Abstract
The current status of peptides that target the mitochondria in the context of cancer is the focus of this review. Chemotherapy and radiotherapy used to kill tumor cells are principally mediated by the process of apoptosis that is governed by the mitochondria. The failure of anticancer therapy often resides at the level of the mitochondria. Therefore, the mitochondrion is a key pharmacological target in cancer due to many of the differences that arise between malignant and healthy cells at the level of this ubiquitous organelle. Additionally, targeting the characteristics of malignant mitochondria often rely on disruption of protein–protein interactions that are not generally amenable to small molecules. We discuss anticancer peptides that intersect with pathological changes in the mitochondrion.
The mitochondrion holds importance among cellular organelles in consideration of selective anticancer therapy because it is the nexus for propagating malignant transformation and controls cell death. The mitochondrion is a universal target in all cancer cells, and new information about functional and structural differences between healthy and malignant cells continues to emerge [1]. Peptides that specifically target malignant mitochondria offer advantages of low toxicity, high specificity and generally increase the range of interactions that are difficult to target with small molecules (e.g., protein–protein, protein–lipid and protein–DNA) [2]. However, the benefits of peptides are offset by difficulties in delivery, such as degradation by proteases and rapid clearance. As such, the delivery of peptide/protein therapeutics is almost exclusively by the parenteral route, but the status quo is actively being challenged by broad efforts (e.g., liposomes, microparticles, nanoparticles, ‘smart’ polymers, hydrogels and chemical modifications to the peptide/protein) to achieve more convenient routes of administration (i.e., oral, nasal and transdermal) and improved pharmacokinetics [3–5]. However, peptides, in many cases, are far from passive in the delivery process and through the maze that is drug delivery (from route of administration to perhaps a target residing within a specific organelle), peptides are employed as the vehicle, homing motif and trans-/intra-cellular passport [6].
There is an emerging view in cancer therapy that the way in which a cancer cell dies (i.e., immunogenic cell death) is important for a durable response [7]. However, traditional chemo therapy and immune response are in conflict because chemotherapy (e.g., DNA-damaging agents) often induces the substantial destruction of immune effectors [8]. However, targeting the mitochondria may be the key to initiate or manipulate the type of death a cell undergoes and commit a cancer cell to ‘immunogenic apoptosis’ [9]. Pairing therapeutic peptides targeting malignant mitochondria with existing small-molecule chemo therapy could lower dose requirements and curb traditional treatment-associated toxicity [10]. Thereby, combined therapy could spare the immune system, reduce patient side effects, sensitize cancer cells to death and establish a durable response [11].
The mitochondrion is now dually famous as both the cellular ‘powerhouse’ and as the central regulator of the cell death pathway that bears its name. The outer membrane of the mitochondrion (OMM) is generally permeable to metabolites and ions, while the inner membrane (IMM) maintains a tightly regulated ion flux. Respiration-driven efflux of protons across the IMM during oxidative phosphorylation sets up a large electrochemical gradient composed of a difference in both the voltage (Δψm) and the concen tration of ions across the membrane (ΔpIon). This electrochemical gradient is the driving force for the production of ATP. Importantly, the negative interior, called the mitochondrial matrix (where the citric acid cycle and fatty acid β-oxidation transpire), allows the accumulation of molecules with cationic character. The intermembrane space (IMS) hosts an array of cytotoxic proteins that are released upon OMM permeabilization [12].
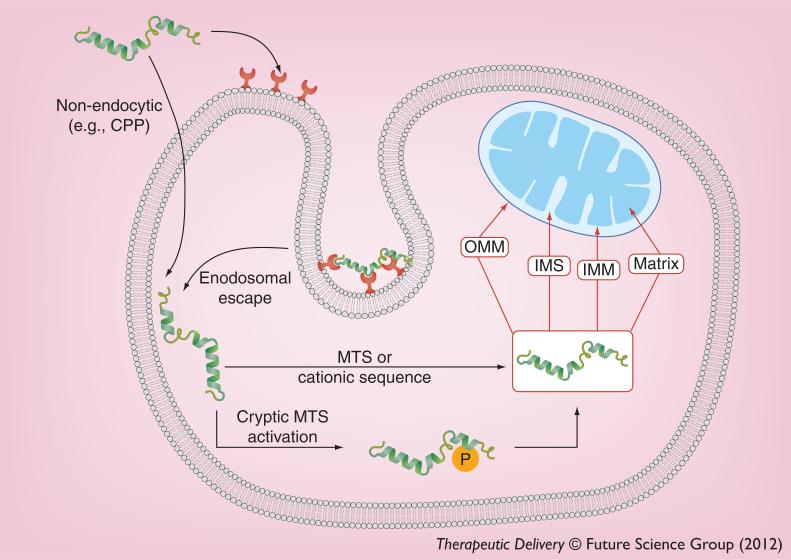
Newer cancer therapies are expected to kill malignant cells while producing minimal or no toxicity to normal cells. This expectation is borne out of the tremendous pace at which our understanding of tumor-specific molecular profiles has occurred in the last decade. The identification of tumor markers has lead to the development of new selective cancer therapies such as trastuzumab, which targets the over expression of HER2/neu in 30% of patients with breast cancer [13]. However, the search is on for more global cancer markers with the hope of generating a more generic cancer therapy. Peptides could properly be referred to as ‘smart’ nanoparticles because they are capable of encoding targeting, entry and therapeutic modality into one customizable unit [14]. To this end, one of the emerging strategies is the therapeutic targeting of proteins that affect multiple downstream pathways (i.e., so-called nodal proteins) in cancer signaling [15]. In this respect, the mitochondrion has become the focus of much interest for cancer therapy as it is the central ‘node’ of cell death induction (Figure 1) [11].
Figure 1. Targeting the mitochondria of malignant cells with peptides.
Selective targeting of the mitochondria of malignant cells can be achieved by engineering motifs that drive mitochondrial accumulation of the toxic peptide, based on differences that commonly exist between cancerous and healthy cells. Internalization of the peptides can be mediated based on the nature of the peptide (e.g., CPPs) and its interaction with the plasma membrane or via receptor complex endocytosis (e.g., tumor-homing motif). The internal milieu of malignant cells (e.g., pro-oxidative environment or upregulated enzymes, such as kinases) can provide the opportunity to customize the mitochondrial targeting of a peptide. For instance, a cryptic MTS can be employed that requires phosphorylation prior to mitochondrial trafficking. Depending on the sequence, mitochondriotoxic peptides can be ‘addressed’ to individual or multiple submitochondrial locations.
CPP: Cell-penetrating peptide; IMM: Inner mitochondrial membrane; IMS: Intermitochondrial membrane space; MTS: Mitochondrial targeting sequence; OMM: Outer mitochondrial membrane; P: Phosphate.
The mitochondria of tumor cells are structurally and functionally different from those of untransformed cells [6]. The alterations in malignant mitochondria or the metabolic pathways governing mitochondrial function vary by cancer type and level of disease progression [16]. Mitochondrial adaptations that take place in cancer range from subtle (e.g., isoform switching) to profound dysfunction [17]; yet, any deviation between healthy and malignant cells is an opportunity to be therapeutically leveraged. Many of the current and emerging therapeutic mitochondrial targets are protein–protein/lipid/DNA interactions that are less amenable to small-molecule intervention. Peptide (and protein) thera peutics, as a class of drugs, are well suited to exploit the often subtle differences between healthy and diseased mitochondria. This review focuses on current and potential mitochondrial anticancer targets in connection with therapeutic peptides.
Mitochondrial cell death pathway
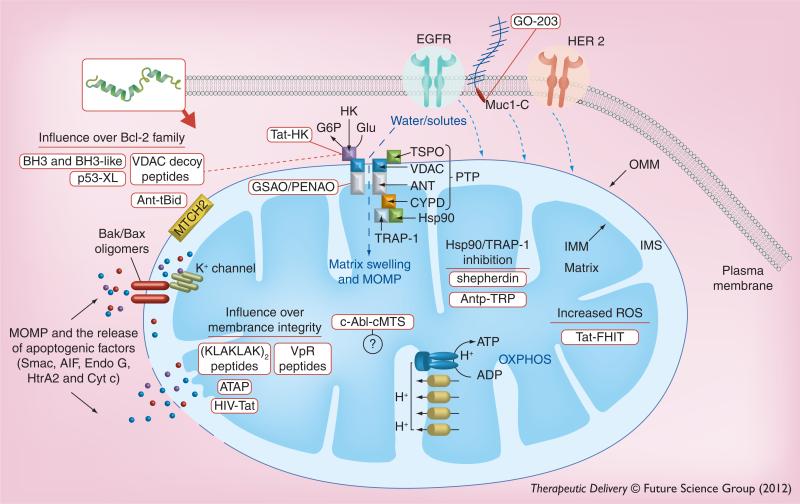
Cell death is generally categorized into either subtypes of stepwise regulated programmed cell death (PCD) or a passive disintegration termed necrosis [18]. PCD that is intracellular in origin is known as the intrinsic apoptotic or mitochondrial cell death pathway. However, the mitochondria ultimately play a key role in all active PCD cell death pathways [18]. Most cytotoxic anticancer agents, in spite of having diverse mechanisms of action, cause death through the mitochondrial pathway [19]. More precisely, death induction signaling from chemotherapy funnels down to the activation of mitochondrial outer membrane permeabilization (MOMP), which, in turn, is controlled by the balance between positive or negative regulators of OMM integrity (Figure 2) [4]. In many cancers (e.g., leukemia, melanoma and hepatocellular carcinoma), resistance to chemotherapy has been directly attributed to the overexpression of the anti-apoptotic B-cell lymphoma-2 (Bcl-2) family members [20].
Figure 2. Mitochondriotoxic peptides facilitate mitochondrial dysfunction by either direct or indirect induction of mitochondrial outer membrane permeabilization through Bcl-2 family interactions, permeability transition pore complex opening or membrane disruption.
MOMP initiated by manipulation of Bcl-2 family members converges on Bax and Bak homo- or hetero-oligomerization in the OMM to form pores that allow the cytosolic release of IMS proteins such as cytochrome c, Smac and AIF. Bax activation also inhibits the mitoKv1.3 IMM channel, leading to IMM hyperpolarization and increased ROS production. Upon PTP opening, there is an unregulated influx of cytosolic solute, loss of Δψm and mitochondrial swelling. Mitochondrial swelling bursts the OMM and releases the pro-death IMS proteins. The PTP is minimally comprised of the VDAC, ANT and CYPD. PTP opening can be suppressed by interaction with anti-apoptotic Bcl-2 family members, HK, TSPO, and the heat shock proteins Hsp90 and TRAP-1. MOMP and release of IMS apoptogenic factors can also occur through the direct disruption of membrane integrity. Therapeutic peptides are represented in red boxes. Solid red lines show peptide target. Dashed lines indicate peptides that have multiple targets.
AIF: Apoptosis-inducing factor; ANT: Adenine nucleotide translocase; ATAP: Amphipathic tail-anchoring peptide; Cyt c: Cytochrome c; Endo G: Endonuclease G; HK: Hexokinase; IMM: Inner mitochondrial membrane; IMS: Intermitochondrial membrane space; MOMP: Mitochondrial outer membrane permeabilization; MUC1: Mucin 1; OMM: Outer mitochondrial membrane; OXPHOS: Oxidative phosphorylation; PTP: Permeability transition pore complex; ROS: Reactive oxygen species; Smac: Second mitochondria-derived activator of caspases.
The Bcl-2 family of proteins are categorized as members with four Bcl-2 homology (BH) domains (BH1–4) or with only one BH domain, the BH3-only proteins. All of the BH3-only proteins (e.g., PUMA, NOXA, Bad, Bim and Bid) are classified as pro-apoptotic, while Bcl-2 proteins with four BH3 domains are anti-apoptotic (e.g., Bcl-2, Bcl-xL, Bcl-w, Mcl-1 and Bfl1) with the exceptions of Bak and Bax. MOMP proceeds by activation and oligomerization of Bax and/or Bak ‘effectors’ to form pores in the mitochondrial outer membrane allowing the release of pro-apoptotic factors such as cytochrome c, AIF, second mitochondria-derived activator of caspase (Smac), endonucleases and HtrA2. Upon release of the apoptotic factors from the IMS, cytosolic caspases are activated and the cell is committed to destruction [21].
Bak is constitutively found at the OMM while Bax is cytosolic, until activated, whereupon it translocates via an N-terminal mitochondrial targeting sequence (MTS) to the OMM. The anti-apoptotic members of the Bcl-2 family, such as Bcl-2 and Bcl-xL, have been found to localize at the OMM, the endoplasmic reticulum and nuclear membranes [21]. Bax or Bak monomers or hetero-oligomers of both proteins form aqueous channels in the OMM. The activation of Bak and Bax may be initiated by many different stimuli including direct interaction with p53 [22], BH3-only proteins such as Bid or Bim and post-translational modifications [23]. Bax inhibition of an IMM potassium channel (mitoKv1.3) also seems to be crucial to cytochrome c, AIF and Endo G release from the IMS, unlike Smac and HtrA2, which are released independently of mitoKv1.3 function. It has been suggested that the release of cytochrome c, AIF, and Endo G are all dependent on a mechanism based on increased reactive oxygen species (ROS), and that this coincides with the inhibition of the mitoKv1.3 channel [24]. ROS increase is a central event in the release of cytochrome c from the IMS, where it is bound to the IMM in association with the unique mitochondrial phospholipid cardiolipin [21]. However, it remains unclear whether the efflux of cytochrome c occurs solely by oligomeric Bax and other protein channels, and/or by the activation of a matrix to cytosol spanning permeability transition pore (PTP) and cristae remodeling [25,26].
The mitochondrial PTP may generally function as a mitochondrial Ca2+ release channel to regulate physiological Ca2+ dynamics; however, it also plays an important role in apoptosis. The PTP is comprised of the TSPO (formerly named peripheral-type benzodiazepine receptor) located at the outer/inner mitochondrial membrane contact sites, the VDAC, and the ANT. The anti-apoptotic Bcl-xL interacts with the ANT, and it has been proposed that this interaction can prevent cell death. In addition, Bcl-2 and Bax may modulate VDAC activity [24].
In some cases, nonselective toxins kill malignant cells more readily than healthy cells [27]. This phenomenon is analogous to nonspecific cytotoxic drugs that attack common targets such as DNA or microtubule formation, but which nonetheless kill rapidly proliferating tumor cells preferentially. A recent study correlates cytotoxic chemotherapy efficacy with mitochondrial priming of malignant cell mitochondria [28]. However, as for classical chemotherapy, any agent that is nonselective runs the risk of toxic side effects. The same reasons that make mitochondria an excellent anticancer target (i.e., cell death and oncogenic transformation functions) also suggests indiscriminate mitochondrial toxins will be unacceptable as chemotherapeutics. The strategy of mitochondrial targeting with intent to cause dysfunction ought to include a ‘cancerotropic’ modality. Depending upon the mitochondrial toxin and type of malignancy, the mitochondria of cancer cells may be self-selecting by way of being in a so-called ‘primed’ state such that the therapeutic index between healthy and cancerous cells for a nonspecific mitochondrial toxin may be very broad. This is unlikely to be true across the typical heterogeneity of cancer, however, and developing selective agents is a prudent course.
Peptide therapeutics can be readily designed to minimize off-target effects in three ways: first, by including a targeting moiety selective to cancer cells coupled to nondiscriminating mitochondriolytic entities; second, by using an agent that has a mechanism of action based on structural and/or functional differences inherent between healthy and malignant mitochondria; or third, using a therapeutic entity toxic to mitochondria, but that remains spatially segregated from the mitochondria unless intracellular pathologic conditions common to cancer cells initiate mitochondrial translocation. Ideally, a combination of these approaches could be used to minimize off-target effects (Figure 1).
Malignant mitochondria (the tumor signature)
Recognition of a difference between the mitochondrial function of healthy cells and those of malignant cells was made by Otto Warburg in the late 1920s. Warburg discovered malignant cells produce ATP via glycolysis even under aerobic conditions (i.e., the so-called Warburg effect) [12]. Warburg's attempt to attack malignant mitochondria on a bioenergetic basis was unsuccessful because the glycolytic shift in malignant cells is not (as presumed by Warburg) due to a fundamental dysfunction of mitochondrial oxidative phosphorylation, but instead related to changes in metabolic signaling pathways [1]. However, recent discoveries (including the mitochondrion as a central regulator of cell death) concerning bioenergetic alterations and malignant transformation have brought Warburg's general strategy back into favor [12]. In addition to corrupted bioenergetic pathways, mitochondrial proteins responsible for controlling the processes of apoptosis/necrosis, respiration and mitochondrial membrane integrity are being exploited for the selective destruction of malignant cells over healthy cells [11].
Even if mitochondrial function is not funda mentally impaired in cancer cells, mitochondrial activity is altered by environmental changes (e.g., low oxygen) and genetic reprogramming [1]. Rapidly expanding tumor cells, in addition to adapting for continued survival, also exploit adaptive mechanisms to meet the vast energetic requirements for proliferation. Metabolic reprogramming is a way to increase ATP production in an oxygen-independent manner while supplying the necessary constituents for anabolism. The shift between glycolysis and oxidative phosphorylation is controlled by two enzymes, mitochondrial pyruvate dehydrogenase and lactate dehydrogenase, which control the catabolic fate of pyruvate [1]. HIF-1 plays a key role in metabolic adaption either to hypoxia itself or through loss-of-function mutations in oxygen-sensing genes. In connection to pyruvate catabolism, HIF-1 upregulates PDK1, which inactivates PDH and suppresses oxidative metabolism of pyruvate in the tricarboxylic acid cycle. This glycolytic shift may also serve to protect cancer cells from producing cytotoxic levels of ROS [12]. The success of a small-molecule inhibitor of PDK1 (dicholoro-acetate) has validated targeting cancer energy metabolism. This drug specifically acts on the mitochondria of cancer cells without disturbing the physiology of non-malignant cells [29].
Other mitochondrial changes can also attend the metabolic adaptations in malignancy that impact mitochondrial function and can reinforce metastatic potential. For instance, increased superoxide formation (ROS), increased mitochondrial membrane potential, isoform switching and alterations in ion channel expression levels have been linked to perpetuating malignancy or chemotherapeutic resistance [30].
Mitochondrial reactive oxygen species
Circumstances dealing with ROS and malignancy often seem contradictory [31]. Increased ROS levels caused by oncogenic processes and perpetuating tumorigenicity seem inconsistent with therapeutic ROS generation leading to cell death induction [32]. Elevated intracellular ROS can lead to the activation of transcription factors (e.g., HIF-1 and NF-κB), leading to a host of protein expression profiles that contribute to malignancy such as proliferation, survival and metastasis [31]. This situation leads to a seeming ‘ROS paradox’ where many antineoplastic agents exert their cytotoxic effects through the generation of ROS [33]. The underlying mechanisms for the essential ROS-mediated killing by chemotherapeutics and radiation therapy is not well understood [31]. However, increased ROS magnitude from therapeutic treatment adds to the already high oxidative burden of the cancer cell overwhelming the antioxidant capacity and, thereby, causing cell death [34]. Moreover, the elevated ROS condition is likely to contribute to cytochrome c mobilization in malignant cells [35]. Unfortunately, the heterogeneity of cancer cell survival mechanisms can also operate by strongly suppressing ROS production, as occurs in resistant breast cancer tumor stem cells [32]. This often occurs through the overexpression of mitochondrial ROS scavenging enzymes such as peroxiredoxin 3, thioredoxin reductase and/or manganese superoxide dismutase (MnSOD) [36].
The targeted delivery of a therapeutic fusion protein to the mitochondria has been used to exploit the production of cytotoxic levels of ROS in cancer models [37]. One such protein that can generate ROS is FHIT. Across a variety of cancer types, apoptosis is induced by transfection or transduction of the FHIT gene. The FHIT is a tumor suppressor that exerts its pro-apoptotic action (in a complex with ferrodoxin reductase) at the mitochondria, by generating ROS and stimulating calcium uptake [38]. Recently, a HIV-Tat-derived protein transduction domain fusion with FHIT (TAT–FHIT) was used to induce apoptosis in hepatocellular carcinoma cells (Table 1) [37].
Table 1.
Pro-apoptotic anticancer peptides.
| Peptide/protein | Mitochondrial target | Mode of action | Ref. |
|---|---|---|---|
| HIV-1 Tat | Mitochondrial membranes | MOMP | [43] |
| TAT-HK | HK isoform 2 associated with OMM | Disruption of HK association with OMM and disinhibition of PTP opening | [92] |
| TAT-FHIT | Ferrodoxin reductase | Increased ROS and calcium flux | [37] |
| HIV-VpR | VDAC/ANT/Bax-mediated IMM and OMM permeabilization; reduced expression of Mfn2 | MOMP via disinhibition of PTP complex and/or Bax activation; interruption of mitochondrial–endoplasmic reticulum interaction | [60,61] |
| TEAM-VP (RGD-VpR) | VDAC/ANT-mediated IMM and OMM permeabilization | MOMP | [94] |
| BHAP (bifunctional: contains an AHNP anti-HER2 motif and [KLAKLAK]2) | Mitochondrial membranes | MOMP | [68] |
| RGD-4C-GG-D(KLAKLAK)2 | Mitochondrial membranes | MOMP | [67,69] |
| ATAP | OMM | MOMP | [63] |
| Ant-BH3 | Bcl-xL | MOMP | [73] |
| LHRH-BH3 | Bcl-xL and Bcl-2 | MOMP | [74] |
| SAHBs (e.g., BIM-SAHB) | Anti-apoptotic and effector (Bak/Bax) Bcl-2 family proteins at the OMM | MOMP | [76] |
| 072RB (Bim-derived peptide fused to Ant internalization sequence) | (Bcl-xL) but likely other anti-apoptotic and effector (Bak/Bax) Bcl-2 family proteins at the OMM | Bax activation and MOMP | [77] |
| IP3R decoy peptides | Bcl-2 (on endoplasmic reticulum) | Pro-apoptotic calcium release | [81] |
| shepherdin | Mitochondrial Hsp90 and TRAP-1 | Disinhibition of PTP opening and mitochondrial depolarization | [132] |
| Antp-TRP | Hsp90 | Apoptosis | [133] |
| Ant-tBid (111-125) and Ant-tBid (59-73) | MTCH2 interaction at OMM | Apoptosis | [120] |
| GSAO and PENAO | ANT | PTP opening and mitochondrial depolarization | [95] |
| VDAC decoy peptides | Multiple targets: HK, Bcl-2, and Bcl-xL | Mitochondrial sensitization to apoptotic stimuli | [98] |
| p53-XL (p53 targeted to the OMM) | Interaction with Bcl-2 family members: Bcl-xL, Bak and Bax | MOMP | [114] |
| c-Abl-cMTS (matrix targeted c-Abl) | Unknown | Apoptosis | [119] |
| GO-203 | C-terminal portion of MUC1 | Disrupts MUC1-C ROS suppression | [58] |
Ant or Antp: Antennapedia helix III, also called Penetratin; ANT: Adenine nucleotide translocase; BHAP: Bifunctional, HER2-blocking and apoptosis-inducing peptide; cMTS: Cryptic mitochondrial translocation sequence; FHIT: Fragile histidine triad; GSAO: 4-(N-[S-glutathionylacetyl]amino)phenylarsenoxide; HK: Hexokinase; IMM: Inner mitochondrial membrane; IP3R: Inositol 1,4,5-trisphosphate receptor; LHRH: Luteinizing hormone-releasing hormone; Mfn2: Mitofusin 2; mitoKv1.3: Mitochondrial voltage-gated potassium channel 1.3; MOMP: Mitochondrial outer membrane permeabilization; OMM: Outer mitochondrial membrane; PENAO: (4-[N-(S-penicillaminylacetyl)amino]phenylarsonous acid); PTP: Permeability transition pore; ROS: Reactive oxygen species; SAHB: Stabilized α-helices of Bcl-2 family protein; TAT or Tat: Transactivator of transcription; tBid, truncated Bid; TEAM-VP: Targeted to endothelial apoptogenic mitochondrio-active VpR-derived peptide; VpR: Viral protein R; Δψm,: Potential difference across inner mitochondrial membrane.
Mitochondrial DNA
The mitochondrion, like the nucleus, but unlike any other organelle, contains DNA [34]. The mitochondrial DNA is thought to be particularly vulnerable to ROS-mediated damage due to its close proximity to ROS production via the electron transport chain and less robust DNA repair mechanisms, as compared with the nuclear system of DNA repair [39]. The susceptibility of mitochondrial DNA oxidative damage may be a key contributor to tumor cell formation where the overproduction of mitochondrial ROS can lead to a vicious cycle that increases metastatic potential. To this point, tumor cell development is associated with impaired production of electron transport chain proteins, which, in turn, are less efficient and generate increased ROS [30,33].
Mitochondrial membrane potential
Tumor progression and expansion has been correlated to increased mitochondrial membrane potential (Δψm). Within tumor cell sub populations, the higher the Δψm, the more likely the contribution to tumor progression and expansion [40]. In human epidermoid adenocarcinoma and hepatoma cancer cells, a higher Δψm defines resistance to cisplatin [41]. As the Δψm increases, intracellular cationic entities will increasingly permeate the mitochondria. Therefore, even slight gains in Δψm may confer a greater killing capacity for cationic agents (including peptidessuch as shepherdin) in cancer cells [40].
Isoform switching
Malignant cells adapt in many ways, including isoform switching at the mitochondria to compensate for bioenergetic demands (e.g., M2 isoform of pyruvate kinase [17]) and to survive harsh environmental conditions [42]. For instance, as a potential way of reducing the ROS burden, cellular adaptation to low oxygen conditions is often accompanied by isoform switching of the mitochondrial respiratory chain via activity of the hypoxia transcription factor, HIF-1 [42]. In some forms of cancer, HIF-1 induces a more efficient COX4-2 expression and the degradation of COX4-1 isoform, thereby allowing respiration to proceed with less ROS produced as a byproduct [42].
Interestingly, Lecoeur et al. showed that a synthetic HIV-Tat protein (86 residues) inhibits COX activity in disrupted mitochondria. The HIV-Tat protein also caused loss of Δψm and cytochrome c release, independent of PTP and Bcl-2 family status [43]. Furthermore, an acetylated HIV-Tat (residue 28) was shown to stimulate the mitochondrial translocation of the pro-apoptotic Bim [43]. HIV-Tat may be a useful platform for future investigations into MOMP/PTP independent targeting of malignant mitochondria. The toxic consequences of HIV-Tat are related to another mitochondriotoxic HIV protein (HIV-1 viral protein R or VpR; Table 1), which has been used to target the mitochondria of cancer cells [44].
Mitochondrial ion channels
Potassium channels, components of the permeability transition pore, and mitochondrial calcium uniporter, are mitochondrial ion channels that have been recognized as therapeutically important targets in cancer [45]. Peptide toxins have long been used for pharmacological targeting of ion channels. Some of these may have utility in targeting aberrantly expressed mitochondrial potassium channels such as the mitoKv1.3 [45]. The expression profiles of voltage-gated potassium (Kv) channels, which have a role in controlling membrane potential, are remodeled in the majority of cancers with a correlation between Kv overexpression and grade of tumor malignancy in certain tumor types [45]. Mitochondrial potassium (MitoK+) channels are also found to play important roles in both cyto-protection and apoptosis (recently reviewed in [46]). Owing to this, mitoK+ channels are considered promising therapeutic targets in cancer [47]. The IMM-localized Kv1.3 is the best characterized mitochondrial Kv, and the Kv1.3 channel (mitochondrial and plasma membrane) is characterized by a very selective and potent peptide toxin pharmacology [25].
Interestingly, mitoKv1.3 is inhibited by an interaction within the IMS with the OMM spanning pro-apoptotic Bax, leading to hyperpolarization of the IMM, resulting in increased ROS production, loss of cytochrome c, propagation of mitochondrial dysfunction and apoptosis [25]. Bax inhibition of mitoKv1.3 was dependent on a specific, highly conserved Bax residue, Lys-128. In spite of Bax inhibition of mitoKv1.3 being required for efficient induction of apoptosis, the absence of the mitoKv1.3 can cause resistance to apoptosis [25]. Szabo et al. recently reported that the action of Bax at the OMM can be substituted by peptide toxins of the mitoKv1.3 channel [46]. The steadily increasing recognition of the role of MitoK+ channels in cancer and mitochondrial function puts a new emphasis on the importance of these channels in the development of targeted anticancer therapy (e.g., the MitoK+ channel, TASK-3, is indispensable for survival of melanoma cells) [48,49].
After decades of searching, the mitochondrial calcium channel (mitochondrial calcium uniporter [MCU]) has been newly discovered [50]. Increased expression of the MCU increases mitochondrial calcium accumulation, and sensitizes cells to apoptotic stimuli [50]. Since calcium is one of the triggers (ROS being another) for PTP opening, this channel is highly relevant in pathological conditions, such as cancer [51]. The MCU will undoubtedly be intensely focused on as a target for modulating cell death [51].
Plasma membrane to mitochondria protein translocation
Tumor cells reprogram intracellular mitochondrial trafficking as a mechanism to escape apoptosis [52]. Several cell-surface proteins translocate to the mitochondria and elicit distinct biological functions in malignant cells [52]. For instance, EGFR, MUC1-C (C-terminal portion of MUC-1) [53] and HER2 aberrantly localize to the mitochondria and antagonize apop tosis (Figure 2). The EGFR family (e.g., EGFR/ERBB1, HER2/ERBB2 and HER3/ERBB3) are often overexpressed and associated with progression, proliferation and drug resistance in many forms of cancer [54]. However, EGFR tyrosine kinase inhibitor (TKI; e.g., gefitinib, lapatinib and erlotinib) and monoclonal antibody anti-EGFR thera peutic agents have met with only modest efficacy in clinical trials [52]. Unfortunately, the process of mitochondrial translocation and antagonism of apoptosis by EGFR is actually enhanced by TKI treatment [55]. At the mitochondria, EGFR interacts with and inhibits the pro-apoptotic PUMA [55]. The consequences are reduced efficacy of EGFR inhibitors and increased apoptotic threshold of cancer cells [52]. Interestingly, in clinical trials, pretreatment (more so than concomitant treatment) of medulloblastoma and glioblastoma cells with obatoclax (GX15-070), a small-molecule inhibitor of Bcl-xL and Mcl-1, led to a greater than additive enhancement of TKI lapatinib-induced cell death [56], indicating that the disruption of the protein–protein interaction between EGFR and PUMA could be an important therapeutic modality in glioblastoma multiforme and possibly other cancers [55].
Abnormal expression of MUC1 in multiple types of cancers can be targeted with a peptide inhibitor, GO-203, which is in Phase I clinical trials. GO-203 blocks the pleiotropic MUC1-C oncogene causing a derepression of ROS production [57,58].
Mitochondrial membranes
One of the key differences between malignant and healthy mitochondria may be the lipid composition of the OMM [26]. Mitochondrial membrane composition, and the spatial organization of its components, may fundamentally affect the processes of apoptosis [21]. For instance, in healthy cells the cholesterol content in OMM (and possibly the shape) of the mitochondria may prevent Bax activation [21]. Cardiolipin, a phospholipid found exclusively in mitochondrial membranes (primarily the IMM), interacts with the α-helices of tBid [59]. A protein–lipid complex containing cardiolipin/tBid (also, the protein MTCH2/MIMP, see section titled ‘MTCH2’) at the OMM are thought to facilitate pore formation by Bax oligomers [21]. However, the exact protein SAR is not well understood in the context of proteins becoming embedded in the dynamic mitochondrial membrane environment, making the precise mechanistic elucidation of MOMP a challenge [21]. As mentioned previously, Bax and Bak OMM localization in ‘primed’ malignant mitochondria is not random, but likely coincides with mitochondrial fission sites for optimal MOMP formation [21].
Membrane disrupting mitochondriotoxic peptides
The 96-amino acid HIV-VpR protein, comprised of three α-helical domains, can integrate into and permeabilize mitochondrial membranes reducing the Δψm, causing fragmentation and cristae swelling [60]. In addition, HIV-VpR can influence apoptosis by interactions with ANT, VDAC and/or Bax [61]. In contrast with other viral proteins that target the mitochondria, the HIV-VpR lacks a canonical MTS, and its association with mitochondria is also independent of the translocase of the outer membrane (TOM) complex [62].
An amphipathic tail-anchoring peptide (ATAP) derived from Bcl-2 family member Bfl-1 was effective in inducing MOMP, possibly by homo-oligomerization and pore formation. A green fluorescent protein-fused ATAP was shown to target the mitochondria (via an intrinsic MTS) [63]. However, as with other pore-forming peptides, ensuring selective targeting to tumor cells will be a priority consideration for future therapeutic development.
Various cationic peptides exhibit an immediate cytotoxic effect against cancer cells, in many cases by binding and disrupting the plasma membrane [64,65]. However, due to the difference in potential at the mitochondria versus the plasma membrane, cationic peptides selectively disrupt mitochondrial membranes at concentrations hundreds of times lower than what is required to interfere with plasma membrane integrity [66]. As one of the pathological hallmarks of cancer is a stably increased Δψm, even modest gains in attraction of cationic mitochondriolytic peptides, although non selective, may confer a greater killing capacity for cancer cells [40]. A common mitochondriotoxic peptide motif is the (KLAKLAK)2 (synthetic derivative of a natural antimicrobial peptide), which causes mitochondrial membrane damage and subsequent triggering of apoptosis. The (KLAKLAK)2 peptide does not efficiently cross the plasma membranes of eukaryotic cells and has low toxicity, unless it is coupled to selective targeting domains allowing internalization by cells [67]. For instance, the RGD-4C-GGD-(KLAKLAK)2 or ‘BHAP’ (HER-2-blocking and apoptosis-inducing peptide; exocyclic AHNP motif fused to [KLAKLAK]2) hybrid peptides allow targeting to endothelial cells of the angiogenic vasculature [67] or HER2-positive cells, respectively [68,69].
Screening is also ongoing for oncolytic peptides to be found among antimicrobial or host-defense peptides. Evolutionarily conserved mitochondriotoxic pore-forming peptides (e.g., colicins and translocation domain of diph theria toxin) exhibit a structural similarity to Bax and Bak [21].
Bcl-2 family
The anti-apoptotic members of the Bcl-2 family (e.g., Mcl-1, Bcl-2 and Bcl-xL) are often relegated to merely having a redundant role in apoptosis. In recent years, however, each Bcl-2 family member (anti- and pro-apoptotic) have been found to have their own specialized niches within the apoptotic program (and in non-apoptotic cellular functions) [70]. For instance, each anti-apoptotic Bcl-2 family member binds a distinct subset of the pro-apoptotic Bcl-2 members [71]. This class of mitochondrial proteins is considered to have tremendous potential for both small-molecule and peptide-targeted therapies [11]. BH3 domains of pro-apoptotic Bcl-2 family members or small-molecule BH3 mimetics are currently used to neutralize the anti-apoptotic action of Bcl-xL, Bcl-w and/or Bcl-2, thereby shifting the balance of power on the OMM from anti-apoptotic to pro-apoptotic to provoke MOMP [72]. BH3 mimetics (e.g., ABT-263 [navitoclax], ABT-737 and GX15-070 [obatoclax]) are currently being evaluated in Phase I/II clinical trials for patients with hematologic malignancies. However, thrombocytopenia is a major side effect of this class of drugs due to their ability to antagonize the prosurvival function of Bcl-xL in platelets [72].
Early work targeting the Bcl-2 family employed a BH3-only domain (Bak) fused to either a cell-penetrating peptide (CPP; derived from the Antennapedia protein [73]) or recep tor-specific sequence from LHRH, creating Ant-BH3 or LHRH-BH3, respectively [74]. There are several variations on conformationally constrained BH3-only domains. For example ‘hydrocarbon-stapled’ peptides containing BH3 domains (stabilized α-helices of Bcl-2 family proteins [SAHBs]; Bim-SAHB) are currently being developed as anticancer agents [75,76]. In addition, modifications of the BH3 domain using natural and non-natural amino acids to improve stability and enhance target specificity have been used as with the Bim-BH3 mimic, 072RB [77]. As certain neoplasias are likely the consequence of mutations or aberrant regulation exclusively of Bim [78], the restoration of a ‘therapeutic’ Bim is an attractive possibility.
Bcl-2 itself is not a strictly mitochondrial protein and has several prosurvival functions, including acting as a pro-angiogenic signaling molecule. Bcl-2 also controls cytoplasmic calcium levels via its BH4 domain in an interaction with inositol 1,4,5-trisphosphate receptors (IP3Rs) on the endoplasmic reticulum. This interaction suppresses pro-apoptotic calcium signaling and buffers cells against pro-apoptotic stimuli [79]. The apoptotic suppression by the BH4 domain of Bcl-2 is more potent than that of the well-recognized prosurvival BH4 domain from Bcl-xL against staurosporine treatment [80]. The BH4 domain of Bcl-2 has been targeted with IP3R-mimetic peptides to trigger pro-apoptotic calcium signaling in malignant lymphocytes (in chronic lymphocytic leukemia) with suppressed IP3R Ca2+ signaling [81]. Importantly, different iterations of the IP3R decoy peptide could alter the magnitude of the released calcium, and the calcium release was sufficient to kill these apoptosis-resistant lymphocytes [81].
Noxa's role in establishing the rate at which apoptosis occurs, in addition to its key role in changing the balance of autophagic processes toward cell death rather than survival, could provide a unique activity for a BH3-only protein if developed as a therapeutic entity [82]. For instance, Noxa influences the kinetics in ABT-737- and TRAIL-induced apoptosis, since knockdown of Noxa delays the velocity of cell death induction in primary glioblastoma cultures derived from patient samples and in an in vivo tumor model [83]. In light of Noxa's ability to alter the rapidity of cell death, specifically targeting Noxa may be a means to manage the type of cell death a cell undergoes (i.e., immunogenic apoptosis) [84].
Mcl-1 is commonly associated with tumor cell resistance in several cancers and certain therapies (radiation) can inadvertently cause resistance by increasing Mcl-1 expression [80,85]. The corollary is that suppression of Mcl-1 restores sensitivity to apoptotic inducers. Introducing the Mcl-1 BH3 helix was sufficient to inhibit Mcl-1 and permit apoptosis [86] whereas several attempts to inactivate positive regulators (e.g., GSK-3β) upstream of Mcl-1 have been largely unsuccessful. Mcl-1, however, is not blocked by the BH3 mimetic ABT-737 [72].
Permeability transition pore
Along with the Bcl-2 family, the members of the PTP complex control mitochondrial membrane integrity. Therefore, the PTP is a key node for MOMP [87]. Not surprisingly, dys regulation of the PTP is another mechanism allowing cancer cells to escape death [88]. The PTP complex spans from the OMM to the matrix and primarily is thought to include: cyclophilin D, a mitochondrial matrix constituent that is a peptidyl-prolyl cis–trans isomerase (and target for inhibition of cyclosporine A); the ANT, an IMM protein, which is an example of isoform switching in cancer, through the overexpression of a MOMP-resistant isoform ANT2 [87]; and the multifunctional VDAC, also called mitochondrial porin, which is localized on the OMM and serves as a key regulator of mitochondrial metabolite flux and apoptosis (Figure 2) [89]. The PTP can also include the channel-like TSPO (formerly called the peripheral benzodiazepine receptor), which contacts the VDAC and ANT from the OMM where it is localized and serves to transport cholesterol and other lipophilic molecules across the IMS, in addition to possibly facilitating cytosolic to mitochondrial communication [90]. Since TSPO basal expression is altered in a number of human pathologies including cancer, it is a promising drug target for a number of therapeutic applications [91]. Another mitochondrial peculiarity that arises in cancer is the association of hexokinase (HK; isoforms I or II) with the PTP at the surface of the OMM with VDAC. Upon displacement of HK from PTP interaction (i.e., VDAC) on the mitochondrial surface by treatment with either small molecules or peptides (e.g., TAT-HK peptide [92]), MOMP and apoptosis ensues [87,93]. One of the functions of ANT, particularly relevant in cancer, is the provision of ATP to HK. This promulgates the pathologic metabolic shift to glucose and stabilizes the closed conformation of the PTP complex [87].
A peptide derived from the mitochondriotoxic HIV Vpr (Vpr67-82) was fused to a homing/internalization peptide sequence RGD (cysteine-cyclized peptide frame holds the RGD motif in a restricted conformation; also used with (KLAKLAK)2, see above, section ‘Membrane disrupting mitochondriotoxic peptides’) that recognizes the integrin αvβ3 receptor, which is often upregulated in invasive tumor types. This fusion peptide (TEAM-VP) selectively induced IMM and OMM permeabilization in a mechanism associated with VDAC and ANT interaction [94]. Interestingly, this group also noted that mitochondrial fission occurred with administration of TEAM-VP [94].
Two peptide trivalent arsenical agents that target ANT are 4-(N-(S-glutathionylacetyl) amino) phenylarsonous acid (GSAO) and 4-(N-(S-penicillaminylacetyl)amino (PENAO) phenylarsonous acid, a cysteine mimetic analog of GSAO that is less prone to cellular efflux). ANT becomes inactivated upon the interaction between its matrix side cysteine residues and the arsenical component of GSAO or PENAO. The inactivation of ANT permits opening of the PTP and mitochondrial depolarization. Both GSAO and PENAO are currently in Phase I clinical trials for patients with advanced solid tumors [95,96].
VDAC activity can be modulated by a variety of proteins, notably members of the Bcl-2 family. The pro-apoptotic Bax and tBid, upon interaction with VDAC, increase the channel pore size [97]. In contrast, in cancer, VDAC is usually inhibited by the anti-apoptotic Bcl-2 proteins (e.g., Bcl-2 and Bcl-xL) and HK I and II. Shoshan-Barmatz et al. have developed a set of VDAC, mimetic peptides to relieve the anti-apoptotic pressure on VDAC [98]. This resensitizes the malignant cell to death signaling. VDAC-derived peptides have been optimized to act as VDAC decoys for HK, Bcl-2 and Bcl-xL to restore apoptotic sensitivity to cancer cells. The addition of a CPP motif to VDAC-mimetic peptides was effective in several cancer cell lines, including B-cell chronic lymphocytic leukemia patient samples and chemoresistant cell lines [98]. Pharmacologic manipulation of the PTP/VDAC interactions may be an avenue to potentiate the efficacy of conventional chemotherapy [98].
Recently, nicotinic acetylcholine receptors (nAChRs) have been found in the OMM and interact with VDAC to regulate PTP function in response to apoptogenic stimuli (e.g., calcium overload or H2O2) [99]. Typically, nAChRs are associated with the cell plasma membrane in synaptic transmission in the muscle and nervous system [99]. However, with the discovery of nAChRs at the OMM, paired with the wealth of pharmacological agents known to modulate this class of channel, it is likely that mitochondrial nAChRs will become a prime therapeutic target [99].
Mitochondrial fission & fusion dynamics
There is a link between Bak and Bax, proapoptotic members of the Bcl-2 family and the processes of mitochondrial fission in apoptosis [21]. To this point, Bax, Bak and possibly Bcl-xL are required for normal mitochondrial dynamics [100]. However, a direct mechanistic tie remains controversial between MOMP and the processes of mitochondrial fission. Upon apoptotic induction, the mitochondrial fission protein Drp1 is recruited, along with Bax, to the OMM. Both active Bax and Bak form foci at mitochondrial fission sites [21].
One of the mechanisms whereby cancer cells evade death is by exploiting the pathway known as autophagy, which is used by both normal and cancer cells to sustain metabolism and adapt to stressful conditions [101,102]. Under conditions of starvation, a cell can typically engage this lysosomal catabolic route to sustain metabolic demands [103]. Autophagy is, paradoxically, considered both a cell death and cell-survival mechanism; however, in the context of malignancy the true function of autophagy is thwarted and is therefore considered an escape route allowing survival [103]. The engagement of this catabolic pathway is dependent on the mitochondria. Mitochondrial morphology determines the cellular response to autophagy [104] such that mitochondrial fusion spares the cell from death. Elongated mitochondria escape autophagic degradation and exhibit optimized ATP production. Conversely, when elongation is blocked either genetically or pharmacologically, the mitochondria consume ATP and trigger starvation-induced death [104]. This autophagic mitochondrial fusion is driven by phosphorylation events and cytoplasmic retention of the profission Drp1 [105]. Since mitochondrial fusion is integral to cell survival in the autophagic pathway, proteins that are central to this process are viable therapeutic targets. Mitofusins (Mfn1 and Mfn2) and Opa1 are OMM and IMM localized proteins, respectively, which are essential for mitochondrial fusion [21]. Mfn2 in particular has been focused on as a therapeutic target because of its role in mitochondrial metabolism and calcium homeostasis [60,106].
The HIV Vpr (96 residues) has been shown to induce host cell death by increasing OMM permeability through interactions with VDAC and ANT [94], as well as by disturbing the OMM integrity [60] as mentioned above. However, HIV Vpr also depletes Mfn2 (an essential GTPase for the fusion of mitochondrial membranes), leading to mitochondrial membrane deformation, loss of Δψm and cell death [60]. The loss of functional Mfn2 via HIV Vpr and consequent reduction in the frequency of fusion among mitochondria suggest that Vpr may confer a unique therapeutic potential in the context of pathologic autophagy [60]. The mitofusin proteins are also of interest because they help to coordinate mitochondria-to-mitochondria docking, as well as tethering the mitochondria and endoplasmic reticulum together to coordinate calcium flow [60]. The intersection between mitochondrial dynamics and apoptosis may be a central leverage point for inducing an immunogenic type of cell death [84].
Pro-apoptotic mitochondrial targeting proteins dysregulated in malignancy
p53
p53 is a critical tumor suppressor protein that is dysregulated in over 50% of cancers [107]. Recapitulating its function is central to many anti-tumor therapies. Under a wide variety of cell-stress conditions, p53 accumulates in the nucleus and mitochondria (reviewed in [108]). At the mitochondria, p53 is found within multiple compartments, such as the OMM, IMS and matrix [109]. Mitochondrial p53 regulates apoptosis by acting in different cellular compartments by: directly modifying propensity for MOMP by interacting with proteins of Bcl-2 family; inhibiting MnSOD activity; and regulating mitochondrial physiology in proliferative conditions via interaction with mitofilin [110]. Mitofilin is a multifunctional regulator of mitochondrial architecture and protein biogenesis [111] and has critical functions in mitochondrial morphology and mitochondrial fusion and fission (as well as protein import), specifically in the formation of tubular cristae and cristae junctions [112]. Finally, there is increasing evidence for the role of p53 in metabolism regulation and mitochondrial respiration, which could be a major component of p53 oncosuppressive function [109,110]. An advantage of targeting p53 to the mitochondria (versus the nucleus) may be the avoidance of nuclear negative regulators of p53, such as HDM2 and FGF1, which inhibit p53's nuclear function and are often highly expressed in certain types of cancer [113]. Recent work in our laboratory has shown that targeting p53 to the OMM of a breast cancer cell line (T47D) using the MTS derived from Bcl-xL triggered a rapid induction of apoptosis [114].
c-Abl
Chronic myelogenous leukemia is defined by the expression of the oncoprotein Bcr-Abl through a reciprocal chromosomal translocation in a hematopoietic stem cell fusing the ABL and BCR genes [115], creating the constitutively active Bcr-Abl tyrosine kinase. Bcr-Abl thwarts the normal prodeath functions of c-Abl. c-Abl has been termed a ‘mitochondrial wracking factor’ [116] because active c-Abl translocation to the mitochondria induces cell death [117,118]. Artificially targeting c-Abl (as a fusion to a cryptic MTS) to the mitochondria of leukemia cells (K562) recapitulated c-Abl's pro-apoptotic function [119].
MTCH2
Recent work has demonstrated the importance of the MTCH2/MIMP that resides on the surface of the OMM in tBid-mediated apoptosis [120]. MTCH2 facilitates mitochondrial recruitment of tBid (in conjunction with OMM cardiolipin, see section titled ‘Mitochondrial membranes’) whereupon tBid activates Bax [21]. MTCH2 is a conserved protein that belongs to the mitochondrial carrier protein family, such as ANT. Unlike ANT, however, MTCH2 is localized to the OMM instead of the IMM [121]. Peptides derived from the MTCH2 and tBid binding interface and fused to the Antennapedia CPP were able to induce apoptosis in cancer cells. While the mechanism of action is not yet clear, it is interesting that the tBid/MTCH2 interface-derived peptide does not include the BH3 domain of tBid [120,121].
Upregulated mitochondrial survival factors
MnSOD
The exclusively mitochondrial MnSOD is a key enzyme involved in superoxide radical scavenging [122]. In the context of oncogenic progression, MnSOD has a bimodal expression profile (permitting oncogenic levels of ROS to persist but preventing overwhelming ROS toxicity), where its expression is lower at early stages of malignancy and higher at later stages [123]. Inhibition of aberrantly elevated MnSOD is considered an effective strategy to selectively kill cancer cells and diminish resistance to standard chemotherapy [124]. Mitochondrial p53 inactivates MnSOD, leading to mitochondrial dysfunction and initiation of apoptosis [122,125]. Additionally, targeting NF-κB with an inhibitory peptide suppressed MnSOD expression in aggressive breast cancer cell lines MDA-MB231 and SKBR3 [124].
TRAP-1
TRAP-1 is a mitochondrial-compartmentalized Hsp90-like chaperone that is strongly cytoprotective [126]. High expression of TRAP-1 is consistently associated with malignancy and with resistance to chemotherapy, while corresponding healthy tissues exhibit very low to undetectable levels of TRAP-1 [126]. TRAP-1 associates with cyclophilin D, a component of the PTP, and inhibits PTP opening (as does aberrantly localized mitochondrial Hsp90; Figure 2). The ATPase activity of TRAP-1 can be inhibited by small molecules (e.g., geldanamycin), but none of these inhibitors could access the mitochondrial compartment. However, a peptide (shepherdin, antagonist of the interaction between Hsp90 and the IAP survivin) was able to target and inhibit mitochondrial TRAP-1, inducing MOMP and cell death selectively in cancer cells [126]. Furthermore, the scope of TRAP-1/mitochondrial Hsp90 inhibition extended to the induction of a mitochondrial specific unfolded protein response program, which can engage an autophagic type cell death mechanism [126].
PDK1
In many cancers the transcription and survival factor HIF-1 increases expression of the mitochondrial PDK1 [11]. PDK1 inhibits pyruvate dehydrogenase, which suppresses the tricarboxylic acid cycle [12]. Consequently, this reinforces the Warburg effect or glycolytic shift in cancer cells and may also protect cells from cytotoxic levels of ROS [12]. Inhibition of this mitochondrial kinase with a small molecule (dichloroacetate [DCA]) has met with good success with low/no toxicity to non-malignant cells [11]. DCA is an excellent example of an agent acting specifically on the mitochondria of cancer cells without perturbing the physiology of non-malignant cells [29].
Survival factors that have special relevance when localized to the mitochondria
Hsp90
Hsp90 is widely regarded as a prosurvival factor in cancer that affects multiple downstream pathways (i.e., Hsp90 is a ‘nodal’ target [127]). Overexpression of Hsp90 is typical in cancer cells, in addition to an activity increase of nearly 100-fold [128]. Hsp90 stabilizes inhibitors of apoptosis such as survivin, inhibits MOMP via cyclophilin D [126], stimulates nitric oxide production through association with nitric oxide synthase [129] and has a role in the mitochondrial unfolded protein response [130]. However, small-molecule antagonists targeting Hsp90 ATPase have a low clinical response in patients [131]. It has been confirmed that this is primarily due to the inability of the agents to access the mitochondrial compartment [131]. A pool of mitochondrial Hsp90 was unexpectedly found localized to the mitochondria in prostate cancer samples while investigating the differential expression of TRAP-1 in cancer and healthy tissue [126]. As with TRAP-1, the mitochondrial Hsp90 was undetectable in the normal corresponding tissue. Several peptides have been developed to target Hsp90, including shepherdin (modeled on the interface between Hsp90 and the IAP survivin [132]) and Antp-TPR (modeled on the interface between Hsp90 and Hsp90 cofactor, Hop [133]). It has become clear that targeting the mitochondrial pool of Hsp90 is key for therapeutic efficacy, with applicability in genetically disparate tumors, and provides a basis for tumor versus normal tissue selectivity in vivo [130].
Peptides are uniquely suited to target malignant mitochondria
Current clinical cancer trials with agents targeting the mitochondria include obatoclax (small-molecule BH3 mimetic) and the mitochondrial PDHK1 inhibitor DCA, but as of yet, there are no peptides in this category [134]. However, mitochondrial targeting antioxidant peptides are in Phase II clinical trials for the indication of acute myocardial infarction [135]. Targeted peptides in cancer have primarily centered on tumor vasculature, inhibition of IAPs [136], monoclonal antibodies, anticancer vaccines [137] and plasma membrane disrupting peptides [138]. Why are there no peptides targeted to the mitochondria for anticancer therapy? The technical problems of intracellular delivery and drug optimization are still formidable obstacles and, until recently, intracellular protein–protein interactions have been considered to be ‘the most intractable of undruggable targets’ [139].
Peptides have the distinct advantage of being able to discriminate between closely related targets with high affinity binding, and low or no toxic effects [140]. Evolutionarily refined natural peptides are a tremendous resource in the pursuit of selective anticancer agents that have the potential to extend the range of druggable targets beyond the scope of small molecules. The rapid development of synthetic peptides is a means to harness this evolutionarily cultivated specificity, subtlety and low toxicity by bridging the problems of stability and delivery [139]. Nonetheless, therapeutic peptide development is often complementary to improvement of targeted small molecules. In many cases, high affinity natural peptides (endogenous protein domains) already exist for many proteins of interest making library screening often un necessary [140]. Conversely, high affinity natural peptides can be used to efficiently screen small molecules for activity. For instance, a high-throughput screen for the anti-apoptotic Bcl-2 family member Bfl-1 used BH3-only domain binding to assess the displacement efficacy of small molecules [141]. Going a step further, an iterative process of rational (e.g., BH3-only peptide) design can be used to refine and optimize target binding as a means to enhance screening for increasingly potent small molecules [142].
Targeting peptides intracellularly
In order to reach the mitochondria, peptides and proteins must have a means to penetrate the plasma membrane. Cell-penetrating peptides (also called protein transduction domains) are highly versatile, nontoxic sequences (as compared with liposomes and polymers) for intracellular delivery [143]. CPPs generally come in two flavors, either as short amino acid sequences that are mainly composed of arginine, lysine and histidine (cationic character mediates the interaction of the peptide with anionic/acidic motifs on the plasma membrane in a receptor-independent fashion), or as amphipathic peptides (lipophilic and hydrophilic tails) that mediate peptide translocation across the plasma membranes. However, the exact mechanism of uptake is controversial for this type of CPP [143]. Recently, a new class of CPPs with delocalized lipophilic cations incorporated into the peptide sequence have been created to specifically facilitate drug delivery to the mitochondria [144]. Finally, CPPs have been widely used in animal models and can achieve systemic delivery in vivo (therefore deliverable to any type of cancer) [143]. However, optimization testing is required for every modification because the orientation of peptide, cargo and linkages can have a significant impact on therapeutic peptide efficacy [64,143].
Peptide toxicity: immunogenicity
Immunogenicity of protein (and to a limited degree, peptide) therapeutics pose a risk that can possibly compromise safety and alter pharmaco kinetics and bioavailability of the agent [145]. The fast growing number of therapeutic proteins and related immunogenicity data shows that most of them induce formation of anti drug anti bodies. Additionally, in silico assessment of peptide immunogenicity risk is becoming more common [145]. In the case where anti drug anti bodies takes the form of neutralizing antibodies, these neutralizing antibodies have a direct effect on the effector-function of the therapeutic proteins [146]. Of special concern are fusion proteins composed of a foreign and self-protein, because of the potential of the foreign moiety to provoke an immune response to the self-protein (epitope spreading) [147]. Risk of immunogenic response (especially non-human peptides, such as enfuvirtide) can be mitigated by re-engineering to reduce the size and/or elimination of MHC-II epitopes [147] through standard humanization techniques. Standard strategies for increasing half-life can also reduce immunogenicity, such as PEGylation or the introduction of non-natural amino acids [143].
Conclusion & future perspective
While it is unclear if mitochondrial dysfunction directly contributes to tumorigenesis (or if mitochondrial dysfunction is co-opted in the process of cancer metabolic transformation), the key clinical point is that the mitochondria of cancer cells, as opposed to normal cells, are ripe for selective therapeutic targeting to overcome common mechanisms of resistance and augment current anticancer strategies [1]. Many of the current and emerging mitochondrial targets are dependent upon disruption of protein–protein interactions. There has been a general lack of progress in developing small molecules targeting protein–protein interactions and, unlike therapeutic monoclonal antibodies, most peptides can function in an intracellular environment and are more amenable to synthesis/modification and delivery [148]. Currently, an advancing developmental congruence exists between peptide drug design and delivery modalities for peptides and proteins. Several recent reviews [3–6] cover important aspects of these advances and are illustrative of the creative energy being applied to the problems associated with peptide drug delivery.
Peptides can harness the culmination of evolutionary processes that dictate specificity of interaction [139]. Using peptides as therapeutics meets the highest standard (and arguably the most natural) for candidates to achieve the specificity and subtlety of interaction small molecules cannot. Even between highly homologous binding targets, peptides can discriminate due to their high-level complexity [149]. Discovery of locations of protein–protein interaction sites can lead to a further refinement of targeted peptides and/or new screening targets for small-molecule intervention. Multifunctional (e.g., targeting moiety combined with bioactive domain) peptides or multivalency (e.g., more than binding modality) can be readily engineered, while undesirable features (e.g., immunogenicity) can be virtually eliminated [148]. Peptides offer low toxicity and highly malleable formats for creating tunable, multifunctional therapeutics [148]. Peptides have advantages over large proteins (avoiding the necessity to employ complex cellular systems, problems of characterization and higher immunogenic potential), small molecules (toxicity, target promiscuity, poor ability to target protein–protein and protein–lipid interactions) and DNA/RNA aptamers (require rounds of screening using large libraries and purified target) [139].
Some of the most promising mitochondrial targeted peptides are derivatives of a parent protein [150]. Moreover, tremendous effort has been channeled into industrial-scale development and production of synthetic peptides in recent years, ramping up the capability to make larger and more complex peptides with improved physiochemical properties [148]. Furthermore, proteins, especially enzymes, have been in use as drugs for many years and biologics are making an increasing contribution to the number of new drug approvals [151]. A variety of US FDA approved biologics have been in use for over three decades, and the number of biologics continues to steadily increase [152].
Drug efficacy and drug resistance are ultimately reconciled at the level of the mitochondrion, and many therapeutic efforts define success by achieving Bak/Bax oligomerization and MOMP [1]. In addition, it is not uncommon for resistance to be exacerbated by the very agents used to try to kill the malignant cells (i.e., induction of EGFR mitochondrial localization [52] or radiation therapy-induced resistance via Mcl-1 stabilization [153]). Therefore, strategies that engage the mitochondria directly bypass problems of resistance and upstream prosurvival pressures [11]. For instance, if malignant cells overcome therapeutically induced ROS via upregulated anti-oxidant mechanisms or RAF signaling, targeting MOMP using BH3-only peptides could restore cell death [154]. Another complementary approach is to test the status of the mitochondria (e.g., overexpression of anti-apoptotic factors) to tailor therapeutic intervention. Recently, ‘BH3 profiling’ (originally defined as a pattern of mitochondrial apoptotic sensitivity based on peptides derived from BH3-only proteins [155]) has been performed as a companion diagnostic for determining the probability of drug efficacy (e.g., bortezomib) across cancer patients [19]. Combining mitochondrially targeted peptides with conventional chemo therapy may be a powerful way to reduce the standard chemotherapeutic dose and associated toxicity, potentiate synergistic killing of tumor cells [156], reduce resistance and elicit an immunogenic response to the malignant tissue [9].
The term ‘mitocan’ has recently been coined to describe therapeutic agents within the rapidly developing and burgeoning field of mitochondrial-focused anticancer therapy [157]. In the near future, we can expect peptide therapeutics to comprise an ever larger segment of the mitocan arsenal.
Key Terms.
Immunogenic apoptosis:
Manner in which a cell dies dictates whether or not an immune response will be mounted against intracellular antigens. When a dying cell shows phenotypic characteristics, such as surface exposure of calreticulin, heat shock protein release and secreted ATP, these so-called ‘danger signals’ on tumor cells can elicit a protective anti-tumor immune response.
Isoform switching: Example of a qualitative variation in gene expression that aids in the adaptation to a changing environment. Isoforms are proteins generated from diverse primary sequences that may have unique traits but maintain the same function.
Programmed cell death:
The intrinsic apoptotic or mitochondrial pathway is one subtype of programmed cell death characterized by mitochondrial outer membrane permeabilization, irreversible loss of Δψm, release of IMS proteins, and respiratory chain inhibition.
Mitochondrial priming:
Relative mitochondrial apoptotic threshold of a tumor type based on a challenge with pro-apoptotic BH3-only proteins or a BH3 mimetic. An enhanced clinical response to standard antineoplastic therapy is correlated with higher mitochondrial priming.
Key Term.
Warburg effect: Cellular metabolic shift to glycolysis and cytosolic lactic acid fermentation in the presence of oxygen (aerobic glycolysis). Aerobic glycolysis is less efficient at producing ATP than the mitochondrial oxidation of pyruvate in the tricarboxylic acid cycle, but confers a proliferative advantage by supplying anabolic carbons.
Executive summary.
Mitochondrial cell death pathway & malignant mitochondria
■ Cancer therapy is dependent on permeabilization of the mitochondrial outer membrane. Directly targeting the mitochondria in cancer is an excellent universal therapeutic strategy, especially because there are ‘targetable’ differences between malignant and healthy mitochondria.
Peptides are uniquely suited to target malignant mitochondria
■ Therapeutic peptide agents have the potential to open new vistas of traditionally ‘undruggable’ targets (e.g., protein–protein interactions) and offer a subtlety and specificity of target interaction, which cannot be matched by small molecules, all while decreasing side effects. Rapid advances in therapeutic peptide design are likely to soon surmount the major problems of stability and delivery.
The mitochondria & immunogenic apoptosis
■ The type of death a cell undergoes may be central to achieving a durable response to anticancer therapy. Targeting the mitochondria may be a means to kill cancer cells, but also be able to modulate the killing to invoke an ‘immunogenic apoptosis’. Pairing peptide therapeutics with traditional chemotherapy may be a way to preserve the immune effector cells (by using a sub-immune toxic chemotherapeutic dose) to achieve tumor killing, while engaging an anti-tumor immune response.
Acknowledgments
This work was funded by NIH R01-CA129528 and by an AFPE Pre-Doctoral Fellowship (JE Constance)
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1■■.Ralph SJ, Rodriguez-Enriquez S, Neuzil J, Saavedra E, Moreno-Sanchez R. The causes of cancer revisited: “mitochondrial malignancy” and ROS-induced oncogenic transformation – why mitochondria are targets for cancer therapy. Mol. Aspects Med. 2010;31(2):145–170. doi: 10.1016/j.mam.2010.02.008. [Comprehensive review of the reciprocal role between mitochondria and oncogenic transformation.] [DOI] [PubMed] [Google Scholar]
- 2.McCarty MF. Targeting multiple signaling pathways as a strategy for managing prostate cancer: multifocal signal modulation therapy. Integr. Cancer Ther. 2004;3(4):349–380. doi: 10.1177/1534735404270757. [DOI] [PubMed] [Google Scholar]
- 3.Mahato RI, Narang AS, Thoma L, Miller DD. Emerging trends in oral delivery of peptide and protein drugs. Crit. Rev. Ther. Drug Carrier Syst. 2003;20(2–3):153–214. doi: 10.1615/critrevtherdrugcarriersyst.v20.i23.30. [DOI] [PubMed] [Google Scholar]
- 4.Tan ML, Choong PF, Dass CR. Recent developments in liposomes, microparticles and nanoparticles for protein and peptide drug delivery. Peptides. 2010;31(1):184–193. doi: 10.1016/j.peptides.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat. Rev. Immunol. 2009;9(5):353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ieva E, Trapani A, Cioffi N, Ditaranto N, Monopoli A, Sabbatini L. Analytical characterization of chitosan nanoparticles for peptide drug delivery applications. Anal. Bioanal. Chem. 2009;393(1):207–215. doi: 10.1007/s00216-008-2463-4. [DOI] [PubMed] [Google Scholar]
- 7.Torchilin VP. Tat peptide-mediated intracellular delivery of pharmaceutical nanocarriers. Adv. Drug Deliv. Rev. 2008;60(4–5):548–558. doi: 10.1016/j.addr.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Martins I, Michaud M, Sukkurwala AQ, et al. Premortem autophagy determines the immunogenicity of chemotherapy-induced cancer cell death. Autophagy. 2012;8(3) doi: 10.4161/auto.19009. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 9.Gogvadze V, Zhivotovsky B, Orrenius S. The warburg effect and mitochondrial stability in cancer cells. Mol. Aspects Med. 2010;31(1):60–74. doi: 10.1016/j.mam.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Wemeau M, Kepp O, Tesniere A, et al. Calreticulin exposure on malignant blasts predicts a cellular anticancer immune response in patients with acute myeloid leukemia. Cell Death Dis. 2010;1:e104. doi: 10.1038/cddis.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bitler BG, Schroeder JA. Anti-cancer therapies that utilize cell penetrating peptides. Recent Pat. Anticancer Drug Discov. 2010;5(2):99–108. doi: 10.2174/157489210790936252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12■■.Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov. 2010;9(6):447–464. doi: 10.1038/nrd3137. [Comprehensive review of small molecules targeted to the mitochondria for cancer therapy.] [DOI] [PubMed] [Google Scholar]
- 13.Hortobagyi GN. Trastuzumab in the treatment of breast cancer. N. Engl. J. Med. 2005;353(16):1734–1736. doi: 10.1056/NEJMe058196. [DOI] [PubMed] [Google Scholar]
- 14.Nakase I, Konishi Y, Ueda M, Saji H, Futaki S. Accumulation of arginine-rich cell-penetrating peptides in tumors and the potential for anticancer drug delivery in vivo. J. Control. Release. 2012;159(2):181–188. doi: 10.1016/j.jconrel.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 15■.Christofk HR, Vander Heiden MG, Harris MH, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452(7184):230–233. doi: 10.1038/nature06734. [Key example of isoform switching of a mitochondrial protein and implications in cancer.] [DOI] [PubMed] [Google Scholar]
- 16.Mossalam M, Dixon AS, Lim CS. Controlling subcellular delivery to optimize therapeutic effect. Therapeutic Delivery. 2010;1(1):169–193. doi: 10.4155/tde.10.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seyfried TN, Shelton LM. Cancer as a metabolic disease. Nutr. Metab. (Lond.) 2010;27(7):7. doi: 10.1186/1743-7075-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18■■.Chonghaile TN, Sarosiek KA, Vo TT, et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science. 2011;334(6059):1129–1133. doi: 10.1126/science.1206727. [Provides insight into the role of the Bcl-2 family in the control over cell death in cancer chemotherapeutic responses.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galluzzi L, Kroemer G. Necroptosis: a specialized pathway of programmed necrosis. Cell. 2008;135(7):1161–1163. doi: 10.1016/j.cell.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Atay C, Ugurlu S, Ozoren N. Shock the heat shock network. J. Clin. Invest. 2009;119(3):445–448. doi: 10.1172/JCI38681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21■.Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell. 2011;21(1):92–101. doi: 10.1016/j.devcel.2011.06.017. [Provides insight into the connection between mitochondrial morphological dynamics and the Bcl-2 family proteins.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chipuk JE, Kuwana T, Bouchier-Hayes L, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303(5660):1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 23.Kutuk O, Letai A. Regulation of Bcl-2 family proteins by posttranslational modifications. Curr. Mol. Med. 2008;8(2):102–118. doi: 10.2174/156652408783769599. [DOI] [PubMed] [Google Scholar]
- 24■.Szabo I, Soddemann M, Leanza L, Zoratti M, Gulbins E. Single-point mutations of a lysine residue change function of Bax and Bcl-xL expressed in Bax- and Bak-less mouse embryonic fibroblasts: novel insights into the molecular mechanisms of Bax-induced apoptosis. Cell Death Differ. 2011;18(3):427–438. doi: 10.1038/cdd.2010.112. [Provides a mechanistic connection between mitochondrial potassium channels and the pro-apoptotic protein Bax.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szabo I, Bock J, Grassme H, et al. Mitochondrial potassium channel Kv1.3 mediates Bax-induced apoptosis in lymphocytes. Proc. Natl Acad. Sci. USA. 2008;105(39):14861–14866. doi: 10.1073/pnas.0804236105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cipolat S, Rudka T, Hartmann D, et al. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell. 2006;126(1):163–175. doi: 10.1016/j.cell.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 27.Liu T, Hannafon B, Gill L, Kelly W, Benbrook D. Flex-Hets differentially induce apoptosis in cancer over normal cells by directly targeting mitochondria. Mol. Cancer Ther. 2007;6(6):1814–1822. doi: 10.1158/1535-7163.MCT-06-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffin C, Karnik A, McNulty J, Pandey S. Pancratistatin selectively targets cancer cell mitochondria and reduces growth of human colon tumor xenografts. Mol. Cancer Ther. 2011;10(1):57–68. doi: 10.1158/1535-7163.MCT-10-0735. [DOI] [PubMed] [Google Scholar]
- 29.Vella S, Conti M, Tasso R, Cancedda R, Pagano A. Dichloroacetate inhibits neuroblastoma growth by specifically acting against malignant undifferentiated cells. Int. J. Cancer. 2012;130(7):1484–1493. doi: 10.1002/ijc.26173. [DOI] [PubMed] [Google Scholar]
- 30.Bellance N, Lestienne P, Rossignol R. Mitochondria: from bioenergetics to the metabolic regulation of carcinogenesis. Front. Biosci. 2009;14:4015–4034. doi: 10.2741/3509. [DOI] [PubMed] [Google Scholar]
- 31.Gupta SC, Hevia D, Patchva S, Park B, Koh W, Aggarwal BB. Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid. Redox Signal. 2012;16(11):1295–1322. doi: 10.1089/ars.2011.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diehn M, Cho RW, Lobo NA, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458(7239):780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishikawa K, Takenaga K, Akimoto M, et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320(5876):661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 34.Bair JS, Palchaudhuri R, Hergenrother PJ. Chemistry and biology of deoxynyboquinone, a potent inducer of cancer cell death. J. Am. Chem. Soc. 2010;132(15):5469–5478. doi: 10.1021/ja100610m. [DOI] [PubMed] [Google Scholar]
- 35.Atlante A, Calissano P, Bobba A, Azzariti A, Marra E, Passarella S. Cytochrome c is released from mitochondria in a reactive oxygen species (ROS)-dependent fashion and can operate as a ROS scavenger and as a respiratory substrate in cerebellar neurons undergoing excitotoxic death. J. Biol. Chem. 2000;275(47):37159–37166. doi: 10.1074/jbc.M002361200. [DOI] [PubMed] [Google Scholar]
- 36.Song IS, Kim HK, Jeong SH, et al. Mitochondrial peroxiredoxin III is a potential target for cancer therapy. Int. J. Mol. Sci. 2011;12(10):7163–7185. doi: 10.3390/ijms12107163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu GR, Qin WW, Li JP, et al. HIV-TAT-fused FHIT protein functions as a potential pro-apoptotic molecule in hepatocellular carcinoma cells. Biosci. Rep. 2012;32(3):271–279. doi: 10.1042/BSR20110033. [DOI] [PubMed] [Google Scholar]
- 38.Martin J, St-Pierre MV, Dufour JF. Hit proteins, mitochondria and cancer. Biochim. Biophys. Acta. 2011;1807(6):626–632. doi: 10.1016/j.bbabio.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Svilar D, Goellner EM, Almeida KH, Sobol RW. Base excision repair and lesion-dependent subpathways for repair of oxidative DNA damage. Antioxid. Redox Signal. 2011;14(12):2491–2507. doi: 10.1089/ars.2010.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40■.Houston MA, Augenlicht LH, Heerdt BG. Stable differences in intrinsic mitochondrial membrane potential of tumor cell subpopulations reflect phenotypic heterogeneity. Int. J. Cell Biol. 2011;6(9):e25207. doi: 10.1155/2011/978583. [Provides insight into the pathophysiological increase in mitochondrial transmembrane potential in malignant cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xue X, You S, Zhang Q, et al. Mitaplatin increases sensitivity of tumor cells to cisplatin by inducing mitochondrial dysfunction. Mol. Pharm. 2012;9(3):634–644. doi: 10.1021/mp200571k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129(1):111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 43.Lecoeur H, Borgne-Sanchez A, Chaloin O, et al. HIV-1 Tat protein directly induces mitochondrial membrane permeabilization and inactivates cytochrome c oxidase. Cell Death Dis. 2012;3:E282. doi: 10.1038/cddis.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nonaka M, Hashimoto Y, Takeshima SN, Aida Y. The human immunodeficiency virus type 1 Vpr protein and its carboxy-terminally truncated form induce apoptosis in tumor cells. Cancer Cell Int. 2009;12(9):20. doi: 10.1186/1475-2867-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arcangeli A, Crociani O, Lastraioli E, Masi A, Pillozzi S, Becchetti A. Targeting ion channels in cancer: a novel frontier in antineoplastic therapy. Curr. Med. Chem. 2009;16(1):66–93. doi: 10.2174/092986709787002835. [DOI] [PubMed] [Google Scholar]
- 46.Szabo I, Leanza L, Gulbins E, Zoratti M. Physiology of potassium channels in the inner membrane of mitochondria. Pflugers Arch. 2012;463(2):231–246. doi: 10.1007/s00424-011-1058-7. [DOI] [PubMed] [Google Scholar]
- 47.Cardoso AR, Queliconi BB, Kowaltowski AJ. Mitochondrial ion transport pathways: role in metabolic diseases. Biochim. Biophys. Acta. 2010;1797(6–7):832–838. doi: 10.1016/j.bbabio.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 48.Felipe A, Bielanska J, Comes N, et al. Targeting the voltage-dependent k(+) channels kv1.3 and kv1.5 as tumor biomarkers for cancer detection and prevention. Curr. Med. Chem. 2012;19(5):661–674. doi: 10.2174/092986712798992048. [DOI] [PubMed] [Google Scholar]
- 49.Kosztka L, Rusznak Z, Nagy D, et al. Inhibition of TASK-3 (KCNK9) channel biosynthesis changes cell morphology and decreases both DNA content and mitochondrial function of melanoma cells maintained in cell culture. Melanoma Res. 2011;21(4):308–322. doi: 10.1097/CMR.0b013e3283462713. [DOI] [PubMed] [Google Scholar]
- 50.De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476(7360):336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong R, Steenbergen C, Murphy E. Mitochondrial permeability transition pore and calcium handling. Methods Mol. Biol. 2012;810:235–242. doi: 10.1007/978-1-61779-382-0_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao X, Zhu H, Ali-Osman F, Lo HW. EGFR and EGFRvIII undergo stress- and EGFR kinase inhibitor-induced mitochondrial translocalization: a potential mechanism of EGFR-driven antagonism of apoptosis. Mol. Cancer. 2011;10:26. doi: 10.1186/1476-4598-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ren J, Bharti A, Raina D, Chen W, Ahmad R, Kufe D. MUC1 oncoprotein is targeted to mitochondria by heregulin-induced activation of c-Src and the molecular chaperone HSP90. Oncogene. 2006;25(1):20–31. doi: 10.1038/sj.onc.1209012. [DOI] [PubMed] [Google Scholar]
- 54.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat. Rev. Cancer. 2007;7(3):169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 55.Zhu H, Cao X, Ali-Osman F, Keir S, Lo HW. EGFR and EGFRvIII interact with PUMA to inhibit mitochondrial translocalization of PUMA and PUMA-mediated apoptosis independent of EGFR kinase activity. Cancer Lett. 2010;294(1):101–110. doi: 10.1016/j.canlet.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cruickshanks N, Hamed H, Bareford MD, et al. Lapatinib and obatoclax kill tumor cells through blockade of ERBB1/3/4 and through inhibition of BCL-XL and MCL-1. Mol. Pharmacol. 2012;81(5):748–758. doi: 10.1124/mol.112.077586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yin L, Kufe D. MUC1-C oncoprotein blocks terminal differentiation of chronic myelogenous leukemia cells by a ROS-mediated mechanism. Genes Cancer. 2011;2(1):56–64. doi: 10.1177/1947601911405044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yin L, Kosugi M, Kufe D. Inhibition of the MUC1-C oncoprotein induces multiple myeloma cell death by down-regulating TIGAR expression and depleting NADPH. Blood. 2012;119(3):810–816. doi: 10.1182/blood-2011-07-369686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim TH, Zhao Y, Ding WX, et al. Bidcardiolipin interaction at mitochondrial contact site contributes to mitochondrial cristae reorganization and cytochrome C release. Mol. Biol. Cell. 2004;15(7):3061–3072. doi: 10.1091/mbc.E03-12-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60■.Huang CY, Chiang SF, Lin TY, Chiou SH, Chow KC. HIV-1 Vpr triggers mitochondrial destruction by impairing Mfn2-mediated ER-mitochondria interaction. PLoS One. 2012;7(3):E33657. doi: 10.1371/journal.pone.0033657. [Offers mechanistic insight into HIV-Vpr peptide's multifunctional pro-apoptotic role.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sabbah EN, Druillennec S, Morellet N, Bouaziz S, Kroemer G, Roques BP. Interaction between the HIV-1 protein Vpr and the adenine nucleotide translocator. Chem. Biol. Drug Des. 2006;67(2):145–154. doi: 10.1111/j.1747-0285.2006.00340.x. [DOI] [PubMed] [Google Scholar]
- 62.Boya P, Pauleau AL, Poncet D, Gonzalez-Polo RA, Zamzami N, Kroemer G. Viral proteins targeting mitochondria: controlling cell death. Biochim. Biophys. Acta. 2004;1659(2–3):178–189. doi: 10.1016/j.bbabio.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 63.Ko JK, Choi KH, Peng J, et al. Amphipathic tail-anchoring peptide and Bcl-2 homology domain-3 (BH3) peptides from Bcl-2 family proteins induce apoptosis through different mechanisms. J. Biol. Chem. 2011;286(11):9038–9048. doi: 10.1074/jbc.M110.198457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papo N, Shai Y. New lytic peptides based on the d.l-amphipathic helix motif preferentially kill tumor cells compared to normal cells. Biochemistry. 2003;42(31):9346–9354. doi: 10.1021/bi027212o. [DOI] [PubMed] [Google Scholar]
- 65.Madan V, Sanchez-Martinez S, Carrasco L, Nieva JL. A peptide based on the pore-forming domain of pro-apoptotic poliovirus 2B viroporin targets mitochondria. Biochim. Biophys. Acta. 2010;1798(1):52–58. doi: 10.1016/j.bbamem.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 66.Szewczyk A, Wojtczak L. Mitochondria as a pharmacological target. Pharmacol. Rev. 2002;54(1):101–127. doi: 10.1124/pr.54.1.101. [DOI] [PubMed] [Google Scholar]
- 67.Ellerby HM, Arap W, Ellerby LM, et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nat. Med. 1999;5(9):1032–1038. doi: 10.1038/12469. [DOI] [PubMed] [Google Scholar]
- 68.Fantin VR, Berardi MJ, Babbe H, Michelman MV, Manning CM, Leder P. A bifunctional targeted peptide that blocks HER-2 tyrosine kinase and disables mitochondrial function in HER-2-positive carcinoma cells. Cancer Res. 2005;65(15):6891–6900. doi: 10.1158/0008-5472.CAN-05-0395. [DOI] [PubMed] [Google Scholar]
- 69.Smolarczyk R, Cichon T, Graja K, Hucz J, Sochanik A, Szala S. Antitumor effect of RGD-4C-GG-D(KLAKLAK)2 peptide in mouse B16(F10) melanoma model. Acta Biochim. Pol. 2006;53(4):801–805. [PubMed] [Google Scholar]
- 70.Galluzzi L, Kepp O, Trojel-Hansen C, Kroemer G. Non-apoptotic functions of apoptosis-regulatory proteins. EMBO Rep. 2012;13(4):322–330. doi: 10.1038/embor.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rudner J, Elsaesser SJ, Muller AC, Belka C, Jendrossek V. Differential effects of anti-apoptotic Bcl-2 family members Mcl-1, Bcl-2, and Bcl-xL on celecoxib-induced apoptosis. Biochem. Pharmacol. 2010;79(1):10–20. doi: 10.1016/j.bcp.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 72.Schoenwaelder SM, Jackson SP. Bcl-xL-inhibitory BH3 mimetics (ABT-737 or ABT-263) and the modulation of cytosolic calcium flux and platelet function. Blood. 2012;119(5):1320–1321. doi: 10.1182/blood-2011-10-387399. author reply 1321–1322. [DOI] [PubMed] [Google Scholar]
- 73.Holinger EP, Chittenden T, Lutz RJ. Bak BH3 peptides antagonize Bcl-xL function and induce apoptosis through cytochrome c-independent activation of caspases. J. Biol. Chem. 1999;274(19):13298–13304. doi: 10.1074/jbc.274.19.13298. [DOI] [PubMed] [Google Scholar]
- 74.Dharap SS, Minko T. Targeted proapoptotic LHRH-BH3 peptide. Pharm. Res. 2003;20(6):889–896. doi: 10.1023/a:1023839319950. [DOI] [PubMed] [Google Scholar]
- 75■.Pitter K, Bernal F, Labelle J, Walensky LD. Dissection of the BCL-2 family signaling network with stabilized α-helices of BCL-2 domains. Methods Enzymol. 2008;446:387–408. doi: 10.1016/S0076-6879(08)01623-6. [Example of conformationally constrained BH3 peptide mimetics using hydrocarbon stapling.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Katz SG, Labelle JL, Godes M, Fisher J, Bird GH, Walensky LD. A stapled BIM BH3 helix restores apoptosis in bim-null mantle cell lymphoma.. Presented at: American Society of Hematology Annual Meeting; Atlanta, GA, USA. 4–7 December 2010. [Google Scholar]
- 77.Ponassi R, Biasotti B, Tomati V, et al. A novel Bim-BH3-derived Bcl-XL inhibitor: biochemical characterization, in vitro, in vivo and ex vivo anti-leukemic activity. Cell Cycle. 2008;7(20):3211–3224. doi: 10.4161/cc.7.20.6830. [DOI] [PubMed] [Google Scholar]
- 78.Gangoda L, Moujalled D, Lee Y, Rahimi A, Puthalakath H. Cell Death in Cancer. De Rode Hoed; Amsterdam, The Netherlands: 2012. Analysis of the role of Bim as a tumor suppressor in Carney complex syndrome. [Google Scholar]
- 79.Rong YP, Bultynck G, Aromolaran AS, et al. The BH4 domain of Bcl-2 inhibits ER calcium release and apoptosis by binding the regulatory and coupling domain of the IP3 receptor. Proc. Natl Acad. Sci. USA. 2009;106(34):14397–14402. doi: 10.1073/pnas.0907555106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iqbal S, Zhang S, Driss A, et al. PDGF upregulates Mcl-1 through activation of β-catenin and HIF-1α-dependent signaling in human prostate cancer cells. PLoS One. 2012;7(1):E30764. doi: 10.1371/journal.pone.0030764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81■.Zhong F, Harr MW, Bultynck G, et al. Induction of Ca(2)+-driven apoptosis in chronic lymphocytic leukemia cells by peptide-mediated disruption of Bcl-2-IP3 receptor interaction. Blood. 2011;117(10):2924–2934. doi: 10.1182/blood-2010-09-307405. [Provides insight into the wide cellular homeostatic regulatory and anti-apoptotic role of the Bcl-2 protein.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Elgendy M, Sheridan C, Brumatti G, Martin SJ. Oncogenic Ras-induced expression of Noxa and Beclin-1 promotes autophagic cell death and limits clonogenic survival. Mol. Cell. 2011;42(1):23–35. doi: 10.1016/j.molcel.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 83.Garg AD, Krysko DV, Verfaillie T, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 2012;31(5):1062–1079. doi: 10.1038/emboj.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cristofanon S, Fulda S. Cell Death in Cancer. De Rode Hoed; Amsterdam, The Netherlands: 2012. ABT-737 promotes tBid mitochondrial insertion to enhance TRAIL-induced apoptosis in glioblastoma cells. [Google Scholar]
- 85.Glaser SP, Lee EF, Trounson E, et al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes Dev. 2012;26(2):120–125. doi: 10.1101/gad.182980.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stewart ML, Fire E, Keating AE, Walensky LD. The MCL-1 BH3 helix is an exclusive MCL-1 inhibitor and apoptosis sensitizer. Nat. Chem. Biol. 2010;6(8):595–601. doi: 10.1038/nchembio.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Galluzzi L, Kepp O, Tajeddine N, Kroemer G. Disruption of the hexokinase-VDAC complex for tumor therapy. Oncogene. 2008;27(34):4633–4635. doi: 10.1038/onc.2008.114. [DOI] [PubMed] [Google Scholar]
- 88.Brenner C, Grimm S. The permeability transition pore complex in cancer cell death. Oncogene. 2006;25(34):4744–4756. doi: 10.1038/sj.onc.1209609. [DOI] [PubMed] [Google Scholar]
- 89.Shoshan-Barmatz V, De Pinto V, Zweckstetter M, Raviv Z, Keinan N, Arbel N. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol. Aspects Med. 2010;31(3):227–285. doi: 10.1016/j.mam.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 90.Rupprecht R, Papadopoulos V, Rammes G, et al. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2010;9(12):971–988. doi: 10.1038/nrd3295. [DOI] [PubMed] [Google Scholar]
- 91.Taliani S, Pugliesi I, Da Settimo F. Structural requirements to obtain highly potent and selective 18 kDa translocator protein (TSPO) Ligands. Curr. Top Med. Chem. 2011;11(7):860–886. doi: 10.2174/156802611795165142. [DOI] [PubMed] [Google Scholar]
- 92.Chiara F, Castellaro D, Marin O, et al. Hexokinase II detachment from mitochondria triggers apoptosis through the permeability transition pore independent of voltage-dependent anion channels. PLoS One. 2008;3(3):E1852. doi: 10.1371/journal.pone.0001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Masgras I, Rasola A, Bernardi P. Induction of the permeability transition pore in cells depleted of mitochondrial DNA. Biochim. Biophys. Acta. 2012 doi: 10.1016/j.bbabio.2012.02.022. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 94.Borgne-Sanchez A, Dupont S, Langonne A, et al. Targeted Vpr-derived peptides reach mitochondria to induce apoptosis of αVβ3-expressing endothelial cells. Cell Death Differ. 2007;14(3):422–435. doi: 10.1038/sj.cdd.4402018. [DOI] [PubMed] [Google Scholar]
- 95.Park D, Chiu J, Perrone GG, Dilda PJ, Hogg PJ. The tumour metabolism inhibitors GSAO and PENAO react with cysteines 57 and 257 of mitochondrial adenine nucleotide translocase. Cancer Cell Int. 2012;12(1):11. doi: 10.1186/1475-2867-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dilda PJ, Decollogne S, Weerakoon L, et al. Optimization of the antitumor efficacy of a synthetic mitochondrial toxin by increasing the residence time in the cytosol. J. Med. Chem. 2009;52(20):6209–6216. doi: 10.1021/jm9008339. [DOI] [PubMed] [Google Scholar]
- 97.Banerjee J, Ghosh S. Bax increases the pore size of rat brain mitochondrial voltage-dependent anion channel in the presence of tBid. Biochem. Biophys. Res. Commun. 2004;323(1):310–314. doi: 10.1016/j.bbrc.2004.08.094. [DOI] [PubMed] [Google Scholar]
- 98.Shoshan-Barmatz V, Ben-Hail D. VDAC, a multi-functional mitochondrial protein as a pharmacological target. Mitochondrion. 2012;12(1):24–34. doi: 10.1016/j.mito.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 99.Gergalova G, Lykhmus O, Kalashnyk O, et al. Mitochondria express α7 nicotinic acetylcholine receptors to regulate Ca accumulation and cytochrome c release: study on isolated mitochondria. PLoS One. 2012;7(2):E31361. doi: 10.1371/journal.pone.0031361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Karbowski M, Norris KL, Cleland MM, Jeong SY, Youle RJ. Role of Bax and Bak in mitochondrial morphogenesis. Nature. 2006;443(7112):658–662. doi: 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]
- 101.Rosenfeldt MT, Ryan KM. The multiple roles of autophagy in cancer. Carcinogenesis. 2011;32(7):955–963. doi: 10.1093/carcin/bgr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.White EJ, Martin V, Liu JL, et al. Autophagy regulation in cancer development and therapy. Am. J. Cancer Res. 2011;1(3):362–372. [PMC free article] [PubMed] [Google Scholar]
- 103.Hoyer-Hansen M, Jaattela M. Autophagy: an emerging target for cancer therapy. Autophagy. 2008;4(5):574–580. doi: 10.4161/auto.5921. [DOI] [PubMed] [Google Scholar]
- 104.Gomes LC, Di Benedetto G, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell. Biol. 2011;13(5):589–598. doi: 10.1038/ncb2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Blackstone C, Chang CR. Mitochondria unite to survive. Nat. Cell Biol. 2011;13(5):521–522. doi: 10.1038/ncb0511-521. [DOI] [PubMed] [Google Scholar]
- 106.Zorzano A, Sebastian D, Segales J, Palacin M. The molecular machinery of mitochondrial fusion and fission: an opportunity for drug discovery? Curr. Opin. Drug Discov. Devel. 2009;12(5):597–606. [PubMed] [Google Scholar]
- 107.Mossalam M, Matissek KJ, Okal A, Constance JE, Lim CS. Direct induction of apoptosis using an optimal mitochondrially targeted p53. Mol. Pharm. 2012;9(5):1449–1458. doi: 10.1021/mp3000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ferecatu I, Bergeaud M, Rodriguez-Enfedaque A, et al. Mitochondrial localization of the low level p53 protein in proliferative cells. Biochem. Biophys. Res. Commun. 2009;387(4):772–777. doi: 10.1016/j.bbrc.2009.07.111. [DOI] [PubMed] [Google Scholar]
- 109.Bergeaud M, Mathieu L, Le Floch N, Mignotte B, Rincheval V, Vayssiere JL. Cell Death in Cancer. De Rode Hoed; Amsterdam, The Netherlands: 2012. Localization and function of p53 protein at mitochondria in proliferative cells. [Google Scholar]
- 110.Von Der Malsburg K, Muller JM, Bohnert M, et al. Dual role of mitofilin in mitochondrial membrane organization and protein biogenesis. Dev. Cell. 2011;21(4):694–707. doi: 10.1016/j.devcel.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 111.Alirol E, Martinou JC. Mitochondria and cancer: is there a morphological connection? Oncogene. 2006;25(34):4706–4716. doi: 10.1038/sj.onc.1209600. [DOI] [PubMed] [Google Scholar]
- 112.Bouleau S, Parvu-Ferecatu I, Rodriguez-Enfedaque A, et al. Fibroblast growth factor 1 inhibits p53-dependent apoptosis in PC12 cells. Apoptosis. 2007;12(8):1377–1387. doi: 10.1007/s10495-007-0072-x. [DOI] [PubMed] [Google Scholar]
- 113.Mossalam M, Matissek KJ, Okal A, Constance JE, Lim CS. Direct induction of apoptosis using an optimal mitochondrially targeted p53. Mol. Pharm. 2012;9(5):1449–1458. doi: 10.1021/mp3000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Barnes DJ, Palaiologou D, Panousopoulou E, et al. Bcr-Abl expression levels determine the rate of development of resistance to imatinib mesylate in chronic myeloid leukemia. Cancer Res. 2005;65(19):8912–8919. doi: 10.1158/0008-5472.CAN-05-0076. [DOI] [PubMed] [Google Scholar]
- 115.Wang JY. Nucleo-cytoplasmic communication in apoptotic response to genotoxic and inflammatory stress. Cell Res. 2005;15(1):43–48. doi: 10.1038/sj.cr.7290263. [DOI] [PubMed] [Google Scholar]
- 116.Ito Y, Pandey P, Mishra N, et al. Targeting of the c-Abl tyrosine kinase to mitochondria in endoplasmic reticulum stress-induced apoptosis. Mol. Cell. Biol. 2001;21(18):6233–6242. doi: 10.1128/MCB.21.18.6233-6242.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lasfer M, Davenne L, Vadrot N, et al. Protein kinase PKCd and c-Abl are required for mitochondrial apoptosis induction by genotoxic stress in the absence of p53, p73 and Fas receptor. FEBS Lett. 2006;580(11):2547–2552. doi: 10.1016/j.febslet.2006.03.089. [DOI] [PubMed] [Google Scholar]
- 118.Constance JE, Despres SD, Nishida A, Lim CS. Selective targeting of c-Abl via a cryptic mitochondrial targeting signal activated by cellular redox status in leukemic and breast cancer cells. Pharm. Res. 2012;29(8):2317–2328. doi: 10.1007/s11095-012-0758-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Katz C, Zaltsman-Amir Y, Mostizky Y, Kollet N, Gross A, Friedler A. Molecular basis of the interaction between the pro apoptotic tBID protein and mitochondrial carrier homologue 2 (MTCH2): key players in the mitochondrial death pathway. J. Biol. Chem. 2012;287(18):15016–15023. doi: 10.1074/jbc.M111.328377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zaltsman Y, Shachnai L, Yivgi-Ohana N, et al. MTCH2/MIMP is a major facilitator of tBID recruitment to mitochondria. Nat. Cell. Biol. 2010;12(6):553–562. doi: 10.1038/ncb2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Holley AK, Dhar SK, Xu Y, St Clair DK. Manganese superoxide dismutase: beyond life and death. Amino Acids. 2012;42(1):139–158. doi: 10.1007/s00726-010-0600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pedram A, Razandi M, Wallace DC, Levin ER. Functional estrogen receptors in the mitochondria of breast cancer cells. Mol. Biol. Cell. 2006;17(5):2125–2137. doi: 10.1091/mbc.E05-11-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ennen M, Minig V, Grandemange S, et al. Regulation of the high basal expression of the manganese superoxide dismutase gene in aggressive breast cancer cells. Free Radic. Biol. Med. 2011;50(12):1771–1779. doi: 10.1016/j.freeradbiomed.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 124.Zhao Y, Chaiswing L, Velez JM, et al. p53 translocation to mitochondria precedes its nuclear translocation and targets mitochondrial oxidative defense protein-manganese superoxide dismutase. Cancer Res. 2005;65(9):3745–3750. doi: 10.1158/0008-5472.CAN-04-3835. [DOI] [PubMed] [Google Scholar]
- 125.Altieri DC, Stein GS, Lian JB, Languino LR. TRAP-1, the mitochondrial Hsp90. Biochim. Biophys. Acta. 2012;1823(3):767–773. doi: 10.1016/j.bbamcr.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dai C, Whitesell L. HSP90: a rising star on the horizon of anticancer targets. Future Oncol. 2005;1(4):529–540. doi: 10.2217/14796694.1.4.529. [DOI] [PubMed] [Google Scholar]
- 127.Kamal A, Thao L, Sensintaffar J, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425(6956):407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- 128.Xu H, Shi Y, Wang J, et al. A heat shock protein 90 binding domain in endothelial nitric-oxide synthase influences enzyme function. J. Biol. Chem. 2007;282(52):37567–37574. doi: 10.1074/jbc.M706464200. [DOI] [PubMed] [Google Scholar]
- 129.Siegelin MD, Dohi T, Raskett CM, et al. Exploiting the mitochondrial unfolded protein response for cancer therapy in mice and human cells. J. Clin. Invest. 2011;121(4):1349–1360. doi: 10.1172/JCI44855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kang BH, Tavecchio M, Goel HL, et al. Targeted inhibition of mitochondrial Hsp90 suppresses localised and metastatic prostate cancer growth in a genetic mouse model of disease. Br. J. Cancer. 2011;104(4):629–634. doi: 10.1038/bjc.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Plescia J, Salz W, Xia F, et al. Rational design of shepherdin, a novel anticancer agent. Cancer Cell. 2005;7(5):457–468. doi: 10.1016/j.ccr.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 132.Horibe T, Kawamoto M, Kohno M, Kawakami K. Cytotoxic activity to acute myeloid leukemia cells by Antp-TPR hybrid peptide targeting Hsp90. J. Biosci. Bioeng. 2012 doi: 10.1016/j.jbiosc.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 133.Seigneuric R, Gobbo J, Colas P, Garrido C. Targeting cancer with peptide aptamers. Oncotarget. 2011;2(7):557–561. doi: 10.18632/oncotarget.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Szeto HH, Schiller PW. Novel therapies targeting inner mitochondrial membrane – from discovery to clinical development. Pharm. Res. 2011;28(11):2669–2679. doi: 10.1007/s11095-011-0476-8. [DOI] [PubMed] [Google Scholar]
- 135.Weiss A, Brill B, Borghouts C, Delis N, Mack L, Groner B. Survivin inhibition by an interacting recombinant peptide, derived from the human ferritin heavy chain, impedes tumor cell growth. J. Cancer Res. Clin. Oncol. 2012;138(7):1205–1220. doi: 10.1007/s00432-012-1195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shirakata T, Oka Y, Nishida S, et al. WT1 peptide therapy for a patient with chemotherapy-resistant salivary gland cancer. Anticancer Res. 2012;32(3):1081–1085. [PubMed] [Google Scholar]
- 137.Sinthuvanich C, Veiga AS, Gupta K, Gaspar D, Blumenthal R, Schneider JP. Anticancer ss-hairpin peptides: membrane-induced folding triggers activity. J. Am. Chem. Soc. 2012;134(14):6210–6217. doi: 10.1021/ja210569f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Verdine GL. Drugging the ‘undruggable’. Harvey Lect. 2006;102:1–15. [PubMed] [Google Scholar]
- 139.Borghouts C, Kunz C, Groner B. Current strategies for the development of peptide-based anti-cancer therapeutics. J. Pept. Sci. 2005;11(11):713–726. doi: 10.1002/psc.717. [DOI] [PubMed] [Google Scholar]
- 140.Zhai D, Godoi P, Sergienko E, et al. High-throughput fluorescence polarization assay for chemical library screening against anti-apoptotic Bcl-2 family member Bfl-1. J. Biomol. Screen. 2012;17(3):350–360. doi: 10.1177/1087057111429372. [DOI] [PubMed] [Google Scholar]
- 141.Verdine GL, Walensky LD. The challenge of drugging undruggable targets in cancer: lessons learned from targeting BCL-2 family members. Clin. Cancer Res. 2007;13(24):7264–7270. doi: 10.1158/1078-0432.CCR-07-2184. [DOI] [PubMed] [Google Scholar]
- 142.Bolhassani A. Potential efficacy of cell-penetrating peptides for nucleic acid and drug delivery in cancer. Biochim. Biophys. Acta. 2011;1816(2):232–246. doi: 10.1016/j.bbcan.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 143.Kelley SO, Stewart KM, Mourtada R. Development of novel peptides for mitochondrial drug delivery: amino acids featuring delocalized lipophilic cations. Pharm. Res. 2011;28(11):2808–2819. doi: 10.1007/s11095-011-0530-6. [DOI] [PubMed] [Google Scholar]
- 144.Moreau V, Fleury C, Piquer D, et al. PEPOP: computational design of immunogenic peptides. BMC Bioinformatics. 2008;9:71. doi: 10.1186/1471-2105-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Van Walle I, Gansemans Y, Parren PW, Stas P, Lasters I. Immunogenicity screening in protein drug development. Expert Opin. Biol. Ther. 2007;7(3):405–418. doi: 10.1517/14712598.7.3.405. [DOI] [PubMed] [Google Scholar]
- 146.Chirino AJ, Ary ML, Marshall SA. Minimizing the immunogenicity of protein therapeutics. Drug Discov. Today. 2004;9(2):82–90. doi: 10.1016/S1359-6446(03)02953-2. [DOI] [PubMed] [Google Scholar]
- 147.Watt PM. Screening for peptide drugs from the natural repertoire of biodiverse protein folds. Nat. Biotechnol. 2006;24(2):177–183. doi: 10.1038/nbt1190. [DOI] [PubMed] [Google Scholar]
- 148.Braun CR, Mintseris J, Gavathiotis E, Bird GH, Gygi SP, Walensky LD. Photoreactive stapled BH3 peptides to dissect the BCL-2 family interactome. Chem. Biol. 2010;17(12):1325–1333. doi: 10.1016/j.chembiol.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Valero JG, Sancey L, Kucharczak J, et al. Bax-derived membrane-active peptides act as potent and direct inducers of apoptosis in cancer cells. J. Cell. Sci. 2011;124(Pt 4):556–564. doi: 10.1242/jcs.076745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Trusheim M, Aitken MC, Berndt ER. Characterizing markets for biopharmaceutical innovations: Do biologics differ from small molecules? (Article 4). Forum Health Econ. Policy. 2010;13(1):16014. [Google Scholar]
- 151.Kozlowski S, Woodcock J, Midthun K, Sherman RB. Developing the nation's biosimilars program. N. Engl. J. Med. 2011;365(5):385–388. doi: 10.1056/NEJMp1107285. [DOI] [PubMed] [Google Scholar]
- 152.Trivigno D, Essmann F, Huber S, Rudner J. Cell Death in Cancer. De Rode Hoed; Amsterdam, The Netherlands: 2012. The deubiquitinase USP9x controls Mcl-1 levels in response to ionizing radiation. [Google Scholar]
- 153.Naumann I, Kappler R, von Schweinitz D, Debatin KM, Fulda S. Bortezomib primes neuroblastoma cells for TRAIL-induced apoptosis by linking the death receptor to the mitochondrial pathway. Clin. Cancer Res. 2011;17(10):3204–3218. doi: 10.1158/1078-0432.CCR-10-2451. [DOI] [PubMed] [Google Scholar]
- 154.Certo M, Del Gaizo Moore V, Nishino M, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9(5):351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 155.Vrielink J, Heins MS, Setroikromo R, et al. Synthetic constrained peptide selectively binds and antagonizes death receptor 5. FEBS J. 2010;277(7):1653–1665. doi: 10.1111/j.1742-4658.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- 156.Neuzil J, Wang XF, Dong LF, Low P, Ralph SJ. Molecular mechanism of ‘mitocan’-induced apoptosis in cancer cells epitomizes the multiple roles of reactive oxygen species and Bcl-2 family proteins. FEBS Lett. 2006;580(22):5125–5129. doi: 10.1016/j.febslet.2006.05.072. [DOI] [PubMed] [Google Scholar]
- 157.Chipuk JE, Green DR. Dissecting p53-dependent apoptosis. Cell Death Differ. 2006;13(6):994–1002. doi: 10.1038/sj.cdd.4401908. [DOI] [PubMed] [Google Scholar]