Abstract
Objective
To determine whether maternal/fetal SNPs in candidate genes are associated with spontaneous preterm labor/delivery.
Study Design
A genetic association study was conducted in 223 mothers and 179 fetuses [preterm labor with intact membranes who delivered <37 weeks (PTB)], and 599 mothers and 628 fetuses (normal pregnancy): 190 candidate genes and 775 SNPs were studied. Single locus/haplotype association analyses were performed; FDR was used to correct for multiple testing (q*=0.15)].
Results
1) The strongest single locus associations with PTB were IL6R (fetus: p=0.000148) and TIMP2 (mother: p=0.000197), remaining significant after correction for multiple comparisons; 2) Global haplotype analysis indicated an association between a fetal DNA variant in IGF2 and maternal COL4A3 (global p=0.004 and 0.007, respectively).
Conclusion
A SNP involved in controlling fetal inflammation (IL6R) and DNA variants in maternal genes encoding for proteins involved in extracellular matrix biology approximately doubled the risk of PTB.
Keywords: Chorioamnionitis, DNA variants, extracellular matrix, genetic association study, genomics, genotype, haplotype, high dimensional biology, IL-6, parturition, prematurity, SNP
INTRODUCTION
Preterm birth (PTB) is the leading cause of perinatal morbidity and mortality worldwide.1 The frequency of PTB varies from 5–12% depending on geographical region.2;3 PTB may be the result of spontaneous preterm labor, with intact or ruptured membranes, or indicated PTB (for fetal or maternal indications).
Genetic predisposition for PTB4–6 has been hypothesized based upon: 1) demonstration of familial aggregation;7–11 2) measures of heritability;11–15 3) identification of disease-susceptibility genes; and 4) racial disparity in preterm birth rate16–19 that may be related to frequency differences in risk predisposing alleles.20–24 Familial aggregation, defined as the co-occurrence of a trait in members of a family that can not be readily accounted for by chance, has been demonstrated for PTB.7–11 Studies in twins have demonstrated a heritability ranging from 17–40%.9;10 Polymorphisms in several genes have been studied and many have identified DNA variants associated with spontaneous preterm labor with and without intact membranes as well as in PTB.21;24–97
The purpose of this genetic association study was to identify DNA variants in the maternal and fetal genomes that can alter the risk for spontaneous preterm labor and delivery with intact membranes. Seven hundred seventy five single nucleotide polymorphisms (SNPs) from 190 candidate genes, which have been implicated in the mechanisms of disease responsible for spontaneous preterm labor, preterm prelabor rupture of membranes (pPROM), small-for-gestational age (SGA), and preeclampsia, were analyzed. The study was conducted in a Hispanic population at a single site from Chile that has an estimated PTB rate of 6%98;99 and with extreme care to phenotypic characterization.
MATERIALS AND METHODS
Study Design
This was a case-control study that included patients with spontaneous preterm labor and intact membranes who delivered a preterm neonate (mothers: 223 and fetuses: 179) and controls (mothers: 599 and fetuses: 628). Spontaneous preterm labor was defined by the presence of regular uterine contractions occurring at a frequency of at least two every 10 minutes who delivered preterm (<37 weeks). The control group included women who delivered at term (37–42 weeks of gestation) without complications of pregnancy including preterm labor with term delivery, preeclampsia, eclampsia, HELLP syndrome, pPROM, SGA, large-for-gestational age neonates, fetal demise, placental abruption, placenta previa, or chorioamnionitis.
Patients of Hispanic origin were recruited at the Sotero del Rio Hospital, in Puente Alto, Chile. All eligible mothers were enrolled in a research protocol, which requested permission to collect DNA from the mother and her neonate for research purposes. The exclusion criteria, beside those explained above for controls, included: 1) known major fetal chromosomal and/or structural anomalies; 2) multiple pregnancy; 3) serious medical illness (chronic renal failure, congestive heart failure, connective tissue disorders, etc.); 4) refusal to provide written informed consent; and 5) a clinical emergency, which prevented counseling of the patient about participation in the study, such as fetal distress or maternal hemorrhage. A blood sample was obtained from the mother at the time of enrollment in the protocol, and from the umbilical cord (blood of fetal origin) after delivery. Demographic and clinical characteristics of the mothers were obtained from a data collection form administered by trained medical and paramedical personnel. The collection of samples and their utilization for research purposes was approved by the Institutional Review Boards of the Sotero del Rio Hospital, Santiago, Chile (an affiliate of the Pontificia Catholic University of Santiago, Chile), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Genotyping
Candidate genes were selected for analysis based on biological plausibility for a role in preterm labor and other pregnancy complications including SGA, pPROM and preeclampsia. Genes involved in processes such as the control of the immune response (pattern recognition receptors, cytokines, chemokines and their respective receptors), uteroplacental ischemia, or angiogenesis were considered appropriate candidates for this study. A complete list of the 190 genes and all SNPs genotyped are included in the supplemental materials (Supplemental Table 1).
SNP discovery within the candidate genes was performed by DNA sequencing at Genaissance Pharmaceuticals, Inc. (New Haven, CT, USA) using its Index Repository, which includes a total of 93 subjects with Native American, Hispanic/Latino, European, Asian, and African-American ancestry.100 To determine which individuals in the Genaissance Index Repository were most representative of the genetic variation observed in the Chilean population, 96 unrelated Chilean individuals who are representative of the patient cohort were sequenced for 16 DNA fragments. A subset of 42 subjects from the Index Repository that is heavily weighted with the Native American and Hispanic/Latino subjects (although European, Asian, and African-American subjects contributed to the subset as well) was determined to be most representative of the variation for the Chilean population. This was based on the correlation in the minor allele frequencies for the SNPs in 16 DNA fragments that were sequenced in both the Index Repository and the sample of patients from Chile (mothers with the same ethnicity delivered at the same hospital). This subset of 42 individuals was used to select polymorphisms for the candidate genes. The selection was performed by applying the Shannon-Wiener diversity metric to this subset as previously described,101 and the SNPs selected for genotyping were intended to capture at least 90% of the haplotypic diversity of each gene, covering variation in the coding regions, 100 bases at each end of the introns, 1000 bases upstream of the start codon, and 100 bases downstream of the stop codon.101
Template DNA for genotyping was obtained by whole-genome amplification102 of genomic DNA isolated from blood using an automated DNA isolation protocol (BioRobot 9604, Qiagen, Valencia, CA, USA). Genotyping was carried out using the MassARRAY TM System (Sequenom, Inc., San Diego, CA, USA) at the high-throughput genotyping facility at Genaissance. Each genotyping assay involved PCR amplification from template DNA in a target region defined by specific primers for the respective polymorphic sites, purification of the amplification product, annealing of the indicated extension primer to one strand of the amplification product adjacent to the polymorphic site, extending the primer by one nucleotide using the MassEXTEND TM reaction (Sequenom, Inc., San Diego, CA, USA), and detection of the allele-specific extension product by mass spectrometry.103
Quality Control
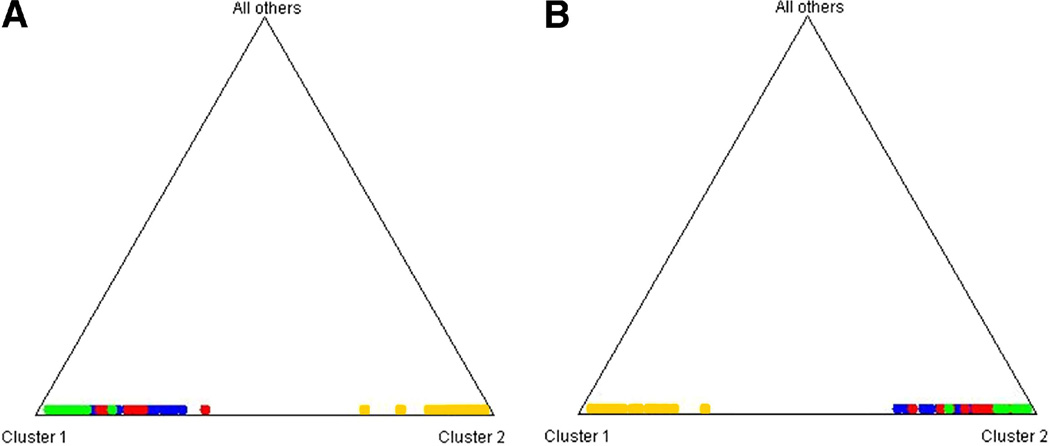
Univariate and multivariate distributions were evaluated for each variable to identify significant outliers. The values of the outliers were removed only if found to be incorrect on reexamination. Each SNP was verified to ensure genetic consistency between the genotypes of mother and offspring. Numerous programs are available for detecting relationship errors;104–108 however, to produce accurate results, these programs require genotyping for a larger proportion of the genome than was available in this study. Therefore, we considered the number of Mendelian inconsistencies between mother and offspring to identify potential relationship errors (e.g. sample mix-ups or mislabeling). When an inconsistency for an individual marker was observed, those genotypes were removed at that marker. In the case of multiple inconsistencies in a given pair, the pair was excluded for further analysis (10 pairs in controls and 5 pairs in cases). Finally, we assessed the presence of genotyping errors. In some instances, genotyping errors will lead to Mendelian inconsistencies, which can be easily identified and removed from the analysis. However, most genotyping errors for SNPs will be Mendelian consistent.109 For example, with mother-offspring pedigree structures, genotyping errors where a homozygous individual has been mistyped as a heterozygous individual will never lead to a Mendelian inconsistency for SNPs. Deviations from Hardy-Weinberg equilibrium (HWE) may indicate the presence of a genotyping error or hidden population stratification,110 or reflect a biological effect such as natural selection (or other evolutionary force) and/or the association of disease and genotype.111–115 Tests for deviations from HWE were performed for mothers and offspring separately and again separately for diagnostic subgroups. Because it is currently unclear how to unequivocally distinguish between deviations from HWE due to genotyping error, and deviations from HWE due to biological causes, such as location at or near a disease susceptibility locus, we noted SNPs that deviate from HWE, but we did not remove them from the analysis. If necessary, we could follow-up these observations with additional testing. In this study, no SNPs found to be associated with spontaneous preterm labor/delivery with intact membranes deviated from HWE in both cases and controls. We tested for population stratification in cases and controls using STRUCTURE.116 We used the CEU and Asian (CHB and JPT) populations as reference populations because Hispanic populations in the Americas, including those in Chile, are the products of admixture between European and Native American populations, and Native American populations are most recently derived from Asia. The analyses show that the Chilean samples, both cases and controls, cluster within the HapMap European (CEU) samples, indicating that our cases and controls do not represent stratified populations (Figure 1).
Figure 1.
STRUCTURE116 analysis of spontaneous preterm labor/delivery with intact membranes samples to detect population stratification. A) Each mother is denoted as a character with cases in RED and controls in GREEN. Also included are samples from HapMap (Phase 3). These samples are the European (CEU) (BLUE) and Asian samples (Chinese and Japanese, YELLOW) to test the hypothesis of population stratification. Clustering at the vertices indicate genetic similarity and little stratification. The samples from Chile cluster within the space defined by the European samples, supporting the hypotheses of little or no genetic stratification. B) The same analyses conducted with fetal samples included in the current study using the criteria and color coding as in (A). As with the maternal there is no evidence of substantial population stratification.
Statistical analysis
Continuous demographic and clinical characteristics of cases and controls (gestational age, birth weight, maternal age, and body mass index (BMI)) were tested for normality using Shapiro-Wilks test. All measurements deviated significantly from normality; therefore, Mann-Whitney two-sample rank sum tests were used for case-control comparisons. χ2 tests were used to test for differences in parity, Apgar scores at 1 and 5 minutes, smoking, and differences in fetal gender between cases and controls. Stata 10.0 statistical software (StataCorp, College Station, TX, USA) was used for all analyses.
Single locus tests of association
Statistical tests for single locus association and for deviations from HWE were calculated using PLINK software.117 Deviations from HWE in cases and controls were determined using χ2 test. Single locus tests of association were performed with logistic regression using an additive genotypic model where the minor allele was coded as the risk allele. Standard summary statistics, odds ratios (OR) and confidence intervals (CI) were reported for these tests of association. Prior to performing single locus and haplotype analyses, linkage disequilibrium (LD) based SNP pruning was performed using PLINK software, with a cutoff of r2 = 0.8. Of the 775 SNPs that passed quality control, we analyzed 697 maternal and 645 fetal SNPs. We excluded a small number of X chromosome SNPs for fetal data, as neonates included in the study were both male and female. This accounted for the most of the difference in the number of SNPs tested in mothers and fetuses.
Haplotype tests of association
Haplotype analyses were performed on genes with at least 1 significantly associated SNP (p< 0.01) and at least two SNPs in the same gene. Haplotype frequencies, as well as haplotype-based association analyses for the dichotomous PTB outcome with 2 and 3 marker sliding windows, were calculated using PLINK software. Only haplotypes that had a frequency of ≥ 0.05 were analyzed, and only SNPs that had less than 5% missing data were used. The strongest associated haplotype windows are reported (p≤ 0.05) and only these were analyzed for haplotype-specific effects. We present the calculation of OR for each haplotype (using the most common haplotype as referent), as well as determination of case and control haplotype frequencies. Standard summary statistics for pairwise LD, D’ and r2, were calculated using Haploview.118;119 Haplotype blocks were assigned using the D’ confidence interval algorithm created by Gabriel et al.120 D is the standard population genetic measure of LD that calculates the deviation of haplotype frequencies from that expected if there were random combinations of alleles at two loci. Since D is allele frequency dependent, D’ is often used because it normalizes the LD (to a range of 0 to 1) based on actual allele frequencies. In contrast, r2 is the standard correlation statistic based on the population distribution of two SNPs. All three of these measures reflect similar patterns of LD.
Histologic chorioamnionitis analysis
All statistically significant single locus and haplotype associations were further analyzed for allele and haplotype differences between patients with spontaneous preterm labor and delivery (<34 weeks of gestation) with histological chorioamnionitis alone or with funisitis and term controls (delivered >37 weeks without histologic chorioamnionitis or funisitis). The purpose of these analyses was to further evaluate whether histologic chorioamnionitis was driving the observed associations.
Multiple testing corrections
A false discovery rate (FDR) correction was performed to adjust for multiple comparisons using a q* of 0.15 in single locus tests of association.121 The q* indicates the expected proportion of results that are identified as interesting that are actually false. This is in contrast to α (typically set to 0.05), which indicates the probability of obtaining a false positive result among all tests performed. FDR is used to measure global error, that is, the expected number of false rejections of the null hypothesis among the total number of rejections. The critical significance level was calculated by ranking the results by p values and then multiplying this rank by q* divided by the total number of tests. Although the choice of q* is somewhat arbitrary, a q* of 0.15 was chosen to be permissive enough in our analyses not to run the risk of eliminating potentially interesting findings due to a Type II error.
Multi-locus analysis
Spontaneous preterm labor with intact membranes is a complex phenotype with no clear pattern of inheritance in pedigrees. Therefore, it is reasonable to assume that multiple variants, either alone or interacting, predispose to this phenotype. Because of this complexity it is important to explore genetic models that do not assume independent single locus effects as such analyses have the potential to reveal genetic models hidden by single locus analyses. Such analyses have previously revealed associations undetected by single locus analyses for hypertension,122 as well as associations with preterm birth.123 One method that is designed to discover of complex models is Multifactor Dimensionality Reduction (MDR). We performed exploratory multi-locus analyses using MDR to identify interactions among maternal, fetal, and maternal/fetal SNPs. MDR has been previously described by Ritchie et al.124 and is available as open source software at www.epistasis.org. Briefly, MDR is a non-parametric (does not assume any statistical model) and model free (no assumption mode of genetic inheritance) unique tool for identifying gene-gene interactions. MDR collapses all of the genetic data into two categories (high and low risk) by comparing all single locus and all multi-locus combinations, and then categorizing each genotype into either high-risk or low-risk on the basis of the ratio of cases to controls that have that genotype. MDR ultimately selects one genetic model, either single or multi-locus, that most successfully predicts phenotype or disease status. Analyses were performed: 1) separately for maternal and fetal data (tag SNPs only); and 2) combined for available maternal and fetal paired DNA samples [e.g. instead of an individual having 751 SNPs (the number of SNPs that completely overlapped for maternal and fetal DNAs), this increased the number of SNPs analyzed to 1502]. Data were analyzed for two- and three-way interactions with 10-fold cross-validation and average balanced accuracy as the metrics for evaluating a model.125 Several filtering steps and parameters were explored and are described on Table 1. The MDR algorithm was implemented with the full array of tag SNPs as well as after filtering, using the Tuned ReliefF (TuRF) approach as described in detail by Moore and White.126 TuRF is a modification of ReliefF. Briefly, ReliefF is a method that estimates the quality of attributes (e.g. SNPs) through a nearest neighbor algorithm that selects neighbors from the same and different classes based on the values of the attributes (in this case genotypes).127 TuRF is a method that systematically removes attributes (e.g. SNPs) that poorly differentiate cases and controls.126 ReliefF and similar computational algorithms have been developed, primarily in computer science and data mining fields, to provide means to screen attributes/variables on a rational basis that relate to quality in terms of ability to classify an outcome. These algorithms allow the reduction in importance of those attributes that are irrelevant to an outcome and will generate noise to any subsequent analyses. Many of the developed algorithms assume independent effects, and are therefore not appropriate when there are interactions among variables that affect outcome/disease. ReliefF does not make this assumption. It is an extension of Relief and we will briefly describe Relief in order to provide a better understanding of the attribute election process. In Relief, an instance (in this case an individual, either case or control) is randomly selected and then the two nearest neighbors (the nearest case and the nearest control, based on the multilocus genotype data). Attributes that are shared with those of the same status are increased in value and those that are shared among individual of opposite classes are devalued. Unshared values of an attribute in two cases are similarly decreased in value. This is repeated a preset number of times. ReliefF has the added feature of choosing just a single individual of the same and single individual of the opposite status an arbitrary number of individuals is selected to assess the attributes. In our case we use a sample of 10 nearest cases and 10 nearest controls on the genotype space. TuRF goes a step further and instead of just changing the attributes (e.g., SNP) values, it systematically removes attributes (e.g. SNPs) that poorly differentiate cases and controls. The motivation behind this algorithm is that the ReliefF estimates of the true associating SNPs will improve as the non-associating SNPs are removed from the dataset. In addition, SNPs were filtered based on results of the single SNP analyses and only SNPs that had a marginal p value of ≤0.1 were included, or only those with a p value <0.05 were analyzed separately. Permutation testing with 1,000 permutations was used to determine statistical significance of all MDR models.
Table 1.
Parameters explored in MDR analyses
| Algorithm | SNPs included | Population |
|---|---|---|
| Balanced Accuracy | all tag SNPs | Maternal |
| tag SNPs with p < 0.05 | ||
| tag SNPs with p < 0.10 | ||
| all tag SNPs | Fetal | |
| tag SNPs with p < 0.05 | ||
| tag SNPs with p < 0.10 | ||
| all tag SNPs | Maternal-Fetal Combined | |
| tag SNPs with p < 0.05 | ||
| tag SNPs with p < 0.10 | ||
| Balanced Accuracy with TuRF with 10 SNP filter | all tag SNPs | Maternal |
| Fetal | ||
| Maternal-Fetal Combined | ||
MDR as described above is ideal for a balanced data set where the number of cases and controls are the same or close to the same. However, computational methods have been developed since the initial development of MDR to test for prediction accuracies in an imbalanced data set, such as ours.125 The method, termed balanced accuracy, corrects for imbalanced data by taking an average of the sensitivity and specificity and is defined as the arithmetic mean of sensitivity and specificity. We tested for balanced accuracy in this manuscript.
Bioinformatics Tools
The SNPper (http://snpper.chip.org) database using dbSNP Build 125 was used to determine marker positions (bp), marker function, and identify amino acid changes.
Pathway analysis
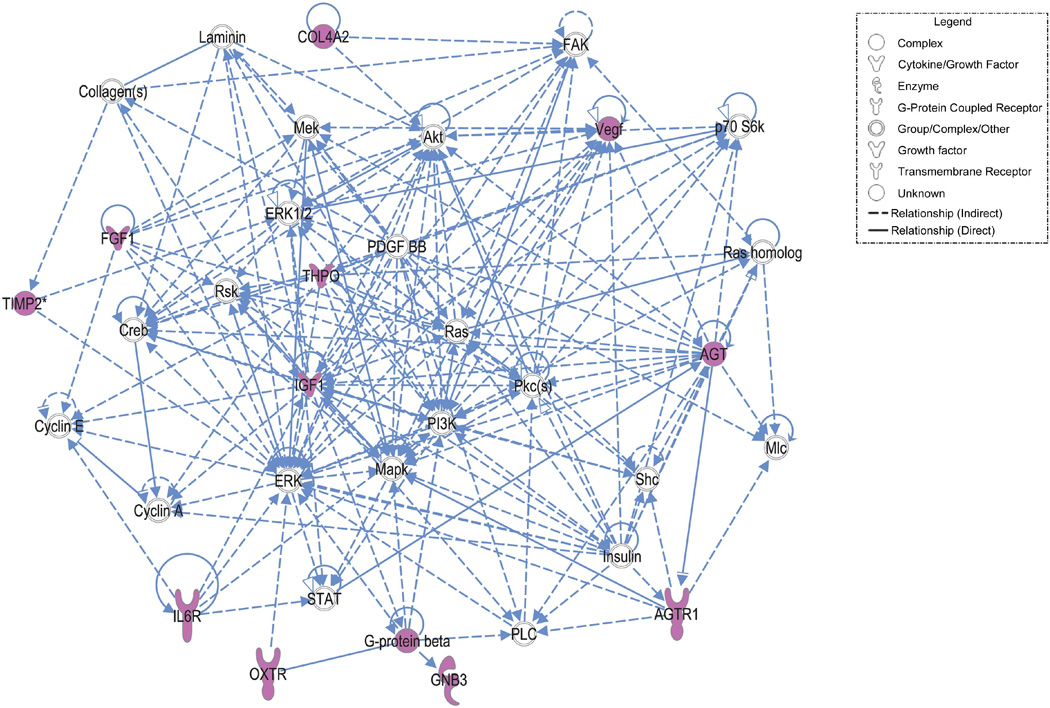
As with the multilocus analyses, it is important to provide a more comprehensive assessment of our results in terms of pathway involvement in spontaneous preterm labor and delivery with intact membranes because it likely that single genes are not by themselves adequate to affect disease risk, but multiple genes within a single pathway may affect function enough to increase risk. Therefore, as part of hypothesis generating exercise we perform pathway based analyses using Ingenuity Pathway Analysis (IPA) (Ingenuity Systems, Inc., Redwood City, CA, USA).19;128–131 Specifically, IPA was used to examine whether the SNPs found to be putatively associated with spontaneous preterm labor and delivery with intact membranes mapped to different biological networks and disease functions. The genes with variants that were significantly associated with PTB (p<0.05) were entered into the IPA analysis tool. These genes were termed "focus genes." The IPA software was used to measure associations of these molecules with other molecules, their network interactions, and biological functions stored in its knowledge base. The knowledge base encompasses relationships between proteins, genes, cells, tissues, xenobiotics, and diseases. The information is scientist-curated, updated, and integrated from the published literature and other databases such as OMIM, GO, and KEGG. Our focus genes served as seeds for the IPA algorithm, which recognizes functional networks by identifying interconnected molecules, including molecules not among the focus genes from the IPA knowledge base. The software illustrates the networks graphically, and calculates a score for each network, which represents the approximate "fit" between the eligible focus molecules and each network. The network score is based on the hypergeometric distribution and is reported as the –log (Fisher's exact test result).
We also used IPA to identify functionally related genes that correspond to canonical pathways. We determined enrichment of our focus genes among the > 200 well-characterized metabolic and cell signaling “canonical” pathways curated by IPA scientists from journal articles, text books, and KEGG Ligand. The Fischer’s exact test was used to calculate a p value representing the probability that the association between the genes in our dataset and the canonical pathway is explained by chance alone.
RESULTS
Significant differences between cases and controls were identified for gestational age at delivery (p<0.0001), birth weight (p<0.0001), Apgar scores at 1 (p<0.0001) and 5 minute (p <0.0001) (Table 2). However, we did not observe significant differences between cases and controls for BMI and smoking status, two variables that have been previously associated with risk for PTB.132–136
Table 2.
Demographic and clinical characteristics of the study population
| Variable | Cases (n = 223) |
Controls (n = 599) |
p-value |
|---|---|---|---|
| Parity (number of previous pregnancies) | 1 [0–2] | 1 [0–1] | 0.193 |
| Maternal age (years) | 24 [19–32] | 24 [20–30] | 0.984 |
| BMI | 23 [21–26] | 24 [22–26] | 0.455 |
| Smoking | 13% | 14% | 0.506 |
| Gestational age at delivery (weeks) | 34 [30–35] | 40 [39–41] | <0.0001 |
| Birth weight (grams) | 2200 [1680–2600] | 3440 [3230–3650] | <0.0001 |
| 1st minute Apgar score | 8 [5–9] | 9 [9–9] | <0.0001 |
| 5th minute Apgar score | 9 [18–9] | 9 [9–9] | <0.0001 |
Results are expressed as median (25th to 75th percentile)
BMI: body mass index
Single locus association
SNPs associated with spontaneous preterm labor/delivery with intact membranes in maternal and fetal DNA (p <0.01) are presented in Table 3. There were no statistically significant deviations from HWE in controls for any of these DNA variants. The most significant association in maternal DNA was observed at a synonymous coding SNP (S101S) in the gene for the tissue inhibitor of metalloproteinase 2 (TIMP2) rs2277698 (OR = 1.98 [95% CI 1.38–2.83], p= 0.000197) (Table 4). The minor allele frequency for this SNP (A) was 0.13 in cases and 0.07 in controls. This SNP was significant after FDR correction for multiple testing.
Table 3.
Gene summary information strongest associations (p < 0.01)
| Population | Gene Name | Gene Code | rs # | Chromosome | Position (bp) | Function |
|---|---|---|---|---|---|---|
| Maternal | Interleukin 6 receptor isoform 1 | IL6R | rs8192282 | 1 | 152668303 | Coding Exon (A/A 31) |
| Alpha 3 type IV collagen isoform 1 | COL4A3 | rs1882435 | 2 | 227810996 | Intron | |
| Lactotransferrin | LTF | rs2269435 | 3 | 46462007 | Intron | |
| Fibroblast growth factor 1 (acidic) isoform 1 | FGF1 | rs34003 | 5 | 141955251 | Intron | |
| Guanine nucleotide-binding protein, beta-3 | GNB3 | rs5440 | 12 | 6819160 | Promoter | |
| Insulin-like growth factor 1 (somatomedin C) | IGF1 | rs5742612 | 12 | 101398994 | Promoter | |
| Tissue inhibitor of metalloproteinase 2 | TIMP2 | rs2277698 | 17 | 74378612 | Coding Exon (S/S 101) | |
| Fetal | Coagulation factor III precursor | F3 | rs610277 | 1 | 94771147 | Intron |
| Natriuretic peptide receptor A/guanylate cyclase | NPR1 | rs3891075 | 1 | 151924811 | Intron | |
| Interleukin 6 receptor isoform 1 precursor | IL6R | rs8192282 | 1 | 152668303 | Coding Exon (A/A 31) | |
| Tenascin R (restrictin, janusin) | TNR | rs2228359 | 1 | 173591274 | Coding Exon (D/D 1079) | |
| Interleukin 1, beta proprotein | IL1B | rs1143643 | 2 | 113304773 | Intron | |
| Fibronectin 1 isoform 3 preproprotein | FN1 | rs2304573 | 2 | 215951895 | Intron | |
| Interleukin 2 precursor | IL2 | rs2069772 | 4 | 123592583 | Intron | |
| Fibroblast growth factor 1 (acidic) isoform 1 | FGF1 | rs34003 | 5 | 141955251 | Intron | |
| Interferon gamma receptor 1 | IFNGR1 | rs1887415 | 6 | 137560931 | Coding Exon (L/P 467) | |
| Insulin-like growth factor 2 | IGF2 | rs3213225 | 11 | 2113112 | Intron | |
| Alpha 1 type IV collagen preproprotein | COL4A1 | rs16975492 | 13 | 109631703 | Coding Exon (P/P 710) | |
| Tissue inhibitor of metalloproteinase 2 | TIMP2 | rs2277698 | 17 | 74378612 | Coding Exon (S/S 101) | |
Table 4.
SNPs found to be significantly associated with spontaneous preterm labor/delivery in single locus tests of association (p < 0.01)
| Population | Gene Code | rs# | Minor Allele |
Minor Allele Frequency |
OR1 | 95% CI2 | p-value | ||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Lower | Upper | ||||||
|
Maternal (Cases n = 223; Controls n = 99) |
IL6R | rs8192282 | A | 0.13 | 0.08 | 1.70 | 1.19 | 2.41 | 0.003 |
| COL4A3 | rs1882435 | A | 0.33 | 0.26 | 1.40 | 1.10 | 1.79 | 0.007 | |
| LTF | rs2269435 | C | 0.38 | 0.3 | 1.39 | 1.10 | 1.76 | 0.007 | |
| FGF1 | rs34003 | G | 0.32 | 0.41 | 0.69 | 0.54 | 0.87 | 0.002 | |
| GNB3 | rs5440 | G | 0.36 | 0.43 | 0.73 | 0.58 | 0.92 | 0.007 | |
| IGF1 | rs5742612 | C | 0.06 | 0.10 | 0.56 | 0.36 | 0.87 | 0.009 | |
| TIMP2 | rs2277698 | A | 0.13 | 0.07 | 1.98 | 1.38 | 2.83 | 0.000197* | |
|
Fetal (Cases n = 179; Controls n = 628) |
F3 | rs610277 | C | 0.10 | 0.01 | 2.47 | 1.31 | 4.67 | 0.005 |
| NPR1 | rs3891075 | T | 0.09 | 0.07 | 2.04 | 1.27 | 3.30 | 0.003 | |
| IL6R | rs8192282 | A | 0.13 | 0.10 | 2.07 | 1.42 | 3.02 | 0.000148* | |
| TNR | rs2228359 | T | 0.41 | 0.3 | 1.47 | 1.14 | 1.91 | 0.004 | |
| IL1B | rs1143643 | A | 0.2 | 0.23 | 1.55 | 1.18 | 2.03 | 0.002 | |
| FN1 | rs2304573 | C | 0.62 | 0.49 | 0.70 | 0.54 | 0.91 | 0.008 | |
| IL2 | rs2069772 | G | 0.00 | 0.13 | 0.53 | 0.35 | 0.80 | 0.003 | |
| FGF1 | rs34003 | G | 0.30 | 0.42 | 0.67 | 0.51 | 0.87 | 0.003 | |
| IFNGR1 | rs1887415 | C | 0.04 | 0.07 | 0.50 | 0.32 | 0.79 | 0.003 | |
| IGF2 | rs3213225 | C | 0.09 | 0.36 | 0.67 | 0.51 | 0.89 | 0.005 | |
| COL4A1 | rs16975492 | A | 0.41 | 0.27 | 0.67 | 0.51 | 0.89 | 0.006 | |
| TIMP2 | rs2277698 | A | 0.13 | 0.14 | 1.82 | 1.27 | 2.62 | 0.001 | |
None of these markers had statistically significant deviations from HWE in cases or controls.
OR is the Odds Ratio for the additive genotypic model
95% CI is the 95% confidence interval of the Odds Ratio.
Significant after FDR correction.
The most significant association observed in fetal DNA was also a synonymous SNP (A31A) in the interleukin 6 receptor 1 (IL6R) rs8192282 (OR = 2.07 [95% CI 1.42–3.02], p= 0.000148) (Table 4). The minor allele frequency for this SNP (A) was 0.13 for cases and 0.10 for controls. This SNP also remained statistically significant after FDR correction.
Additional SNPs that were associated with spontaneous preterm labor/delivery with intact membranes at a p value of < 0.05 are presented in Tables 5a and 5b. Although we have not emphasized these findings in the present report, in some instances these SNPs represent associations in genes previously reported that may lend support to previous findings, or may be additional SNPs in genes reported with a p value < 0.01. Such findings strengthen the likelihood of an association because it will be based on multiple SNPs for the same gene (e.g. collagen type IV, LTF and TIMP2 in mothers and IGF2, IL2, TIMP2, collagen type IV, and TNR in fetuses).
Table 5.
Single SNP associations (0.01 < p < 0.05)
| a. Maternal DNA | ||||||
|---|---|---|---|---|---|---|
| Gene | SNP | A1 | OR | L95 | U95 | P |
| AGTR1 | rs5183 | G | 2.748 | 1.215 | 6.215 | 0.01522 |
| HSPG2 | rs2229489 | A | 1.947 | 1.135 | 3.34 | 0.01559 |
| SELE | rs5356 | C | 1.348 | 1.056 | 1.72 | 0.01641 |
| MMP16 | rs3739382 | T | 2.564 | 1.185 | 5.544 | 0.01675 |
| AGT | rs4762 | T | 1.433 | 1.062 | 1.934 | 0.01854 |
| COL4A2 | rs2296852 | A | 0.5672 | 0.3521 | 0.9137 | 0.01975 |
| LTF | rs1475967 | T | 0.7642 | 0.6093 | 0.9585 | 0.02 |
| COL4A4 | rs3752896 | G | 1.306 | 1.042 | 1.638 | 0.02074 |
| IL18 | rs549908 | C | 1.328 | 1.043 | 1.691 | 0.0212 |
| MMP10 | rs486055 | A | 1.764 | 1.085 | 2.867 | 0.02204 |
| SERPINE1 | GNSC_629203538 | T | 2.397 | 1.131 | 5.081 | 0.02251 |
| THPO | rs2280740 | A | 1.493 | 1.054 | 2.114 | 0.02398 |
| LTF | rs2239692 | G | 1.407 | 1.045 | 1.894 | 0.02442 |
| VEGFC | rs7664413 | A | 0.7468 | 0.575 | 0.9698 | 0.02851 |
| F3 | rs610277 | C | 2.131 | 1.07 | 4.241 | 0.03129 |
| TIMP2 | rs55743137 | C | 1.358 | 1.023 | 1.803 | 0.03435 |
| CRHR2 | rs8192498 | A | 2.51 | 1.051 | 5.996 | 0.03836 |
| IL12B | GNSC_632292870 | G | 2.662 | 1.042 | 6.801 | 0.04081 |
| SELE | rs1076637 | A | 1.278 | 1.008 | 1.619 | 0.04256 |
| REN | rs3730103 | G | 1.663 | 1.017 | 2.72 | 0.04264 |
| IL10 | rs1800872 | A | 1.268 | 1.008 | 1.596 | 0.04267 |
| COL5A2 | rs6750027 | A | 1.324 | 1.009 | 1.738 | 0.04308 |
| MMP10 | rs17860949 | T | 1.454 | 1.011 | 2.091 | 0.04343 |
| HTR2A | rs6314 | T | 0.5693 | 0.3274 | 0.9899 | 0.04594 |
| TBXAS1 | rs3735355 | A | 0.4793 | 0.2323 | 0.9887 | 0.0465 |
| TLR2 | rs5743700 | T | 0.3845 | 0.1499 | 0.9861 | 0.04669 |
| IL1B | rs1143634 | T | 0.7206 | 0.5213 | 0.9961 | 0.0473 |
| THBS4 | rs2241826 | G | 1.32 | 1.003 | 1.738 | 0.0478 |
| LTF | GNSC_617330042 | A | 1.39 | 1.003 | 1.927 | 0.04825 |
| OXTR | GNSC_631469608 | A | 1.575 | 1.003 | 2.473 | 0.04862 |
| b. Fetal DNA | ||||||
|---|---|---|---|---|---|---|
| Gene | SNP | A1 | OR | L95 | U95 | P |
| IL10RA | rs17121493 | G | 1.815 | 1.155 | 2.854 | 0.009803 |
| TIMP2 | rs55743137 | C | 1.494 | 1.097 | 2.033 | 0.01073 |
| TLR1 | rs5743553 | A | 2.728 | 1.252 | 5.946 | 0.01159 |
| COL4A3 | rs1882435 | A | 1.392 | 1.074 | 1.805 | 0.01253 |
| COL4A4 | rs3752896 | A | 0.7424 | 0.5823 | 0.9464 | 0.0162 |
| CETP | rs891144 | T | 1.603 | 1.083 | 2.371 | 0.01821 |
| COL4A2 | rs7338575 | C | 1.369 | 1.055 | 1.778 | 0.01837 |
| SERPINE1 | rs6092 | A | 1.629 | 1.081 | 2.455 | 0.0198 |
| IL4R | rs1029489 | A | 0.7479 | 0.5852 | 0.9559 | 0.02034 |
| AGTR1 | rs5183 | G | 2.555 | 1.151 | 5.673 | 0.02113 |
| NPPA | rs5068 | C | 0.256 | 0.07936 | 0.8259 | 0.0226 |
| HSPG2 | GNSC_634098268 | T | 1.406 | 1.049 | 1.885 | 0.02264 |
| ELN | rs2301995 | A | 1.44 | 1.05 | 1.974 | 0.02347 |
| COL4A2 | rs12873113 | T | 1.45 | 1.045 | 2.011 | 0.02617 |
| IGF2R | rs894817 | A | 1.316 | 1.032 | 1.679 | 0.02698 |
| IRS1 | rs1801278 | A | 1.762 | 1.055 | 2.945 | 0.03053 |
| COL4A3 | rs56326869 | C | 0.1134 | 0.01558 | 0.8255 | 0.03161 |
| COL4A4 | rs3752895 | C | 0.7748 | 0.6118 | 0.9813 | 0.03433 |
| IL9R | GNSC_14430798 | C | 0.4446 | 0.2085 | 0.9483 | 0.03596 |
| LPA | rs4708871 | G | 2.195 | 1.05 | 4.587 | 0.03655 |
| REN | rs3730103 | G | 1.753 | 1.033 | 2.975 | 0.03763 |
| ELN | rs17855988 | C | 1.631 | 1.028 | 2.589 | 0.03784 |
| IL2 | rs2069771 | T | 2.655 | 1.05 | 6.71 | 0.03908 |
| TIMP1 | rs11551797 | T | 0.4538 | 0.2141 | 0.9618 | 0.03924 |
| COL4A5 | rs28465565 | C | 0.4558 | 0.2153 | 0.965 | 0.04006 |
| IL3RA | GNSC_649033887 | A | 0.3728 | 0.1447 | 0.9606 | 0.04104 |
| SERPINC1 | rs677 | C | 1.601 | 1.017 | 2.521 | 0.04197 |
| TNR | rs1385540 | T | 1.37 | 1.01 | 1.858 | 0.04276 |
| IL1R1 | rs3917318 | G | 1.288 | 1.008 | 1.647 | 0.04315 |
| FIGF | GNSC_634828146 | C | 0.4742 | 0.2289 | 0.9824 | 0.04468 |
| IGF2 | rs2230949 | T | 1.568 | 1.01 | 2.434 | 0.04508 |
| F7 | rs6046 | A | 1.382 | 1.004 | 1.901 | 0.04705 |
| COL4A2 | rs391859 | A | 0.7216 | 0.522 | 0.9975 | 0.04827 |
| COL4A6 | GNSC_634841755 | A | 0.4671 | 0.2187 | 0.9975 | 0.04925 |
| COL4A4 | rs1800516 | C | 0.3023 | 0.09162 | 0.9977 | 0.04956 |
Haplotype association
Haplotype analyses of genes with at least one significant SNP (p < 0.05) and two SNPs in the gene identified four genes in maternal samples [alpha 3 type IV collagen isoform 1 (COL4A3), interleukin 6 receptor (IL6R), lactotransferrin (LTF), and fibroblast growth factor 1 (acidic) isoform 1 (FGF1)] and three genes in fetal samples [insulin-like growth factor 2 (IGF2), interleukin 2 (IL2), and alpha 1 type IV collagen preprotein (COL4A1)] that were associated with the risk of spontaneous preterm labor/delivery with intact membranes (Table 6; Figure 2).
Table 6.
Most Significantly Associating Haplotypes (Global p < 0.05)
| Population | Gene | Markers | Haplotype | Haplotype Frequency |
OR | 95%CI | p-value | ||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Lower | Upper | ||||||
| Maternal | COL4A3 | rs1882435-rs10178458-rs55997063 | Global p-value | - | - | 0.007 | |||
| CTT | 0.08 | 0.11 | 0.8 | 0.53 | 1.21 | 0.279 | |||
| ACT | 0.31 | 0.23 | 1.48 | 1.14 | 1.90 | 0.002 | |||
| CCT (referent) | 0.60 | 0.66 | - | - | - | - | |||
| IL6R | rs8192282-rs7521458 | Global p-value | - | - | 0.043 | ||||
| AT | 0.12 | 0.08 | 1.51 | 1.04 | 2.20 | 0.023 | |||
| GT | 0.22 | 0.25 | 0.88 | 0.67 | 1.15 | 0.339 | |||
| GC (referent) | 0.66 | 0.66 | - | - | - | - | |||
| LTF | rs2269435-rs2239692 | Global p-value | - | - | 0.013 | ||||
| CG | 0.18 | 0.13 | 1.53 | 1.12 | 2.09 | 0.005 | |||
| CA | 0.19 | 0.16 | 1.32 | 0.98 | 1.78 | 0.058 | |||
| TA (referent) | 0.63 | 0.70 | - | - | - | - | |||
| FGF1 | rs34003-rs17217240-rs9324894 | Global p-value | - | - | 0.022 | ||||
| TGT | 0.07 | 0.07 | 0.87 | 0.54 | 1.36 | 0.526 | |||
| GGC | 0.29 | 0.37 | 0.69 | 0.53 | 0.88 | 0.002 | |||
| TGC (referent) | 0.64 | 0.56 | - | - | - | - | |||
| Fetal | IGF2 | rs3213225-rs3213223 | Global p-value | - | - | 0.004 | |||
| TT | 0.23 | 0.07 | 2.78 | 1.96 | 3.94 | <0.0001 | |||
| CC | 0.10 | 0.36 | 0.24 | 0.16 | 0.35 | <0.0001 | |||
| TC (referent) | 0.67 | 0.57 | - | - | - | - | |||
| IL2 | rs2069772-rs2069771-rs2069762 | Global p-value | - | - | 0.005 | ||||
| ACG | 0.19 | 0.41 | 0.26 | 0.19 | 0.35 | <0.0001 | |||
| GCT | 0.00 | 0.14 | - | - | - | - | |||
| ACT (referent) | 0.81 | 0.45 | - | - | - | - | |||
| COL4A1 | rs685884-GNSC_633869360 | Global p-value | - | - | 0.012 | ||||
| TA | 0.09 | 0.05 | 1.12 | 0.69 | 1.8 | 0.608 | |||
| CG | 0.19 | 0.49 | 0.24 | 0.18 | 0.33 | <0.0001 | |||
| TG (referent) | 0.73 | 0.46 | - | - | - | - | |||
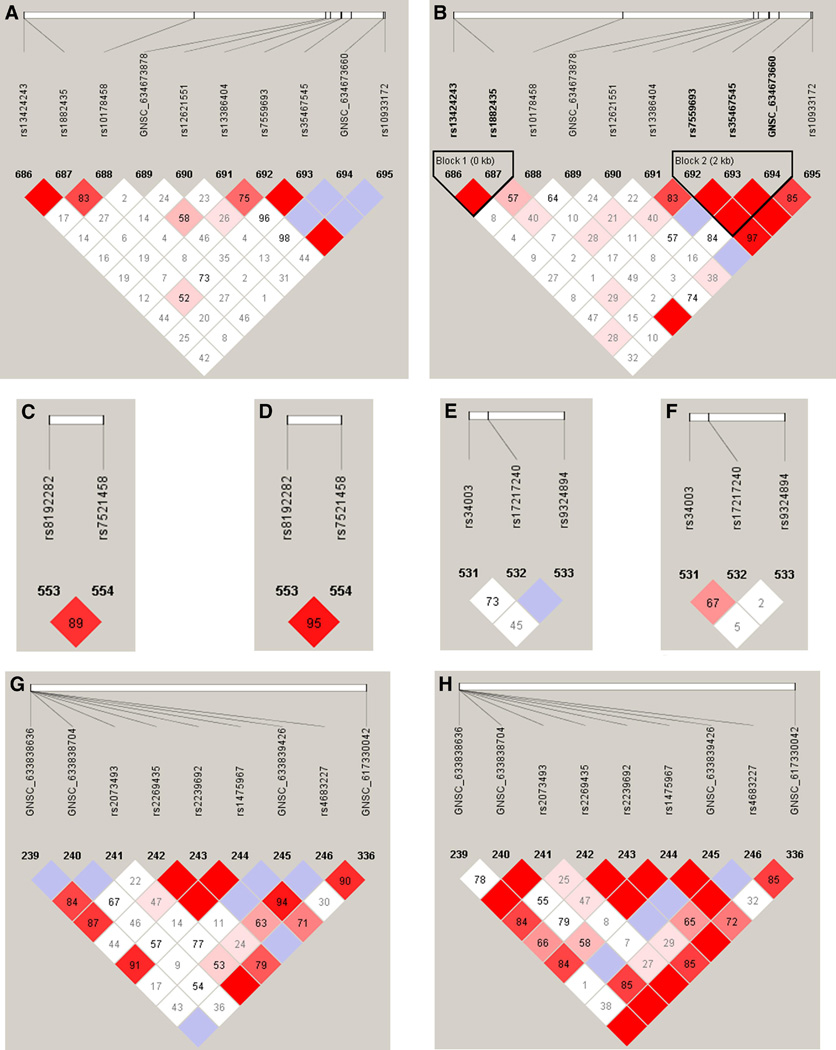
Figure 2.
Patterns of linkage disequilibrium for genes associated in haplotype analyses of maternal samples in this study. Case and control patterns of LD are shown separately. Haploview119 plots are presented with the SNPs and relative locations within the gene across the top of each panel. The bottom of each figure shows the pair-wise linkage disequilibrium values of all SNPs as measured by D’. D’ is a standardized measure of linkage disequilibrium D’ values are given in each diamond. D’ between each pairwise combination of SNPs is given in each diamond on a scale of 0–100. A value of 100 represents maximum possible linkage disequilibrium. Specifically, in panel (a) the D’ between rs1882435 (687) and rs35467545 (693) can be seen by tracing down from each SNP to the diamond where the lines intersect. In this case, D’ is 52. Solid red diamonds represent a D’ of 100 and blue diamonds are values with low logarithm of the odds (LOD) scores (<2) and hence unreliable values. White diamonds represent evidence of strong recombination and hence little LD. a. COL4A3 cases; b. COL4A3 controls; c. IL6R cases; d. IL6R controls; e. FGF1 cases; f. FGF1 controls; g.LTF cases; h. LTF controls.
The most significant haplotype association for maternal DNA (p < 0.01) was for COL4A3 at rs1882435-rs10178458-rs55997063 (global p = 0.007; Table 6). This haplotype included COL4A3 SNP rs1882435, a SNP that independently associated with spontaneous preterm labor/delivery (p = 0.007; Table 6), where the A allele was the risk allele. Upon examination of the individual haplotypes, it was clear that all statistically significant haplotypes for rs1882435-rs10178458-rs55997063 contained the rs1882435 risk allele. Examination of the LD plot for COL4A3 demonstrated that rs10178458 is in moderate LD with rs1882432, but the pattern of LD appears different between cases and controls (Figure 2a; 2b).
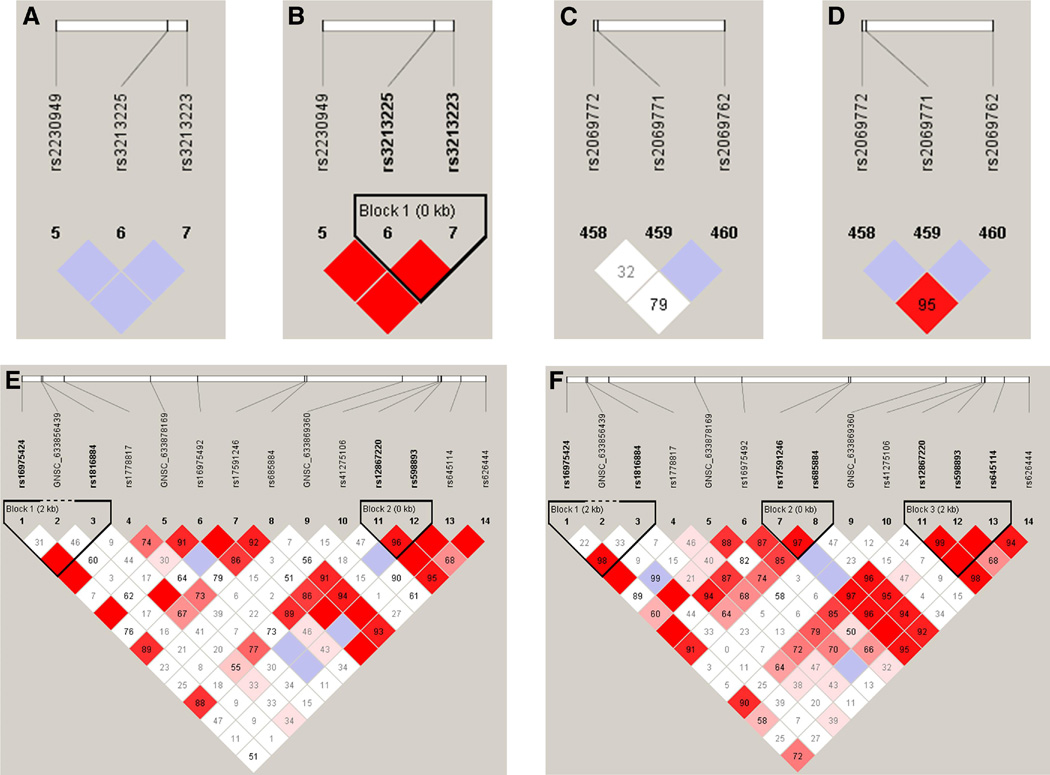
The most significant associations for fetal DNA (p < 0.01) were for IGF2 and IL2 (Table 6; Figure 3). The haplotypes for IGF2 were at rs3213225-rs3213223 (global p = 0.004) and for IL2 at rs2069772-rs2069771-rs2069762 (global p = 0.005) (Table 6). Both of these haplotypes contained a SNP that had a marginal effect; however, the IGF2 rs3213225-rs3213223 had a global haplotype p value that was slightly more significant than the individual main effect (IGF2 rs3213225, p = 0.005; Table 6), and the associations at individual haplotypes suggest that the risk allele for rs3213225 is not driving the association at this haplotype. Further examination of the LD plot suggests that there is strong LD (as measured by D’) between rs3213225-and rs3213223 in controls but not cases (Figure 3a; 3b).
Figure 3.
Patterns of linkage disequilibrium for genes associated in haplotype analyses of fetal samples in this study. Case and control patterns of LD are shown separately. Haploview119 plots are presented with the SNPs and relative locations within the gene across the top of each panel. The bottom of each figure shows the pair-wise linkage disequilibrium values of all SNPs as measured by D’. D’ is a standardized measure of linkage disequilibrium on a scale of 0–100. D’ values are given in each diamond. A value of 100 represents maximum possible linkage disequilibrium. Solid red diamonds represent a D’ of 100 and blue diamonds are values with low logarithm of the odds (LOD) scores (<2) and hence unreliable values. White diamonds represent evidence of strong recombination and hence little LD. a. IGF2 cases; b. IGF2 controls; c. IL2 cases; d. IL2 controls; e. COL4A1 cases; f. COL4A1 controls.
Histologic chorioamnionitis
Sub-analyses of all statistically significant single locus and haplotype associations for differences between cases with histologic chorioamnionitis and controls (Tables 7 and 8) demonstrated a decrease in the OR for the maternal sample TIMP2 rs2277698 with the OR dropping from 1.98 (Table 4) to 1.49, as well as a loss of statistical significance (Table 7). However, there was an increase in the OR for fetal sample IL6R rs8192282 association (OR changing from 2.07 to 2.94) and this SNP remained statistically significant (p = 0.045). No significant global haplotype p values (Table 8) were observed for any of the maternal sample haplotypes that demonstrated significant association in Table 6. There were not enough fetal cases (n=14) with histologic chorioamnionitis to perform haplotype analyses of these samples. Overall, we caution that the interpretation of these findings should be tempered by the small samples sizes.
Table 7.
Analysis of histologic chorioamnionitis for significantly associated SNPs
| Population | Gene Code | rs# | OR1 | 95% CI2 | p-value | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
|
Maternal (Cases n=30 Controls n=464) |
IL6R | rs8192282 | 1.82 | 0.87 | 3.80 | 0.110 | |
| COL4A3 | rs1882435 | 1.03 | 0.57 | 1.89 | 0.912 | ||
| LTF | rs2269435 | 0.95 | 0.54 | 1.67 | 0.845 | ||
| FGF1 | rs34003 | 0.59 | 0.33 | 1.05 | 0.074 | ||
| GNB3 | rs5440 | 0.43 | 0.24 | 0.79 | 0.006 | ||
| IGF1 | rs5742612 | 0.50 | 0.15 | 1.65 | 0.256 | ||
| TIMP2 | rs2277698 | 1.49 | 0.62 | 3.61 | 0.377 | ||
|
Fetal (Cases n=14 Controls n=465) |
F3 | rs610277 | 3.78 | 0.79 | 18.01 | 0.096 | |
| NPR1 | rs3891075 | 1.68 | 0.36 | 7.76 | 0.505 | ||
| IL6R | rs8192282 | 2.94 | 1.02 | 8.43 | 0.045 | ||
| TNR | rs2228359 | 1.01 | 0.42 | 2.42 | 0.979 | ||
| IL1B | rs1143643 | 1.70 | 0.74 | 3.92 | 0.212 | ||
| FN1 | rs2304573 | 1.23 | 0.54 | 2.82 | 0.626 | ||
| IL2 | rs2069772 | 0.22 | 0.03 | 1.59 | 0.133 | ||
| FGF1 | rs34003 | 0.43 | 0.16 | 1.18 | 0.101 | ||
| IFNGR1 | rs1887415 | 0.60 | 0.14 | 2.58 | 0.495 | ||
| IGF2 | rs3213225 | 0.73 | 0.30 | 1.79 | 0.496 | ||
| COL4A1 | rs16975492 | 0.62 | 0.24 | 1.56 | 0.307 | ||
| TIMP2 | rs2277698 | 2.14 | 0.72 | 6.34 | 0.169 | ||
OR is the Odds Ratio for the additive genotypic model;
95% CI is the 95% confidence interval of the Odds Ratio
Table 8.
Analysis of histologic chorioamnionitis for significantly associated haplotypes
| Population | Gene | Markers | Haplotype | Haplotype Frequency |
OR | 95%CI | p-value | ||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Lower | Upper | ||||||
| Maternal | COL4A3 | rs1882435-rs10178458-rs55997063 | Global p-value | 0.914 | |||||
| CTT | 0.09 | 0.11 | 0.77 | 0.23 | 2.00 | 0.581 | |||
| ACT | 0.23 | 0.23 | 0.98 | 0.48 | 1.88 | 0.953 | |||
| CCT (referent) | 0.69 | 0.66 | |||||||
| IL6R | rs8192282-rs7521458 | Global p-value | 0.229 | ||||||
| AT | 0.15 | 0.09 | 1.97 | 0.80 | 4.37 | 0.079 | |||
| GT | 0.27 | 0.25 | 1.19 | 0.60 | 2.25 | 0.584 | |||
| GC (referent) | 0.58 | 0.66 | |||||||
| LTF | rs2269435-rs2239692 | Global p-value | 0.403 | ||||||
| CG | 0.17 | 0.12 | 1.35 | 0.59 | 2.84 | 0.405 | |||
| CA | 0.12 | 0.17 | 0.68 | 0.25 | 1.57 | 0.357 | |||
| TA (referent) | 0.72 | 0.71 | |||||||
| FGF1 | rs34003-rs17217240-rs9324894 | Global p-value | 0.103 | ||||||
| GGT | 0.01 | 0.06 | 0.21 | 0.01 | 1.32 | 0.098 | |||
| TGT | 0.04 | 0.06 | 0.62 | 0.12 | 2.05 | 0.435 | |||
| GGC | 0.26 | 0.35 | 0.56 | 0.28 | 1.06 | 0.060 | |||
| TGC (referent) | 0.69 | 0.53 | |||||||
There were only 14 cases available to do haplotype analyses for the sub-analysis of histologic chorioamnionitis. These were not enough cases to do haplotype analyses under the same criteria used for haplotype analyses onTable 4.
MDR analysis
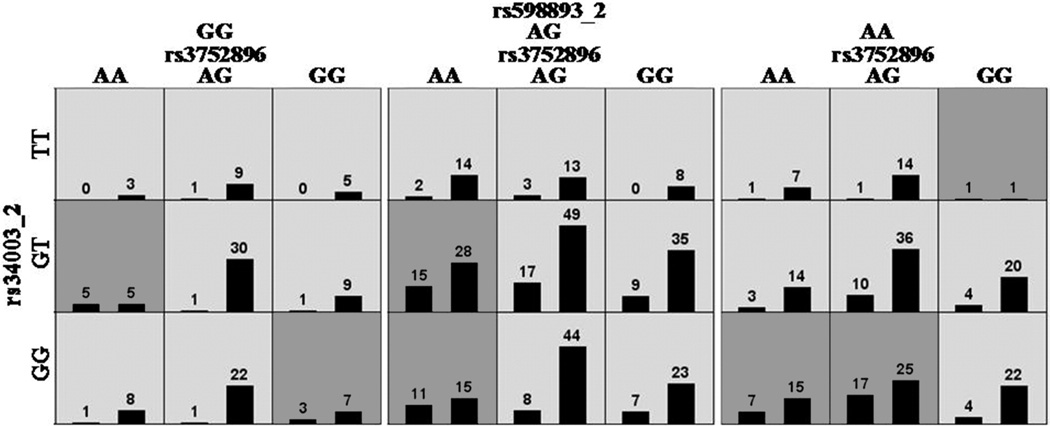
Exploratory MDR analyses were performed using different filtering approaches. The only statistically significant associations were in the analyses in which maternal and fetal genotypes were combined after filtering for SNPs with statistically significant main effects (both p< 0.05 and p< 0.10) (Table 9). The only model with a p value < 0.05 was found in analyses of SNPs filtered for main effects with a p< 0.10 (Table 9c, Figure 4). This model included rs3752896_ maternal, rs34003_fetal, and rs598893_fetal, and had a testing balanced accuracy of 0.62 (p= 0.043), but a cross validation consistency of only 6/10, suggesting that this model is unlikely to generalize. The maternal SNP, rs3752896, is an intronic SNP in collagen type IV alpha 4 (COL4A4) and the two fetal SNPs are both intronic and include a SNP (rs34003) in fibroblast growth factor 1 (FGF1) and a SNP (rs598893) in COL4A1. Only fetal SNP, rs34003, was among the most associated SNPs in the single locus analysis (Table 4). Two other models are worth mentioning: 1) a maternal model using TuRF that included rs2069762-rs5369-GNSC_30713312, SNPs in IL2, Endothelin1 (EDN1) and IL6, respectively (Testing Balanced Accuracy = 0.61; p = 0.057, CVC = 9/10) (Table 9a); and 2) a fetal model following TuRF filtering that included rs2304573-rs2621208-rs2252070, SNPs in Fibronectin 1 (FN1), COL1A2 and MMP13, respectively (Testing Balanced Accuracy = 0.62, p = 0.063, CVC = 8/10) (Table 9b).
Table 9.
Summary of MDR analyses
| a. PTB Maternal | ||||
|---|---|---|---|---|
| Algorithm | Model | Testing Balanced Accuracy |
Cross Validation Consistency |
p-value |
| Balanced Accuracy with all tag SNPS | rs2069762 | 0.55 | 6/10 | 0.377 |
| rs2069762 rs17689966 | 0.59 | 6/10 | 0.132 | |
| rs3917318rs2069762 rs7338575 | 0.55 | 2/10 | 0.397 | |
| Balanced Accuracy with TuRF – 10 SNPS | rs2069762 | 0.55 | 10/10 | 0.321 |
| GNSC_635148713 rs2069762 | 0.55 | 5/10 | 0.330 | |
| rs2069762rs5369 GNSC_30713312 | 0.61 | 9/10 | 0.057 | |
| Balanced Accuracy with Tag SNPs with p < 0.05 | rs1882435 | 0.56 | 9/10 | 0.331 |
| rs2269435 rs5440 | 0.56 | 3/10 | 0.306 | |
| rs1882435rs34003 rs10459953 | 0.59 | 4/10 | 0.139 | |
| BalancedAccuracy with Tag SNPs with p < 0.10 | rs1882435 | 0.56 | 8/10 | 0.356 |
| rs2269435 rs5440 | 0.53 | 2/10 | 0.579 | |
| rs1882435rs34003 Rs10459953 | 0.55 | 2/10 | 0.406 | |
| b. PTB Fetal | ||||
|---|---|---|---|---|
| Algorithm | Model | Testing Balanced Accuracy |
Cross Validation Consistency |
p-value |
| Balanced Accuracy with all tag SNPS | rs2069762 | 0.54 | 4/10 | 0.581 |
| rs3752896 rs2069762 | 0.59 | 5/10 | 0.204 | |
| rs1250215rs3752896 rs2069762 | 0.56 | 2/10 | 0.371 | |
| Balanced Accuracy with TuRF – 10 SNPS | rs2069762 | 0.55 | 8/10 | 0.432 |
| rs2304573 rs2621208 | 0.61 | 10/10 | 0.085 | |
| rs2304573 rs2621208 rs2252070 | 0.62 | 8/10 | 0.063 | |
|
Balanced Accuracy with Tag SNPs with p< 0.05 |
rs2069772 | 0.56 | 8/10 | 0.292 |
| rs2069772 rs16975492 | 0.59 | 5/10 | 0.174 | |
| rs34003 rs321225 rs16975492 | 0.53 | 3/10 | 0.634 | |
| Balanced Accuracy with Tag SNPs with p < 0.10 | rs2069772 | 0.56 | 8/10 | 0.292 |
| rs2069772 rs1800692 | 0.54 | 4/10 | 0.554 | |
| rs2069772 rs2146323 rs3213225 | 0.55 | 4/10 | 0.414 | |
| c. PTB Maternal-Fetal Combined | ||||
|---|---|---|---|---|
| Algorithm | Model | Testing Balanced Accuracy |
Cross Validation Consistency |
p-value |
| Balanced Accuracy with all tag SNPS | rs2069762 | 0.55 | 6/10 | 0.461 |
| rs2069762_2 rs2274849 | 0.58 | 5/10 | 0.254 | |
| Balanced Accuracy with TuRF – 10 SNPS | rs2069762_2 | 0.53 | 8/10 | 0.614 |
| rs2069762_2 rs16145 | 0.62 | 9/10 | 0.084 | |
| rs2069762_2 rs16145 rs2243290 | 0.51 | 4/10 | 0.943 | |
| Balanced Accuracy with Tag SNPs with p < 0.05 | rs8192282_2 | 0.61 | 7/10 | 0.050 |
| rs5356 rs1882435_2 | 0.58 | 2/10 | 0.160 | |
| rS1882435_2 rs16975492_2 rs2277698 | 0.54 | 1/10 | 0.456 | |
| Balanced Accuracy with Tag SNPs with p < 0.10 | rs8192282_2 | 0.61 | 7/10 | 0.050 |
| rs1143643_2 rs7338575_2 | 0.54 | 2/10 | 0.522 | |
| rs3752896 rs34003_2 rs598893_2 | 0.62 | 6/10 | 0.043 | |
Bold indicates a statistically significant interaction (permutation p < 0.05).
Combined analysis consisted of matching fetal to maternal individuals and adding the SNPs to the analysis (e.g., instead of an individual having 751 SNPs this would increase to 1449). Any results with a “_2” indicates that the genotypes were fetal. Any maternal or fetal genotypes without matches were removed from the combined analysis.
Figure 4.
MDR model for a three-way interaction involving maternal SNP rs3752896 (COL4A4) and fetal SNPs rs34003 (FGF1) and rs598893 (COL4A1). Each panel represents a three locus genotype; the genotype for each SNP is labeled on the figure. Each large square (3 × 3 box) represents a different genotype for rs598893_2 (GG on left, AG in the middle, and AA on the right). Within each square, each row of cells delineates rs34003_2 genotypes (top TT, middle GT and bottom GG) and each column the rs3752896 genotypes. Therefore, each small cell describes a single and unique three locus genotype. Within each cell are two bars that represent the number of cases with this genotype (left hand bar) and number of controls (right hand bar). Each multilocus cell is denoted as “high risk” (dark gray) or “low risk” (light gray) for spontaneous preterm labor/delivery with intact membranes. Risk status is determined by the ratio of cases to controls adjusted by the number of cases and controls studied. The testing average balanced accuracy is 62% (p-value = 0.043) with a cross-validation consistency of 6 out of 10.
Pathway analysis
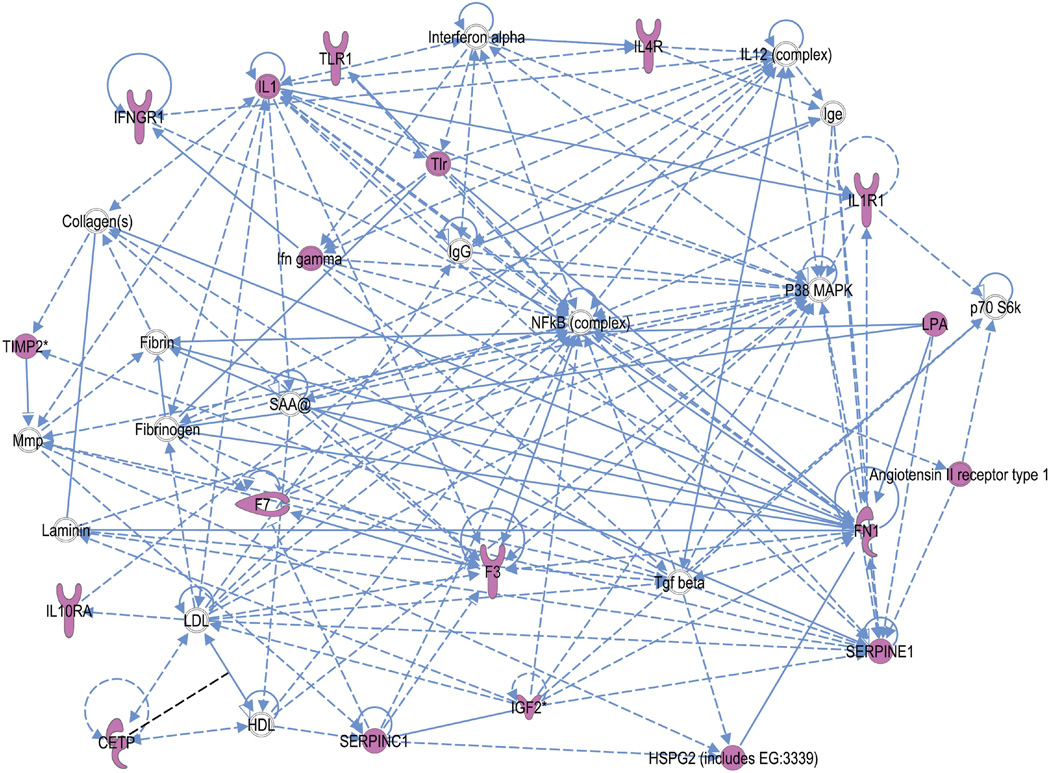
In mothers, the IPA network algorithm discovered that the focus molecules were significantly interconnected in four networks, scoring 12 to 20 (p= 10−12 to p= 10−20) (Table 10). Using the “overlapping network” feature of IPA, we found these networks were joined together by a few molecules, namely TIMP-2 as well as several network partners supplied by the IPA knowledge base (e.g. MMPs and ACAN, which is involved in cell adhesion and physiologic functions associated with extracellular matrix). An example of the top scoring network is shown in Figure 5. Using the fetal genes as input, the IPA network algorithm discovered that fetal focus genes are also highly interconnected. It modeled these into three networks, scoring 7 to 35 (Table 11). In contrast to the maternal model, these networks did not have overlapping molecules joining them, though TIMP2 did play a role in the top-ranked network along with several inflammatory genes (including TLR) and coagulation factors (Figure 6).
Table 10.
IPA networks of maternal genes
| Network | Molecules* | Calculated Score |
Focus molecules |
Top Functions of Network |
|---|---|---|---|---|
| 1 | AGT, AGTR1, Akt, COL4A2, Collagen(s), Creb, Cyclin A, Cyclin E, ERK, ERK1/2, FAK, FGF1, G-protein beta, GNB3, IGF1, IL6R, Insulin, Laminin, Mapk, Mek, Mlc, OXTR, p70 S6k, PDGF BB, PI3K, Pkc(s), PLC, Ras, Ras homolog, Rsk, Shc, STAT, THPO, TIMP2, Vegf | 20 | 10 | Cardiovascular System Development and Function, Tissue Morphology, Cellular Growth and Proliferation |
| 2 | Angiotensin II receptor type 1, C8, Calcineurin protein(s), Cyclooxygenase, F3, Fibrinogen, Ifn gamma, Ige, IgG, Ikb, IKK (complex), IL1, IL10, IL18, IL12 (complex), Il12 receptor, IL12B, Interferon alpha, LDL, MHC Class II, Mmp, MMP16, Nfat (family), NFkB (complex), NfkB-RelA, Nos, P38 MAPK, SELE, SERPINE1, Sod, STAT5a/b, Tgf beta, Tlr, TLR2, VEGFC | 18 | 9 | Cell-mediated Immune Response, Cellular Movement, Hematological System Development and Function |
| 3 | ACAN, acetaldehyde, ADAMTS1, Ap1, Calmodulin, Caspase, COL4A3, COL4A4 (includes EG:1286), FGFR1, FSH, Histone h3, Hsp70, HTR2A, IL17A (includes EG:3605), IL1B, ITGA3, Jnk, LRPAP1, LTF, MIF, MMP3, MMP7, MMP13, platelet activating factor, PTH, RAPGEF1, REN, SNCG, THBS4, Timp, TIMP2, Tnf receptor, Trypsin, xanthine, XDH | 16 | 8 | Connective Tissue Disorders, Genetic Disorder, Cellular Movement |
| 4 | ACAN, ADAM17, AFP, alcohol, CCL7, COL5A2, CRHR2, CRK, CXCL12, EGFR, FCER2, Fgfr, FGFR1, HMGA2, HSPG2 (includes EG:3339), IL6, IL17A (includes EG:3605), ITGA5, Mmp, MMP3, MMP7, MMP10, MMP13, MMP17, NQO1, platelet activating factor, PLC gamma, PTPN6, SAA2, TBXAS1, testosterone, Timp, TIMP2, TIMP4, TNF | 12 | 6 | Cellular Movement, Connective Tissue Disorders, Genetic Disorder |
Focus molecules are underlined.
Figure 5.
Connection map for the first ranked network generated by IPA from maternal focus gene input. The biomarkers passing the p < 0.05 significance threshold (focus molecules, depicted in pink) were entered in to the IPA software for an unsupervised functional analysis to discern regulatory networks involving these molecules. The asterisk indicates that there was more than one SNP probe for the gene tested and the most significant value was placed into the analysis. Solid lines show direct interaction (binding/physical contact); dashed line, indirect interaction supported by the literature but possibly involving one or more intermediate molecules that have not been investigated definitively. Molecular interactions involving only binding are connected with a line without an arrowhead since directionality cannot be inferred.
Table 11.
IPA networks of fetal genes
| Network | Molecules* | Calculated Score |
Focus molecules |
Top Functions of Network |
|---|---|---|---|---|
| 1 | Angiotensin II receptor type 1, CETP, Collagen(s),F3, F7, Fibrin, Fibrinogen, FN1, HDL, HSPG2 (includes EG:3339), Ifn gamma, IFNGR1, Ige, IGF2, IgG, IL1, IL10RA, IL12 (complex), IL1R1, IL4R, Interferon alpha, Laminin, LDL, LPA, Mmp, NFkB (complex), P38 MAPK, p70 S6k, SAA@, SERPINC1, SERPINE1, Tgf beta, TIMP2, Tlr, TLR1 | 35 | 15 | Cardiovascular Disease, Hematological Disease, Infectious Disease |
| 2 | AGTR1, Akt, Ap1, COL4A1, COL4A2, COL4A3, Creb, Cyclin A, Cyclin E, ERK, ERK1/2, FAK, FGF1, IGF2R, IL2, IL6R, Insulin, IRS1, MAP2K1/2, Mapk, Mek, MHC Class II, NPPA, NPR1, PDGF BB, PI3K, Pkc(s), PLC, Ras, REN, Shc, Sos, STAT, TCR, Vegf | 27 | 12 | Cardiovascular Disease, Cardiovascular System Development and Function, Embryonic Development |
| 3 | ADAM8, BDNF, C11ORF82, CARD18, CHAT, Ck2, COL4A4 (includes EG:1286), CXCL6, ELN, FSH, GBP6, GNL1, H2-Q4, Histone h3, IL1/IL6/TNF, IL17B, IL17C, IL1B, Jnk, MHC CLASS I D2D ANTIGEN, MIF, PENK, PLD3, Rac, SAA2, SCUBE1, SCUBE2, SLC14A1, SLC5A2, SLC5A4B, SLFN12L, TMEM176B, TNF, TNR, TREM3 | 7 | 4 | Cancer, Cell Cycle, Cell-To-Cell Signaling and Interaction |
Focus molecules are underlined.
Figure 6.
Connection map for the first ranked network generated by IPA from fetal focus gene input. The biomarkers passing the p < 0.05 significance threshold (focus molecules, depicted in pink) were entered in to the IPA software for an unsupervised functional analysis to discern regulatory networks involving these molecules. The asterisk indicates that there was more than one SNP probe for the gene tested and the most significant value was placed into the analysis. Solid lines show direct interaction (binding/physical contact); dashed line, indirect interaction supported by the literature but possibly involving one or more intermediate molecules that have not been investigated definitively. Molecular interactions involving only binding are connected with a line without an arrowhead since directionality cannot be inferred. The symbol, “SAA@” indicates serum amyloid, which includes SAA1, SAA2, SAA3P, and SAA4.
Our focus genes were identified by IPA as part of several well-established canonical signaling pathways. In mothers, the IPA algorithm identified the “hepatic fibrosis/hepatic stellate cell activation” as the best scoring of the canonical pathways involving the “focus genes” (p = 4.9 × 10−12). This pathway was also the best scoring of the canonical pathways in the fetal genes (p = 9.1 × 10−14). The coagulation system was the second ranked canonical pathway for the fetal genes (p = 1.0 × 10−6).
COMMENT
Principal findings of the study
We report the results of a large, carefully phenotyped genetic association study of women with preterm labor and delivery with intact membranes and their offspring in a single Hispanic population. This study identified maternal and fetal DNA variants that predispose to preterm labor with intact membranes leading to preterm delivery. The main observations were: 1) a SNP in TIMP2 in mothers was significantly associated with the phenotype; 2) a SNP in IL6R in fetuses increased the risk for preterm labor/delivery; 3) haplotypes for COL4A3 in the mother and IGF2 and IL2 in the fetus were associated with preterm labor/delivery with intact membranes; 4) pathway analysis suggested that maternal and fetal genes involved in the control of inflammation and extracellular matrix metabolism are involved in the biological processes that predispose to this pregnancy outcome; and 5) importantly, TIMP2 was consistently found in the different networks (maternal and fetal) associated with preterm labor/delivery with intact membranes. Collectively, these findings support the concept that there is a genetic component in the risk of spontaneous preterm labor/delivery with intact membranes in this population.
Single locus analysis for mothers
The observed association between TIMP2 and spontaneous preterm labor with intact membranes is novel and implicates extracellular matrix metabolism as an important factor predisposing to preterm parturition. TIMP2 is expressed in the amnion137 and plays an important role in the regulation of the activity of matrix degrading enzymes. At low concentrations, TIMP2 can enhance activation of MMP2, while at high concentrations it inhibits the activity of MMP2.138;139 Similarly, higher concentrations of TIMP2 inhibit MMP9 (gelatinase B) activity. MMP2 is a constitutive enzyme, while MMP9 is inducible in response to infection.140 Both have been found in amniotic fluid141–145 and the activity and concentration of MMP9 is increased in the amniotic fluid of women with preterm labor and intact membranes, as well as in women with preterm PROM.141;143 We have previously reported that amniotic fluid TIMP2 concentrations are low in women with spontaneous labor (term and preterm), with intact or ruptured membranes, regardless of the microbial status of the amniotic cavity.144 A decrease in TIMP2 is thought to favor the activity of MMPs, promoting extracellular matrix degradation associated with labor. In addition, TIMP2 has been implicated in the metabolism of collagen IV, a component of the basement membrane region of the amnion/chorion and uterine cervix.146–148 Moreover, TIMP2 has been found to inhibit angiogenesis through mechanisms that are independent of MMPs.149 We have recently reported that a subset of women who develop preterm labor with intact membranes have an increased plasma concentration of anti-angiogenic factors prior to the development of this complication.150 In a parallel study, we have found a significant association between the same TIMP2 SNP and pPROM (submitted for publication). Collectively, this evidence provides biological support for the association of a TIMP2 SNP with preterm labor/delivery with intact membranes.
Single locus analysis in fetuses demonstrates an association between preterm labor/delivery and IL6 receptor
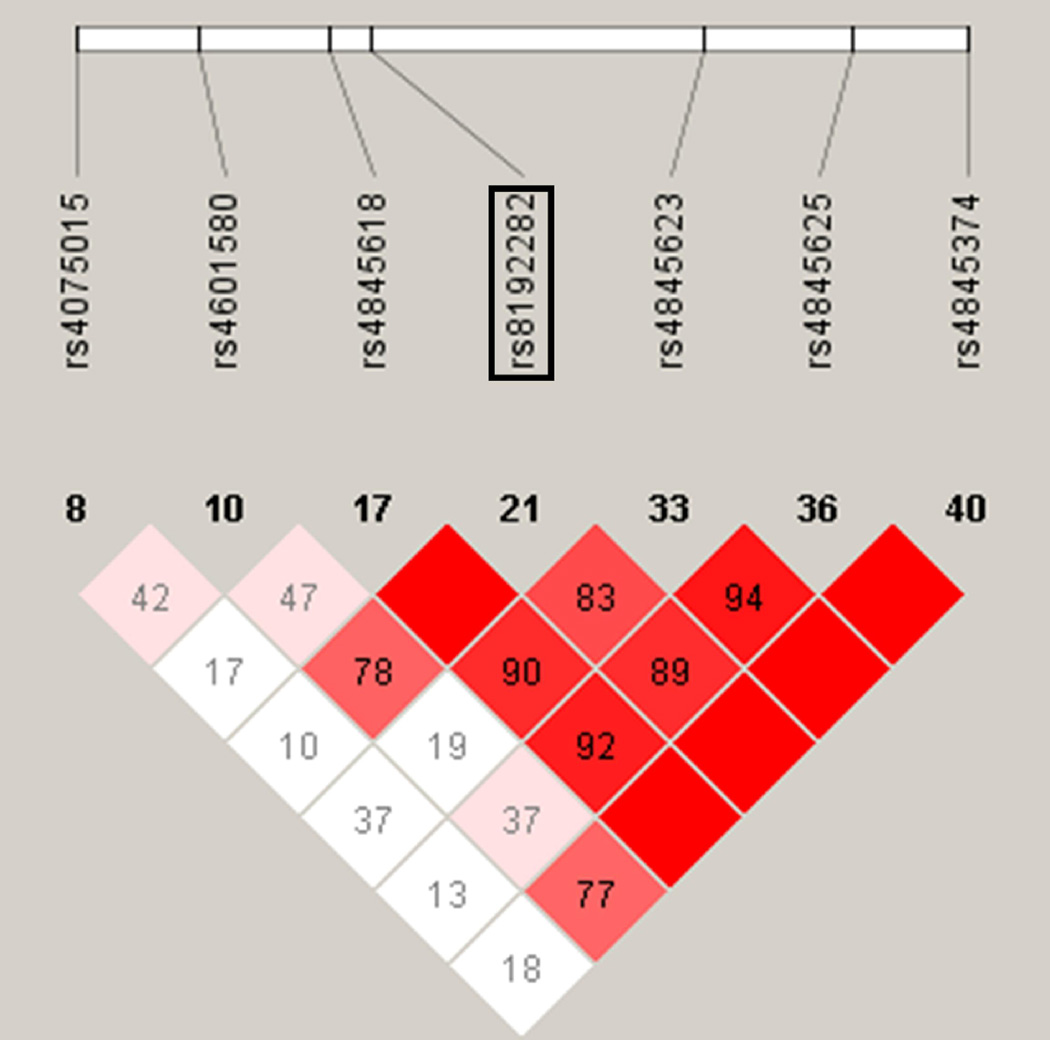
This is the first demonstration of an association between IL6R and spontaneous preterm labor with intact membranes in Hispanics. This observation is consistent with previous reports of IL6R associations with spontaneous PTB in individuals of European and African descent.89 The SNP found to be associated in our study was rs8192282. In contrast, the positive results of Velez et al.89 were based on multiple haplotypes in the IL6R gene in both European-American and African-American populations. However, the SNPs found to be significant in the previous study89 are in strong LD in Caucasian HapMap samples with the SNP we found in the current study (Figure 7; D’ = 0.78–1.00). The consistency of the association between IL6R and spontaneous preterm labor/delivery among different ethnic populations lends support to the importance of this finding.
Figure 7.
Linkage disequilibrium pattern of IL6R region in the HapMap CEU samples of IL6R SNPs from the current study and that of Velez et al.89 The Haploview figure is as described in Supplemental Figure 2. The SNP that associated with spontaneous preterm labor/delivery with intact membranes in the current study (rs 8192282) is denoted by a rectangle surrounding it in the top of the panel. All other SNPs are from associating haplotypes in Velez et al.89
Although IL-6 has been considered neither sufficient nor necessary for preterm delivery in murine models,151 the IL-6/IL-6R system has been previously implicated in preterm labor.152–154 The median amniotic fluid concentration of IL-6 in the midtrimester of pregnancy is higher in women destined to have a pregnancy loss or a preterm delivery than that of women who deliver at term.155–157 Moreover, among patients with preterm labor and intact membranes, amniotic fluid IL-6 concentrations are associated with preterm delivery, the amniocentesis-to-delivery interval, the likelihood of delivery within 48 hours and 7 days, as well as with neonatal morbidity and mortality.158;159 IL-6 is a major mediator of the acute phase response to infection and tissue injury and is differentially expressed by intrauterine tissues in the context of labor (term and preterm).22;160;161 Indeed, the fetal concentration of this cytokine has been used to define the fetal inflammatory response syndrome, which is associated with the onset of preterm labor, fetal multisystemic organ involvement, and neonatal morbidity.159;162–167 In addition, DNA variants in the IL-6 gene have been associated with sepsis, brain injury and cerebral palsy, specially in preterm neonates.168–171 IL-6 mediates its biological functions through the IL-6R. A cleaved form of the IL-6R can be found in biological fluids and tissues. The amniotic fluid concentrations of the soluble IL-6R are elevated in preterm labor with intra-amniotic infection.154 It is of relevance to the findings of this study that Velez et al.24;89 reported that amniotic fluid IL-6 concentrations were strongly associated with IL6R haplotypes in mothers and fetuses in a Caucasian population in the US. The effects of IL-6R genetic variants on IL-6 concentrations has also been reported in non-pregnant subjects and in one case it was found to be more important than IL-6 DNA variants themselves in determining IL-6 concentrations. This finding is consistent with our observation of both IL-6 and IL-6R associations in previous studies.172;173 Pathway analyses indicated that IL6R operates in a network entailing other fetal genes associated with spontaneous preterm labor/delivery with intact membranes, including collagens and IL2. Therefore, the statistical findings in this study, the consistency of the findings among ethnic groups, and the biological role of IL-6 in preterm labor indicate the importance of the IL-6/IL-6R system in the predisposition to spontaneous preterm labor/delivery.
Haplotype analyses suggest novel genes predisposing to preterm labor/delivery
Maternal haplotype analysis found that haplotypes in four genes were associated with preterm labor/delivery: COL4A3, IL6R, LTF, and FGF1 (Table 6). The most significant global association was in the COL4A3 gene. One haplotype (ACT) was associated with a 48% increased risk of preterm labor/delivery. Collagen IV is a major component of the basement membrane of several organs, including the uterine cervix.146–148;174 Treatment of cervical smooth muscle cells with TNF-alpha results in increased MMP-9 mRNA expression, induction of MMP-9 production, and decreased TIMP-2 secretion.175 Therefore, our findings with haplotype analysis for collagen IV and the single locus association in the mother supports the relationship between structural proteins of the extracellular matrix (collagen IV) and a regulator of its degradation (TIMP2) lending substantial biological plausibility to both associations.
The significant association between a haplotype in IL6R in the mothers and spontaneous preterm labor/delivery further supports the role of the IL6/IL6R system in the predisposition for this phenotype. In addition, the IL6R SNP rs8192282 in mothers was of marginal significance, even though it did not remain after multiple testing corrections (Table 5).A maternal haplotype in lactoferrin (LTF) was significantly associated with predisposition to preterm labor/delivery. This protein is a major component of the innate immune system and has antimicrobial properties, including the ability to protect against biofilm infections. Lactoferrin has been reported to prevent the formation of biofilms by Pseudomonas.176 Biofilms have been recently reported in the amniotic cavity177 and it is possible that a deficiency in the concentration of this protein may predispose to biofilm formation in this unique niche and its consequences, such as the difficulty in obtaining a positive culture and refractoriness to treatment.178–180 A proposed mechanism of action for lactoferrin to inhibit biofilm formation is via iron chelation and stimulation of a specialized form of bacterial-surface motility (twitching), thereby, preventing the formation of cell clusters and biofilms.176 This protein is normally present in the amniotic fluid and its concentration increases with gestational age and decreases at the time of the onset of spontaneous labor.181 Another important aspect of lactoferrin is that it downregulates pro-inflammatory cytokines and the production of reactive oxygen radicals. We have previously reported that the amniotic fluid concentrations of lactoferrin are increased in women with preterm labor and intra-amniotic infection.181
A maternal haplotype for fibroblast growth factor 1 (FGF1) was also associated with the risk of spontaneous preterm labor/delivery. This protein is very pleiotropic, and has been implicated in vascularization.182 A subgroup of patients with spontaneous preterm labor/delivery are thought to have vascular lesions as a primary mechanism of disease.183 It is possible that defective angiogenesis plays a role in some cases of preterm labor.150 Further studies are required to explore the role of FGF1 in this condition.
Three genes in fetal DNA had significant haplotype associations: IGF2, IL2, and COL4A1. The association with COL4A1 in the fetus is consistent with the finding of a haplotype in COL4A3 in the mother in this study, and TIMP2, as discussed above. The role of IL2 in preterm parturition is less clear. This cytokine is a T cell growth factor that has been reported to stimulate prostaglandin production by chorion.184;185 However, a role for IL-2 in parturition is not well established. It is possible that this cytokine may play a role in tolerance regulation of the fetal allograft. Finally, fetal haplotypes in IGF2 were found to be a large modifier of the risk of preterm delivery; one haplotype increased the risk, and another one decreased the risk. This gene was studied because we were interested in examining the relationship between DNA variants and SGA. Spontaneous preterm labor has been associated with SGA,186 and also with accelerated fetal growth.187;188 The finding reported herein calls for further examination of the role of insulin-like growth factor 2 and pathway in spontaneous preterm parturition.
Exploratory Analysis
Spontaneous preterm parturition is syndromic in nature, and multiple mechanisms of disease are likely to be involved.189–191 Discovery tools employing unbiased methods have been used to study complex problems in Obstetrics.192–195 To address the complexity of the genetic predisposition to spontaneous preterm labor and delivery, we performed two exploratory analyses. MDR explicitly addressed the potential role of interactions among genes (maternal, fetal, and maternal-fetal). The results of these analyses were not compelling in identifying complex genetic models (e.g. epistasis). Although such gene-gene interactions have been previously reported for spontaneous preterm birth involving IL6, IL6R, and TNF-alpha, these were in the mother in Caucasian individuals.123 Therefore, our negative results may reflect the different ethnic group under study, different DNA variants between studies, and the sensitivity of interaction tests to changes in allele frequencies.196 However, it is of note that the models we detected, although not significant, did include SNPs in genes that are in extracellular matrix metabolism and inflammatory pathways, especially IL6, MMP13, and several collagen genes.
Additionally, there is an increasing realization of the importance of pathways in predisposition to complex diseases. We utilized IPA to examine the contribution of genetic variants in determining networks and disease functions. The prominence of hepatic fibrosis among established signaling pathways in both maternal and fetal analysis presumably reflects involvement of inflammatory cascades and extracellular matrix remodeling. The findings suggest that maternal and fetal gene variants involving inflammation and extracellular matrix metabolism pathways predispose to spontaneous preterm labor/delivery. These findings are consistent with a large body of literature supporting this view.
Strengths and limitations of the study
The strengths of our study include a well-defined phenotype (spontaneous preterm labor and delivery with intact membranes) and a relatively homogeneous population. This is the largest study to examine the genetic predisposition to spontaneous preterm birth in Hispanics. Moreover, the study includes both maternal and fetal DNA and a relatively large number of genes and DNA variants. The number of DNA variants selected was estimated to cover 90% of the coding DNA variation in the candidate genes. Importantly, we identified that both maternal and fetal DNA variants contributed to modify the risk. Limitations of these types of studies are that confirmation of the findings is required and that we have not examined the effect of environmental factors,50;197;198 which are known to play a role in the risk of preterm delivery. In addition, functional studies are needed to assess the precise physiologic implications of the DNA variants and their iterations identified in this study.
Conclusion
This large genetic association study of candidate genes involved in adverse pregnancy outcome revealed that both maternal and fetal DNA variants are associated with spontaneous preterm delivery.
Supplementary Material
Acknowledgment
This research was supported (in part) by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Footnotes
This work was presented in Abstract form at the 30th Annual Meeting of the Society for Maternal-Fetal Medicine in Chicago, IL, February 1–6, 2010, and was the recipient of the March of Dimes Award for Best Research in Prematurity.
Reference List
- 1.McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N.Engl.J.Med. 1985;312:82–90. doi: 10.1056/NEJM198501103120204. [DOI] [PubMed] [Google Scholar]
- 2.Slattery MM, Morrison JJ. Preterm delivery. Lancet. 2002;360:1489–1497. doi: 10.1016/S0140-6736(02)11476-0. [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biggio J, Christiaens I, Katz M, Menon R, Merialdi M, Morken NH, et al. A call for an international consortium on the genetics of preterm birth. Am J Obstet.Gynecol. 2008;199:95–97. doi: 10.1016/j.ajog.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhari BP, Plunkett J, Ratajczak CK, Shen TT, DeFranco EA, Muglia LJ. The genetics of birth timing: insights into a fundamental component of human development. Clin.Genet. 2008;74:493–501. doi: 10.1111/j.1399-0004.2008.01124.x. [DOI] [PubMed] [Google Scholar]
- 6.Plunkett J, Borecki I, Morgan T, Stamilio D, Muglia LJ. Population-based estimate of sibling risk for preterm birth, preterm premature rupture of membranes, placental abruption and pre-eclampsia. BMC.Genet. 2008;9:44. doi: 10.1186/1471-2156-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porter TF, Fraser AM, Hunter CY, Ward RH, Varner MW. The risk of preterm birth across generations. Obstet.Gynecol. 1997;90:63–67. doi: 10.1016/S0029-7844(97)00215-9. [DOI] [PubMed] [Google Scholar]
- 8.Winkvist A, Mogren I, Hogberg U. Familial patterns in birth characteristics: impact on individual and population risks. Int.J.Epidemiol. 1998;27:248–254. doi: 10.1093/ije/27.2.248. [DOI] [PubMed] [Google Scholar]
- 9.Clausson B, Lichtenstein P, Cnattingius S. Genetic influence on birthweight and gestational length determined by studies in offspring of twins. BJOG. 2000;107:375–381. doi: 10.1111/j.1471-0528.2000.tb13234.x. [DOI] [PubMed] [Google Scholar]
- 10.Treloar SA, Macones GA, Mitchell LE, Martin NG. Genetic influences on premature parturition in an Australian twin sample. Twin.Res. 2000;3:80–82. doi: 10.1375/136905200320565526. [DOI] [PubMed] [Google Scholar]
- 11.Ward K, Argyle V, Meade M, Nelson L. The heritability of preterm delivery. Obstet.Gynecol. 2005;106:1235–1239. doi: 10.1097/01.AOG.0000189091.35982.85. [DOI] [PubMed] [Google Scholar]
- 12.Wilcox AJ, Skjaerven R, Lie RT. Familial patterns of preterm delivery: maternal and fetal contributions. Am J Epidemiol. 2008;167:474–479. doi: 10.1093/aje/kwm319. [DOI] [PubMed] [Google Scholar]
- 13.Boyd HA, Poulsen G, Wohlfahrt J, Murray JC, Feenstra B, Melbye M. Maternal contributions to preterm delivery. Am J Epidemiol. 2009;170:1358–1364. doi: 10.1093/aje/kwp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plunkett J, Feitosa MF, Trusgnich M, Wangler MF, Palomar L, Kistka ZA, et al. Mother's genome or maternally-inherited genes acting in the fetus influence gestational age in familial preterm birth. Hum.Hered. 2009;68:209–219. doi: 10.1159/000224641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinberg CR, Shi M. The genetics of preterm birth: using what we know to design better association studies. Am J Epidemiol. 2009;170:1373–1381. doi: 10.1093/aje/kwp325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Savitz DA. Preterm birth subtypes among blacks and whites. Epidemiology. 1992;3:428–433. doi: 10.1097/00001648-199209000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Kistka ZA, Palomar L, Lee KA, Boslaugh SE, Wangler MF, Cole FS, et al. Racial disparity in the frequency of recurrence of preterm birth. Am J Obstet.Gynecol. 2007;196:131–136. doi: 10.1016/j.ajog.2006.06.093. [DOI] [PubMed] [Google Scholar]
- 18.Palomar L, DeFranco EA, Lee KA, Allsworth JE, Muglia LJ. Paternal race is a risk factor for preterm birth. Am J Obstet.Gynecol. 2007;197:152–157. doi: 10.1016/j.ajog.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 19.Menon R, Pearce B, Velez DR, Merialdi M, Williams SM, Fortunato SJ, et al. Racial disparity in pathophysiologic pathways of preterm birth based on genetic variants. Reprod.Biol.Endocrinol. 2009;7:62. doi: 10.1186/1477-7827-7-62. 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen DP, Genc M, Vardhana S, Babula O, Onderdonk A, Witkin SS. Ethnic differences of polymorphisms in cytokine and innate immune system genes in pregnant women. Obstet.Gynecol. 2004;104:293–300. doi: 10.1097/01.AOG.0000133486.85400.5e. [DOI] [PubMed] [Google Scholar]
- 21.Menon R, Velez DR, Thorsen P, Vogel I, Jacobsson B, Williams SM, et al. Ethnic differences in key candidate genes for spontaneous preterm birth: TNF-alpha and its receptors. Hum.Hered. 2006;62:107–118. doi: 10.1159/000096301. [DOI] [PubMed] [Google Scholar]
- 22.Menon R, Merialdi M, Lombardi SJ, Fortunato SJ. Differences in the placental membrane cytokine response: a possible explanation for the racial disparity in preterm birth. Am.J.Reprod.Immunol. 2006;56:112–118. doi: 10.1111/j.1600-0897.2006.00394.x. [DOI] [PubMed] [Google Scholar]
- 23.Menon R, Williams SM, Fortunato SJ. Amniotic fluid interleukin-1beta and interleukin-8 concentrations: racial disparity in preterm birth. Reprod.Sci. 2007;14:253–259. doi: 10.1177/1933719107301336. [DOI] [PubMed] [Google Scholar]
- 24.Velez DR, Menon R, Thorsen P, Jiang L, Simhan H, Morgan N, et al. Ethnic differences in interleukin 6 (IL-6) and IL6 receptor genes in spontaneous preterm birth and effects on amniotic fluid protein levels. Ann.Hum.Genet. 2007;71:586–600. doi: 10.1111/j.1469-1809.2007.00352.x. [DOI] [PubMed] [Google Scholar]
- 25.Dizon-Townson DS, Major H, Varner M, Ward K. A promoter mutation that increases transcription of the tumor necrosis factor-alpha gene is not associated with preterm delivery. Am.J.Obstet.Gynecol. 1997;177:810–813. doi: 10.1016/s0002-9378(97)70273-4. [DOI] [PubMed] [Google Scholar]
- 26.Roberts AK, Monzon-Bordonaba F, Van Deerlin PG, Holder J, Macones GA, Morgan MA, et al. Association of polymorphism within the promoter of the tumor necrosis factor alpha gene with increased risk of preterm premature rupture of the fetal membranes. Am J Obstet Gynecol. 1999;180:1297–1302. doi: 10.1016/s0002-9378(99)70632-0. [DOI] [PubMed] [Google Scholar]
- 27.Aidoo M, McElroy PD, Kolczak MS, Terlouw DJ, ter Kuile FO, Nahlen B, et al. Tumor necrosis factor-alpha promoter variant 2 (TNF2) is associated with pre-term delivery, infant mortality, and malaria morbidity in western Kenya: Asembo Bay Cohort Project IX. Genet.Epidemiol. 2001;21:201–211. doi: 10.1002/gepi.1029. [DOI] [PubMed] [Google Scholar]
- 28.Ferrand PE, Fujimoto T, Chennathukuzhi V, Parry S, Macones GA, Sammel M, et al. The CARD15 2936insC mutation and TLR4 896 A>G polymorphism in African Americans and risk of preterm premature rupture of membranes (PPROM) Mol.Hum.Reprod. 2002;8:1031–1034. doi: 10.1093/molehr/8.11.1031. [DOI] [PubMed] [Google Scholar]
- 29.Ferrand PE, Parry S, Sammel M, Macones GA, Kuivaniemi H, Romero R, et al. A polymorphism in the matrix metalloproteinase-9 promoter is associated with increased risk of preterm premature rupture of membranes in African Americans. Mol.Hum.Reprod. 2002;8:494–501. doi: 10.1093/molehr/8.5.494. [DOI] [PubMed] [Google Scholar]
- 30.Fujimoto T, Parry S, Urbanek M, Sammel M, Macones G, Kuivaniemi H, et al. A single nucleotide polymorphism in the matrix metalloproteinase-1 (MMP-1) promoter influences amnion cell MMP-1 expression and risk for preterm premature rupture of the fetal membranes. J.Biol.Chem. 2002;277:6296–6302. doi: 10.1074/jbc.M107865200. [DOI] [PubMed] [Google Scholar]
- 31.Genc MR, Gerber S, Nesin M, Witkin SS. Polymorphism in the interleukin-1 gene complex and spontaneous preterm delivery. Am.J.Obstet.Gynecol. 2002;187:157–163. doi: 10.1067/mob.2002.122407. [DOI] [PubMed] [Google Scholar]
- 32.Landau R, Xie HG, Dishy V, Stein CM, Wood AJ, Emala CW, et al. beta2-Adrenergic receptor genotype and preterm delivery. Am.J.Obstet.Gynecol. 2002;187:1294–1298. doi: 10.1067/mob.2002.128524. [DOI] [PubMed] [Google Scholar]
- 33.Lorenz E, Hallman M, Marttila R, Haataja R, Schwartz DA. Association between the Asp299Gly polymorphisms in the Toll-like receptor 4 and premature births in the Finnish population. Pediatr.Res. 2002;52:373–376. doi: 10.1203/00006450-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Ozkur M, Dogulu F, Ozkur A, Gokmen B, Inaloz SS, Aynacioglu AS. Association of the Gln27Glu polymorphism of the beta-2-adrenergic receptor with preterm labor. Int J Gynaecol Obstet. 2002;77:209–215. doi: 10.1016/s0020-7292(02)00035-8. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, et al. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. 2002;287:195–202. doi: 10.1001/jama.287.2.195. [DOI] [PubMed] [Google Scholar]
- 36.Chen D, Hu Y, Wu B, Chen L, Fang Z, Yang F, et al. Tumor necrosis factor-alpha gene G308A polymorphism is associated with the risk of preterm delivery. Beijing Da.Xue.Xue.Bao. 2003;35:377–381. [PubMed] [Google Scholar]
- 37.Kalish RB, Vardhana S, Gupta M, Chasen ST, Perni SC, Witkin SS. Interleukin-1 receptor antagonist gene polymorphism and multifetal pregnancy outcome. Am.J.Obstet.Gynecol. 2003;189:911–914. doi: 10.1067/s0002-9378(03)00770-1. [DOI] [PubMed] [Google Scholar]
- 38.Simhan HN, Krohn MA, Roberts JM, Zeevi A, Caritis SN. Interleukin-6 promoter-174 polymorphism and spontaneous preterm birth. Am J Obstet Gynecol. 2003;189:915–918. doi: 10.1067/s0002-9378(03)00843-3. [DOI] [PubMed] [Google Scholar]
- 39.Witkin SS, Vardhana S, Yih M, Doh K, Bongiovanni AM, Gerber S. Polymorphism in intron 2 of the fetal interleukin-1 receptor antagonist genotype influences midtrimester amniotic fluid concentrations of interleukin-1beta and interleukin-1 receptor antagonist and pregnancy outcome. Am J Obstet Gynecol. 2003;189:1413–1417. doi: 10.1067/s0002-9378(03)00630-6. [DOI] [PubMed] [Google Scholar]
- 40.Amory JH, Adams KM, Lin MT, Hansen JA, Eschenbach DA, Hitti J. Adverse outcomes after preterm labor are associated with tumor necrosis factor-alpha polymorphism-863, but not-308, in mother-infant pairs. Am.J.Obstet.Gynecol. 2004;191:1362–1367. doi: 10.1016/j.ajog.2004.05.067. [DOI] [PubMed] [Google Scholar]
- 41.Annells MF, Hart PH, Mullighan CG, Heatley SL, Robinson JS, Bardy P, et al. Interleukins-1, -4, -6, -10, tumor necrosis factor, transforming growth factor-beta, FAS, and mannose-binding protein C gene polymorphisms in Australian women: Risk of preterm birth. Am.J.Obstet.Gynecol. 2004;191:2056–2067. doi: 10.1016/j.ajog.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 42.Bessler H, Osovsky M, Sirota L. Association between IL-1ra gene polymorphism and premature delivery. Biol.Neonate. 2004;85:179–183. doi: 10.1159/000075578. [DOI] [PubMed] [Google Scholar]
- 43.Chen D, Hu Y, Chen C, Yang F, Fang Z, Wang L, et al. Polymorphisms of the paraoxonase gene and risk of preterm delivery. Epidemiology. 2004;15:466–470. doi: 10.1097/01.ede.0000129509.59912.b2. [DOI] [PubMed] [Google Scholar]
- 44.Doh K, Sziller I, Vardhana S, Kovacs E, Papp Z, Witkin SS. Beta2-adrenergic receptor gene polymorphisms and pregnancy outcome. J.Perinat.Med. 2004;32:413–417. doi: 10.1515/JPM.2004.138. [DOI] [PubMed] [Google Scholar]
- 45.Genc MR, Onderdonk AB, Vardhana S, Delaney ML, Norwitz ER, Tuomala RE, et al. Polymorphism in intron 2 of the interleukin-1 receptor antagonist gene, local midtrimester cytokine response to vaginal flora, and subsequent preterm birth. Am.J.Obstet.Gynecol. 2004;191:1324–1330. doi: 10.1016/j.ajog.2004.05.074. [DOI] [PubMed] [Google Scholar]
- 46.Hao K, Wang X, Niu T, Xu X, Li A, Chang W, et al. A candidate gene association study on preterm delivery: application of high-throughput genotyping technology and advanced statistical methods. Hum.Mol.Genet. 2004;13:683–691. doi: 10.1093/hmg/ddh091. [DOI] [PubMed] [Google Scholar]
- 47.Hartel CH, Finas D, Ahrens P, Kattner E, Schaible T, Muller D, et al. Polymorphisms of genes involved in innate immunity: association with preterm delivery. Mol Hum.Reprod. 2004;10:911–915. doi: 10.1093/molehr/gah120. [DOI] [PubMed] [Google Scholar]
- 48.Kalish RB, Vardhana S, Gupta M, Perni SC, Witkin SS. Interleukin-4 and-10 gene polymorphisms and spontaneous preterm birth in multifetal gestations. Am.J.Obstet.Gynecol. 2004;190:702–706. doi: 10.1016/j.ajog.2003.09.066. [DOI] [PubMed] [Google Scholar]
- 49.Kalish RB, Vardhana S, Gupta M, Perni SC, Chasen ST, Witkin SS. Polymorphisms in the tumor necrosis factor-alpha gene at position-308 and the inducible 70 kd heat shock protein gene at position +1267 in multifetal pregnancies and preterm premature rupture of fetal membranes. Am.J.Obstet.Gynecol. 2004;191:1368–1374. doi: 10.1016/j.ajog.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Macones GA, Parry S, Elkousy M, Clothier B, Ural SH, Strauss JF., III A polymorphism in the promoter region of TNF and bacterial vaginosis: preliminary evidence of gene-environment interaction in the etiology of spontaneous preterm birth. Am.J.Obstet.Gynecol. 2004;190:1504–1508. doi: 10.1016/j.ajog.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 51.Moore S, Ide M, Randhawa M, Walker JJ, Reid JG, Simpson NA. An investigation into the association among preterm birth, cytokine gene polymorphisms and periodontal disease. BJOG. 2004;111:125–132. doi: 10.1046/j.1471-0528.2003.00024.x-i1. [DOI] [PubMed] [Google Scholar]
- 52.Nukui T, Day RD, Sims CS, Ness RB, Romkes M. Maternal/newborn GSTT1 null genotype contributes to risk of preterm, low birthweight infants. Pharmacogenetics. 2004;14:569–576. doi: 10.1097/00008571-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 53.Papazoglou D, Galazios G, Koukourakis MI, Kontomanolis EN, Maltezos E. Association of-634G/C and 936C/T polymorphisms of the vascular endothelial growth factor with spontaneous preterm delivery. Acta Obstet Gynecol Scand. 2004;83:461–465. doi: 10.1111/j.0001-6349.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- 54.Resch B, Gallistl S, Kutschera J, Mannhalter C, Muntean W, Mueller WD. Thrombophilic polymorphisms--factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations--and preterm birth. Wien.Klin.Wochenschr. 2004;116:622–626. doi: 10.1007/s00508-004-0223-9. [DOI] [PubMed] [Google Scholar]
- 55.Valdez LL, Quintero A, Garcia E, Olivares N, Celis A, Rivas F, Jr, et al. Thrombophilic polymorphisms in preterm delivery. Blood Cells Mol Dis. 2004;33:51–56. doi: 10.1016/j.bcmd.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 56.Wang H, Parry S, Macones G, Sammel MD, Ferrand PE, Kuivaniemi H, et al. Functionally significant SNP MMP8 promoter haplotypes and preterm premature rupture of membranes (PPROM) Hum.Mol Genet. 2004;13:2659–2669. doi: 10.1093/hmg/ddh287. [DOI] [PubMed] [Google Scholar]
- 57.Crider KS, Whitehead N, Buus RM. Genetic variation associated with preterm birth: a HuGE review. Genet.Med. 2005;7:593–604. doi: 10.1097/01.gim.0000187223.69947.db. [DOI] [PubMed] [Google Scholar]
- 58.Engel SA, Erichsen HC, Savitz DA, Thorp J, Chanock SJ, Olshan AF. Risk of spontaneous preterm birth is associated with common proinflammatory cytokine polymorphisms. Epidemiology. 2005;16:469–477. doi: 10.1097/01.ede.0000164539.09250.31. [DOI] [PubMed] [Google Scholar]
- 59.Fuks A, Parton LA, Polavarapu S, Netta D, Strassberg S, Godi I, et al. Polymorphism of Fas and Fas ligand in preterm premature rupture of membranes in singleton pregnancies. Am.J.Obstet.Gynecol. 2005;193:1132–1136. doi: 10.1016/j.ajog.2005.05.082. [DOI] [PubMed] [Google Scholar]
- 60.Hartel C, von OS, Koch J, Ahrens P, Kattner E, Segerer H, et al. Polymorphisms of haemostasis genes as risk factors for preterm delivery. Thromb.Haemost. 2005;94:88–92. doi: 10.1160/TH04-10-0653. [DOI] [PubMed] [Google Scholar]
- 61.Johnson WG, Scholl TO, Spychala JR, Buyske S, Stenroos ES, Chen X. Common dihydrofolate reductase 19-base pair deletion allele: a novel risk factor for preterm delivery. Am.J.Clin.Nutr. 2005;81:664–668. doi: 10.1093/ajcn/81.3.664. [DOI] [PubMed] [Google Scholar]
- 62.Kalish RB, Nguyen DP, Vardhana S, Gupta M, Perni SC, Witkin SS. A single nucleotide A>G polymorphism at position-670 in the Fas gene promoter: relationship to preterm premature rupture of fetal membranes in multifetal pregnancies. Am.J.Obstet.Gynecol. 2005;192:208–212. doi: 10.1016/j.ajog.2004.06.106. [DOI] [PubMed] [Google Scholar]
- 63.Bodamer OA, Mitterer G, Maurer W, Pollak A, Mueller MW, Schmidt WM. Evidence for an association between mannose-binding lectin 2 (MBL2) gene polymorphisms and pre-term birth. Genet.Med. 2006;8:518–524. doi: 10.1097/01.gim.0000232478.43335.19. [DOI] [PubMed] [Google Scholar]
- 64.Engel SM, Olshan AF, Siega-Riz AM, Savitz DA, Chanock SJ. Polymorphisms in folate metabolizing genes and risk for spontaneous preterm and small-for-gestational age birth. Am.J.Obstet.Gynecol. 2006;195:1231–1211. doi: 10.1016/j.ajog.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 65.Erichsen HC, Engel SA, Eck PK, Welch R, Yeager M, Levine M, et al. Genetic variation in the sodium-dependent vitamin C transporters, SLC23A1, and SLC23A2 and risk for preterm delivery. Am.J.Epidemiol. 2006;163:245–254. doi: 10.1093/aje/kwj035. [DOI] [PubMed] [Google Scholar]
- 66.Kalish RB, Vardhana S, Normand NJ, Gupta M, Witkin SS. Association of a maternal CD14-159 gene polymorphism with preterm premature rupture of membranes and spontaneous preterm birth in multi-fetal pregnancies. J.Reprod.Immunol. 2006;70:109–117. doi: 10.1016/j.jri.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 67.Kerk J, Dordelmann M, Bartels DB, Brinkhaus MJ, Dammann CE, Dork T, et al. Multiplex measurement of cytokine/receptor gene polymorphisms and interaction between interleukin-10 (−1082) genotype and chorioamnionitis in extreme preterm delivery. J.Soc.Gynecol.Investig. 2006;13:350–356. doi: 10.1016/j.jsgi.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 68.Mattar R, de Souza E, Daher S. Preterm delivery and cytokine gene polymorphisms. J.Reprod.Med. 2006;51:317–320. [PubMed] [Google Scholar]
- 69.Murtha AP, Nieves A, Hauser ER, Swamy GK, Yonish BA, Sinclair TR, et al. Association of maternal IL-1 receptor antagonist intron 2 gene polymorphism and preterm birth. Am.J.Obstet.Gynecol. 2006;195:1249–1253. doi: 10.1016/j.ajog.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 70.Speer EM, Gentile DA, Zeevi A, Pillage G, Huo D, Skoner DP. Role of single nucleotide polymorphisms of cytokine genes in spontaneous preterm delivery. Hum.Immunol. 2006;67:915–923. doi: 10.1016/j.humimm.2006.08.291. [DOI] [PubMed] [Google Scholar]
- 71.Wang H, Parry S, Macones G, Sammel MD, Kuivaniemi H, Tromp G, et al. A functional SNP in the promoter of the SERPINH1 gene increases risk of preterm premature rupture of membranes in African Americans. Proc.Natl.Acad.Sci.U.S.A. 2006;103:13463–13467. doi: 10.1073/pnas.0603676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ameglio F, Vento G, Romagnoli C, Giardina B, Capoluongo E. Association of MBL2 variants with early preterm delivery. Genet.Med. 2007;9:136–137. doi: 10.1097/GIM.0b013e31802fba43. [DOI] [PubMed] [Google Scholar]
- 73.Chen BH, Carmichael SL, Shaw GM, Iovannisci DM, Lammer EJ. Association between 49 infant gene polymorphisms and preterm delivery. Am.J.Med.Genet.A. 2007;143A:1990–1996. doi: 10.1002/ajmg.a.31868. [DOI] [PubMed] [Google Scholar]
- 74.Ehn NL, Cooper ME, Orr K, Shi M, Johnson MK, Caprau D, et al. Evaluation of fetal and maternal genetic variation in the progesterone receptor gene for contributions to preterm birth. Pediatr.Res. 2007;62:630–635. doi: 10.1203/PDR.0b013e3181567bfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gibson CS, MacLennan AH, Dekker GA, Goldwater PN, Dambrosia JM, Munroe DJ, et al. Genetic polymorphisms and spontaneous preterm birth. Obstet.Gynecol. 2007;109:384–391. doi: 10.1097/01.AOG.0000252712.62241.1a. [DOI] [PubMed] [Google Scholar]
- 76.Grisaru-Granovsky S, Tevet A, Bar-Shavit R, Salah Z, Elstein D, Samueloff A, et al. Association study of protease activated receptor 1 gene polymorphisms and adverse pregnancy outcomes: results of a pilot study in Israel. Am.J.Med.Genet.A. 2007;143A:2557–2563. doi: 10.1002/ajmg.a.31985. [DOI] [PubMed] [Google Scholar]
- 77.Meirhaeghe A, Boreham CA, Murray LJ, Richard F, Davey SG, Young IS, et al. A possible role for the PPARG Pro12Ala polymorphism in preterm birth. Diabetes. 2007;56:494–498. doi: 10.2337/db06-0915. [DOI] [PubMed] [Google Scholar]
- 78.Steffen KM, Cooper ME, Shi M, Caprau D, Simhan HN, Dagle JM, et al. Maternal and fetal variation in genes of cholesterol metabolism is associated with preterm delivery. J Perinatol. 2007;27:672–680. doi: 10.1038/sj.jp.7211806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stonek F, Hafner E, Philipp K, Hefler LA, Bentz EK, Tempfer CB. Methylenetetrahydrofolate reductase C677T polymorphism and pregnancy complications. Obstet Gynecol. 2007;110:363–368. doi: 10.1097/01.AOG.0000270122.13198.6f. [DOI] [PubMed] [Google Scholar]
- 80.Valdez-Velazquez LL, Quintero-Ramos A, Perez SA, Mendoza-Carrera F, Montoya-Fuentes H, Rivas F, Jr, et al. Genetic polymorphisms of the renin-angiotensin system in preterm delivery and premature rupture of membranes. J Renin.Angiotensin.Aldosterone.Syst. 2007;8:160–168. doi: 10.3317/jraas.2007.026. [DOI] [PubMed] [Google Scholar]
- 81.Chaves JH, Babayan A, Bezerra CM, Linhares IM, Witkin SS. Maternal and neonatal interleukin-1 receptor antagonist genotype and pregnancy outcome in a population with a high rate of pre-term birth. Am J Reprod.Immunol. 2008;60:312–317. doi: 10.1111/j.1600-0897.2008.00625.x. [DOI] [PubMed] [Google Scholar]
- 82.Diaz-Cueto L, Dominguez-Lopez P, Cantillo-Cabarcas J, Perez-Figueroa G, Arechavaleta-Velasco M, Arechavaleta-Velasco F. Progesterone receptor gene polymorphisms are not associated with preterm birth in a Hispanic population. Int.J.Gynaecol.Obstet. 2008;103:153–157. doi: 10.1016/j.ijgo.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 83.Fortunato SJ, Menon R, Velez DR, Thorsen P, Williams SM. Racial disparity in maternal-fetal genetic epistasis in spontaneous preterm birth. Am.J.Obstet.Gynecol. 2008;198:666–669. doi: 10.1016/j.ajog.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 84.Hollegaard MV, Grove J, Thorsen P, Wang X, Mandrup S, Christiansen M, et al. Polymorphisms in the tumor necrosis factor alpha and interleukin 1-beta promoters with possible gene regulatory functions increase the risk of preterm birth. Acta Obstet.Gynecol.Scand. 2008;87:1285–1290. doi: 10.1080/00016340802468340. [DOI] [PubMed] [Google Scholar]
- 85.Rey G, Skowronek F, Alciaturi J, Alonso J, Bertoni B, Sapiro R. Toll receptor 4 Asp299Gly polymorphism and its association with preterm birth and premature rupture of membranes in a South American population. Mol Hum.Reprod. 2008;14:555–559. doi: 10.1093/molehr/gan049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Suh YJ, Kim YJ, Park H, Park EA, Ha EH. Oxidative stress-related gene interactions with preterm delivery in Korean women. Am J Obstet Gynecol. 2008;198:541–547. doi: 10.1016/j.ajog.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 87.Uma R, Forsyth JS, Struthers AD, Fraser CG, Godfrey V, Murphy DJ. Correlation of angiotensin converting enzyme activity and the genotypes of the I/D polymorphism in the ACE gene with preterm birth and birth weight. Eur J Obstet Gynecol Reprod Biol. 2008;141:27–30. doi: 10.1016/j.ejogrb.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 88.Velez DR, Fortunato SJ, Thorsen P, Lombardi SJ, Williams SM, Menon R. Preterm birth in Caucasians is associated with coagulation and inflammation pathway gene variants. PLoS.One. 2008;3:e3283. doi: 10.1371/journal.pone.0003283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Velez DR, Fortunato SJ, Williams SM, Menon R. Interleukin-6 (IL-6) and receptor (IL6-R) gene haplotypes associate with amniotic fluid protein concentrations in preterm birth. Hum.Mol Genet. 2008;17:1619–1630. doi: 10.1093/hmg/ddn049. [DOI] [PubMed] [Google Scholar]
- 90.Velez DR, Menon R, Simhan H, Fortunato S, Canter JA, Williams SM. Mitochondrial DNA variant A4917G, smoking and spontaneous preterm birth. Mitochondrion. 2008;8:130–135. doi: 10.1016/j.mito.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 91.Gargano JW, Holzman CB, Senagore PK, Reuss ML, Pathak DR, Friderici KH, et al. Polymorphisms in thrombophilia and renin-angiotensin system pathways, preterm delivery, and evidence of placental hemorrhage. Am.J.Obstet.Gynecol. 2009;201:317–319. doi: 10.1016/j.ajog.2009.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kramer MS, Kahn SR, Rozen R, Evans R, Platt RW, Chen MF, et al. Vasculopathic and thrombophilic risk factors for spontaneous preterm birth. Int.J.Epidemiol. 2009;38:715–723. doi: 10.1093/ije/dyp167. [DOI] [PubMed] [Google Scholar]
- 93.Kwon HS, Sohn IS, Lee JY, Lee SJ, Kim SN, Kim BJ. Intercellular adhesion molecule-1 K469E polymorphism in Korean patients with spontaneous preterm delivery. Int.J.Gynaecol.Obstet. 2009;104:37–39. doi: 10.1016/j.ijgo.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 94.Sata F, Toya S, Yamada H, Suzuki K, Saijo Y, Yamazaki A, et al. Proinflammatory cytokine polymorphisms and the risk of preterm birth and low birthweight in a Japanese population. Mol Hum.Reprod. 2009;15:121–130. doi: 10.1093/molehr/gan078. [DOI] [PubMed] [Google Scholar]
- 95.Tsai HJ, Yu Y, Zhang S, Pearson C, Ortiz K, Xu X, et al. Association of genetic ancestry with preterm delivery and related traits among African American mothers. Am J Obstet Gynecol. 2009;201:94–10. doi: 10.1016/j.ajog.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Velez DR, Fortunato S, Thorsen P, Lombardi SJ, Williams SM, Menon R. Spontaneous preterm birth in African Americans is associated with infection and inflammatory response gene variants. Am J Obstet Gynecol. 2009;200:209–227. doi: 10.1016/j.ajog.2008.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yu Y, Tsai HJ, Liu X, Mestan K, Zhang S, Pearson C, et al. The joint association between F5 gene polymorphisms and maternal smoking during pregnancy on preterm delivery. Hum.Genet. 2009;124:659–668. doi: 10.1007/s00439-008-0589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gonzalez R, Merialdi M, Lincetto O, Lauer J, Becerra C, Castro R, et al. Reduction in neonatal mortality in Chile between 1990 and 2000. Pediatrics. 2006;117:e949–e954. doi: 10.1542/peds.2005-2354. [DOI] [PubMed] [Google Scholar]
- 99.Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, Rubens C, Menon R, Van Look PF. WHO systematic review on maternal mortality and morbidity: The global burden of preterm birth. The Bulletin of the World Health Organization. 2009 doi: 10.2471/BLT.08.062554. Ref Type: Report. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stephens JC, Schneider JA, Tanguay DA, Choi J, Acharya T, Stanley SE, et al. Haplotype variation and linkage disequilibrium in 313 human genes. Science. 2001;293:489–493. doi: 10.1126/science.1059431. %20. [DOI] [PubMed] [Google Scholar]
- 101.Judson R, Salisbury B, Schneider J, Windemuth A, Stephens JC. How many SNPs does a genome-wide haplotype map require? Pharmacogenomics. 2002;3:379–391. doi: 10.1517/14622416.3.3.379. [DOI] [PubMed] [Google Scholar]
- 102.Kuivaniemi H, Yoon S, Shibamura H, Skunca M, Vongpunsawad S, Tromp G. Primer-extension preamplified DNA is a reliable template for genotyping. Clin.Chem. 2002;48:1601–1604. [PubMed] [Google Scholar]
- 103.Winkelmann BR, Hoffmann MM, Nauck M, Kumar AM, Nandabalan K, Judson RS, et al. Haplotypes of the cholesteryl ester transfer protein gene predict lipid-modifying response to statin therapy. Pharmacogenomics.J. 2003;3:284–296. doi: 10.1038/sj.tpj.6500195. [DOI] [PubMed] [Google Scholar]
- 104.Boehnke M, Cox NJ. Accurate inference of relationships in sib-pair linkage studies. Am.J.Hum.Genet. 1997;61:423–429. doi: 10.1086/514862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Goring HH, Ott J. Relationship estimation in affected sib pair analysis of late-onset diseases. Eur.J.Hum.Genet. 1997;5:69–77. [PubMed] [Google Scholar]
- 106.Olson JM. Relationship estimation by Markov-process models in a sib-pair linkage study. Am.J.Hum.Genet. 1999;64:1464–1472. doi: 10.1086/302360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Epstein MP, Duren WL, Boehnke M. Improved inference of relationship for pairs of individuals. Am.J.Hum.Genet. 2000;67:1219–1231. doi: 10.1016/s0002-9297(07)62952-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abecasis GR, Cherny SS, Cookson WO, Cardon LR. GRR: graphical representation of relationship errors. Bioinformatics. 2001;17:742–743. doi: 10.1093/bioinformatics/17.8.742. [DOI] [PubMed] [Google Scholar]
- 109.Douglas JA, Skol AD, Boehnke M. Probability of detection of genotyping errors and mutations as inheritance inconsistencies in nuclear-family data. Am.J.Hum.Genet. 2002;70:487–495. doi: 10.1086/338919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee WC. Detecting population stratification using a panel of single nucleotide polymorphisms. Int.J.Epidemiol. 2003;32:1120. doi: 10.1093/ije/dyg301. [DOI] [PubMed] [Google Scholar]
- 111.Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat.Genet. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 112.Nielsen DM, Ehm MG, Weir BS. Detecting marker-disease association by testing for Hardy-Weinberg disequilibrium at a marker locus. Am.J.Hum.Genet. 1998;63:1531–1540. doi: 10.1086/302114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wittke-Thompson JK, Pluzhnikov A, Cox NJ. Rational inferences about departures from Hardy-Weinberg equilibrium. Am.J.Hum.Genet. 2005;76:967–986. doi: 10.1086/430507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Trikalinos TA, Salanti G, Khoury MJ, Ioannidis JP. Impact of violations and deviations in Hardy-Weinberg equilibrium on postulated gene-disease associations. Am.J.Epidemiol. 2006;163:300–309. doi: 10.1093/aje/kwj046. [DOI] [PubMed] [Google Scholar]
- 115.Ryckman KK, Jiang L, Li C, Bartlett J, Haines JL, Williams SM. A prevalence-based association test for case-control studies. Genet.Epidemiol. 2008;32:600–605. doi: 10.1002/gepi.20342. [DOI] [PubMed] [Google Scholar]
- 116.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am.J.Hum.Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Devlin B, Risch N. A comparison of linkage disequilibrium measures for fine-scale mapping. Genomics. 1995;29:311–322. doi: 10.1006/geno.1995.9003. %20; [DOI] [PubMed] [Google Scholar]
- 119.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 120.Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 121.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav.Brain Res. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 122.Williams SM, Ritchie MD, Phillips JA, III, Dawson E, Prince M, Dzhura E, et al. Multilocus analysis of hypertension: a hierarchical approach. Hum.Hered. 2004;57:28–38. doi: 10.1159/000077387. [DOI] [PubMed] [Google Scholar]
- 123.Menon R, Velez DR, Simhan H, Ryckman K, Jiang L, Thorsen P, et al. Multilocus interactions at maternal tumor necrosis factor-alpha, tumor necrosis factor receptors, interleukin-6 and interleukin-6 receptor genes predict spontaneous preterm labor in European-American women. Am.J.Obstet.Gynecol. 2006;194:1616–1624. doi: 10.1016/j.ajog.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 124.Ritchie MD, Hahn LW, Roodi N, Bailey LR, Dupont WD, Parl FF, et al. Multifactor-dimensionality reduction reveals high-order interactions among estrogen-metabolism genes in sporadic breast cancer. Am.J.Hum.Genet. 2001;69:138–147. doi: 10.1086/321276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Velez DR, White BC, Motsinger AA, Bush WS, Ritchie MD, Williams SM, et al. A balanced accuracy function for epistasis modeling in imbalanced datasets using multifactor dimensionality reduction. Genet.Epidemiol. 2007;31:306–315. doi: 10.1002/gepi.20211. [DOI] [PubMed] [Google Scholar]
- 126.Moore JH, White BC. Tuning ReliefF for Genome-Wide Genetic Analysis. In: Marchiori E, Moore JH, Rajapakse JC, editors. Evolutionary Computation, Machine Learning and Data Mining in Bioinformatics. Heidelberg: Springer Berlin; 2007. pp. 166–175. [Google Scholar]
- 127.Kira K, Rendell LA. A Practical Approach to Feature Selection. In: Sleeman D, Edwards P, editors. Proceedings of the Ninth International Workshop on Machine Learning. Morgan-Kaufman; Aberdeen: 1992. pp. 249–256. [Google Scholar]
- 128.Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, et al. A network-based analysis of systemic inflammation in humans. Nature. 2005;437:1032–1037. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- 129.Loza MJ, McCall CE, Li L, Isaacs WB, Xu J, Chang BL. Assembly of inflammation-related genes for pathway-focused genetic analysis. PLoS.One. 2007;2:e1035. doi: 10.1371/journal.pone.0001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kuijl C, Savage ND, Marsman M, Tuin AW, Janssen L, Egan DA, et al. Intracellular bacterial growth is controlled by a kinase network around PKB/AKT1. Nature. 2007;450:725–730. doi: 10.1038/nature06345. [DOI] [PubMed] [Google Scholar]
- 131.Winn VD, Haimov-Kochman R, Paquet AC, Yang YJ, Madhusudhan MS, Gormley M, et al. Gene expression profiling of the human maternal-fetal interface reveals dramatic changes between midgestation and term. Endocrinology. 2007;148:1059–1079. doi: 10.1210/en.2006-0683. [DOI] [PubMed] [Google Scholar]
- 132.Goldenberg RL, Tamura T. Prepregnancy weight and pregnancy outcome. JAMA. 1996;275:1127–1128. [PubMed] [Google Scholar]
- 133.Andres RL, Day MC. Perinatal complications associated with maternal tobacco use. Semin.Neonatol. 2000;5:231–241. doi: 10.1053/siny.2000.0025. [DOI] [PubMed] [Google Scholar]
- 134.Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine.Tob.Res. 2004;6(Suppl 2):S125–S140. S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 135.Hendler I, Goldenberg RL, Mercer BM, Iams JD, Meis PJ, Moawad AH, et al. The Preterm Prediction Study: association between maternal body mass index and spontaneous and indicated preterm birth. Am.J.Obstet.Gynecol. 2005;192:882–886. doi: 10.1016/j.ajog.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 136.Torloni MR, Betran AP, Daher S, Widmer M, Dolan SM, Menon R, et al. Maternal BMI and preterm birth: a systematic review of the literature with meta-analysis. J.Matern.Fetal Neonatal Med. 2009;22:957–970. doi: 10.3109/14767050903042561. [DOI] [PubMed] [Google Scholar]
- 137.Rowe TF, King LA, MacDonald PC, Casey ML. Tissue inhibitor of metalloproteinase-1 and tissue inhibitor of metalloproteinase-2 expression in human amnion mesenchymal and epithelial cells. Am J Obstet.Gynecol. 1997;176:915–921. doi: 10.1016/s0002-9378(97)70621-5. [DOI] [PubMed] [Google Scholar]
- 138.Caterina JJ, Yamada S, Caterina NC, Longenecker G, Holmback K, Shi J, et al. Inactivating mutation of the mouse tissue inhibitor of metalloproteinases-2(Timp-2) gene alters proMMP-2 activation. J Biol.Chem. 2000;275:26416–26422. doi: 10.1074/jbc.M001271200. [DOI] [PubMed] [Google Scholar]
- 139.Worley JR, Thompkins PB, Lee MH, Hutton M, Soloway P, Edwards DR, et al. Sequence motifs of tissue inhibitor of metalloproteinases 2 (TIMP-2) determining progelatinase A (proMMP-2) binding and activation by membrane-type metalloproteinase 1 (MT1-MMP) Biochem.J. 2003;372:799–809. doi: 10.1042/BJ20021573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fortunato SJ, Menon R, Bryant C, Lombardi SJ. Programmed cell death (apoptosis) as a possible pathway to metalloproteinase activation and fetal membrane degradation in premature rupture of membranes. Am J Obstet.Gynecol. 2000;182:1468–1476. doi: 10.1067/mob.2000.107330. [DOI] [PubMed] [Google Scholar]
- 141.Athayde N, Romero R, Gomez R, Maymon E, Pacora P, Mazor M, et al. Matrix metalloproteinases-9 in preterm and term human parturition. J.Matern.Fetal Med. 1999;8:213–219. doi: 10.1002/(SICI)1520-6661(199909/10)8:5<213::AID-MFM3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 142.Riley SC, Leask R, Chard T, Wathen NC, Calder AA, Howe DC. Secretion of matrix metalloproteinase-2, matrix metalloproteinase-9 and tissue inhibitor of metalloproteinases into the intrauterine compartments during early pregnancy. Mol.Hum.Reprod. 1999;5:376–381. doi: 10.1093/molehr/5.4.376. [DOI] [PubMed] [Google Scholar]
- 143.Maymon E, Romero R, Pacora P, Gervasi MT, Gomez R, Edwin SS, et al. Evidence of in vivo differential bioavailability of the active forms of matrix metalloproteinases 9 and 2 in parturition, spontaneous rupture of membranes, and intra-amniotic infection. Am.J.Obstet.Gynecol. 2000;183:887–894. doi: 10.1067/mob.2000.108878. [DOI] [PubMed] [Google Scholar]
- 144.Maymon E, Romero R, Pacora P, Gomez R, Mazor M, Edwin S, et al. A role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2) in human parturition, premature rupture of membranes and intraamniotic infection. J.Perinat.Med. 2001;29:308–316. doi: 10.1515/JPM.2001.044. [DOI] [PubMed] [Google Scholar]
- 145.Lavee M, Goldman S, niel-Spiegel E, Shalev E. Matrix metalloproteinase-2 is elevated in midtrimester amniotic fluid prior to the development of preeclampsia. Reprod.Biol.Endocrinol. 2009;7:85. doi: 10.1186/1477-7827-7-85. 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Frappart L, Berger G, Grimaud JA, Chevalier M, Bremond A, Rochet Y, et al. Basement membrane of the uterine cervix: immunofluorescence characteristics of the collagen component in normal or atypical epithelium and invasive carcinoma. Gynecol.Oncol. 1982;13:58–66. doi: 10.1016/0090-8258(82)90009-9. [DOI] [PubMed] [Google Scholar]
- 147.Stewart CJ, McNicol AM. Distribution of type IV collagen immunoreactivity to assess questionable early stromal invasion. J.Clin.Pathol. 1992;45:9–15. doi: 10.1136/jcp.45.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Miosge N. The ultrastructural composition of basement membranes in vivo. Histol.Histopathol. 2001;16:1239–1248. doi: 10.14670/HH-16.1239. [DOI] [PubMed] [Google Scholar]
- 149.Seo DW, Li H, Guedez L, Wingfield PT, Diaz T, Salloum R, et al. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell. 2003;114:171–180. doi: 10.1016/s0092-8674(03)00551-8. [DOI] [PubMed] [Google Scholar]
- 150.Chaiworapongsa T, Romero R, Tarca A, Pedro KJ, Mittal P, Kwon KS, et al. A subset of patients destined to develop spontaneous preterm labor has an abnormal angiogenic/anti-angiogenic profile in maternal plasma: evidence in support of pathophysiologic heterogeneity of preterm labor derived from a longitudinal study. J.Matern.Fetal Neonatal Med. 2009;22:1122–1139. doi: 10.3109/14767050902994838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yoshimura K, Hirsch E. Interleukin-6 is neither necessary nor sufficient for preterm labor in a murine infection model. J Soc.Gynecol.Investig. 2003;10:423–427. doi: 10.1016/s1071-5576(03)00138-2. [DOI] [PubMed] [Google Scholar]
- 152.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J.Clin.Invest. 1990;85:1392–1400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am.J.Obstet.Gynecol. 1993;169:805–816. doi: 10.1016/0002-9378(93)90009-8. [DOI] [PubMed] [Google Scholar]
- 154.Ghezzi F, Cohen J, Tolosa JE, Gomez R, Mazor M, Berry SM, Romero R. Microbial invasion of the amniotic cavity is associated with increased concentrations of amniotic fluid interleukin-6 soluble receptor. Am.J.Obstet.Gynecol. 1996;174(1 (Pt 2)):402. Ref Type: Abstract. [Google Scholar]
- 155.Wenstrom KD, Andrews WW, Tamura T, DuBard MB, Johnston KE, Hemstreet GP. Elevated amniotic fluid interleukin-6 levels at genetic amniocentesis predict subsequent pregnancy loss. Am.J.Obstet.Gynecol. 1996;175:830–833. doi: 10.1016/s0002-9378(96)80007-x. [DOI] [PubMed] [Google Scholar]
- 156.Wenstrom KD, Andrews WW, Hauth JC, Goldenberg RL, DuBard MB, Cliver SP. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am.J.Obstet.Gynecol. 1998;178:546–550. doi: 10.1016/s0002-9378(98)70436-3. [DOI] [PubMed] [Google Scholar]
- 157.Yoon BH, Oh SY, Romero R, Shim SS, Han SY, Park JS, et al. An elevated amniotic fluid matrix metalloproteinase-8 level at the time of mid-trimester genetic amniocentesis is a risk factor for spontaneous preterm delivery. Am.J.Obstet.Gynecol. 2001;185:1162–1167. doi: 10.1067/mob.2001.117678. [DOI] [PubMed] [Google Scholar]
- 158.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am.J.Obstet.Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 159.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J.Matern.Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 160.Steinborn A, Geisse M, Kaufmann M. Expression of cytokine receptors in the placenta in term and preterm labour. Placenta. 1998;19:165–170. doi: 10.1016/s0143-4004(98)90005-4. [DOI] [PubMed] [Google Scholar]
- 161.Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M, et al. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am.J.Obstet.Gynecol. 2006;195:394–324. doi: 10.1016/j.ajog.2005.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am.J.Obstet.Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 163.Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am.J.Obstet.Gynecol. 1998;179:186–193. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 164.Romero R, Maymon E, Pacora P, Gomez R, Mazor M, Yoon BH, et al. Further observations on the fetal inflammatory response syndrome: a potential homeostatic role for the soluble receptors of tumor necrosis factor alpha. Am.J.Obstet.Gynecol. 2000;183:1070–1077. doi: 10.1067/mob.2000.108885. [DOI] [PubMed] [Google Scholar]
- 165.Romero R, Espinoza J, Goncalves LF, Gomez R, Medina L, Silva M, et al. Fetal cardiac dysfunction in preterm premature rupture of membranes. J.Matern.Fetal Neonatal Med. 2004;16:146–157. doi: 10.1080/14767050400009279. [DOI] [PubMed] [Google Scholar]
- 166.Kim YM, Romero R, Chaiworapongsa T, Espinoza J, Mor G, Kim CJ. Dermatitis as a component of the fetal inflammatory response syndrome is associated with activation of Toll-like receptors in epidermal keratinocytes. Histopathology. 2006;49:506–514. doi: 10.1111/j.1365-2559.2006.02542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, et al. The fetal inflammatory response syndrome. Clin.Obstet.Gynecol. 2007;50:652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 168.Kilpinen S, Hulkkonen J, Wang XY, Hurme M. The promoter polymorphism of the interleukin-6 gene regulates interleukin-6 production in neonates but not in adults. Eur.Cytokine Netw. 2001;12:62–68. [PubMed] [Google Scholar]
- 169.Harding D, Dhamrait S, Millar A, Humphries S, Marlow N, Whitelaw A, et al. Is interleukin-6 -174 genotype associated with the development of septicemia in preterm infants? Pediatrics. 2003;112:800–803. doi: 10.1542/peds.112.4.800. [DOI] [PubMed] [Google Scholar]
- 170.Harding DR, Dhamrait S, Whitelaw A, Humphries SE, Marlow N, Montgomery HE. Does interleukin-6 genotype influence cerebral injury or developmental progress after preterm birth? Pediatrics. 2004;114:941–947. doi: 10.1542/peds.2003-0494-F. [DOI] [PubMed] [Google Scholar]
- 171.Wu YW, Croen LA, Torres AR, Van De WJ, Grether JK, Hsu NN. Interleukin-6 genotype and risk for cerebral palsy in term and near-term infants. Ann.Neurol. 2009;66:663–670. doi: 10.1002/ana.21766. [DOI] [PubMed] [Google Scholar]
- 172.Rafiq S, Frayling TM, Murray A, Hurst A, Stevens K, Weedon MN, et al. A common variant of the interleukin 6 receptor (IL-6r) gene increases IL-6r and IL-6 levels, without other inflammatory effects. Genes Immun. 2007;8:552–559. doi: 10.1038/sj.gene.6364414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Reich D, Patterson N, Ramesh V, De Jager PL, McDonald GJ, Tandon A, et al. Admixture mapping of an allele affecting interleukin 6 soluble receptor and interleukin 6 levels. Am J Hum.Genet. 2007;80:716–726. doi: 10.1086/513206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Winkler M, Rath W. Changes in the cervical extracellular matrix during pregnancy and parturition. J Perinat.Med. 1999;27:45–60. doi: 10.1515/JPM.1999.006. [DOI] [PubMed] [Google Scholar]
- 175.Watari M, Watari H, DiSanto ME, Chacko S, Shi GP, Strauss JF., III Pro-inflammatory cytokines induce expression of matrix-metabolizing enzymes in human cervical smooth muscle cells. Am J Pathol. 1999;154:1755–1762. doi: 10.1016/S0002-9440(10)65431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Singh PK, Parsek MR, Greenberg EP, Welsh MJ. A component of innate immunity prevents bacterial biofilm development. Nature. 2002;417:552–555. doi: 10.1038/417552a. [DOI] [PubMed] [Google Scholar]
- 177.Romero R, Schaudinn C, Kusanovic JP, Gorur A, Gotsch F, Webster P, et al. Detection of a microbial biofilm in intraamniotic infection. Am.J.Obstet.Gynecol. 2008;198:135. doi: 10.1016/j.ajog.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 179.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–138. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 180.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin.Microbiol.Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pacora P, Maymon E, Gervasi MT, Gomez R, Edwin SS, Yoon BH, et al. Lactoferrin in intrauterine infection, human parturition, and rupture of fetal membranes. Am.J.Obstet.Gynecol. 2000;183:904–910. doi: 10.1067/mob.2000.108882. [DOI] [PubMed] [Google Scholar]
- 182.Hoshino M, Takahashi M, Aoike N. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. J Allergy Clin.Immunol. 2001;107:295–301. doi: 10.1067/mai.2001.111928. [DOI] [PubMed] [Google Scholar]
- 183.Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am.J.Obstet.Gynecol. 1993;168:585–591. doi: 10.1016/0002-9378(93)90499-9. [DOI] [PubMed] [Google Scholar]
- 184.Coulam CH, Edwin SS, LaMarche S, Mitchell MD. Actions of interleukin-2 on chorio-decidual prostaglandin biosynthesis. Prostaglandins. 1993;46:145–156. doi: 10.1016/0090-6980(93)90040-e. [DOI] [PubMed] [Google Scholar]
- 185.Zicari A, Ticconi C, Pasetto N, Losardo A, Salerno A, Pontieri G, et al. Interleukin-2 in human amniotic fluid during pregnancy and parturition: implications for prostaglandin E2 release by fetal membranes. J.Reprod.Immunol. 1995;29:197–208. doi: 10.1016/0165-0378(95)00945-h. [DOI] [PubMed] [Google Scholar]
- 186.Espinoza J, Kusanovic JP, Kim CJ, Kim YM, Kim JS, Hassan SS, et al. An episode of preterm labor is a risk factor for the birth of a small-for-gestational-age neonate. Am.J.Obstet.Gynecol. 2007;196:574–575. doi: 10.1016/j.ajog.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lampl M, Kusanovic JP, Erez O, Espinoza J, Gotsch F, Goncalves L, et al. Early rapid growth, early birth: accelerated fetal growth and spontaneous late preterm birth. Am.J.Hum.Biol. 2009;21:141–150. doi: 10.1002/ajhb.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lampl M, Kusanovic JP, Erez O, Gotsch F, Espinoza J, Goncalves L, et al. Growth perturbations in a phenotype with rapid fetal growth preceding preterm labor and term birth. Am.J.Hum.Biol. 2009;21:782–792. doi: 10.1002/ajhb.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. 17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Romero R. Prenatal medicine: the child is the father of the man. J.Matern.Fetal Neonatal Med. 2009;22:636–639. doi: 10.1080/14767050902784171. 1996. [DOI] [PubMed] [Google Scholar]
- 191.Di Renzo GC. The great obstetrical syndromes. J.Matern.Fetal Neonatal Med. 2009;22:633–635. doi: 10.1080/14767050902866804. [DOI] [PubMed] [Google Scholar]
- 192.Romero R, Kuivaniemi H, Tromp G. Functional genomics and proteomics in term and preterm parturition. J Clin.Endocrinol.Metab. 2002;87:2431–2434. doi: 10.1210/jcem.87.6.8689. [DOI] [PubMed] [Google Scholar]
- 193.Romero R, Kuivaniemi H, Tromp G, Olson J. The design, execution, and interpretation of genetic association studies to decipher complex diseases. Am.J.Obstet.Gynecol. 2002;187:1299–1312. doi: 10.1067/mob.2002.128319. [DOI] [PubMed] [Google Scholar]
- 194.Romero R, Tromp G. High-dimensional biology in obstetrics and gynecology: functional genomics in microarray studies. Am J Obstet.Gynecol. 2006;195:360–363. doi: 10.1016/j.ajog.2006.06.077. [DOI] [PubMed] [Google Scholar]
- 195.Romero R, Espinoza J, Gotsch F, Kusanovic JP, Friel LA, Erez O, et al. The use of high-dimensional biology (genomics, transcriptomics, proteomics, and metabolomics) to understand the preterm parturition syndrome. BJOG. 2006;113(Suppl 3):118–135. doi: 10.1111/j.1471-0528.2006.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Greene CS, Penrod NM, Williams SM, Moore JH. Failure to replicate a genetic association may provide important clues about genetic architecture. PLoS.One. 2009;4:e5639. doi: 10.1371/journal.pone.0005639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Romero R, Chaiworapongsa T, Kuivaniemi H, Tromp G. Bacterial vaginosis, the inflammatory response and the risk of preterm birth: a role for genetic epidemiology in the prevention of preterm birth. Am J Obstet.Gynecol. 2004;190:1509–1519. doi: 10.1016/j.ajog.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 198.Merialdi M, Murray JC. The changing face of preterm birth. Pediatrics. 2007;120:1133–1134. doi: 10.1542/peds.2007-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.