh.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valayannopoulos V, Brassier A, Chabli A, Caillaud C, Lemoine M, Odent T, Arnoux JB, de Lonlay P. Enzyme replacement therapy for lysosomal storage disorders. Arch Pediatr. 2011;18(10):1119–1123. doi: 10.1016/j.arcped.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Weinreb N, Barranger J, Packman S, Prakash-Cheng A, Rosenbloom B, Sims K, Angell J, Skrinar A, Pastores GM. Imiglucerase (Cerezyme) improves quality of life in patients with skeletal manifestations of Gaucher disease. Clin Genet. 2007;71(6):576–588. doi: 10.1111/j.1399-0004.2007.00811.x. [DOI] [PubMed] [Google Scholar]
- 45.Zimran A. Velaglucerase alfa: a new option for Gaucher disease treatment. Drugs Today (Barc) 2011;47(7):515–529. doi: 10.1358/dot.2011.47.7.1608922. [DOI] [PubMed] [Google Scholar]
- 46.Abian O, Alfonso P, Velazquez-Campoy A, Giraldo P, Pocovi M, Sancho J. Therapeutic Strategies for Gaucher Disease: Miglustat (NB-DNJ) as a Pharmacological Chaperone for Glucocerebrosidase and the Different Thermostability of Velaglucerase Alfa and Imiglucerase. Mol Pharm. 2011;8(6):2390–2397. doi: 10.1021/mp200313e. [DOI] [PubMed] [Google Scholar]
- 47.Larsen SD, Wilson MW, Abe A, Shu L, George CH, Kirchhoff P, Showalter HD, Xiang J, Keep RF, Shayman JA. Property-based design of a glucosylceramide synthase inhibitor that reduces glucosylceramide in the brain. J Lipid Res. 2012;53(2):282–291. doi: 10.1194/jlr.M021261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lukina E, Watman N, Arreguin EA, Banikazemi M, Dragosky M, Iastrebner M, Rosenbaum H, Phillips M, Pastores GM, Rosenthal DI, Kaper M, Singh T, Puga AC, Bonate PL, Peterschmitt MJ. A phase 2 study of eliglustat tartrate (Genz-112638), an oral substrate reduction therapy for Gaucher disease type 1. Blood. 2010;116(6):893–899. doi: 10.1182/blood-2010-03-273151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lukina E, Watman N, Arreguin EA, Dragosky M, Iastrebner M, Rosenbaum H, Phillips M, Pastores GM, Kamath RS, Rosenthal DI, Kaper M, Singh T, Puga AC, Peterschmitt MJ. Improvement in hematological, visceral, and skeletal manifestations of Gaucher disease type 1 with oral eliglustat tartrate (Genz-112638) treatment: 2-year results of a phase 2 study. Blood. 2010;116(20):4095–4098. doi: 10.1182/blood-2010-06-293902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chatterjee S, Cleveland T, Shi WY, Inokuchi J, Radin NS. Studies of the action of ceramide-like substances (D- and L-PDMP) on sphingolipid glycosyltransferases and purified lactosylceramide synthase. Glycoconj J. 1996;13(3):481–486. doi: 10.1007/BF00731481. [DOI] [PubMed] [Google Scholar]
- 51.Shayman JA, Lee L, Abe A, Shu L. Inhibitors of glucosylceramide synthase. Methods Enzymol. 2000;311:373–387. doi: 10.1016/s0076-6879(00)11097-3. [DOI] [PubMed] [Google Scholar]
- 52.Sun Y, Liou B, Xu YH, Quinn B, Zhang W, Hamler R, Setchell KD, Grabowski GA. Gaucher disease: Ex vivo and in vivo effects of isofagomine on acid beta-glucosidase variants and substrate levels. J Biol Chem. 2011;287(6):4275–4287. doi: 10.1074/jbc.M111.280016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenbloom BE, Becker P, Weinreb N. Multiple myeloma and Gaucher genes. Genet Med. 2009;11(2):134. doi: 10.1097/GIM.0b013e318195e2b6. [DOI] [PubMed] [Google Scholar]
- 54.Rosenbloom BE, Weinreb NJ, Zimran A, Kacena KA, Charrow J, Ward E. Gaucher disease and cancer incidence: a study from the Gaucher Registry. Blood. 2005;105(12):4569–4572. doi: 10.1182/blood-2004-12-4672. [DOI] [PubMed] [Google Scholar]
- 55.Zimran A, Ilan Y, Elstein D. Enzyme replacement therapy for mild patients with Gaucher disease. Am J Hematol. 2009;84(4):202–204. doi: 10.1002/ajh.21369. [DOI] [PubMed] [Google Scholar]
- 56.Gadola SD, Silk JD, Jeans A, Illarionov PA, Salio M, Besra GS, Dwek R, Butters TD, Platt FM, Cerundolo V. Impaired selection of invariant natural killer T cells in diverse mouse models of glycosphingolipid lysosomal storage diseases. J Exp Med. 2006;203(10):2293–2303. doi: 10.1084/jem.20060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Speak AO, Salio M, Neville DC, Fontaine J, Priestman DA, Platt N, Heare T, Butters TD, Dwek RA, Trottein F, Exley MA, Cerundolo V, Platt FM. Implications for invariant natural killer T cell ligands due to the restricted presence of isoglobotrihexosylceramide in mammals. Proc Natl Acad Sci U S A. 2007;104(14):5971–5976. doi: 10.1073/pnas.0607285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kitatani K, Sheldon K, Anelli V, Jenkins RW, Sun Y, Grabowski GA, Obeid LM, Hannun YA. Acid beta-glucosidase 1 counteracts p38delta-dependent induction of interleukin-6: possible role for ceramide as an anti-inflammatory lipid. J Biol Chem. 2009;284(19):12979–12988. doi: 10.1074/jbc.M809500200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kitatani K, Sheldon K, Rajagopalan V, Anelli V, Jenkins RW, Sun Y, Grabowski GA, Obeid LM, Hannun YA. Involvement of acid beta-glucosidase 1 in the salvage pathway of ceramide formation. J Biol Chem. 2009;284(19):12972–12978. doi: 10.1074/jbc.M802790200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun Y, Fox T, Adhikary G, Kester M, Pearlman E. Inhibition of corneal inflammation by liposomal delivery of short-chain, C-6 ceramide. J Leukoc Biol. 2008;83(6):1512–1521. doi: 10.1189/jlb.0108076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gomez-Munoz A, Gangoiti P, Granado MH, Arana L, Ouro A. Ceramide-1-phosphate in cell survival and inflammatory signaling. Adv Exp Med Biol. 2010;688:118–130. doi: 10.1007/978-1-4419-6741-1_8. [DOI] [PubMed] [Google Scholar]
- 62.Chapman JV, Gouaze-Andersson V, Messner MC, Flowers M, Karimi R, Kester M, Barth BM, Liu X, Liu YY, Giuliano AE, Cabot MC. Metabolism of short-chain ceramide by human cancer cells—implications for therapeutic approaches. Biochem Pharmacol. 2010;80(3):308–315. doi: 10.1016/j.bcp.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gouaze V, Liu YY, Prickett CS, Yu JY, Giuliano AE, Cabot MC. Glucosylceramide synthase blockade down-regulates P-glycoprotein and resensitizes multidrug-resistant breast cancer cells to anticancer drugs. Cancer Res. 2005;65(9):3861–3867. doi: 10.1158/0008-5472.CAN-04-2329. [DOI] [PubMed] [Google Scholar]
- 64.Gouaze-Andersson V, Yu JY, Kreitenberg AJ, Bielawska A, Giuliano AE, Cabot MC. Ceramide and glucosylceramide upregulate expression of the multidrug resistance gene MDR1 in cancer cells. Biochim Biophys Acta. 2007;1771(12):1407–1417. doi: 10.1016/j.bbalip.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu YY, Gupta V, Patwardhan GA, Bhinge K, Zhao Y, Bao J, Mehendale H, Cabot MC, Li YT, Jazwinski SM. Glucosylceramide synthase upregulates MDR1 expression in the regulation of cancer drug resistance through cSrc and beta-catenin signaling. Mol Cancer. 2010;9:145. doi: 10.1186/1476-4598-9-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barth BM, Gustafson SJ, Young MM, Fox TE, Shanmugavelandy SS, Kaiser JM, Cabot MC, Kester M, Kuhn TB. Inhibition of NADPH oxidase by glucosylceramide confers chemoresistance. Cancer Biol Ther. 2010;10(11):1126–1136. doi: 10.4161/cbt.10.11.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang Y, DiVittore NA, Kaiser JM, Shanmugavelandy SS, Fritz JL, Heakal Y, Tagaram HR, Cheng H, Cabot MC, Staveley-O’Carroll KF, Tran MA, Fox TE, Barth BM, Kester M. Combinatorial therapies improve the therapeutic efficacy of nanoliposomal ceramide for pancreatic cancer. Cancer Biol Ther. 2011;12(7):574–585. doi: 10.4161/cbt.12.7.15971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karahatay S, Thomas K, Koybasi S, Senkal CE, Elojeimy S, Liu X, Bielawski J, Day TA, Gillespie MB, Sinha D, Norris JS, Hannun YA, Ogretmen B. Clinical relevance of ceramide metabolism in the pathogenesis of human head and neck squamous cell carcinoma (HNSCC): attenuation of C(18)-ceramide in HNSCC tumors correlates with lymphovascular invasion and nodal metastasis. Cancer Lett. 2007;256(1):101–111. doi: 10.1016/j.canlet.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koybasi S, Senkal CE, Sundararaj K, Spassieva S, Bielawski J, Osta W, Day TA, Jiang JC, Jazwinski SM, Hannun YA, Obeid LM, Ogretmen B. Defects in cell growth regulation by C18:0-ceramide and longevity assurance gene 1 in human head and neck squamous cell carcinomas. J Biol Chem. 2004;279(43):44311–44319. doi: 10.1074/jbc.M406920200. [DOI] [PubMed] [Google Scholar]
- 70.Mesicek J, Lee H, Feldman T, Jiang X, Skobeleva A, Berdyshev EV, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Ceramide synthases 2, 5, and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cell Signal. 2010;22(9):1300–1307. doi: 10.1016/j.cellsig.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lavie Y, Cao H, Volner A, Lucci A, Han TY, Geffen V, Giuliano AE, Cabot MC. Agents that reverse multidrug resistance, tamoxifen, verapamil, and cyclosporin A, block glycosphingolipid metabolism by inhibiting ceramide glycosylation in human cancer cells. J Biol Chem. 1997;272(3):1682–1687. doi: 10.1074/jbc.272.3.1682. [DOI] [PubMed] [Google Scholar]
- 72.Chapman JV, Gouaze-Andersson V, Karimi R, Messner MC, Cabot MC. P-glycoprotein antagonists confer synergistic sensitivity to short-chain ceramide in human multidrug-resistant cancer cells. Exp Cell Res. 2011;317(12):1736–1745. doi: 10.1016/j.yexcr.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lucci A, Giuliano AE, Han TY, Dinur T, Liu YY, Senchenkov A, Cabot MC. Ceramide toxicity and metabolism differ in wild-type and multidrug-resistant cancer cells. Int J Oncol. 1999;15(3):535–540. doi: 10.3892/ijo.15.3.535. [DOI] [PubMed] [Google Scholar]
- 74.Lucci A, Han TY, Liu YY, Giuliano AE, Cabot MC. Modification of ceramide metabolism increases cancer cell sensitivity to cytotoxics. Int J Oncol. 1999;15(3):541–546. doi: 10.3892/ijo.15.3.541. [DOI] [PubMed] [Google Scholar]
- 75.Borst P, Zelcer N, van Helvoort A. ABC transporters in lipid transport. Biochim Biophys Acta. 2000;1486(1):128–144. doi: 10.1016/s1388-1981(00)00053-6. [DOI] [PubMed] [Google Scholar]
- 76.Weinreb NJ, Barranger JA, Charrow J, Grabowski GA, Mankin HJ, Mistry P. Guidance on the use of miglustat for treating patients with type 1 Gaucher disease. Am J Hematol. 2005;80(3):223–229. doi: 10.1002/ajh.20504. [DOI] [PubMed] [Google Scholar]
- 77.Norris-Cervetto E, Callaghan R, Platt FM, Dwek RA, Butters TD. Inhibition of glucosylceramide synthase does not reverse drug resistance in cancer cells. J Biol Chem. 2004;279(39):40412–40418. doi: 10.1074/jbc.M404466200. [DOI] [PubMed] [Google Scholar]
- 78.Chai L, McLaren RP, Byrne A, Chuang WL, Huang Y, Dufault MR, Pacheco J, Madhiwalla S, Zhang X, Zhang M, Teicher BA, Carter K, Cheng SH, Leonard JP, Xiang Y, Vasconcelles M, Goldberg MA, Copeland DP, Klinger KW, Lillie J, Madden SL, Jiang YA. The chemosensitizing activity of inhibitors of glucosylceramide synthase is mediated primarily through modulation of P-gp function. Int J Oncol. 2011;38(3):701–711. doi: 10.3892/ijo.2010.888. [DOI] [PubMed] [Google Scholar]
- 79.Callaghan R, Higgins CF. Interaction of tamoxifen with the multidrug resistance P-glycoprotein. Br J Cancer. 1995;71(2):294–299. doi: 10.1038/bjc.1995.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 81.Barth BM, Gustafson SJ, Kuhn TB. Neutral sphingomyelinase activation precedes NADPH oxidase-dependent damage in neurons exposed to the proinflammatory cytokine tumor necrosis factor-alpha. J Neurosci Res. 2012;90(1):229–242. doi: 10.1002/jnr.22748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Radin NS, Shayman JA, Inokuchi J. Metabolic effects of inhibiting glucosylceramide synthesis with PDMP and other substances. Adv Lipid Res. 1993;26:183–213. [PubMed] [Google Scholar]
- 83.Abe A, Inokuchi J, Jimbo M, Shimeno H, Nagamatsu A, Shayman JA, Shukla GS, Radin NS. Improved inhibitors of glucosylceramide synthase. J Biochem. 1992;111(2):191–196. doi: 10.1093/oxfordjournals.jbchem.a123736. [DOI] [PubMed] [Google Scholar]
- 84.Tagaram HR, Divittore NA, Barth BM, Kaiser JM, Avella D, Kimchi ET, Jiang Y, Isom HC, Kester M, Staveley-O’Carroll KF. Nanoliposomal ceramide prevents in vivo growth of hepatocellular carcinoma. Gut. 2011;60(5):695–701. doi: 10.1136/gut.2010.216671. [DOI] [PubMed] [Google Scholar]