Abstract
Many epithelial and endothelial cells express a cholinergic autocrine loop in which acetylcholine acts as a growth factor to stimulate cell growth. Cancers derived from these tissues similarly express a cholinergic autocrine loop and ACh secreted by the cancer or neighboring cells interacts with M3 muscarinic receptors expressed on the cancer cells to stimulate tumor growth. Primary proliferative pathways involve MAPK and Akt activation. The ability of muscarinic agonists to stimulate, and M3 antagonists to inhibit tumor growth has clearly been demonstrated for lung and colon cancer. The ability of muscarinic agonists to stimulate growth has been shown for melanoma, pancreatic, breast, ovarian, prostate and brain cancers, suggesting that M3 antagonists will also inhibit growth of these tumors as well. As yet no clinical trials have proven the efficacy of M3 antagonists as cancer therapeutics, though the widespread clinical use and low toxicity of M3 antagonists support the potential role of these drugs as adjuvants to current cancer therapies.
Keywords: Muscarinic receptors, Acetylcholine, M3 muscarinic receptor, Lung cancer, Colon cancer, Therapy
1 Introduction
The majority of cancers derived from epithelial and endothelial cells express muscarinic acetylcholine receptors (mAChR) and activation of the Gq-linked muscarinic receptors (M1, M3 and M5) leads to increased cell proliferation. In addition, many of those cancers also secrete acetylcholine (ACh) which stimulates cell growth; thus for many cancers, ACh acts as an autocrine growth factor. For cancers that do not synthesize ACh, muscarinic receptor activation can also come from neuronal, endocrine or paracrine sources of ACh or from constitutive activity of muscarinic receptors. To a large extent, expression of muscarinic receptors by cancers follows expression of the receptors by the normal tissue, though patterns of both muscarinic receptors and ACh synthesis can change between normal tissues and tumors. The ability of muscarinic activation to stimulate cancer growth clearly suggests that muscarinic antagonists will have the potential to inhibit lung cancer growth.
2 The Non-neuronal Cholinergic Autocrine Loop
The expression of muscarinic receptors in cancer derives from the continued expression of the non-neuronal cholinergic autocrine and paracrine signaling loop that exists in most endothelial and epithelial tissues. The best characterized non-neuronal cholinergic autocrine loop is in lung, and elements of that loop and how they pertain to cancer are discussed below.
2.1 The Cholinergic Autocrine Loop Expressed in Normal Lung
Bronchial epithelial cells (BEC) synthesize and secrete ACh which interacts with mAChR and nicotinic ACh receptors (nAChR) expressed by the BEC (Klapproth et al. 1997; Proskocil et al. 2004; Reinheimer et al. 1998). ACh secretion and signaling by BEC are similar in some ways to cholinergic signaling by neurons and different in other ways (Fig. 1). In BEC, as in neurons, ACh is synthesized from choline and acetyl-CoA by the enzyme choline acetyltransferase (ChAT). ACh secreted by BEC interacts with the same receptors (nAChR and mAChR) as ACh secreted by neurons; and ACh secreted by BEC is inactivated by acetylcholinesterase and butyryl cholinesterase just like neuronal ACh. The key differences between neuronal cholinergic signaling and BEC cholinergic signaling is the transport of choline into the cell, the secretory process and signal transduction mechanisms. Understanding these differences is important as it has implications for how muscarinic receptors stimulate cancer growth and how that stimulation can be potentially targeted. Because the key focus of this chapter is on muscarinic signaling in cancer, detail is provided on how these mechanisms affect cancer growth. A more general discussion of non-neuronal cholinergic signaling is in Wessler and Kirkpatrick (2011).
Fig. 1.
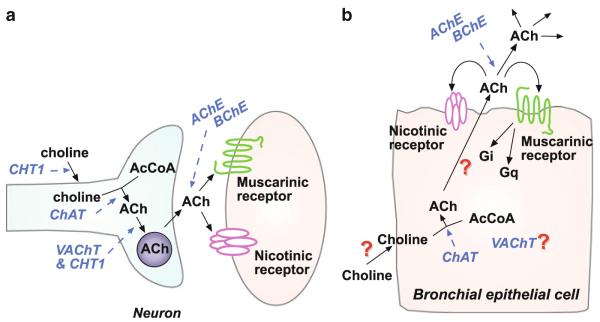
Cholinergic signaling in neurons and bronchial epithelial cells. (a) In neurons, choline for ACh synthesis is transported by the choline high-affinity transporter (CHT1). ACh is then synthesized by the action of choline acetyltransferase (ChAT), and packaged into synaptic vesicles by the action of the vesicular acetylcholine transporter (VAChT) and CHT1. ACh is then secreted by the complex processes that control synaptic release. Released ACh then interacts with postsynaptic nAChR and mAChR as well as presynaptic receptors. Signaling is terminated by acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). Key signal transduction events lead to the generation of action potentials, opening of membrane and internal ion channels, muscle contraction and kinase activation. (b) In bronchial epithelial cells (BEC), though CHT1 is present, CHT1 does not appear necessary for choline transport for ACh synthesis. In BEC, as for neurons, ChAT is utilized for ACh synthesis, though since there are multiple isoforms of ChAT, different splicing products may be utilized in different cell types. Since CHT1 is not required, and BEC do not have synaptic vesicles, the role of VAChT and CHT1 in ACh secretion is unknown, though both are expressed in BEC (Proskocil et al. 2004). ACh released by BEC is inactivated by the same cholinesterases as expressed in neurons. A key difference is that released ACh is not limited just to synaptic communication, but can also signal multiple neighboring cells as a paracrine factor or more distal cells as a hormone
2.2 The Cholinergic Autocrine Loop Expressed in Lung Cancer
The cholinergic autocrine loop expressed in normal BEC is similarly expressed in lung cancers that derive from airway epithelial cells (Song et al. 2003) (Fig. 2). The overwhelming majority of lung cancers derive from airway epithelial cells. Lung cancers are classified as small cell lung carcinoma (SCLC), which accounts for approximately 15–20% of the cases and non-small cell lung carcinoma (NSCLC), which accounts for the remaining 80–85% (Gabrielson 2006). SCLC derives from cells related to pulmonary neuroendocrine cells (Kumar et al. 2009). The two most common forms of NSCLC are squamous cell lung carcinoma (SCC) and lung adenocarcinoma, which together represent at least 80% of all NSCLC (Gabrielson 2006). Based on histology, gene expression and location, SCC is considered to arise from BEC of large airways and adenocarcinoma from epithelial cells of smaller airways (Kumar et al. 2009). These cell types of origin of SCLC, lung adenocarcinoma and SCC all express muscarinic receptors and synthesize ACh, thus not surprisingly the majority of these cancers also synthesize ACh and express muscarinic receptors.
Fig. 2.
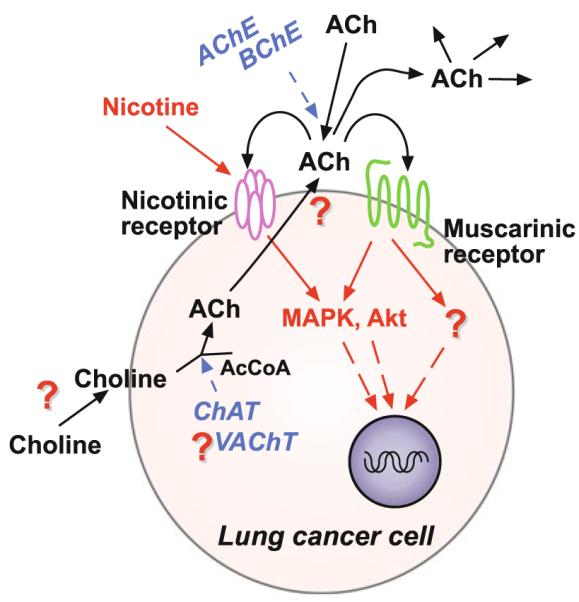
Cholinergic signaling by lung cancer cells. Cholinergic signaling by lung cancer cells is similar to normal bronchial epithelial cells. Steps for ACh synthesis and signal transduction in lung cancer provide the potential steps to target for development of therapies. In particular, inhibition of choline transport and muscarinic receptor antagonists offer unique advantages as discussed in Sect. 5. Targeting proliferative kinase pathways such as MAPK and Akt is an area of major development for cancer therapy in general since so many growth factors activate those pathways
3 M3 Muscarinic Receptors and Lung Cancer Growth
As described above, muscarinic receptors are expressed by lung cancers as part of a cholinergic autocrine loop expressed in both normal and neoplastic lung. Proliferation is stimulated by several mechanisms. First, activation of M3 receptors leads to increased intracellular calcium which in turn leads to activation of Akt and MAPK (Figs. 3 and 4) (Song et al. 2003, 2007) . As shown in Fig. 3, ACh rapidly increases intracellular calcium in lung cancer cell lines and the increase is blocked by M3 antagonists and by knockdown of M3 RNA by siRNAs (Song et al. 2003, 2007). As shown in Fig. 4, M3 receptor activation leads in turn to Akt and MAPK activation, which is also blocked by M3 antagonists. This activation then leads to cell proliferation as shown in Fig. 5, and as for activation of signaling, cell growth can be inhibited by M3 antagonists (Song et al. 2003, 2007).
Fig. 3.
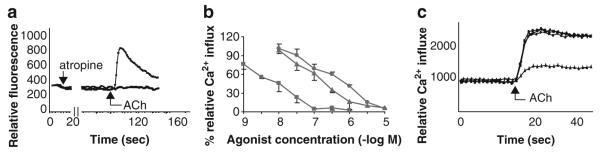
Calcium responses to muscarinic agonists and antagonists in H82 cells. (a) A representative trace of the [Ca2+]I response of H82 cells to ACh in the absence (−) or presence (+) of atropine. (b) Rank order potency of selective muscarinic antagonists to inhibit the [Ca2+]I increase elicited by ACh in H82 cells. Antagonists tested were 4-DAMP (filled square, a selective M3 antagonist), pirenzepine (filled triangle, a selective M1 antagonist) and AFDX 116 (filled circle, a selective M2/M4 antagonist). The rank order potency of these antagonists is most consistent with mediation by the M3 mAChR. (c) siRNA knockdown of M3 mAChR blocked the ACh induced increase in [Ca2+]I but control, M1 and M5 mAChR knockdowns had no effect. Filled circle = control siRNA, filled square = M1 siRNA, filled triangle = M3 siRNA, filled diamond = M5 siRNA. Data are presented as mean ± SE of at least 12 replicates from 3 separate experiments. Modified after Song et al. (2007)
Fig. 4.
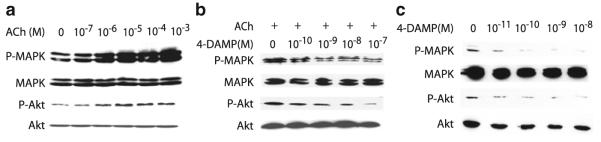
Effect of ACh on phosphorylation of MAPK and Akt in H82 SCLC cells. (a) Western blot showing increased MAPK and Akt phosphorylation induced by concentrations of ACh shown. (b) Western blot showing that phosphorylation of Akt and MAPK induced by 3 × 10−5 M ACh was decreased by the M3 antagonist 4-DAMP in a concentration-dependent fashion. (c) Western blot showing that 4-DAMP alone decreased basal phosphorylation of Akt and MAPK. Modified after Song et al. (2007)
Fig. 5.
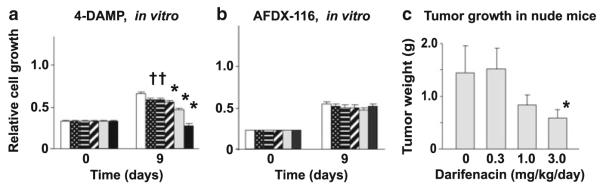
Regulation of H82 cell proliferation by mAChR subtype antagonists. The MTS assay was used to detect H82 cell growth after treatment with 4-DAMP and AFDX-116. (a) The M3 mAChR antagonist 4-DAMP inhibited H82 cell proliferation in a concentration-dependent manner. (b) The M2/M4 selective mAChR antagonist, AFDX 116 had no significant effect on cell growth. All data are expressed as the mean ± SE of 24 replicates of two separate experiments. White column, control; dotted-pattern column, 10−9 M; horizontal-pattern column, 10−8 M; diagonal-pattern column, 10−7 M; gray column, 10−6 M; black column, 10−5 M. *p < 0.001 and †p < 0.05 compared to control at 9 days by Tukey–Kramer multiple comparison test after 2-way ANOVA. (c) Effect of darifenacin on growth of H82 tumor xenografts in nude mice. (c) Tumor weight. *p < 0.05 compared to control by t test. Modified after Song et al. (2007)
As can be seen in Figs. 4 and 5, the addition of M3 antagonists inhibits kinase activation and cell proliferation, in the absence of added ligand. This implies either a role for ACh secreted by the cells into the cell culture medium or constitutive activity of the M3 receptor. Constitutive (unliganded) activity is well established for G-protein coupled receptors in general (Kenakin 2004) and has been specifically demonstrated for the M3 muscarinic receptor (Casarosa et al. 2010). Most likely, both of these mechanisms are involved in muscarinic stimulation of lung cancer growth. M3 antagonists inhibit growth of cell lines in vitro which express very little ChAT (Song et al. 2007) which implies a role for constitutive activity. However, the ability of M3 antagonists to inhibit cell proliferation and generation of IP3 metabolites is decreased (though not eliminated) by the addition of cholinesterase to cell culture medium which implies a role for autocrine cholinergic activation as well (Spindel, unpublished observation). Therefore, in patients, as discussed further below, the growth of lung cancers expressing muscarinic M3 receptors can be stimulated by ACh secreted from the tumor by paracrine sources of ACh from neighboring airway epithelium and by ACh from distal sources present in blood (Fujii et al. 1995).
4 Muscarinic Receptors and Specific Cancers
The ability of ACh to stimulate the growth of normal and neoplastic lung suggests that growth of any cancers that express M3 receptors can potentially be inhibited by muscarinic antagonists and that cancers that express both ACh and M3 receptors should be especially sensitive. As outlined in Table 1, this includes most lung cancers, pancreatic cancer and cervical cancer as analyzed by our laboratory, as well as other cancers as discussed below. Broadening the range of potentially sensitive cancers even further, we have observed that lung cancers that express M1 or M5 receptors can also be inhibited, suggesting that these Gq-linked subtypes may also confer sensitivity to lung cancers (Song et al. 2009).
Table 1.
Frequency of ChAT and M3 coexpression in selected cancers
| Cancer type | N | % M3 | % ChAT | % M3 and chat coexpression |
|---|---|---|---|---|
| Lung (SCLC) | 24 | 70 | 92 | 70 |
| Lung (BAC) | 20 | 85 | 80 | 70 |
| Lung (SCC) | 31 | 71 | 58 | 45 |
| Pancreatic | 32 | 78.1 | 65.6 | 50 |
| Cervical | 14 | 50 | 71 | 43 |
Frequency of M3 mAChR, ChAT and their coexpression in archival samples of SCLC, bronchoalveolar lung carcinoma (BAC), squamous cell lung carcinoma (SCC), pancreatic carcinoma and cervical carcinoma as determined by immunostaining. Sample size of each series as shown (N). Modified after Song et al. (2007)
4.1 Lung Cancer
The initial report of muscarinic receptor expression in normal lung was in 1984 by Whitsett and Hollinger (1984) based on QNB binding. Subsequently, Mak et al. (1992) demonstrated that in airway epithelium, expression of the M3 receptor predominated. Studies by Wessler and co-workers (Klapproth et al. 1997; Reinheimer et al. 1996; Wessler and Kirkpatrick 2001) and Proskocil et al. (2004) then established that airway epithelium also synthesized ACh. Expression of muscarinic receptors in lung cancers was initially shown by Cunningham et al. (1985) and Morin et al. (1987) though effects on proliferation were not clearly determined. Subsequently, studies by Spindel and co-workers (Song et al. 2003, 2007, 2008) demonstrated that the majority of SCLC and NSCLC expressed M3 receptors as shown in Table 1. As discussed above, Song et al. also showed that both SCLC and NSCLC synthesized and secreted ACh (Song et al. 2003, 2007, 2008) which acted as an autocrine growth factor for lung cancers. An example of expression of ChAT and M3 receptors in SCLC tumor and a cell line is shown in Fig. 6.
Fig. 6.
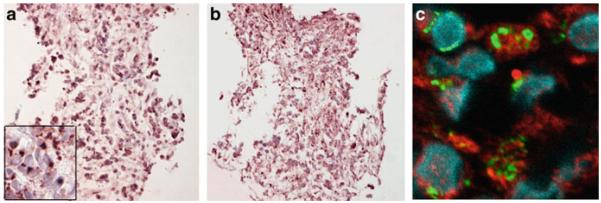
Immunohistochemistry of ChAT and M3 mAChR expression in an SCLC biopsy. (a) ChAT immunostaining (400×, chromogen = VIP), insert box = 1,000×. (b) M3R immunostaining (400×, chromogen = VIP). (c) Confocal image showing coexpression of M3 mAChR (red) and ChAT (green) in tumor cells in same sample as (a) and (b). Modified after Song et al. (2007)
Song et al. (2008) also demonstrated an apparent activation of cholinergic signaling in lung cancer with increased levels of ChAT and ACh, and decreased levels of cholinesterase in lung cancers compared to normal lung. Martinez-Moreno has similarly reported that cholinesterase levels are reduced in lung cancer, thus increasing the available ACh to stimulate tumor growth (de Martinez-Lopez et al. 2008; Martinez-Moreno et al. 2006).
The potential of M3 muscarinic receptor antagonists to inhibit lung cancer growth was demonstrated by Song et al. (2003, 2007, 2008, 2010) who showed that multiple M3 antagonists, including 4-diphenyl-acetoxy-N-methyl-piperidine (4-DAMP), para-fluoro-hexahydrosila-difenidol (P-F-HHSiD), darifenacin and tiotropium all could inhibit lung cancer cell proliferation in vitro and the effectiveness of darifenacin and tiotropium to inhibit lung cancer cell growth in vivo in nude mice was also demonstrated. While most reports suggest that M3 receptors are most important for lung cancer proliferation, Matthiesen et al. (2006) has suggested for lung fibroblasts that M2 receptors may be more important, though Pieper et al. (2007) also support a key role for M3 receptors in mediating lung fibroblast proliferation. It is important to note that fibroblasts are not, however, the primary cell of origin for most lung cancers. Interestingly, in a preliminary observation, Song et al. (2004) has suggested that M2 receptors might play an inhibitory role in the growth of lung cancers. This observation which needs further study would suggest that greater selectivity of M3 over M2 for muscarinic antagonists used for lung cancer therapy would be desirable.
4.2 Skin Cancer
Skin cancer is by far the most common form of cancer. Basal and squamous cell skin carcinomas are most frequent and arise from keratinocytes though rarely cause significant morbidity or mortality. Melanomas arise from melanocytes and while they represent only about 3% of skin cancer, they cause by far the majority of skin cancer morbidity and mortality.
4.2.1 Squamous and Basal Cell Skin Carcinoma
Squamous and basal cell skin carcinomas arise from keratinocytes. As discussed in “Muscarinic Receptor Agonists and Antagonists: Effects on Keratinocyte Function” by Grando (2011), non-neuronal cholinergic signaling by keratinocytes has been extensively described by Grando and co-workers and the ability of acetylcholine and muscarinic receptors to stimulate keratinocyte proliferation and muscarinic antagonists to inhibit proliferation is well characterized (Arredondo et al. 2003; Chernyavsky et al. 2004; Grando et al. 1993, 2006). As squamous and basal cell carcinomas are treated by local curative surgery, the role of muscarinic antagonists to inhibit their growth is not likely to be clinically significant.
4.2.2 Melanoma and Merkel Cell Carcinoma
Expression of M2–M5 muscarinic receptors in normal melanocytes was reported by Buchli et al. (2001). Subsequently multiple reports have established that melanomas primarily express M3 muscarinic receptors (Boss et al. 2005; Lammerding-Koppel et al. 1997; Noda et al. 1998; Oppitz et al. 2008); and, critically, that M3 muscarinic receptors expression appears elevated in leading edges of tumors and in metastases (Lammerding-Koppel et al. 1997; Oppitz et al. 2008). Consistent with this, Boss et al. (2005) have shown that M3 receptors play a role in chemotaxis of melanoma cells. This would suggest a potential for M3 antagonists to inhibit melanoma growth or metastasis, but this remains to be determined.
Merkel cell carcinomas derive from skin neuroendocrine cells and, though relatively rare, can have an aggressive clinical course. By immunohistochemistry, Bowers et al. (2008) reported that 15 of 15 primary cutaneous cases of Merkel cell carcinoma expressed M3 and M5 receptors. Given that the proliferation of other types of neuroendocrine cells such as pulmonary neuroendocrine cells that express muscarinic receptors can be inhibited by M3 antagonists, this would suggest that muscarinic antagonists might also inhibit growth of Merkel Cell Carcinomas, but this again needs to be determined.
4.3 Colon Cancer
Initial reports of muscarinic receptor expression in colon adenocarcinoma were by Frucht et al. (1992) based on the presence N-methylscopolamine and carbamylcholine binding to most colon cancer cell lines. Follow-up studies by Frucht and co-workers (Frucht et al. 1999; Yang and Frucht 2000) demonstrated that the receptors were primarily M3, were increased approximately eightfold in tumor versus normal, and that carbamylcholine stimulated proliferation of colon carcinoma cell lines expressing M3 receptors. Raufman et al. (2003) and Ukegawa et al. (2003) confirmed those findings, again showing the importance of M3 receptors and also demonstrated that the proliferative action of M3 receptors depended in part on the transactivation of EGF receptors. The actual role of M3 receptors in colon cancer development was further confirmed by Raufman et al. (2008) who showed that M3 receptor knockout mice were resistant to the development of colon tumors in the azoxymethane-induced colon neoplasia model. This suggests that M3 receptor antagonists may be useful for colon cancer treatment or chemoprevention.
Showing that the cholinergic autocrine loop also played an important role in colon cancer, Cheng et al. (2008) showed that most colon cancers, such as lung cancers, synthesize ACh and that ChAT expression is higher in colon adenocarcinoma than in normal colon enterocytes. The role of ACh as an autocrine growth factor for colon cancer was further confirmed by showing that the choline transport inhibitor hemicholinium-3 could inhibit growth of colon adenocarcinoma cell lines and that the addition of cholinesterase inhibitors to the cell culture medium could stimulate colon cancer cell growth (Cheng et al. 2008). This observation may be clinically important since the levels of cholinesterase appear decreased in colon cancer (Montenegro et al. 2005). Thus in colon cancer, as in lung cancer, there is upregulation of the cholinergic autocrine loop with increased levels of M3 receptors, increased ChAT expression and decreased cholinesterase expression.
Raufmann et al. have also demonstrated that some bile acids can bind to the M3 receptor; thus bile acids may represent another endogenous ligand to stimulate colon cancer growth through muscarinic receptors (Raufman et al. 2002, 2003).
4.4 Gastric Cancer
M1, M3 and M5 receptors are expressed in normal gastric epithelium consistent with their role in regulation of acid and enzyme secretion (Aihara et al. 2005; Leonard et al. 1991; Xie et al. 2005). Consistent with this, gastric carcinomas also express muscarinic receptors. In early studies, two out of four gastric carcinoma cell lines established by Park et al. expressed muscarinic receptors (Park et al. 1990) though muscarinic subtype was not determined. Subsequently, Kodaira et al. (1999) demonstrated that five out of eight gastric carcinoma cell lines examined expressed M3 receptors and that carbachol treatment stimulated MAP kinase in those cell lines but did not stimulate cell proliferation. This would argue against a proliferative role for muscarinic receptors in stomach cancer.
4.5 Pancreatic Cancer
While a key role for muscarinic receptors in regulating pancreatic endocrine and exocrine function of the pancreas is well established (Gautam et al. 2005, 2006; Williams 2006), muscarinic effects on pancreatic carcinoma are less well defined. In normal pancreas, M3 receptors play a role in regulating insulin and glucagon secretion (Gautam et al. 2006; Gromada and Hughes 2006), while M1 and M3 receptors are involved in acinar secretion (Gautam et al. 2005). In an examination of pancreatic carcinoma cell lines, two out of five lines expressed muscarinic receptors, though the subtype was not identified (Ackerman et al. 1989). Similarly, nafenopin-induced pancreatic carcinomas in rats expressed muscarinic receptors that were linked to calcium mobilization, though the subtype and muscarinic effects on cell proliferation were not determined (Chien and Warren 1985, 1986). Notably, approximately 50% of pancreatic adenocarcinomas examined by Sekhon et al. (2002) expressed ChAT; so depending on the degree of muscarinic receptor expression by pancreatic carcinomas, there is potential for autocrine stimulation. Effects of muscarinic antagonists on pancreatic carcinoma growth have not been characterized.
4.6 Breast Cancer
The degree of expression of muscarinic receptors in breast cancer has not been well characterized. It has, however, been clearly demonstrated that muscarinic activation stimulates growth of MCF-7 human breast carcinoma cells (Jimenez and Montiel 2005; Schmitt et al. 2010). As shown by siRNA studies, stimulation of proliferation is mediated by M3 receptors leading to Erk 1/2 activation with partial dependence on Src and Cam Kinase pathways. Negroni et al. (2010) have also demonstrated the presence of autoantibodies in blood of breast cancer patients that directly stimulates MCF-7 cell proliferation in an M3-dependent manner. Similar M3-dependent stimulation of proliferation has also been observed for mouse breast cancer cell lines (Espanol et al. 2007; Fiszman et al. 2007). Interestingly, Cabello et al. (2001) have demonstrated that organophosphorus pesticides lead to rat mammary tumors perhaps by inhibiting cholinesterase through a muscarinic mechanism since the effect could be blocked by atropine. The potential for cholinergic stimulation to lead to development of breast cancer is further supported by the recent paper linking α9 nAChR to breast cancer (Lee et al. 2010).
4.7 Ovarian Cancer
Initial studies by Batra et al. (1993) showed the presence of muscarinic receptors in ovarian adenocarcinoma with binding profile most consistent with M3 receptors. Studies by Oppitz et al. (2002) reported that 23 of 39 ovarian cancers studied expressed muscarinic receptors. Studies by Mayerhofer and co-workers (Fritz et al.2001; Mayerhofer and Kunz 2005) have clearly demonstrated a clear cholinergic autocrine loop expressed by normal ovary. As for colon and lung, cholinergic agonists stimulate the growth of ovarian cells, which would suggest that muscarinic antagonists might have a beneficial effect in ovarian cancer. Consistent with this, expression of muscarinic receptors by ovarian cancer is associated with decreased patient survival (Oppitz et al. 2002).
4.8 Prostate Cancer
Relatively less is known about the role of muscarinic receptors in prostate cancer and the potential of anti-muscarinics to inhibit cancer growth (Witte et al. 2008). In normal prostate gland epithelium, M1 receptors predominate (Ruggieri et al. 1995) and sparse M2 receptors are found in the stroma (Obara et al. 2000). M1 receptors similarly predominate in benign prostate adenomas and benign prostatic hyperplasia (BPH) (Ruggieri et al. 1995). Luthin et al. (1997) showed that carbachol stimulated proliferation in three out of three prostate carcinoma cell lines (PC3, LnCaP, DU145) tested and based on antagonists, the primary mechanism appeared to be M1 activation of the Erk cascade, though M3 effects could not be excluded. Rayford et al. (1997) similarly showed that carbachol stimulated proliferation of LnCaP cells and that the effect appeared mediated by M3 receptors. Neither the studies by Luthin et al. nor Rayford et al. used siRNA techniques, so determinations of specificity of receptor mediation was based only on relative antagonist potencies. Rayford et al. (1997) also reported that carbachol stimulated the proliferation of primary cultures of normal prostate, BPH and prostate carcinoma. Notably they also reported that the ability of carbachol to stimulated proliferation was significantly increased in prostate carcinoma compared to normal prostate or BPH (Rayford et al. 1997). This suggests that muscarinic antagonists with M3 or M3 combined with M1 selectivity might be helpful for prostate carcinoma therapy. In addition, whether there will be differences between responses of androgen-dependent and -independent prostate carcinomas remains to be determined.
4.9 Brain Cancer (Astrocytoma and Neuroblastoma)
One of the earliest reports on the ability of acetylcholine to stimulate cell proliferation through Gq-linked muscarinic receptors was by Ashkenazi et al. (1989) who reported that carbachol could stimulate proliferation of primary cultures of astrocytes, and astrocyte and neuroblastoma cell lines. This was confirmed by Guizzetti et al. (1996) who also demonstrated that astrocytes expressed M2, M3 and M5 receptors. Wessler et al. (1997) then demonstrated that astrocytes also synthesized acetylcholine, thus establishing that normal astrocytes, such as lung epithelial cells express the cholinergic autocrine loop. As for lung, carbachol activation of M3 receptors leads to rapid activation of MAPK and Akt (Guizzetti and Costa 2001; Tang et al. 2002; Yagle et al. 2001). These data suggest that muscarinic antagonists may have the potential to inhibit growth of both astrocytomas and neuroblastomas.
5 Targeting Muscarinic Signaling for Lung Cancer Therapy
Muscarinic signaling in lung cancer is summarized in Fig. 2 which shows potential levels to target proliferation. Muscarinic activation of lung cancer growth can potentially be targeted at the following levels: (1) by blocking choline transport into the cancer cell; (2) by blocking ChAT activity in the cancer cell; (3) by blocking ACh secretion from the cancer cell; (4) by muscarinic antagonists; and (5) by blocking muscarinic receptor activated proliferative pathways. Some of these approaches are clearly more promising than others.
Clearly of great promise is the use of M3 muscarinic antagonists to block cancer growth. This has been demonstrated in multiple in vitro studies and in limited mouse studies as discussed above. If additional preclinical studies appear promising, then clinical trials should be considered. Given that multiple muscarinic antagonists are already in routine clinical use for overactive bladder and COPD with minimal side effects, the barriers for clinical studies should be relatively low and even small effects of muscarinic antagonists on survival or quality of life would suggest a place for these drugs as adjuvants to existing therapeutic regimes. In addition, because of the common use of these drugs, there may be epidemiologic data that could be mined to determine if there is indeed therapeutic potential for their use as cancer therapeutics.
Blocking choline transport into cancer cells so as to prevent ACh synthesis is potentially promising since the choline transporters used for ACh synthesis by lung cancer cells are different than the transporters used for neuronal ACh synthesis. Neurons use the choline high-affinity transporter (CHT1) to mediate ACh synthesis (Ferguson et al. 2004; Okuda et al. 2000) while cancer cells do not need CHT1 and may use the choline transporter-like proteins (CTL1-5) (Machova et al. 2009; Song and Spindel 2007; Wang et al. 2007). In addition, choline is needed for membrane phospholipids, so this approach would potentially block cancer growth both by limiting ACh synthesis and phospholipid synthesis (Glunde et al. 2006).
Blocking ChAT activity in cancer cells is not likely to be a viable approach as the same enzyme is also used in neurons (Song et al. 2003), thus resulting in impaired neurotransmission, respiration and muscle contraction. Similarly, stimulating cholinesterase activity in the tumors would likely be highly toxic as that would also affect neuronal and muscular neurotransmission. At present very little is known about the regulation of ACh secretion by cancers to determine if that could be successfully targeted. Discussion of strategies to block kinase pathways activated by muscarinic receptors is clearly promising, but is not unique just for muscarinic activation, since multiple factors activate the same pathways in many cancers. Inhibition of these pathways is a major area of cancer therapy development in general (Bennasroune et al. 2004; Engelman 2009; Friday and Adjei 2008; Natoli et al. 2010).
Thus the near term prospects for targeting muscarinic activation of cancer growth rests with muscarinic antagonists and downstream kinase inhibitors. Future approaches will likely include blocking ACh synthesis in cancers by targeting choline transport.
In summary, there are considerable data suggesting that muscarinic receptors may be therapeutically useful as an adjunct to existing cancer therapies. The case is most compelling for M3 antagonists for lung and colon cancer; and additional studies are clearly warranted for melanoma, pancreatic, breast, ovarian, prostate and brain cancers. As for many potential uses of muscarinic antagonists, more selective antagonists with greater ratios of M1, M3 and M5 selectivity relative to M2 and M4 would be desirable, though siRNA-based approaches may solve this problem.
Acknowledgments
This study was supported by NIH grants RR00163 HL087710 and a research grant from Boehringer Ingelheim.
References
- Ackerman MS, Roeske WR, Heck RJ, Korc M. Identification and characterization of muscarinic receptors in cultured human pancreatic carcinoma cells. Pancreas. 1989;4:363–370. doi: 10.1097/00006676-198906000-00014. [DOI] [PubMed] [Google Scholar]
- Aihara T, Nakamura Y, Taketo MM, Matsui M, Okabe S. Cholinergically stimulated gastric acid secretion is mediated by M(3) and M(5) but not M(1) muscarinic acetylcholine receptors in mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1199–G1207. doi: 10.1152/ajpgi.00514.2004. [DOI] [PubMed] [Google Scholar]
- Arredondo J, Hall LL, Ndoye A, Chernyavsky AI, Jolkovsky DL, Grando SA. Muscarinic acetylcholine receptors regulating cell cycle progression are expressed in human gingival keratinocytes. J Periodontal Res. 2003;38:79–89. doi: 10.1034/j.1600-0765.2003.01006.x. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Ramachandran J, Capon DJ. Acetylcholine analogue stimulates DNA synthesis in brain-derived cells via specific muscarinic receptor subtypes. Nature. 1989;340:146–150. doi: 10.1038/340146a0. [DOI] [PubMed] [Google Scholar]
- Batra S, Popper LD, Iosif CS. Characterisation of muscarinic cholinergic receptors in human ovaries, ovarian tumours and tumour cell lines. Eur J Cancer. 1993;29A:1302–1306. doi: 10.1016/0959-8049(93)90078-t. [DOI] [PubMed] [Google Scholar]
- Bennasroune A, Gardin A, Aunis D, Cremel G, Hubert P. Tyrosine kinase receptors as attractive targets of cancer therapy. Crit Rev Oncol Hematol. 2004;50:23–38. doi: 10.1016/j.critrevonc.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Boss A, Oppitz M, Drews U. Muscarinic cholinergic receptors in the human melanoma cell line SK-Mel 28: modulation of chemotaxis. Clin Exp Dermatol. 2005;30:557–564. doi: 10.1111/j.1365-2230.2005.01865.x. [DOI] [PubMed] [Google Scholar]
- Bowers JW, Schlauder SM, Calder KB, Morgan MB. Acetylcholine receptor expression in Merkel cell carcinoma. Am J Dermatopathol. 2008;30:340–343. doi: 10.1097/DAD.0b013e31816797e4. [DOI] [PubMed] [Google Scholar]
- Buchli R, Ndoye A, Arredondo J, Webber RJ, Grando SA. Identification and characterization of muscarinic acetylcholine receptor subtypes expressed in human skin melanocytes. Mol Cell Biochem. 2001;228:57–72. doi: 10.1023/a:1013368509855. [DOI] [PubMed] [Google Scholar]
- Cabello G, Valenzuela M, Vilaxa A, Duran V, Rudolph I, Hrepic N, Calaf G. A rat mammary tumor model induced by the organophosphorous pesticides parathion and malathion, possibly through acetylcholinesterase inhibition. Environ Health Perspect. 2001;109:471–479. doi: 10.1289/ehp.01109471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarosa P, Kiechle T, Sieger P, Pieper MP, Gantner F. The constitutive activity of the human muscarinic M3 receptor unmasks differences in the pharmacology of anticholinergics. J Pharmacol Exp Ther. 2010;333:201–209. doi: 10.1124/jpet.109.163188. [DOI] [PubMed] [Google Scholar]
- Cheng K, Samimi R, Xie G, Shant J, Drachenberg C, Wade M, Davis RJ, Nomikos G, Raufman JP. Acetylcholine release by human colon cancer cells mediates autocrine stimulation of cell proliferation. Am J Physiol Gastrointest Liver Physiol. 2008;295(3):G591–G597. doi: 10.1152/ajpgi.00055.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyavsky AI, Arredondo J, Wess J, Karlsson E, Grando SA. Novel signaling pathways mediating reciprocal control of keratinocyte migration and wound epithelialization through M3 and M4 muscarinic receptors. J Cell Biol. 2004;166:261–272. doi: 10.1083/jcb.200401034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien JL, Warren JR. Differentiation of muscarinic cholinergic receptors in acinar carcinoma of rat pancreas. Cancer Res. 1985;45:4858–4863. [PubMed] [Google Scholar]
- Chien JL, Warren JR. Muscarinic receptor coupling to intracellular calcium release in rat pancreatic acinar carcinoma. Cancer Res. 1986;46:5706–5714. [PubMed] [Google Scholar]
- Cunningham JM, Lennon VA, Lambert EH, Scheithauer B. Acetylcholine receptors in small cell carcinomas. J Neurochem. 1985;45:159–167. doi: 10.1111/j.1471-4159.1985.tb05488.x. [DOI] [PubMed] [Google Scholar]
- de Martinez-Lopez CA, Nieto-Ceron S, Pons-Castillo A, Galbis-Martinez L, Latour-Perez J, Torres-Lanzas J, Tovar-Zapata I, Martinez-Hernandez P, Rodriguez-Lopez JN, Cabezas-Herrera J. Cancer-associated differences in the acetylcholinesterase activity in bronchial aspirates of lung cancer patients. Clin Sci (Lond) 2008;115(8):245–253. doi: 10.1042/CS20070393. [DOI] [PubMed] [Google Scholar]
- Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- Espanol AJ, de la TE, Fiszman GL, Sales ME. Role of non-neuronal cholinergic system in breast cancer progression. Life Sci. 2007;80:2281–2285. doi: 10.1016/j.lfs.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Bazalakova M, Savchenko V, Tapia JC, Wright J, Blakely RD. Lethal impairment of cholinergic neurotransmission in hemicholinium-3-sensitive choline transporter knockout mice. Proc Natl Acad Sci U S A. 2004;101:8762–8767. doi: 10.1073/pnas.0401667101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiszman GL, Middonno MC, De la TE, Farina M, Espanol AJ, Sales ME. Activation of muscarinic cholinergic receptors induces MCF-7 cells proliferation and angiogenesis by stimulating nitric oxide synthase activity. Cancer Biol Ther. 2007;6:1106–1113. doi: 10.4161/cbt.6.7.4330. [DOI] [PubMed] [Google Scholar]
- Friday BB, Adjei AA. Advances in targeting the Ras/Raf/MEK/Erk mitogen-activated protein kinase cascade with MEK inhibitors for cancer therapy. Clin Cancer Res. 2008;14:342–346. doi: 10.1158/1078-0432.CCR-07-4790. [DOI] [PubMed] [Google Scholar]
- Fritz S, Wessler I, Breitling R, Rossmanith W, Ojeda SR, Dissen GA, Amsterdam A, Mayerhofer A. Expression of muscarinic receptor types in the primate ovary and evidence for nonneuronal acetylcholine synthesis. J Clin Endocrinol Metab. 2001;86:349–354. doi: 10.1210/jcem.86.1.7146. [DOI] [PubMed] [Google Scholar]
- Frucht H, Gazdar AF, Park JA, Oie H, Jensen RT. Characterization of functional receptors for gastrointestinal hormones on human colon cancer cells. Cancer Res. 1992;52:1114–1122. [PubMed] [Google Scholar]
- Frucht H, Jensen RT, Dexter D, Yang WL, Xiao Y. Human colon cancer cell proliferation mediated by the M3 muscarinic cholinergic receptor. Clin Cancer Res. 1999;5:2532–2539. [PubMed] [Google Scholar]
- Fujii T, Yamada S, Yamaguchi N, Fujimoto K, Suzuki T, Kawashima K. Species differences in the concentration of acetylcholine, a neurotransmitter, in whole blood and plasma. Neurosci Lett. 1995;201:207–210. doi: 10.1016/0304-3940(95)12180-3. [DOI] [PubMed] [Google Scholar]
- Gabrielson E. Worldwide trends in lung cancer pathology. Respirology. 2006;11:533–538. doi: 10.1111/j.1440-1843.2006.00909.x. [DOI] [PubMed] [Google Scholar]
- Gautam D, Han SJ, Heard TS, Cui Y, Miller G, Bloodworth L, Wess J. Cholinergic stimulation of amylase secretion from pancreatic acinar cells studied with muscarinic acetylcholine receptor mutant mice. J Pharmacol Exp Ther. 2005;313:995–1002. doi: 10.1124/jpet.105.084855. [DOI] [PubMed] [Google Scholar]
- Gautam D, Han SJ, Hamdan FF, Jeon J, Li B, Li JH, Cui Y, Mears D, Lu H, Deng C, Heard T, Wess J. A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 2006;3:449–461. doi: 10.1016/j.cmet.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Glunde K, Ackerstaff E, Mori N, Jacobs MA, Bhujwalla ZM. Choline phospholipid metabolism in cancer: consequences for molecular pharmaceutical interventions. Mol Pharm. 2006;3:496–506. doi: 10.1021/mp060067e. [DOI] [PubMed] [Google Scholar]
- Grando SA. Muscarinic receptor agonists and antagonists: effects on keratinocyte function. In: Fryer AD, editor. Muscarinic receptors, Handbook of experimental pharmacology. Springer; Heidelberg: 2011. [DOI] [PubMed] [Google Scholar]
- Grando SA, Kist DA, Qi M, Dahl MV. Human keratinocytes synthesize, secrete, and degrade acetylcholine. J Invest Dermatol. 1993;101:32–36. doi: 10.1111/1523-1747.ep12358588. [DOI] [PubMed] [Google Scholar]
- Grando SA, Pittelkow MR, Schallreuter KU. Adrenergic and cholinergic control in the biology of epidermis: physiological and clinical significance. J Invest Dermatol. 2006;126:1948–1965. doi: 10.1038/sj.jid.5700151. [DOI] [PubMed] [Google Scholar]
- Gromada J, Hughes TE. Ringing the dinner bell for insulin: muscarinic M3 receptor activity in the control of pancreatic beta cell function. Cell Metab. 2006;3:390–392. doi: 10.1016/j.cmet.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Costa LG. Activation of phosphatidylinositol 3 kinase by muscarinic receptors in astrocytoma cells. Neuroreport. 2001;12:1639–1642. doi: 10.1097/00001756-200106130-00025. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Costa P, Peters J, Costa LG. Acetylcholine as a mitogen: muscarinic receptor-mediated proliferation of rat astrocytes and human astrocytoma cells. Eur J Pharmacol. 1996;297:265–273. doi: 10.1016/0014-2999(95)00746-6. [DOI] [PubMed] [Google Scholar]
- Jimenez E, Montiel M. Activation of MAP kinase by muscarinic cholinergic receptors induces cell proliferation and protein synthesis in human breast cancer cells. J Cell Physiol. 2005;204:678–686. doi: 10.1002/jcp.20326. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Efficacy as a vector: the relative prevalence and paucity of inverse agonism. Mol Pharmacol. 2004;65:2–11. doi: 10.1124/mol.65.1.2. [DOI] [PubMed] [Google Scholar]
- Klapproth H, Reinheimer T, Metzen J, Munch M, Bittinger F, Kirkpatrick CJ, Hohle KD, Schemann M, Racke K, Wessler I. Non-neuronal acetylcholine, a signalling molecule synthezised by surface cells of rat and man. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:515–523. doi: 10.1007/pl00004977. [DOI] [PubMed] [Google Scholar]
- Kodaira M, Kajimura M, Takeuchi K, Lin S, Hanai H, Kaneko E. Functional muscarinic m3 receptor expressed in gastric cancer cells stimulates tyrosine phosphorylation and MAP kinase. J Gastroenterol. 1999;34:163–171. doi: 10.1007/s005350050238. [DOI] [PubMed] [Google Scholar]
- Kumar V, Abbas AK, Fausto N, Aster JC. Robbins and Cotran pathologic basis of disease. 8th edn W.B. Saunders Company; Philadelphia, PA: 2009. [Google Scholar]
- Lammerding-Koppel M, Noda S, Blum A, Schaumburg-Lever G, Rassner G, Drews U. Immunohistochemical localization of muscarinic acetylcholine receptors in primary and meta-static malignant melanomas. J Cutan Pathol. 1997;24:137–144. doi: 10.1111/j.1600-0560.1997.tb01567.x. [DOI] [PubMed] [Google Scholar]
- Lee CH, Huang CS, Chen CS, Tu SH, Wang YJ, Chang YJ, Tam KW, Wei PL, Cheng TC, Chu JS, Chen LC, Wu CH, Ho YS. Overexpression and activation of the {alpha}9-nicotinic receptor during tumorigenesis in human breast epithelial cells. J Natl Cancer Inst. 2010;102:1322–1335. doi: 10.1093/jnci/djq300. [DOI] [PubMed] [Google Scholar]
- Leonard A, Cuq P, Magous R, Bali JP. M3-subtype muscarinic receptor that controls intracellular calcium release and inositol phosphate accumulation in gastric parietal cells. Biochem Pharmacol. 1991;42:839–845. doi: 10.1016/0006-2952(91)90044-6. [DOI] [PubMed] [Google Scholar]
- Luthin GR, Wang P, Zhou H, Dhanasekaran D, Ruggieri MR. Role of m1 receptor-G protein coupling in cell proliferation in the prostate. Life Sci. 1997;60:963–968. doi: 10.1016/s0024-3205(97)00035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machova E, O’Regan S, Newcombe J, Meunier FM, Prentice J, Dove R, Lisa V, Dolezal V. Detection of choline transporter-like 1 protein CTL1 in neuroblastoma x glioma cells and in the CNS, and its role in choline uptake. J Neurochem. 2009;110(4):1297–1309. doi: 10.1111/j.1471-4159.2009.06218.x. [DOI] [PubMed] [Google Scholar]
- Mak JC, Baraniuk JN, Barnes PJ. Localization of muscarinic receptor subtype mRNAs in human lung. Am J Respir Cell Mol Biol. 1992;7:344–348. doi: 10.1165/ajrcmb/7.3.344. [DOI] [PubMed] [Google Scholar]
- Martinez-Moreno P, Nieto-Ceron S, Torres-Lanzas J, Ruiz-Espejo F, Tovar-Zapata I, Martinez-Hernandez P, Rodriguez-Lopez JN, Vidal CJ, Cabezas-Herrera J. Cholinesterase activity of human lung tumours varies according to their histological classification. Carcinogenesis. 2006;27:429–436. doi: 10.1093/carcin/bgi250. [DOI] [PubMed] [Google Scholar]
- Matthiesen S, Bahulayan A, Kempkens S, Haag S, Fuhrmann M, Stichnote C, Juergens UR, Racke K. Muscarinic receptors mediate stimulation of human lung fibroblast proliferation. Am J Respir Cell Mol Biol. 2006;35:621–627. doi: 10.1165/rcmb.2005-0343RC. [DOI] [PubMed] [Google Scholar]
- Mayerhofer A, Kunz L. A non-neuronal cholinergic system of the ovarian follicle. Ann Anat. 2005;187:521–528. doi: 10.1016/j.aanat.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Montenegro MF, Nieto-Ceron S, Ruiz-Espejo F, Paez dlC, Rodriguez-Berrocal FJ, Vidal CJ. Cholinesterase activity and enzyme components in healthy and cancerous human colorectal sections. Chem Biol Interact. 2005;157:158–429. doi: 10.1016/j.cbi.2005.10.091. [DOI] [PubMed] [Google Scholar]
- Morin D, Zini R, Lange F, Lange J, Tillement JP. Alterations of beta-adrenergic, muscarinic cholinergic receptors and imipramine binding sites in human lung tumors. Int J Clin Pharmacol Ther Toxicol. 1987;25:605–608. [PubMed] [Google Scholar]
- Natoli C, Perrucci B, Perrotti F, Falchi L, Iacobelli S. Tyrosine kinase inhibitors. Curr Cancer Drug Targets. 2010;10:462–483. doi: 10.2174/156800910791517208. [DOI] [PubMed] [Google Scholar]
- Negroni MP, Fiszman GL, Azar ME, Morgado CC, Espanol AJ, Pelegrina LT, De la TE, Sales ME. Immunoglobulin G from breast cancer patients in stage I stimulates muscarinic acetylcholine receptors in MCF7 cells and induces proliferation. Participation of nitric oxide synthase-derived nitric oxide. J Clin Immunol. 2010;30:474–484. doi: 10.1007/s10875-010-9370-0. [DOI] [PubMed] [Google Scholar]
- Noda S, Lammerding-Koppel M, Oettling G, Drews U. Characterization of muscarinic receptors in the human melanoma cell line SK-Mel-28 via calcium mobilization. Cancer Lett. 1998;133:107–114. doi: 10.1016/s0304-3835(98)00215-8. [DOI] [PubMed] [Google Scholar]
- Obara K, Arai K, Miyajima N, Hatano A, Tomita Y, Takahashi K. Expression of m2 muscarinic acetylcholine receptor mRNA in primary culture of human prostate stromal cells. Urol Res. 2000;28:196–200. doi: 10.1007/s002400000113. [DOI] [PubMed] [Google Scholar]
- Okuda T, Haga T, Kanai Y, Endou H, Ishihara T, Katsura I. Identification and characterization of the high-affinity choline transporter. Nat Neurosci. 2000;3:120–125. doi: 10.1038/72059. [DOI] [PubMed] [Google Scholar]
- Oppitz M, Mobus V, Brock S, Drews U. Muscarinic receptors in cell lines from ovarian carcinoma: negative correlation with survival of patients. Gynecol Oncol. 2002;85:159–164. doi: 10.1006/gyno.2002.6597. [DOI] [PubMed] [Google Scholar]
- Oppitz M, Busch C, Garbe C, Drews U. Distribution of muscarinic receptor subtype M3 in melanomas and their metastases. J Cutan Pathol. 2008;35:809–815. doi: 10.1111/j.1600-0560.2007.00905.x. [DOI] [PubMed] [Google Scholar]
- Park JG, Frucht H, LaRocca RV, Bliss DP, Jr, Kurita Y, Chen TR, Henslee JG, Trepel JB, Jensen RT, Johnson BE. Characteristics of cell lines established from human gastric carcinoma. Cancer Res. 1990;50:2773–2780. [PubMed] [Google Scholar]
- Pieper MP, Chaudhary NI, Park JE. Acetylcholine-induced proliferation of fibroblasts and myofibroblasts in vitro is inhibited by tiotropium bromide. Life Sci. 2007;80:2270–2273. doi: 10.1016/j.lfs.2007.02.034. [DOI] [PubMed] [Google Scholar]
- Proskocil BJ, Sekhon HS, Jia Y, Savchenko V, Blakely RD, Lindstrom J, Spindel ER. Acetylcholine is an autocrine or paracrine hormone synthesized and secreted by airway bronchial epithelial cells. Endocrinology. 2004;145:2498–2506. doi: 10.1210/en.2003-1728. [DOI] [PubMed] [Google Scholar]
- Raufman JP, Chen Y, Cheng K, Compadre C, Compadre L, Zimniak P. Selective interaction of bile acids with muscarinic receptors: a case of molecular mimicry. Eur J Pharmacol. 2002;457:77–84. doi: 10.1016/s0014-2999(02)02690-0. [DOI] [PubMed] [Google Scholar]
- Raufman JP, Cheng K, Zimniak P. Activation of muscarinic receptor signaling by bile acids: physiological and medical implications. Dig Dis Sci. 2003;48:1431–1444. doi: 10.1023/a:1024733500950. [DOI] [PubMed] [Google Scholar]
- Raufman JP, Samimi R, Shah N, Khurana S, Shant J, Drachenberg C, Xie G, Wess J, Cheng K. Genetic ablation of M3 muscarinic receptors attenuates murine colon epithelial cell proliferation and neoplasia. Cancer Res. 2008;68:3573–3578. doi: 10.1158/0008-5472.CAN-07-6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayford W, Noble MJ, Austenfeld MA, Weigel J, Mebust WK, Shah GV. Muscarinic cholinergic receptors promote growth of human prostate cancer cells. Prostate. 1997;30:160–166. doi: 10.1002/(sici)1097-0045(19970215)30:3<160::aid-pros3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Reinheimer T, Bernedo P, Klapproth H, Oelert H, Zeiske B, Racke K, Wessler I. Acetylcholine in isolated airways of rat, guinea pig, and human: species differences in role of airway mucosa. Am J Physiol. 1996;270:L722–L728. doi: 10.1152/ajplung.1996.270.5.L722. [DOI] [PubMed] [Google Scholar]
- Reinheimer T, Munch M, Bittinger F, Racke K, Kirkpatrick CJ, Wessler I. Glucocorticoids mediate reduction of epithelial acetylcholine content in the airways of rats and humans. Eur J Pharmacol. 1998;349:277–284. doi: 10.1016/s0014-2999(98)00185-x. [DOI] [PubMed] [Google Scholar]
- Ruggieri MR, Colton MD, Wang P, Wang J, Smyth RJ, Pontari MA, Luthin GR. Human prostate muscarinic receptor subtypes. J Pharmacol Exp Ther. 1995;274:976–982. [PMC free article] [PubMed] [Google Scholar]
- Schmitt JM, Abell E, Wagner A, Davare MA. ERK activation and cell growth require CaM kinases in MCF-7 breast cancer cells. Mol Cell Biochem. 2010;335:155–171. doi: 10.1007/s11010-009-0252-9. [DOI] [PubMed] [Google Scholar]
- Sekhon H, Sauer D, Corless CL, Lupo SL, Lindstrom J, Spindel ER. Expression of nicotinic acetylcholine receptors, choline acetyltransferase and lynx1 in pancreatic carcinoma. Proc AACR. 2002;43:A2569. abstract. [Google Scholar]
- Song P, Spindel ER. Novel Na-independent choline transporters mediate choline transport and acetylcholine induced-proliferation in small cell lung carcinoma. Am J Respir Crit Care Med. 2007;175:A47. abstract. [Google Scholar]
- Song P, Sekhon HS, Jia Y, Keller JA, Blusztajn JK, Mark GP, Spindel ER. Acetylcholine is synthesized by and acts as an autocrine growth factor for small cell lung carcinoma. Cancer Res. 2003;63:214–221. [PubMed] [Google Scholar]
- Song P, Sekhon HS, Duan J, Mark GP, Spindel ER. Inhibitory regulation by M2 muscarinic acetylcholine receptors is decreased in lung cancers. Am J Respir Crit Care Med. 2004;169:A290. abstract. [Google Scholar]
- Song P, Sekhon HS, Lu A, Arredondo J, Sauer D, Gravett C, Mark GP, Grando SA, Spindel ER. M3 muscarinic receptor antagonists inhibit small cell lung carcinoma growth and mitogen-activated protein kinase phosphorylation induced by acetylcholine secretion. Cancer Res. 2007;67:3936–3944. doi: 10.1158/0008-5472.CAN-06-2484. [DOI] [PubMed] [Google Scholar]
- Song P, Sekhon HS, Fu XW, Maier M, Jia Y, Duan J, Proskosil BJ, Gravett C, Lindstrom J, Mark GP, Saha S, Spindel ER. Activated cholinergic signaling provides a target in squamous cell lung carcinoma. Cancer Res. 2008;68:4693–4700. doi: 10.1158/0008-5472.CAN-08-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P, Maier M, Olivas AS, Spindel ER. Inhibition of lung cancer cell growth by tiotropium: mechanism of action. Am J Respir Crit Care Med. 2009;179:A2675. abstract. [Google Scholar]
- Song P, Olivas AS, Spindel ER. Tiotropium inhibits growth of squamous cell lung carcinoma (SCC) cell lines in vitro and also inhibits SCC growth in vivo in nude mice by inhalation. Eur Respir J. 2010;36(946S) abstract. [Google Scholar]
- Tang X, Batty IH, Downes CP. Muscarinic receptors mediate phospholipase C-dependent activation of protein kinase B via Ca2+, ErbB3, and phosphoinositide 3-kinase in 1321N1 astrocytoma cells. J Biol Chem. 2002;277:338–344. doi: 10.1074/jbc.M108927200. [DOI] [PubMed] [Google Scholar]
- Ukegawa JI, Takeuchi Y, Kusayanagi S, Mitamura K. Growth-promoting effect of muscarinic acetylcholine receptors in colon cancer cells. J Cancer Res Clin Oncol. 2003;129:272–278. doi: 10.1007/s00432-003-0433-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Li J, Chen F, Zhao Y, He X, Wan D, Gu J. Choline transporters in human lung adenocarcinoma: expression and functional implications. Acta Biochim Biophys Sin (Shanghai) 2007;39:668–674. doi: 10.1111/j.1745-7270.2007.00323.x. [DOI] [PubMed] [Google Scholar]
- Wessler IK, Kirkpatrick CJ. The non-neuronal cholinergic system: an emerging drug target in the airways. Pulm Pharmacol Ther. 2001;14:423–434. doi: 10.1006/pupt.2001.0313. [DOI] [PubMed] [Google Scholar]
- Wessler IK, Kirkpatrick CJ. Activation of muscarinic receptors by non-neuronal acetylcholine. In: Fryer AD, editor. Muscarinic receptors, Handbook of experimental pharmacology. Springer; Heidelberg: 2011. [DOI] [PubMed] [Google Scholar]
- Wessler I, Reinheimer T, Klapproth H, Schneider FJ, Racke K, Hammer R. Mammalian glial cells in culture synthesize acetylcholine. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:694–697. doi: 10.1007/pl00005107. [DOI] [PubMed] [Google Scholar]
- Whitsett JA, Hollinger B. Muscarinic cholinergic receptors in developing rat lung. Pediatr Res. 1984;18:1136–1140. doi: 10.1203/00006450-198411000-00016. [DOI] [PubMed] [Google Scholar]
- Williams JA. Regulation of pancreatic acinar cell function. Curr Opin Gastroenterol. 2006;22:498–504. doi: 10.1097/01.mog.0000239863.96833.c0. [DOI] [PubMed] [Google Scholar]
- Witte LP, Chapple CR, de la Rosette JJ, Michel MC. Cholinergic innervation and muscarinic receptors in the human prostate. Eur Urol. 2008;54:326–334. doi: 10.1016/j.eururo.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Xie G, Drachenberg C, Yamada M, Wess J, Raufman JP. Cholinergic agonist-induced pepsinogen secretion from murine gastric chief cells is mediated by M1 and M3 muscarinic receptors. Am J Physiol Gastrointest Liver Physiol. 2005;289:G521–G529. doi: 10.1152/ajpgi.00105.2004. [DOI] [PubMed] [Google Scholar]
- Yagle K, Lu H, Guizzetti M, Moller T, Costa LG. Activation of mitogen-activated protein kinase by muscarinic receptors in astroglial cells: role in DNA synthesis and effect of ethanol. Glia. 2001;35:111–120. doi: 10.1002/glia.1076. [DOI] [PubMed] [Google Scholar]
- Yang WL, Frucht H. Cholinergic receptor up-regulates COX-2 expression and prostaglandin E(2) production in colon cancer cells. Carcinogenesis. 2000;21:1789–1793. doi: 10.1093/carcin/21.10.1789. [DOI] [PubMed] [Google Scholar]


