Abstract
The purpose of pre-clinical murine model development is to establish that the pathophysiological outcome of our rodent model of radiation-induced lung injury is sufficiently representative of the anticipated pulmonary response in the human population. This objective is based on concerns that the C57BL/6J strain may not be the most appropriate preclinical model of lethal radiation lung injury in humans. In this study, we assessed this issue by evaluating the relationship between morbidity (pulmonary function, histopathologic damage) and mortality among three strains of mice, C57BL/6J, CBA/J, and C57L/J. These different strains display variations in latency and phenotypic expression of radiation-induced lung damage. By comparing the response of each strain to the human pulmonary response, we established an appropriate animal model(s) of human radiation-induced pulmonary injury. Observations in the C57L/J and CBA/J murine models can be extrapolated to the human lung for evaluation of mechanisms of action of radiation as well as future efficacy testing and approving agents that fall under the “Animal Rule” of the US Food and Drug Administration (FDA) (21 CFR Parts 314 and 601).
Keywords: lungs, rodent, radiation damage, dose-response, whole thorax irradiation
Introduction
Deliberate or accidental radiation exposure can lead to life threatening or severely debilitating pulmonary disease when the thorax receives an acute dose in excess of 7.5 Gy(Van Dyk et al. 1981). Currently, the lack of a well-defined animal model limits the degree to which pre-clinical observations can be used to direct improvements in therapeutic measures, resulting in improved outcomes in human patients. A number of species have been used to model clinical radiation pathogenesis in the laboratory, including rodents, mini-pigs, dog, hamsters, rabbits, and others(Hopewell et al. 2000, Sharplin and Franko 1989a, Sharplin and Franko 1989b). However, the genetic and physiologic differences among species and strains used as pre-clinical models impede researchers’ ability to appropriately compare and interpret the outcomes of these studies. There is currently no consensus among the radiobiology community as to which species and strain is most accurate representation of human tissue injury and, therefore, the best model for studying the effects of radiation on healthy lung tissue (Di Carlo et al. 2012).
The most common experimental model used in radiation research is the mouse (Williams et al. 2010). As such, it one of the best characterized species in terms of the pathogenesis of pulmonary injury following thoracic or total body irradiation. Even within the species, however, there is considerable variability in histopathologic sequelae and severity of lung injury between strains in response to wide field, thoracic irradiation (Jackson et al. 2010, Jackson et al. 2011). These differences were first recognized in 1983 by Julian Down and Gordon Steel in a comparison between CBA and C57BL strains (Down and Steel 1983). Franko et al. extended this work, evaluating nine murine strains and characterizing them as being either “fibrosis” or “pneumonitis” prone (Sharplin and Franko 1989a, Sharplin and Franko 1989b). It was these latter studies that led to the subsequent adoption of the C57BL/6 strain as the standard model for both evaluating the molecular mechanisms associated with radiation-induced lung injury and efficacy screening of candidate medical countermeasures (MCM) over the past two decades.
More recently, a number of studies have questioned the use of the C57BL/6J strain as the ‘gold standard’ for pre-clinical research due to the long latency period prior to the development of focal fibrosis (6-9 months), mild pneumonitis, and, most importantly, pleural effusions (Down et al. 1984, Jackson et al. 2010, Travis et al. 1980, Travis and Tucker 1986). Pleural effusions are rarely seen in the human victims of radiation accidents, and when they are, their contribution to morbidity/mortality appears to be minimal (Gross 1977).
During the past several years, there has been a growing interest in re-evaluating many of the common research models used in biomedical research, particularly in radiation-induced tissue injury. This revitalization of interest stems from the FDA requirements for efficacy screening of MCMs, which mandate that testing be accomplished using a well-characterized animal model that reflects the pathogenesis of radiation injury in human lung. The goal of this requirement is to optimize the predictive validity of the pre-clinical findings.
Ideally, an appropriate animal model should exhibit a similar course of disease development as humans, including dose-response, natural history, and histopathology of injury following acute thoracic radiation exposure. The purpose of this project was to thoroughly characterize murine strain differences in pulmonary radioresponse to identify the model that most closely reflects the pathogenesis of radiation toxicity in human lung (Jackson et al. 2010, Jackson et al. 2011).
One of the requirements of the FDA Animal Rule is for the pre-clinical study endpoints to reflect the desired benefit in humans, generally an improvement in survival or a significant reduction in major morbidity (21 CFR Parts 314 and 601). Therefore, in this study, survival was chosen as the primary endpoint. Secondary endpoints included those with clinical correlates to maximize translation of animal efficacy data to clinical parameters. Longitudinal measurements of respiratory function were chosen as the secondary endpoints. Retrospective analysis was performed to determine whether any of the measured endpoints could be useful as early indicators of individual risk for developing lethal lung injury. Finally, lung tissue was harvested to assess histopathologic damage at the time of morbidity/mortality or at the final time point for the in-life portion of the study, depending on which came first.
Materials and Methods
Animals
Female C57L/J, CBA/J, and C57BL/6J mice were purchased 8-10 weeks of age (~20g) from Jackson Labs. Animals were housed 5 per cage at the Duke Genome Science Research Building (GSRBII), a barrier facility, and provided food and water ad libitum. All experiments were performed with prior approval from the Duke University Institutional Animal Care and Use Committee (IACUC).
Radiation
Radiation dose and uniformity of distribution was determined prior to initiation of the study as previously described(McGurk et al. 2012). Animals were allowed to acclimate to the facility for one to two weeks prior to radiation exposure. For irradiation, animals were anesthetized using ketamine (100 mg/kg) and xylazine (10 mg/kg) and irradiated in the prone position with 7.5, 10, 12.5, 15, or 17.5 Gy of 320-kVp X rays (Precision X-ray Inc., North Branford, CT, Filter = 2.00 mm Al, HVL≅1.0mm Cu, dose rate = 69.2 cGy min-1). Radiation was delivered to the thorax through adjustable apertures with 8 mm lead shielding of the head, abdomen, and forelimbs. Sham-irradiated animals were treated in the same way, except that the radiation source was not turned on. To confirm that radiation was confined to the thorax, port films were taken prior to each radiation procedure. After radiation, animals were kept on heating blankets to help maintain normal body temperature until fully awake and mobile, at which time they were returned to the housing facility.
Survival Analysis
Radiation pneumonitis is expected to be the predominant and lethal response between 3 and 6 months after whole thorax irradiation. The clinically relevant measurable parameter for radiation pneumonitis in a mouse model is mortality. All animals were closely monitored for respiratory distress over the first 6 months post-radiation exposure. Moribund mice were euthanized by sodium pentobarbital overdose (>250 mg/kg) per IACUC recommendations. Surviving mice were euthanized at a pre-determined final time point of 182 days. Subsets of mice in each group were euthanized at a pre-determined time point of 10 or 14 weeks (n = 5/group). Body weights were recorded every 2 weeks during the in-life phase until the first sign of weight loss, after which body weights are recorded daily and animals observed for behavioral changes twice per day. Criteria for euthanasia included a combination of animal observation (lethargy, hunchback, ruffled fur) and/or body weight loss >15%. At the time of euthanasia, a final body weight was recorded and final pulmonary function test (whole body plethysmography) performed.
Analysis of Respiratory Function
Pulmonary function was assessed prior to radiation exposure and every two weeks thereafter during the in-life portion of the study using an unrestrained whole body plethysmograph (Buxco Electronics, Wilmington, NC).
Gross Morphology and Histopathology
At the time of euthanasia, a bilateral thoracotomy was performed. Pleural fluid was measured using pre-weighed tissue paper to absorb excess fluid and repeating the weight measurement. The whole lung and heart were excised, rinsed in cold phosphate-buffered saline, and wet lung weights recorded. The left lobe was inflated through the bronchus with 10% neutral buffered formalin, paraffin embedded, and sectioned in 5-μm sections. The right lobe and heart were snap frozen in liquid nitrogen.
Hematoxylin and Eosin (H&E) and Masson’s trichrome staining were performed as previously described(Gauter-Fleckenstein et al. 2008). H&E stained sections were evaluated for inflammatory cells, alveolar capillary distension or congestion, the presence of hyaline membranes, and alveolar wall thickness. Scoring of perivascular and alveolar inflammation was performed as previously described(Ford et al. 2001). Perivascular inflammation was scored on a scale of 0-4 where a score of 0 indicates no cell layers, while 4 denotes four or more layers of inflammatory cells around the vessel. Alveolar inflammation was scored on a scale of 0-5 with 0 being no alveolar inflammation and 5 being complete consolidation of the tissue. Semi-quantitative assessment of the degree of interstitial fibrosis was determined using a predetermined numerical scale of 0-8 based on the Ashcroft scoring method(Ashcroft et al. 1988). The criteria for this scoring were based on histological features such as alveolar wall thickness, fibrotic damage to lung structures, and fibrous lesions. Three independent reviewers scored the tissue.
Statistical Design and Analysis
Statistical analyses were performed and plots generated using SAS version 9.2. Animal identity was included in this analysis to account for inter-animal variations. The proportion of mice that survived for 182-days was computed for each strain and radiation dose. The mean survival times (MST) among decedents were presented by strain (C57BL/6J, C57L/J, CBA/J) and radiation dose (0, 7.5, 10, 12.5, 15, and 17.5 Gy). Kaplan-Meier Survival Curves were used to present survival data by strain and radiation dosage. A time-to-event analysis for overall survival was also performed on the survival data using a Cox proportional hazards regression model to compare the time to death of the strains with radiation dose as a covariate. Secondary parameters (lung weight, breathing rates, etc.) were examined using descriptive statistics (mean, standard deviation, median, maximum, and minimum). All statistical analyses were performed at alpha = 0.05 (significance) and 0.10 (marginal significance) levels. All tests were conducted as two-sided tests.
Results
Kaplan-Meier survival curves for radiation dose-response among three inbred murine strains
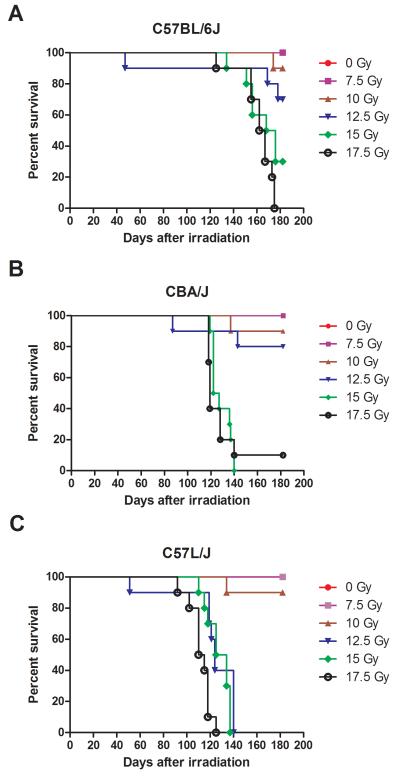
The first step in our efforts to establish an appropriate murine model of human pulmonary radiation injury was to identify the dose response for lethal lung injury in each of three strains (C57L/J, CBA/J, and C57BL/6J). These strains were chosen based on preliminary data(Jackson et al. 2010) that suggests that the pulmonary response to radiation of these mice is comparable to that of humans. For these studies, ten female mice from each strain were grouped and irradiated in the prone position with a single dose ranging from 7.5 to 17.5 Gy (320 kVp X-rays, 2.5 Gy increments) delivered to the whole thorax. Sham-irradiated age and sex-matched mice were included as controls. Figure 1 shows the resulting survival curves for each strain. In this study, a one gray (Gy) increase in the radiation dose significantly increased the odds of death (p < 0.0001) across all strains(Morris and Gardner 1988). There was a significant difference in survival (p=0.0038) between murine strains across all doses. Consistent with our previous results(Jackson et al. 2011), C57L/J mice displayed a significantly higher odds of death compared to C57BL/6J mice (odds ratio = 15.24) or CBA/J mice (odds ratio = 10.26).
Figure 1. Kaplan-Meier Survival Curves.
Female C57BL/6J, CBA/J, and C57L/J mice were irradiated to the whole thorax with a single dose of 7.5-17.5 Gy in 2.5 Gy increments. Animals were followed for up to 182 days post-radiation for survival. There was a significant difference in survival between strains (p < 0.0038). C57L/J mice had significantly higher odds of death when compared to C57BL/6J (odds ratio = 15.24) or CBA/J mice (odds ratio = 10.26). A one Gray increase significantly increased the odds of death in all strains (p < 0.0001).
Comparison of radiation dose response relationships: From rodents to humans
The overall objective here was to assess the natural progression of radiation-induced pulmonary toxicity in three murine strains and identify which response is most comparable to that of humans. In this study, the radiation dose to cause lethality in 50% of mice within the first 180 days (LD50/180) was determined using probit analysis of the dose-response curves (Fig. 1). The survival data was then compared to data derived from non-human primates at the University of Maryland-Baltimore(Garofalo et al. 2012) and published literature describing the human pulmonary dose-response for lethality(Fryer et al. 1978, Van Dyk et al. 1981). Table 1 describes the median survival times and lethal dose for 50% of mice within the first six months post-exposure. The lethal dose for 50 percent of C57BL/6J mice by day 180 (LD50/180) was 13.43 Gy (95% Confidence Interval, 12.3-14.7 Gy). The LD50/180 for CBA/J mice was 13.10 Gy (95% Confidence Interval, 12.1-14.2 Gy). The LD50/180 for C57L/J mice was 10.24 Gy (95% Confidence Interval, 0.2-509 Gy). The inability to establish an LD50/180 for the C57L/J strain is indicative of the steep slope of the dose-response for this strain. Ninety-percent survival was observed at a dose of 10 Gy compared to 0% survival at the next dose of 12.5 Gy in C57L/J mice. The steepness of the dose-response in C57L/J is similar to that observed in human lung where the threshold for clinically symptomatic lung injury is approximately 7.5 Gy with 50% incidence of clinical pneumonitis being approximately 10.60 Gy based on probit analysis of clinical data provided in Van Dyk et al. (Van Dyk et al. 1981).
Table 1. Comparison of radiation dose response for lung injury among murine strains, nonhuman primates, and humans.
| Murine strain |
No. of subjects in study |
Radiation dose |
Percent lethality across all doses |
Mean survival time |
LD50/180 | 95% Confidence Interval |
|---|---|---|---|---|---|---|
| C57BL/6J | 57 | 7.5-17.5 Gy | 35.1 | 162 (±14) days |
13.43 Gy | 12.20-14.69 Gy |
| CBA/J | 55 | 7.5-17.5 | 36.7 | 125.5(±12) days |
13.11 Gy | 12.08-14.21 Gy |
| C57L/J | 60 | 7.5-17.5 Gy | 56.4 | 120 (±17) days |
10.24 Gy | NE |
| NHP # | 46 | 9-12 Gy | 50 | 144.5 days | 10.28 Gy | 9.92-10.67 Gy |
|
No. of subjects |
Radiation doses |
Incidence of pneumonitis |
Time of onset of PN |
TD50/180 |
95% Confidence Interval |
|
| Humans □ | 150 | 0-12.5 Gy | 15% | ~100 days | 10.60 Gy | 9.97-12.05 Gy |
NE = not estimable; Lethality for C57L/J mice was 10% at 10 Gy and 100% at 12.5 Gy. Therefore, a precise LD50/180 was not able to be determined due to the steep dose response for lethal lung injury in this strain. The dose-response study is being repeated with tighter incremental windows;
Non-human primate (NHP)- Data from Garofalo et al. 2012;
Based on probit analysis of data provided in Table 5 of Van Dyke et al 1981, LD=Lethal dose; TD= Tolerated dose; PN = Pneumonitis
Studies are ongoing to explore the dose-response relationships using tighter incremental windows (≤75cGy) to better estimate the lethal dose for 30, 50, and 70% of mice within the first 180 days post-radiation. Despite the limitations our radiation increments impose, these results provide a good estimation of the dose-range expected to cause significant morbidity/mortality in each of the three strains used, allowing for comparison with non-human primates and humans.
In this study, the mean survival times across all doses were 163 days in C57BL/6J mice, 126 days in CBA/J mice and only 121 days in C57L/J mice. In comparison, the mean time-to-onset of acute pneumonitis in humans is approximately 100 days (peak 2-3 months)(Van Dyk et al. 1981) and 145 days in non-human primates (Garofalo et al. 2012) following wide-field radiation exposure to the thorax. Establishing a link between temporal onset and dose-response in each of these rodent strains, as well as in non-human primates and humans is one step towards defining an accurate animal model in which medical countermeasures can be tested with a reasonable expectation that the results will translate to human lung injury.
Temporal onset of respiratory dysfunction coincides with morbidity and/or mortality among strains
To assess the dose related incidence of functional injury, respiratory function was monitored every two weeks throughout the in-life portion of the study using an unrestrained whole body plethysmograph (WBP; Buxco Electronics, Wilmington, NC). A baseline measurement was recorded for each subject prior to irradiation. Following irradiation, we used the WBP to assess respiratory rate, enhanced pause (e.g. airway resistance/air flow), relaxation time, minute volume, peak inspiratory flow, and peak expiratory flow, among other parameters. A number of studies have shown genetic differences among murine strains in airway mechanics(Tankersley et al. 1999), therefore we first evaluated baseline differences in ventilation and respiratory parameters among strains. Although statistically significant differences were observed prior to radiation, none appeared to be associated with radiation tolerance of the lung. Moreover, retrospective analysis of respiratory changes over time found no single respiratory parameter to be useful as an early predictor for individual sensitivity or likelihood for developing lethal lung injury. However, there was a clear difference between survivors and non-survivors in several respiratory parameters following irradiation including enhanced pause (Fig. 2), relaxation time (Fig. 3), and minute volume (Fig. 4).
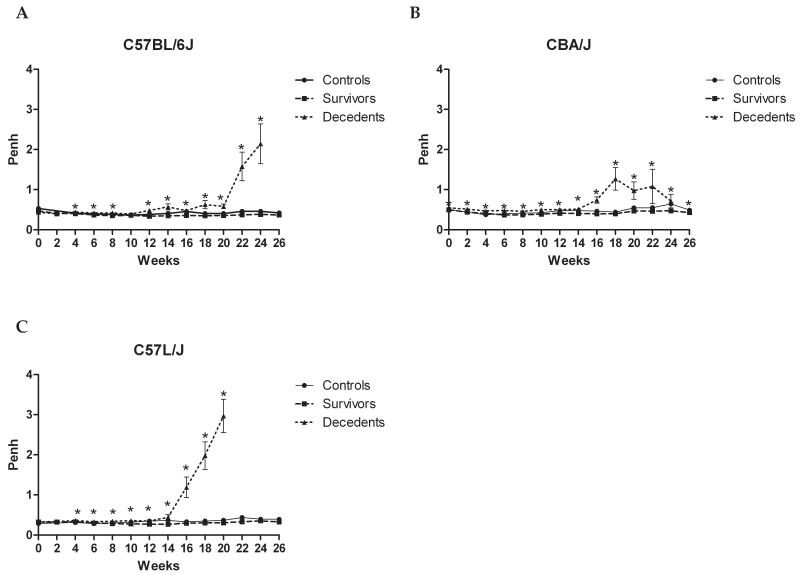
Figure 2. Strain and time-dependent changes in airway resistance (Penh) between non-irradiated controls survivors, and decedents during the twenty-six week (182 day) follow-up period.
Mice received a single dose of 7.5, 10, 12.5, 15, or 17.5 Gy to the whole thorax and were followed for up to 182 days post-irradiation. Age-matched non-irradiated controls were included in the analysis. A clear difference in enhanced pause (Penh), a measurement of airway resistance, is seen between irradiated mice surviving to 182 days (survivors) and those succumbing to radiation-induced lung injury (decedents). Penh rises sharply in decedents, suggesting a reduction in airflow, during the peak phase of injury in C57BL/6J (A), CBA/J (B), and C57L/J (C) mouse strains. The acute increase in Penh occurs approximately 9-14 days prior to individual animal mortality among all strains. This data suggests Penh may be an excellent indicator of morbidity/mortality in mice. Error bars represent SEM. *p<0.05
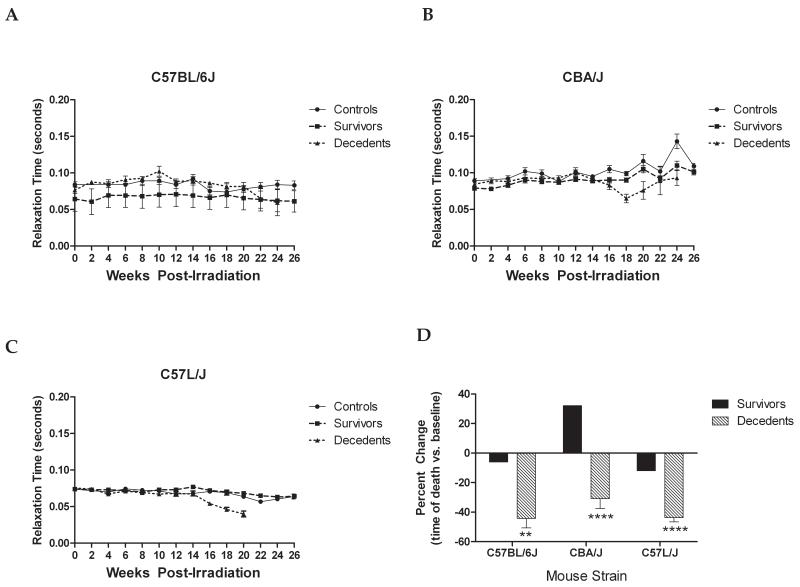
Figure 3. Relaxation time decreases after thoracic irradiation in C57BL/6J (A), CBA/J (B), and C57L/J (C) mice over the course to disease progression.
There is significant impairment in relaxation time among mice succumbing to radiation-induced lung injury as compared to their surviving counterparts (radiation doses: 7.5-17.5 Gy WTLI; non-irradiated controls not shown). The percent change in relaxation time at the time of death compared to pre-irradiation values is presented (D). Error bars represent SEM. **p<0.01, ****p<0.0001
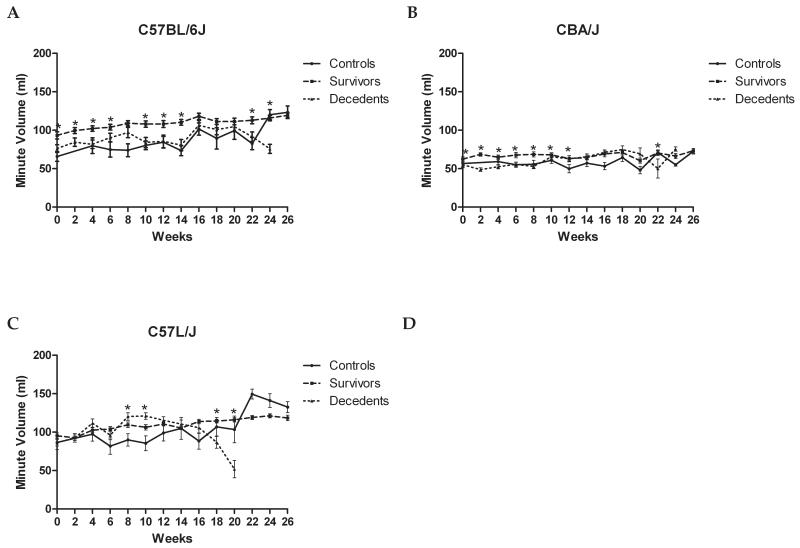
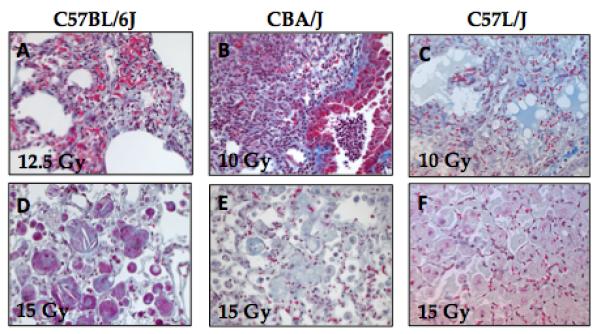
Figure 4. Longitudinal changes in minute volume (MV) is consistent with obliterative bronchiolitis observed in the C57L strains.
The decrease in minute volume suggests a progressive impairment in gas exchange among moribund mice in comparison to their surviving age-matched, irradiated counterparts. The decrease in MV among C57BL/6J (A) and CBA (B) strains is less robust than that among C57L/J mice (C). This is consistent with the observed pathologic injury showing complete consolidation of the alveoli with foamy-lipid laden macrophages and proteinacious edema (D). Error bars represent SEM, *p<0.05
Gross morphology and histopathologic damage
At the time of necropsy, wet lung weights were recorded as an indicator of edema and congestion (Fig. 5). Histopathologic damage and cause of morbidity was dependent on both strain and radiation dose (Fig. 6). As with humans, the lungs of C57L/J mice demonstrated a heavy mononuclear cell infiltrate particularly around the major vessels, congestion of the airways, increase in acidophilic alveolar macrophages and foamy lipid-laden macrophages, type II cell hyperplasia, and hyaline membranes. Inflammation appeared most severe near the airways and disseminated towards the subpleura. Because severe injury was observed at the lower doses of 7.5 and 10 Gy in this strain, it is possible that many of the surviving mice receiving 10 Gy or even 7.5 Gy would have eventually succumbed to acute pneumonitis if the follow-up time had been extended past 182 days (Fig. 6). Collagen deposition was also observed with increased alveolar wall thickness and fibrosis. Comparison of perivascular and alveolar inflammation, fibrosis, and lung weights demonstrates that C57L/J exhibit a more severe inflammatory reaction and greater extent of fibrosis (10-15%) than either CBA/J (5-7% fibrosis) or C57BL/6J (5-10% fibrosis) strain.
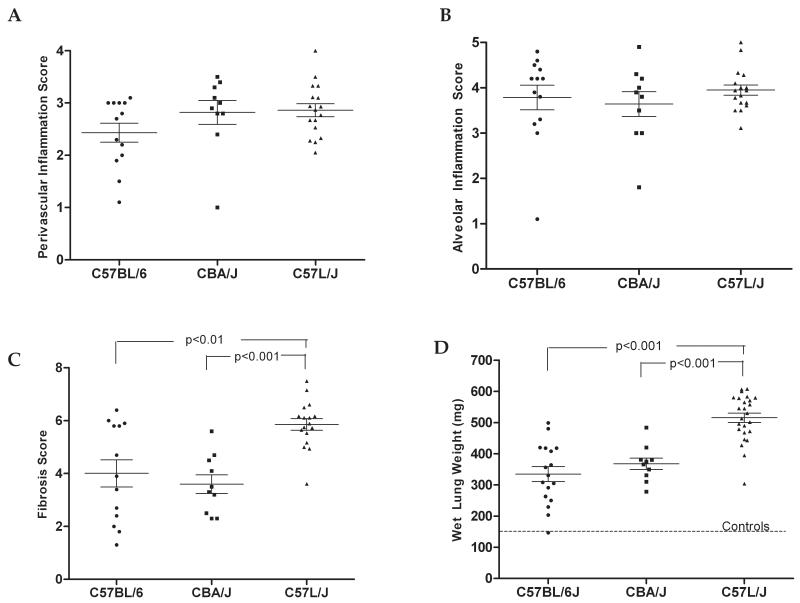
Figure 5. Comparison of pathologic damage among mice at the time of death from lung injury following exposure to 7.5-17.5 Gy WTLI.
A) Perivascular inflammation was scored on a scale of 0-4 with 4 being more than 4 layers of mononuclear lymphocytes surrounding at least one pulmonary blood vessel. Differences in perivascular inflammation were not significant among strains as all displayed some vascular inflammation. However the percentage of vessels with inflammation was greater in C57L/J than in C57BL/6J mice. B) Alveolar inflammation, characterized by eosinophilic and foamy, lipid-laden macrophages, was present in strains at the time of death from radiation-lung damage. C) The severity of fibrosis was greater in C57L/J mice than in CBA/J (p< 0.001) or C57BL/6J mice (p<0.01). D) C57L/J had significantly higher lung weights at doses above than either C57BL/6J or CBA/J. The average lung weight among C57L/J mice exposed to 7.5 Gy was 231.50 mg, which was an 81% increase over the non-IR controls demonstrating the severe sensitivity of this strain to radiation. Among all parameters, there appeared to be greater variability in the extent of lung damage among individual C57BL/6J mice at the time of death when compared to the variability around the mean in either the C57L/J or CBA/J strains. Error bars represent the mean ± SEM.
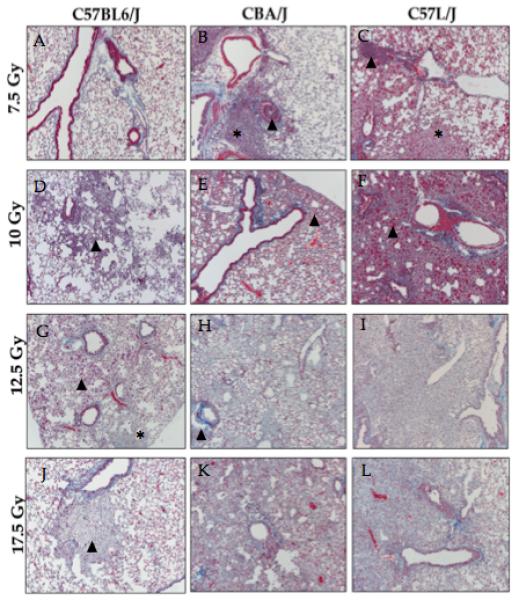
Figure 6. Comparison of histopathologic damage among strains at radiation doses ranging from 7.5 to 17.5 Gy WTLI.
There is significant variation in pathology among strains. A) Lung tissue from C57BL/6J mice appear mostly normal 26 weeks post-WTLI with a single dose of 7.5 Gy, LW: 162 mg. B) Lungs of CBA/J mice display airway congestion (arrowhead), increased alveolar wall thickness, and lymphocytic inflammation (*) 26 weeks post-WTLI (7.5Gy). LW: 168 mg. C) Significant inflammation and edema is observed in the lungs of C57L/J mice after 7.5 Gy (26 weeks post-IR), which is consisted with the elevation in lung weight. Perivascular lymphocytic cuffing (arrowhead) and foamy macrophages (*) are shown. LW: 257 mg. D) Localized alveolar inflammation (arrowhead) is seen in the irradiated lungs of C57BL/6J mice at 26 weeks post-10 Gy WTLI. This is characterized by an increase in alveolar macrophages and thickening of the alveolar walls. LW: 194 mg. E) An organized pneumonia is seen in the lungs of CBA/J mice 26 weeks after 10 Gy WTLI. LW: 171 mg. F) Complete obliteration of the alveoli and airway congestion is seen in the irradiated lung of C57L/J mice after 10 Gy WTLI. Tissue damage is characterized by excessive, contracted fibrosis, alveolar and vascular inflammation, and significant edema. 26 weeks, LW: 342 mg. G) An increase in eosinophilic macrophages (arrowhead) and focal fibrosis (*) is seen 26 weeks-post 12.5 Gy WTLI in C57BL/6J mice. LW: 296 mg. H) Vascular inflammation and collagen deposition are seen in the lungs of CBA/J mice exposed to 12.5 Gy WTLI. 20-week survival, LW: 484 mg. I) Complete fibrotic consolidation of the alveoli 17 weeks post-WTLI with a single dose of 12.5 Gy. LW: 472 mg J) Foamy alveolar macrophages (arrowhead) are seen in the lungs of moribund C57BL/6J mice following WTLI with a single dose of 17.5 Gy. 22-week survival, LW: 356 mg. K) Collagen deposition, increased alveolar wall thickness, and edema/congestion are seen in the lungs of CBA/J mice after 17.5 Gy WTLI. 18-week survival, LW: 420 mg. L) Fibrotic consolidation of the alveoli in the lungs of of C57L/J mice following 17.5 Gy WTLI. 16-week survival, LW: 600 mg. Significant interstitial edema and airway congestion was an overall feature seen in many of the C57L/J mice at all doses. WTLI: whole thorax lung irradiation. LW=lung weight. *15 Gy is not shown.
Evaluation of histopathologic damage at the time of death suggests a reduction in air space to a volume smaller than that required for sufficient pulmonary gas exchange. This was due to accumulation of giant, multinucleated cells and macrophages within the alveoli, gross distortion of the alveoli due to contracted fibrosis, and blockage of airways with an acidophilic crystalline material and mononuclear inflammatory cells in the C57L strains, but not the CBA/J strain. In C57L/J mice, at the time of death, almost all airways appeared to be congested with significant interstitial edema. This was observed to a lesser extent in the CBA/J mice and not readily observed in the C57BL/6J mice. While a similarly significant increase in alveolar macrophage accumulation was observed in CBA/J mice, a similar reduction in minute volume (MV) was not seen. This may be due in part to the difference in type of histopathologic damage. Whereas C57L/J and C57BL/6J demonstrated gross architectural distortion and honeycombing fibrosis in parts of the lung, the CBA/J mice showed thickening of alveolar walls and inflammation, but little to no contracted fibrosis.
Discussion
The mouse is one of the best species for modeling radiation-induced lung injury due to its small size, close DNA sequence similarity to humans (<2.5% difference), and the ability to utilize gene-targeted mutagenesis to study the influence of specific genes or pathways on radiation pneumonitis/fibrosis(Rivera and Tessarollo 2008). Despite these similarities, there have been a number of therapeutic modalities over the years that displayed strong efficacy in pre-clinical models, but for unknown reasons, were ineffective in the clinic(Mestas and Hughes 2004). One of the explanations for the poor translation of promising therapeutics has been that the efficacy of these agents has been evaluated using pre-clinical models that fail to adequately represent the pathophysiology of the disease in humans(Mestas and Hughes 2004). The aim of this study was to identify the murine strain(s) that best emulates the clinical pathophysiology of radiation-induced lung disease, in the hope of improving the ability to translate knowledge gained from animal-models to advancements in clinical therapy(Dodd-o et al. 2006).
The U.S. government is currently funding the development of medical countermeasures (MCM) against radiation-induced lung toxicity for future FDA licensure under the “Animal Rule”. The current study was designed to develop a research platform that meets the FDA Animal Rule criteria for a robust and reliable pre-clinical model of radiation-induced lung injury in the context of supra-therapeutic whole-thorax exposure.
The experimental design was based on our previous studies, which evaluate the pulmonary pathology associated with thoracic irradiation among six of the most commonly used rodent strains in pre-clinical radiobiology research(Jackson et al. 2010, Jackson et al. 2011). Based on those studies(Jackson et al. 2010, Jackson et al. 2011), we identified the CBA/J and C57L/J mouse strains, and to a lesser extent, the C57BL/6J strain, to represent the full spectrum of pulmonary pathology associated with acute radiation exposure to the thorax(Jackson et al. 2010). As a result, these three strains were chosen for further characterization in the current study.
Here, we systematically characterize the pulmonary response to thoracic irradiation at incremental doses between 7.5 and 17.5 Gy. We then compared functional, histopathologic, and survival outcome to a non-human primate model at the University of Maryland and historical evidence from humans in order to determine which strain’s response best mirrored that of humans. As the primary endpoint for MCM efficacy is improvement in survival, our first aim was to determine the dose-response for survival among strains. The mean survival time in this study was 120 (± 17) days in C57L/J mice, 125 (± 12 days) in CBA/J mice, and 162 (± 14) days in C57BL/6J mice. The long latency period in C57BL/6J contrasts with the temporal onset of pneumonitis in humans (100 days) and non-human primates (144 days) (Table 1).
The difference in the lethal dose for 30-70% of mice within 180 days (LD30-70/180) was striking. In C57L/J mice, the dose-response was steep with an LD30/180 of 10.1 Gy and an LD70/180 of 10.3 Gy. In contrast, for C57BL/6J mice, the LD30-70/180 was 12.4-14.6. It should be noted, however, that due to the very steep dose response in C57L/J mice with only one partial response between 0 and 100%, we could not define the LD30-70/180 values according to standard probit analysis, The LD30-70/180 is likely to fall within the range of 10.5-12.0 Gy and demands further studies that employ doses that fall within this tight incremental window for comparison. Nonetheless, the incidence of radiation-pneumonitis in C57L/J mice most closely resembles that observed in non-human primates (NHP). In NHPs, the LD30-70/180 is approximately 9.8-10.7 Gy, and in humans, approximately 9.6-11.6 Gy (determined from data provided in Van Dyk et al.) (Van Dyk et al. 1981). These values with 95% confidence intervals and at different iso-effect levels need to be carefully considered in estimation of dose-modifying factors (DMF) on application of a given radiation mitigator. Of particular concern are the levels of statistical significance around DMF values and the impact of different slopes of the respective dose-response curves.
Another criteria for successful adherence to the FDA Animal Rule is a clear understanding of the natural history of pulmonary pathogenesis following radiation. In the clinic, a decline in pulmonary function following radiotherapy for tumors in and around the thorax appears to be correlated with symptomatic lung damage (cough, dyspnea, etc.)(Marks et al. 2000). In order to identify the analog of this clinical end point in the animal model, we assessed respiratory function non-invasively using an unrestrained, whole body plethysmograph (WBP) system. The advantage of the unrestrained WBP over other, more invasive techniques is the ability to monitor changes in pulmonary function among individual, freely moving mice throughout the course of disease development. The disadvantage of the WBP is that it is less precise when compared to more invasive techniques (Bates and Irvin 2003). As a result, values obtained with WBP are considered to be estimates of lung mechanics rather than absolute values(Hoyt et al. 2007). Here, we found changes in Penh (airway hyperreactivity/air flow; Fig. 2), gas exchange (Fig. 3), and relaxation time (Fig. 4) first occurred two to four weeks prior to animal mortality. The temporal onset was inversely proportional to dose among all strains and was earliest in the more “sensitive” C57L/J strain.
Another respiratory parameter evaluated was “enhanced pause” or Penh. Penh is a dimensionless quantity derived from pressure changes within the plethysmograph chamber(Lundblad et al. 2002). There is a great deal of controversy over the reliability of using Penh as a marker for airway hyperreactivity(Lomask 2006). Numerous studies have alternatively suggested Penh to be a measurement for changes in respiratory patterns rather than pulmonary mechanics. Regardless of the implications of Penh changes, in this study, Penh was a reliable and robust predictor of mortality. A sharp rise in Penh occurred consistently 9-14 days prior to mortality among individual mice (Fig. 2). In our opinion, this consistent occurrence makes Penh a useful marker for euthanasia, as it is an excellent indicator of imminent mortality.
Although there was a clear divergence in pulmonary function between survivors and decedents in the weeks to months following radiation, no single parameter could predict individual risk for developing lethal pneumonitis prior to symptomatic onset.
Pathologic exam was performed on lung specimens collected either at the time of morbidity/mortality or at the pre-determined endpoint of 10, 14, or 26 weeks (data from 10-14 weeks not shown). In humans, acute interstitial pneumonitis develops 2-3 months after radiation exposure and may range from mild inflammation to fulminant organ failure(Gross 1977). After localized high-dose irradiation, the pneumonitis reaction may be followed by progressive fibrosis that can result in significant tissue scarring and contraction, impaired gas exchange, and organ dysfunction(Gross 1977). Histopathologic examination of tissue collected from individuals succumbing to pneumonitis shows epithelial cell hyperplasia, thrombosis and edema, accumulation of foamy, lipid-laden macrophages and mononuclear cellular infiltrates, increased alveolar septa thickness, hyaline membranes, and increased connective tissue density (Gross 1977). In individuals succumbing to lung injury six months after localized exposure, the histopathology is dominated by contracted fibrosis, gross architectural distortion, and reduced perfusion(Gross 1977).
In this study, diffuse interstitial pneumonitis was observed in the lungs of C57L/J and CBA/J mice 26 weeks after radiation exposure at all radiation doses (7.5-17.5 Gy). In contrast, a milder pneumonitis was observed in C57BL/6J and only at the highest radiation doses (≥12.5 Gy) (Fig. 6). Histological findings from C57L/J lungs indicate severe airway obstruction, alveolar edema, and moderate to extensive fibrosis, which likely contribute to the decline in lung function and increased mortality in this strain (Figs. 6 and 7). These mice also had fine, eosinophillic crystalline structures and inflammatory cells of mixed type within almost all of the bronchiolar airways (Fig. 7). Perivascular lymphocytic cuffing that progressed to widespread interstitial inflammation and patchy consolidation of tissue was another prominent feature in the lungs of moribund C57L/J mice. Additionally, we observed that these mice had extreme alveolar inflammation, characterized by eosinophillic and lipid-and hemosiderin-laden alveolar macrophages and giant multinucleate cells (Fig. 7). This inflammation led to complete congestion/consolidation of the alveoli. Organized exudate and parenchymal scarring were also evident; severity increased with dose. Most importantly, in this strain, the entirety of the lung appeared to be severely damaged in moribund mice.
Figure 7. Obliterative bronchiolitis and interstitial pneumonia in C57L/J mice 26 weeks following 7.5 Gy to the whole thorax.
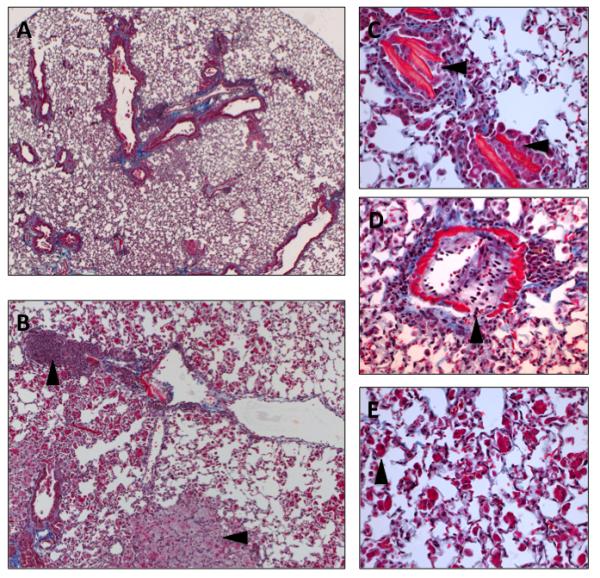
A) Masson’s Trichrome stain of lung tissue 26 weeks after 7.5 Gy whole thorax irradiation in C57L/J mice (5x magnification). B) Higher magnification (10x magnification) shows alveolar inflammation with foamy-macrophages (−>)and perivascular lymphocytic cuffing (*). C) Fine, eosinophillic needle like structures within the terminal bronchioles. D) Breakdown of the vessel wall, inflammation, and fibroproliferation. E) Giant, eosinophilic alveolar macrophages characteristic of alveolar inflammation. C-E, 40x magnification.
In contrast, lungs from the C57BL/6J strain had large areas that appeared normal. In the majority, less than 30% of the lung was damaged. This may be attributed to the delayed progression of lung injury beyond the period of this study as well as the intervening intrusion of lethal pleural effusions(Down 1986). The damage observed in the lungs from these mice included cholesterol clefts, patchy consolidation and inflammation, characteristic of interstitial lung disease, as well as focal tissue scarring and retraction mainly around the subpleura (Fig. 5). While CBA/J mice displayed clear evidence of an organizing pneumonia with thickened alveolar walls, there was very little contracted fibrosis. The lungs of CBA mice showed extensive perivascular and alveolar inflammation, with edema and congestion (Fig. 5).
The mouse remains one of the best small-animal models for human disease. However, it should be noted that there are a number of physiological and immunological dissimilarities between mice and humans that will continue to confound research regardless of strain used. These include differences in innate and adaptive immunity(Mestas and Hughes 2004), lobularity, septa and pleural thickness, and blood supply to the pleura(Williams et al. 2010), Mice also have a shorter life span, are skewed towards a Th1 immunological response, and are less diverse due to singular use of inbred strains(Rivera and Tessarollo 2008). The presence of bronchus-associated lymphoid tissue at sites of bronchial tree bifurcation may also play a role in the immunological responses of lung tissue to pathogenic insults that may not adequately reflect the pathology in humans. These often-overlooked differences are some of the primary reasons there is a widespread failure of therapeutic compounds to successfully achieve the desired benefit in humans.
Despite these complications, mice remain one of the most affordable and practical models for investigation of human pathologies and therapeutic countermeasures. Developing a robust and reproducible model that can be standardized across institutions will help us to better define the complex relationships between molecular and cellular biology, physiologic effects, and pathologic outcomes associated with radiation-induced lung disease. Establishing a superior research platform for MCM development requires a constant flow of information between clinicians and researchers to ensure data can be extrapolated from pre-clinical models to human disease.
This study found significant strain-related differences in radiation-induced lung damage between the C57L/J, CBA/J, and C57BL/6J strains. In this study, we have shown the lungs of C57L/J mice are quite analogous to the lungs of humans and non-human primates with respect to radiation dose-response and pathologic damage following thoracic irradiation. In addition, CBA/J mice remain an excellent model for radiation-pneumonitis without contracted fibrosis.
Strain differences can be exploited to better qualify the molecular mechanisms associated with radiation-induced lung injury and to more efficiently screen medical countermeasures according to the criteria laid out within the FDA Animal Rule. In particular, use of both the C57L/J and CBA/J strains in pre-clinical pulmonary radiobiology studies can enable elucidation of the mechanisms related to pneumonitis versus fibrosis and assist in determining the indication of MCMs.
The broad availability of genetically modified models on C57BL/6J background makes this strain appealing due to the elegance of experimental design that these mice offer. However, in a number of disease-types the C57BL/6J strain is an inferior model as they less adequately reflect the pathogenesis of the disease in humans when compared to other strains(Gueders et al. 2009). The long-latency period and dose-response for lethality in C57BL/6J mice, coupled with the complication of pleural effusions on survival, make this a less than ideal pre-clinical model for lung damage due to acute radiation exposure, as this strain is most dissimilar to humans. While it is recognized that C57L/J mice are difficult to obtain at sufficiently large quantities for optimal statistically powered experimental design, methods exist to overcome these limitations and should be explored.
In conclusion, a broader approach should be taken for the study of radiation-induced lung injury in preclinical models. To improve the extrapolation of results from bench to bedside, models should best reflect the currently known pathogenesis of the disease in humans. This may require backcrossing of carefully selected strains to improve the “humanness” of the model or use of more than one strain in research experiments (e.g. CBA/J or C57L/J). While this is more costly, it will improve the predictive validity of research outcomes and be more cost-effective in the end in both money spent and in lives saved.
Figure 8.
Acknowledgements
The authors wish to thank Dr. Zahid Rabbani for his help in the evaluation of the histological slides. This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN266200500043C.
Footnotes
Conflict of Interest: All authors declare no conflict of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41:467–70. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates JH, Irvin CG. Measuring lung function in mice: the phenotyping uncertainty principle. Journal of applied physiology. 2003;94:1297–306. doi: 10.1152/japplphysiol.00706.2002. [DOI] [PubMed] [Google Scholar]
- Di Carlo AL, Jackson IL, Shah JR, Czarniecki CW, Maidment B, Williams JP. Development and licensure of medical countermeasures to treat lung damage resulting from a radiological or nuclear incident. Radiation Research. 2012 doi: 10.1667/rr2881.1. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd-O JM, Hristopoulos ML, Welsh-Servinsky LE, Tankersley CG, Pearse DB. Strain-specific differences in sensitivity to ischemia-reperfusion lung injury in mice. Journal of applied physiology. 2006;100:1590–5. doi: 10.1152/japplphysiol.00681.2005. [DOI] [PubMed] [Google Scholar]
- Down JD. The nature and relevance of late lung pathology following localised irradiation of the thorax in mice and rats. The British journal of cancer. 1986;(Supplement 7):330–2. [PMC free article] [PubMed] [Google Scholar]
- Down JD, Laurent GJ, Mcanulty RJ, Steel GG. Oxygen-dependent protection of radiation lung damage in mice by WR 2721. International journal of radiation biology and related studies in physics, chemistry, and medicine. 1984;46:597–607. doi: 10.1080/09553008414551791. [DOI] [PubMed] [Google Scholar]
- Down JD, Steel GG. The expression of early and late damage after thoracic irradiation: a comparison between CBA and C57B1 mice. Radiation research. 1983;96:603–10. [PubMed] [Google Scholar]
- Ford JG, Rennick D, Donaldson DD, Venkayya R, Mcarthur C, Hansell E, Kurup VP, Warnock M, Grunig G. Il-13 and IFN-gamma: interactions in lung inflammation. Journal of immunology. 2001;167:1769–77. doi: 10.4049/jimmunol.167.3.1769. [DOI] [PubMed] [Google Scholar]
- Fryer CJ, Fitzpatrick PJ, Rider WD, Poon P. Radiation pneumonitis: experience following a large single dose of radiation. International journal of radiation oncology, biology, physics. 1978;4:931–6. doi: 10.1016/0360-3016(78)90002-0. [DOI] [PubMed] [Google Scholar]
- Garofalo MC, Farese AM, Ward AA, Taylor-Howell C, Cui W, Gibbs A, Prado K, Jackson W, Macvittie TJ. The delayed pulmonary syndrome following acute high dose irradiation: a rhesus macaque model. Health physics. 2012 doi: 10.1097/HP.0b013e3182a32b3f. In Press. [DOI] [PubMed] [Google Scholar]
- Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jiang C, Batinic-Haberle I, Vujaskovic Z. Comparison of two Mn porphyrin-based mimics of superoxide dismutase in pulmonary radioprotection. Free radical biology & medicine. 2008;44:982–9. doi: 10.1016/j.freeradbiomed.2007.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross NJ. Pulmonary effects of radiation therapy. Annals of internal medicine. 1977;86:81–92. doi: 10.7326/0003-4819-86-1-81. [DOI] [PubMed] [Google Scholar]
- Gueders MM, Paulissen G, Crahay C, Quesada-Calvo F, Hacha J, Van Hove C, Tournoy K, Louis R, Foidart JM, Noel A, Cataldo DD. Mouse models of asthma: a comparison between C57BL/6 and BALB/c strains regarding bronchial responsiveness, inflammation, and cytokine production. Inflammation research: official journal of the European Histamine Research Society ... [et al.] 2009;58:845–54. doi: 10.1007/s00011-009-0054-2. [DOI] [PubMed] [Google Scholar]
- Hopewell JW, Rezvani M, Moustafa HF. The pig as a model for the study of radiation effects on the lung. International journal of radiation biology. 2000;76:447–52. doi: 10.1080/095530000138439. [DOI] [PubMed] [Google Scholar]
- Hoyt R, Hawkins J, Clair M, Kennett M. Mouse Physiology. In: FOX J, BARTHOLD S, DAVISSON M, NEWCOMER C, QUIMBY F, SMITH A, editors. The Mouse in Biomedical Research: Diseases. Elsevier; Burlington: 2007. [Google Scholar]
- Jackson IL, Vujaskovic Z, Down JD. Revisiting strain-related differences in radiation sensitivity of the mouse lung: recognizing and avoiding the confounding effects of pleural effusions. Radiation research. 2010;173:10–20. doi: 10.1667/RR1911.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson IL, Vujaskovic Z, Down JD. A further comparison of pathologies after thoracic irradiation among different mouse strains: finding the best preclinical model for evaluating therapies directed against radiation-induced lung damage. Radiation research. 2011;175:510–18. doi: 10.1667/RR2421.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomask M. Further exploration of the Penh parameter. Experimental and toxicologic pathology: official journal of the Gesellschaft fur Toxikologische Pathologie. 2006;57(Suppl 2):13–20. doi: 10.1016/j.etp.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Lundblad LK, Irvin CG, Adler A, Bates JH. A reevaluation of the validity of unrestrained plethysmography in mice. Journal of applied physiology. 2002;93:1198–207. doi: 10.1152/japplphysiol.00080.2002. [DOI] [PubMed] [Google Scholar]
- Marks LB, Fan M, Clough R, Munley M, Bentel G, Coleman RE, Jaszczak R, Hollis D, Anscher M. Radiation-induced pulmonary injury: symptomatic versus subclinical endpoints. International journal of radiation biology. 2000;76:469–75. doi: 10.1080/095530000138466. [DOI] [PubMed] [Google Scholar]
- Mcgurk R, Toncheva G, Jackson IL, Vujaskovic Z. Development of a small animal irradiation platform. Health Physics. 2012 doi: 10.1097/hp.0b013e3182632526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. Journal of immunology. 2004;172:2731–8. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Morris JA, Gardner MJ. Calculating confidence intervals for relative risks (odds ratios) and standardised ratios and rates. British medical journal. 1988;296:1313–6. doi: 10.1136/bmj.296.6632.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J, Tessarollo L. Genetic background and the dilemma of translating mouse studies to humans. Immunity. 2008;28:1–4. doi: 10.1016/j.immuni.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Sharplin J, Franko AJ. A quantitative histological study of strain-dependent differences in the effects of irradiation on mouse lung during the early phase. Radiation research. 1989a;119:1–14. [PubMed] [Google Scholar]
- Sharplin J, Franko AJ. A quantitative histological study of strain-dependent differences in the effects of irradiation on mouse lung during the intermediate and late phases. Radiation research. 1989b;119:15–31. [PubMed] [Google Scholar]
- Tankersley CG, Rabold R, Mitzner W. Differential lung mechanics are genetically determined in inbred murine strains. Journal of applied physiology. 1999;86:1764–9. doi: 10.1152/jappl.1999.86.6.1764. [DOI] [PubMed] [Google Scholar]
- Travis EL, Down JD, Holmes SJ, Hobson B. Radiation pneumonitis and fibrosis in mouse lung assayed by respiratory frequency and histology. Radiation research. 1980;84:133–43. [PubMed] [Google Scholar]
- Travis EL, Tucker SL. The relationship between functional assays of radiation response in the lung and target cell depletion. The British journal of cancer. 1986;(Supplement 7):304–19. [PMC free article] [PubMed] [Google Scholar]
- Van Dyk J, Keane TJ, Kan S, Rider WD, Fryer CJ. Radiation pneumonitis following large single dose irradiation: a re-evaluation based on absolute dose to lung. International journal of radiation oncology, biology, physics. 1981;7:461–7. doi: 10.1016/0360-3016(81)90131-0. [DOI] [PubMed] [Google Scholar]
- Williams JP, Brown SL, Georges GE, Hauer-Jensen M, Hill RP, Huser AK, Kirsch DG, Macvittie TJ, Mason KA, Medhora MM, Moulder JE, Okunieff P, Otterson MF, Robbins ME, Smathers JB, Mcbride WH. Animal models for medical countermeasures to radiation exposure. Radiation research. 2010;173:557–78. doi: 10.1667/RR1880.1. [DOI] [PMC free article] [PubMed] [Google Scholar]