Abstract
Social support has been shown to influence health outcomes in later life. In this study, we focus on social engagement as an umbrella construct that covers select social behaviors in a lifespan sample that included oldest-old adults, a segment of the adult population for whom very little data currently exist. We examined relationships among social engagement, positive health behaviors, and physical health to provide new evidence that addresses gaps in the extant literature concerning social engagement and healthy aging in very old adults. Participants were younger (21–59 years), older (60–89 years), and oldest-old (90–97 years) adults (N = 364) in the Louisiana Healthy Aging Study (LHAS). Linear regression analyses indicated that age, gender, and hours spent outside of the house were significantly associated with self-reported health. The number of clubs and hours outside of home were more important factors in the analyses of objective health status than positive health behaviors, after considering age group and education level. These data strongly suggest that social engagement remains an important determinant of physical health into very late adulthood. The discussion focuses on practical applications of these results including social support interventions to maintain or improve late life health.
Keywords: Social engagement, social relations, positive health behaviors, physical health, healthy aging
The association between social relations and health is well documented and has been a topic of interest in the scientific community for many years (Berkman, 1995; Burg & Seeman, 1994; House, Landis, & Umberson, 1988). From a theoretical perspective, there are many reasons why one might expect to observe significant relationships among social relations and health. For instance, social relations, such as family, friends and others important to the individual, may boost one’s feelings of self-worth and mastery which are needed for health maintenance and well-being (Antonucci, 2001; Antonucci & Jackson, 1987). These persons may also assist in the attainment of health-related goals (VonDras & Madey, 2004), or serve as agents of social control to promote healthy lifestyles and discourage unhealthy behaviors (e.g., Tucker, Klein, & Elliott, 2004). Other evidence has shown that social networks affect health behaviors with links to biological and behavioral characteristics such as obesity (Christakis & Fowler, 2007). The purpose of the present research was to examine hypothesized relationships among social engagement, health promoting behaviors, and physical health in a sample of adults who ranged in age from 21 to over 90 years. Given the current demographic trends that predict dramatic growth in the “oldest-old” segment of the adult population, isolating variables that may foster health and promote the retention of physical function into late adulthood is a critical and timely challenge.
The social convoy model (Kahn & Antonucci, 1980) holds that social support is an important determinant of well-being throughout life, from infancy to old age. One’s convoy, or personal network of family, friends, and other people, is seen as the vehicle through which social support is given and received. In particular, social support may promote well-being and may also protect against a variety of other life stresses, including stress related to aging. The concept of social support has also been viewed as a part of the broader concept of social relations which are best understood within a life span perspective (Antonucci & Akiyama, 1995). A number of researchers have examined social support influences on well-being in late adulthood. For example, Finch, Okun, Barrera, Zautra, and Reich (1989) found that while positive social relationships were linked to well-being, negative social relationships were related to both well-being and increased psychological distress in an older adult sample, implying that the influence of social connections in late life depends on the qualities of the relationship. Krause (2005) found non-equivalent effects of social support where the negative effects of financial strain were offset by emotional support in the oldest-old (persons over age 85 years) but not for young-old adults, underscoring the importance of considering age specific subgroups with the older adult population.
Researchers have also studied the influence of social factors on physical health with mixed results. Some have found that higher levels of social integration (e.g., Seeman, 1996), social support given to others (e.g., Ostir, Simonsick, Kasper, & Guralnik, 2002), and social engagement (e.g., Walter-Ginzburg, Blumstein, & Modan, 2002) were associated with better physical functioning and reduced risk of mortality, while others report ambiguous or no effects (e.g., Bisschop, Kriegsman, van Tilburg, et al., 2003; Vaillant, Meyer, Mukamal, & Soldz, 1998). Unger, McAvay, Bruce, Berkman and Seeman (1999) examined the relationship between social support and self-reported physical functioning (indexed by the Nagi physical functioning scale) in 850 high-functioning male and female participants aged 70–79. They found that participants with an increased number of social ties showed significantly less functional decline. Interactions suggested that the positive effect of social networks were more beneficial for those with a low physical functioning score and for men compared to women. Based on these results, the authors concluded that the favorable effect of positive social networks might not be equivalent for all older adults.
An important aspect of the social relations and health dynamic concerns the role of positive health behaviors. In particular, physical declines may be lessened through health promoting behaviors, such as not smoking, moderation in alcohol use, and exercising (Centers for Disease Control and Prevention, 2007). Proper diet and adequate physical activity are widely recognized as important and linked to a variety of health benefits, such as reduced chronic disability, enhanced immune, cardiovascular and muscular functioning, and improved emotional status (U.S. Department of Health and Human Services, 1996). Conversely, a poor diet, sedentary lifestyle, and other health risks such as obesity may promote the occurrence of age-related diseases that contribute to disability in adulthood (Reynolds, Saito, & Crimmins, 2005). Michael, Colditz, Coakley, and Kawachi (2000) measured health behaviors (smoking, alcohol consumption, exercise, and BMI), social network characteristics (marital status, sociability, church group membership, and membership in other community organizations), and self-reported physical functioning (indexed by the SF-36 Health Survey) in 56,436 women aged 55–72 years. They found that elements of a woman’s social network and health behaviors were significantly correlated with self-reported levels of physical functioning. All positive health behaviors were associated with higher levels of physical functioning. Aspects of social support were positively correlated with physical health after controlling for health behaviors and demographic variables. Together, Michael et al. (2000) and Unger et al.’s (1999) results imply that an individual’s social involvement and health promoting behaviors are crucial factors for successful aging.
In the present research, we focus on the relationships among social engagement, positive health behaviors and physical health in the oldest-old (defined as 90 years and older) and two younger reference groups (age 21 to 59 and 60 to 89 years). The first goal of the study was to examine age and gender differences in social engagement, positive health behaviors and physical health. This study extends previous research in this area in two important ways. First, we sample from a broader age range than has been used in prior studies with older adults (Michal et al., 2000; Unger et al., 1999). Second, we adopt a multidimensional perspective on physical health that includes measures of self-reported health and objective health status. The inclusion of multiple health indicators was desirable to provide a more complete analysis of the hypothesized associations among age, gender, social engagement and health variables in adulthood. Based on previous research, we expected to observe significant age (e.g., Michael et al., 2000) and gender differences (e.g., Unger et al., 1999) in self-reported physical health ratings. We also expected significant age and gender differences in objective health status (Anderson & Smith, 2005).
The second goal of this study concerned predictors of physical health. We expected that social engagement and positive health behaviors would be significantly associated with self-reported physical health and objective health status after considering age and other salient individual difference characteristics. Such a pattern of outcomes would be consistent with the convoy model of social relations (cf. Antonucci, Ajrouch, & Birditt, 2006; Levitt, Weber, & Guacci, 1993). These outcomes would also provide new evidence of the beneficial effects of social involvement and positive health behaviors for successful aging (cf. Rowe & Kahn, 1997).
Method
Participants
A total of 364 individuals participated in this study. There were 115 younger adults (M = 43.7 years, SD = 10.4 years, age range 21 to 59 years), 129 older adults (M = 74.7 years, SD = 8.2 years, age range 60 to 89 years); and 120 oldest-old adults (M = 91.6 years, SD = 1.72 years, age range 90 to 97 years). All were drawn from the Louisiana Healthy Aging Study (LHAS), a multidisciplinary study of the determinants of longevity conducted in collaboration with researchers from Louisiana State University (LSU), LSU Health Sciences Center in New Orleans, Tulane University, the Pennington Biomedical Research Center, University of Pittsburgh, and the University of Alabama at Birmingham. LHAS participants were sampled randomly from the Voters Registration 2000 files for those age 20 to 64 years old and from the Medicare Beneficiary Enrollment Data file of the Center for Medicare and Medicaid Services (CMS) for participants age 65 years and older who live within a 40-mile radius of Baton Rouge (surrounding 8 parishes) constituting the Greater Baton Rouge community. All scored at least a 25 or higher on the Mini-Mental State Exam (MMSE; Folstein, Folstein, & McHugh, 1975).
Materials
Social engagement was defined as perceived social support (i.e., satisfaction with support received from others for dealing with problems that arise in everyday life rated on a four-point Likert scale), presence of a confidant (i.e., someone you can talk to about issues that concern you rated as yes or no) and social activities (i.e., the number of club or organization memberships and number of hours spent outside the home, both rated on separate four-point Likert scales). Positive health behaviors were self-reported alcohol use and tobacco use, both rated on three-point Likert scales, taken from a medical history administered by the nurse on intake interview. Self-reported physical health was assessed using the Medical Outcomes Study Short Form-36 (SF-36; Ware & Sherbourne, 1992; Ware, 2000). The SF-36 is comprised of eight health indicators, including physical functioning (PF), role limitations due to physical health problems (RP), bodily pain (BP), perceptions of general health (GH), vitality (VT), social functioning (SF), role limitations due to emotional health problems (RE), and mental health (MH). SF-36 scores range from 0 (lowest functioning) to 100 (highest functioning). Subscales are combined to form composite mental (MCS) and physical component scores (PCS). Normative data yields a mean of 50 and a standard deviation of 10 for the physical and mental health composite scores (Ware, Kosinski, & Keller, 1994). The PCS scores served as a measure of self-reported physical health in this study. Objective health status was based on a cumulative index reflecting the presence of six chronic conditions which were documented in the participants’ medical history assessed by the LHAS nurse. These conditions were high cholesterol, hypertension, diabetes, arthritis, cancer, and heart problems. Our rationale for selecting these six conditions was to provide a broad assessment of health, ranging from mild conditions (e.g., high cholesterol) to more serious illnesses (e.g., cancer and heart problems). For each participant, scores of 0 (absence) and 1 (presence) were assigned for each individual health condition. Condition scores were summed to create the chronic 6 composite index of health (range from 0 to 6).
Statistical analyses were conducted to examine the effects of age (categorized), gender and their interaction effect on social engagement, positive health behaviors, and the two physical health dimensions, the first goal of the study. Multivariate ANOVAs were conducted to determine the best predictive model based on demographic and social network variables, and positive health behaviors for self-reported physical health (SF-36 PCS) and objective health status (chronic 6), the second goal of the study. In all regression analyses, we considered a linear model with three main groups of responses. The first group of variables included demographic characteristics (age group, gender, education level, marital status). The second group characterized social engagement variables (social support, clubs/memberships in social organizations, hours spent outside of home, and presence of a confidant). The third group characterized positive health behaviors (tobacco usage and alcohol usage). These ten variables (factors) were used in all-possible-submodels factor selection analysis1. All statistical analyses were conducted with SAS Version 9.1.3, statistical software package (SAS Institute Inc., Cary, NC, USA).
Procedure
Informed consent was obtained for all participants at the beginning of the session. The procedures used in this study were reviewed and approved by the institutional review boards of the Pennington Biomedical Research Center (PBRC) and Louisiana State University in Baton Rouge, LA. Testing occurred across one or two sessions scheduled within approximately a 4 to 6 week period. For those between the ages of 21 to 89 years, all sessions were held at the PBRC. For persons 90 years of age and over, the first session was held in their home and a second was held at the PBRC. All participants were compensated at least $50 each for their voluntary participation.
Results
Analyses of Individual Difference Characteristics
Individual difference and health characteristics of the sample appear in Tables 1a (continuous variables) and 1b (categorical variables). One-way analyses of variance (ANOVAs) and chi square tests of independence (when indicated) were conducted on these data with age group as a between group factor. As can be seen in Table 1a, oldest-old adults’ mean MMSE score was lower than the younger and older adults’ scores (p < 0.01 for each comparison). The oldest-old adults’ mean vocabulary score was lower than the other two age groups who did not differ from each other. Most participants (92.03%) were within the normal range on the Geriatric Depression Scale (GDS; Sheikh & Yesavage, 1986) at the time of testing2. The oldest-old adults’ mean GDS score was numerically higher than the other groups, yet well below the cutoff score of 5 representing mild depression. As can be seen in Table 1b, younger adults were more likely to rate never married and the oldest-old were more likely to rate widowed compared to the other age groups. Participants’ responses to three self-reported health questions from the Older American Resources and Services Multidimensional Functional Assessment Questionnaire (Duke University Center for the Study of Aging and Human Development, 1975) indicated that most were generally in good health. Health at the present time and age group were significantly associated. For health prevents activities, the oldest-old adults rated their health as standing in the way of doing things they want to do more often than the other age groups. For health compared to others, oldest-old adults rated their health as better than their age mates more often than did the younger groups. This finding is not surprising in that the oldest-old are survivors. Anecdotally, many of them commented that their age mates had died, so their health status was obviously better by comparison.
Table 1a.
Individual Difference and Health Characteristics – continuous responses
| Younger adults (n = 115) | Older adults (n = 129) | Oldest-old adults (n = 120) | |||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | F-value | P-value | |
| Age | 43.68 (10.41) | 74.71 (8.18) | 91.57 (1.72) | ||
| MMSEa | 29.4 (1.1) | 28.8 (1.2) | 27.5 (1.5) | 65.96 | < 0.0001 |
| Vocabularyb | 24.1 (7.4) | 24.3 (7.5) | 22.1 (7.2) | 3.32 | 0.04 |
| GDSc | 1.85 (2.48) | 1.43 (1.65) | 2.44 (2.26) | 6.83 | 0.0012 |
| Chronic 6d | 0.81 (0.92) | 2.27 (1.22) | 2.45 (1.22) | 68.83 | <0.0001 |
Notes.
Mini-Mental State Exam (Folstein, Folstein, & McHugh, 1975).
A short-form of the Wechsler Adult Intelligence Scale Vocabulary subtest (Jastak & Jastak, 1965).
Geriatric Depression Scale (Sheikh & Yesavage, 1986).
Chronic 6 is a cumulative index of objective health status based on the presence of six conditions (high cholesterol, hypertension, diabetes, arthritis, cancer, and heart problems).
Table 1b.
Individual Difference and Health Characteristics – categorical responses
| Younger adults (n = 115) | Older adults (n = 129) | Oldest-old adults (n = 120) | ||
|---|---|---|---|---|
| % | P-value (relation with age group) | |||
| Years of Education | ||||
| At most high school | 24.35 | 31.01 | 39.17 | |
| Partial college or training | 35.65 | 30.23 | 30.00 | |
| College degree | 26.96 | 24.81 | 20.83 | |
| Graduate degree | 13.04 | 13.95 | 10.00 | |
| Chi-square test for independence | 0.35 | |||
| Marital status | ||||
| Never married | 13.04 | 3.1 | 0.83 | |
| Married | 76.52 | 65.12 | 23.33 | |
| Divorced or separated | 10.43 | 10.85 | 0.83 | |
| Widowed | 0.00 | 20.93 | 75.00 | |
| Chi-square test for independence | < 0.0001 | |||
| Health at the present time | ||||
| Excellent | 22.61 | 21.71 | 16.67 | |
| Good | 59.13 | 62.02 | 59.17 | |
| Fair | 14.78 | 16.28 | 24.17 | |
| Poor | 3.48 | 0.00 | 0.00 | |
| Chi-square test for independence | 0.04 | |||
| Health prevents activities | ||||
| Not at all | 60.00 | 48.84 | 32.50 | |
| A little/some | 35.65 | 41.09 | 46.67 | |
| A great deal | 4.35 | 10.08 | 20.83 | |
| Chi-square test for independence | < 0.0001 | |||
| Health compared to others | ||||
| Better than | 37.39 | 65.89 | 86.67 | |
| The same as | 47.83 | 32.56 | 11.67 | |
| Worse | 14.78 | 1.55 | 1.67 | |
| Chi-square test for independence | < 0.0001 | |||
| Type of chronic conditions | ||||
| Heart problems | 4.35 | 33.33 | 40.00 | <0.0001 |
| Hypertension | 18.26 | 58.91 | 71.67 | <0.0001 |
| High cholesterol | 23.48 | 43.41 | 32.50 | 0.004 |
| Diabetes | 5.22 | 12.40 | 12.50 | 0.11 |
| Arthritis | 27.83 | 68.99 | 65.83 | <0.0001 |
| Cancer | 1.74 | 10.08 | 22.50 | < 0.0001 |
Analyses of Age and Gender Differences
Social Engagement Variables
A generalized linear model analysis with age group and gender as factors yielded significant main effects of age group for each of the social engagement variables (p < 0.05 for all), with no other significant effects. A more detailed analysis of comparisons between age groups was carried out by specific contrast estimates and corresponding χ2 tests. As can be seen in Table 2, the majority of participants in each age group reported having a confidant, although the oldest-old were less likely to have one than young (p < 0.01) and old adults (p < 0.05) who did not differ from each other (p = 0.41). As anticipated, for social support, we found a statistically significant difference between the young and old and the young and oldest-old (p < 0.0001 for both) but no significant difference between the old and oldest-old (p = 0.29). For number of clubs and social organizations, the difference between the young and old was highly significant (p < 0.0001) as was the difference between the young and oldest-old (p = 0.006), and between the old and oldest-old (p = 0.024). Similarly, all comparisons between age groups for number of hours spent outside of home were statistically significant (p < 0.0001 for each).
Table 2.
Social Engagement Variables and Positive Health Behaviors
| Younger adults (n = 115) | Older adults (n = 129) | Oldest-old adults (n = 120) | |
|---|---|---|---|
| % | |||
| Confidant | 95.65 | 93.02 | 83.33 |
| Social Support | |||
| Very satisfied | 49.57 | 77.52 | 83.33 |
| Fairly satisfied | 36.52 | 20.16 | 15.00 |
| A little satisfied | 6.96 | 1.55 | 1.67 |
| Not satisfied | 6.96 | 0.78 | 0.00 |
| Number of clubs and social organizations | |||
| None | 14.78 | 3.10 | 9.17 |
| Between 1–3 | 79.13 | 69.77 | 71.67 |
| Between 4–6 | 4.35 | 19.38 | 15.83 |
| More than 6 | 1.74 | 7.75 | 3.33 |
| Number of hours per week spent outside of home | |||
| None | 0.87 | 0.78 | 3.33 |
| Between 1–5 | 3.48 | 17.05 | 35.00 |
| Between 6–12 | 12.17 | 23.26 | 29.17 |
| Between 13–19 | 15.65 | 16.28 | 15.00 |
| More than 19 | 67.83 | 42.64 | 17.50 |
| Positive health behaviors | |||
| Tobacco Use | |||
| Current | 15.65 | 4.65 | 1.67 |
| Former | 33.04 | 48.84 | 48.33 |
| Never | 51.30 | 46.51 | 50.00 |
| Alcohol Use | |||
| Current | 76.52 | 54.26 | 55.00 |
| Former | 15.65 | 24.81 | 20.00 |
| Never | 7.83 | 20.93 | 25.00 |
Positive Health Behaviors
To examine the influence of age and gender on positive health behaviors, tobacco use and alcohol use were analyzed separately by a generalized linear model approach. The χ2 test yielded a non-significant age group effect for tobacco use (Table 2). However, males and females significantly differed with males using tobacco more often than females, χ2 (1) = 19.8, p < .0001. The age group main effect was significant for alcohol use, χ2 (2) = 16.96, p = 0.0002, indicating the highest proportion of current alcohol users and the lowest proportion of never users among the younger adults with no evidence for a difference between the old and oldest–old. A marginally significant gender main effect occurred favoring females having higher proportion among current alcohol users and lower among never users compared to males, χ2 (1) = 3.07, p = 0.08. The age group-by-gender interaction effect was non-significant for the two variables.
Physical Health
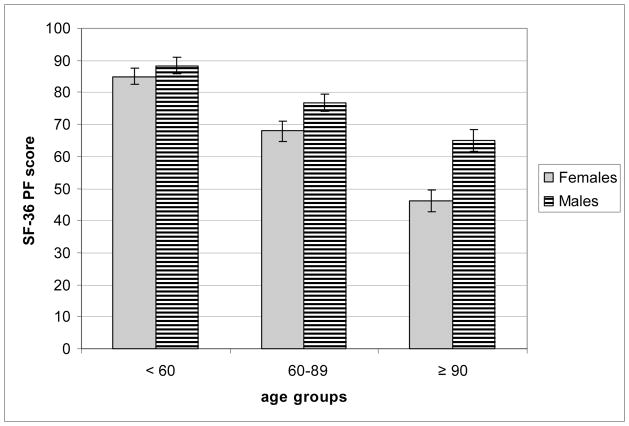
Self-reported health and objective health status mean scores by age group and gender appear in Table 3. Analyses of the SF-36 PCS scores yielded significant main effects of age group (p < 0.0001) and gender (p <0.002). The age group-by-gender interaction was non-significant, so interpretative caution is warranted. Nonetheless, a more detailed analysis revealed a significant gender difference for the oldest-old (p < 0.007) and the old (p < 0.046) but not for the young (p = 0.47). To provide further insight into this finding, we conducted a follow-up analysis on the SF-36 PF subscale scores as a measure of self-reported physical functioning. Analyses of SF-36 PF scores yielded significant main effects of age group (p < 0.0001) and gender (p < 0.0001) with males performing better on this index of physical function than females. The age group-by-gender interaction was also significant (p < 0.04) which is shown in Figure 1. Pairwise comparisons confirmed a statistically significant gender difference for the oldest-old (p < 0.0001) and old (p < 0.033) but not for the young (p = 0.46).
Table 3.
Dimensions of Physical Health by Age Group and Gender
| Younger adults (n = 115) | Older adults (n = 129) | Oldest-old adults (n = 120) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| SF-36 PCS scorea | ||||||
| Males | 50.62 | 7.6 | 46.47 | 8.58 | 42.32 | 8.75 |
| Females | 49.27 | 9.32 | 43.08 | 10.55 | 37.34 | 10.99 |
| Mean | 49.76 | 8.72 | 44.66 | 9.80 | 39.46 | 10.36 |
| Number of chronic conditionsb | ||||||
| Males | 0.81 | 0.86 | 2.18 | 1.35 | 2.29 | 1.3 |
| Females | 0.81 | 0.95 | 2.35 | 1.1 | 2.57 | 1.16 |
| Mean | 0.81 | 0.92 | 2.27 | 1.22 | 2.45 | 1.22 |
Notes.
SF-36 Physical Health Composite Score.
Cumulative number of chronic conditions(0–6).
Figure 1.
The age group x gender interaction effect for the SF-36 PF subscale scores
For objective health status, analyses of the chronic 6 health index yielded only a significant age group main effect, F (2, 358) = 68.83, p < .0001. The six chronic disease variables, each dichotomous, were then analyzed by logistic regression with respect to age group and gender with the interaction term included in each model. As expected, a significant age group main effect occurred on each response, except diabetes, χ2 (2) = 3.85, p = 0.146. To be precise, the age group main effect was significant for cancer χ2 (2) = 26.39, p < .0001, arthritis, χ2 (2) = 50.98, p < .0001, heart problems, χ2 (2) = 52.22, p < .0001, high blood pressure, χ2 (2) = 72.63, p < .0001, and high cholesterol, χ2 (2) = 8.88, p = .011. In addition, the main effect of gender was significant for diabetes, χ2 (1) = 5.53, p =.019, high blood pressure, χ2 (1) = 5.41, p = .02, and arthritis, χ2 (1) = 4.06, p = .044. The age group-by-gender interaction was non-significant in all of the responses.
Regression Analysis
SF-36 PCS
The all-possible-submodels factor selection method yielded a 5 factor model that appears in Table 4. The 5 factor model was the best model with the lowest MSE among all 10 models with the highest R2. Demographic factors in the best predictive model are age group, gender, and education level, and from among the social engagement variables, they are social support and hours spent outside of home. None of the positive health behaviors were included in the best model. To verify that the additive model does not leave out important interaction terms, we tested this additive model against a model with these same five factors with all possible two-way interactions included. The F-test comparing the restricted model against the full model yielded F(55, 295) = 0.86, p = 0.75, confirming that the additive model is a satisfactory predictive model. The demographic factors were included into the model first. For this model, R2 = 0.19, MSE = 90.55, and SSR = 7633.62, and the model F-test, F(6,357)=14.05, p < 0.0001. The F-test testing the significance of the added factors, the social support and hours spent outside of the house simultaneously, yielded F(7, 350) = 2.244, p = 0.03. On the basis of these findings, we may conclude that after controlling for the demographic factors age group, gender, and education level, the social engagement variables, social support and hours spent outside of the house, contribute significantly to the model. After refining the analysis, we examined the importance of the two social engagement variables separately (controlling for the three demographic factors). It is well known, that the order of entering the factors into the model makes a difference in testing for model increment. The first factor entering the model after the three demographic factors was social support. The F-test testing the significance of the model increment is F(3, 354) = 1.59824, p = 0.189. After adding the hours spent outside of the home, the test of model increment, F(4,350)=2.71, p = 0.03. If we reverse the order and add first hours spent outside of the house, the F-test of the model increment is F(4, 353) =3.057, p = 0.017. After adding the social support factor, the model increment test yields F(3, 350) = 1.16, p = 0.326. Hence we may conclude that in the best additive predictive model for SF-36 (PCS), when controlling for demographic factors (age group, gender, and education level), hours spent outside of the house is more important than social support.
Table 4.
Best additive predictive model for SF-36 PCS
| Predictive factors | F-statistics (ndf, ddf) | p-value | Proportion of explained variability |
|---|---|---|---|
| Age group | 18.61 (3, 350) | <0.0001 | 84.6% |
| Gender | 3.67 (1, 350) | 0.056 | |
| Education level | 1.41 (3, 350) | 0.24 | |
| Social support | 1.16 (3, 350) | 0.326 | 15.4% (after controlling for demographics) |
| Hours | 2.71 (4, 350) | 0.03 |
R2 = 0.226, MSE = 88.395, F-test, F(13, 350) = 7.85, p < 0.0001
Objective Health Status
For the chronic 6 health status index, among all additive models, the 6 factor model was the best predictive model (see Table 5) 3. The next step in the analysis was establishing the importance of added groups of factors, in a precise order, that is due to demographic variables in the model (age group, education level), next due to social engagement variables (clubs, hours spent outside of home) and the last due to health positive behaviors (alcohol, tobacco usage). In the model with age group and education level factors only, R2 = 0.299, MSE = 1.280, F(5, 358) = 30.48 with p < 0.0001, and the model sum of squares, SSR = 195.193. After adding the social engagement variables (clubs, hours spent outside of house) into the model, the analysis yielded R2 = 0.338, MSE = 1.23, and SSR = 221.008. The F-test testing whether the additional factors in the model are simultaneously statistically significant yielded F(7, 351) = 2.9919, p < 0.005. Adding the third group of factors, the positive health behavior variables (alcohol and tobacco usage), yielded R2 = 0.351, MSE = 1.223, and SSR = 229.335. The F-test testing the increment in the model yielded F(4, 347) = 1.7, p = 0.149. After controlling for the demographic variables (age group, education level), we reversed the order and as the next group we considered the positive health behavior variables (alcohol, tobacco usage). The analysis of the model with the four factors, age group, education level, alcohol and tobacco usage yielded R2 = 0.314, MSE = 1.266, and SSR =205.328. The F-test of adding the factors resulted in F(4,354) = 2.0, p = 0.094. Now after adding the third group of factors, the social engagement variables (clubs and hours spent outside home), the F-test testing the increment of the model is F(7, 347) = 2.8, p = 0.0075. Hence it is reasonable to conclude that the two social predictors, clubs and hours spent outside of home, are more important factors in predicting objective health status (chronic 6) than the positive health behavior factors, even after controlling for age group and education level.
Table 5.
Best additive predictive model for number of chronic conditions
| Predictive factors | F-statistic (ndf, ddf) | p-value | Proportion of explained variability |
|---|---|---|---|
| Age group | 31.27 (2, 347) | <0.0001 | 85.11% |
| Education level | 1.64 (3, 347) | 0.18 | |
| Tobacco usage | 2.41 (2, 347) | 0.09 | 4.42% (after controlling for demographics) |
| Alcohol usage | 1.23 (2, 347) | 0.29 | |
| Clubs | 3.61 (3, 347) | 0.0135 | 10.47% (after controlling for demographics and tobacco and alcohol usage) |
| Hours | 2.73 (4, 347) | 0.029 |
R2=0.35, MSE = 1.223, F(16, 347) = 11.72, p < 0.0001
General Discussion
The first goal of this study was to address associations among age, gender, social engagement, positive health behaviors and physical health. We observed age effects in all of the social engagement measures. Most reported having a confidant, although the oldest-old were less likely to do so than younger and older adults (see Table 2), possibly due to the higher incidence of widowhood in this age group compared to the other groups (see Table 1b). Most were fairly to very satisfied with the social support they received for dealing with day to day problems. Older adults (62.5%) were roughly five times more likely and the oldest-old adults twice as likely (25.0%) to report membership in more than 6 clubs and social organizations compared to the younger adults (12.5%). This result was surprising in that we had anticipated the opposite trend -- a reduction in the breadth of social involvement (i.e., fewer club memberships) with advanced age -- based on Carstensen’s (1991) socioemotional selectivity theory, which holds that older people view time as limited and are more selective in their choices of social interactions (see also Carstensen, 1992). Interpretative caution is warranted, as we estimated social engagement based on the number of clubs and social organizations, which does not capture the extent of participation or direct involvement in group activities. It is also possible that younger respondents may have competing professional and personal obligations that may limit their participation in outside clubs and social organizations.
Regarding positive health behaviors, most reported that they had never used tobacco or were former users. Very few of the current tobacco users were oldest-old adults (7.7%) compared to older adults (23.1%) and younger adults (69.2%) who smoke, although the numeric differences for tobacco use among the age groups were not statistically significant, so interpretative caution is warranted. In contrast, the gender effect in tobacco use was significant, favoring males (see also Unger et al., 1999). For alcohol use, the two older groups indicated that they never used alcohol or were former users significantly more often compared to the younger adult group. Taken together, the pattern of outcomes from the analyses of positive health behaviors implies that reduced levels of tobacco and alcohol use are associated with longevity and healthy aging. This aspect of the data is also consistent with prior research which has shown that older adults have fewer negative health behaviors and experience attempts by others to change unhealthy behaviors less often than do younger and middle age adults (Tucker et al., 2004).
Self-reported health was assessed using the SF-36 PCS, which is a composite score based on several subscales that is influenced the most by measures of perceptions of physical functioning, ability to fulfill roles because of physical health problems, bodily pain and general health. We found significant age and gender differences in the SF-36 PCS, consistent with prior research which documents lower health-related quality of life for women than men (Kaplan, Anderson, & Wingard, 1991). Follow-up analyses on the SF-36 PF subscale confirmed that the gender difference, favoring males, was smallest (and non-significant) for the younger adults and increased in size (significantly so) for the older adults with the largest difference noted for the oldest-old adults (see Fig. 1). Prior research has shown that older women experience greater difficulty on physical function measures than men, possibly due to greater reported discomfort with physical activity (see Merrill, Seeman, Kasl, & Berkman, 1997; Wood et al., 2005). Our results are consistent with this notion. Wood et al. (2005) have suggested that gender differences in physical functioning may be due to older women’s tendency to have lower body weight and strength than older men. Other evidence has shown that age-related declines in upper body flexibility were associated with lower health-related quality of life using the SF-36 PCS (see Fabre et al., 2007). The present results, among these others, underscore the important role of physical function in fostering health-related quality of life in late adulthood.
Objective health status was estimated using a cumulative index that reflected the presence of six chronic conditions. Inclusion of the objective health index is a strength of the present study given that chronic conditions are a critical component of physical health and an important focus of gerontological research and geriatric practice (see Steinhagen-Thiessen & Borchelt, 1999, for discussion). Our results yielded age group differences for 5 of the 6 conditions (not diabetes), consistent with national trends that show an increase in the number of chronic conditions after age 65 (Centers for Disease Control, 2007). We also found gender differences on 3 of the 6 conditions. The reported prevalence of hypertension and arthritis were both greater for women than men. In contrast, rates of diabetes favored men over women, consistent with the male predominance in Type II diabetes documented in the medical literature (see Gale & Gillespie, 2001, for discussion).
The second goal of the study concerned predictors of two dimensions of physical health, self-reported physical health and objective health status, which were analyzed separately using linear regression techniques to permit a comprehensive overview of the different aspects of health. Regression analyses revealed that age, gender, and social engagement (hours spent outside of the house) were associated with self-reported health as indexed by the SF-36 PCS scores. The best model for predicting self-reported health accounted for only 22.6% of the variance, however (see Table 4). By comparison, the same sets of variables (demographic factors, social engagement variables, positive health behaviors) accounted for relatively greater proportions of variance in the best model obtained for objective health status (35.0%; see Table 5). Critically, social engagement (indexed by hours outside of the home) was significantly associated with both physical health dimensions after age, gender and demographic factors were entered into the model. For objective health status, the contribution of both social engagement indices (hours outside of the home and the number of clubs and social organizations) remained significant after controlling for age, gender, and demographic factors. Prior epidemiological research has shown that social integration is associated with reduced mortality risk and better mental health (see House et al., 1988; Seeman, 1996, for discussion). The present results are in line with epidemiological studies and underscore the important role of social engagement across these physical health dimensions. One should note that social engagement, as conceptualized in this study, may reflect the health benefits of social interaction coupled with higher functional status. Another possibility is that the current social engagement measures are proxy variables for activity level or everyday functioning. Previous research has shown that greater social activity was associated with greater life satisfaction and reduced risk of functional decline and mortality over a 6-year period (Menec, 2003). Thus, careful examination of activity level influences along with variations in functional status on physical health is a potentially important direction for future research with very old adults.
A well-known demographic reality concerns the projected growth of the elderly population, with the greatest increases expected for those persons age 85 years and older (often called the “oldest-old”). Mortality deceleration, referring to the increased likelihood of survival after age 80 today (Vaupel et al., 1998), has numerous social, economic, and public policy implications. The results of this study have shown that the association between social involvement and health persists into very late life. Considering applied implications, other researchers have discussed the potential to improve physical health of adults by instituting social support interventions (Hogan, Linden, & Najarian, 2002; Cohen, 2004; Uchino, 2009). These interventions may be of most relevance to older adults, as this segment of the population is more likely to experience health-related declines resulting in a need for assistance and/or support. Social support interventions work by either expanding the support within an existing network or through the development of novel networks for those persons without an existing one. It is through the development of these networks that one can generate an increase of instrumental (material aids), emotional, and informational support resources, which may in turn impact physical health. Cohen (2004) suggests that support has the potential to lessen illness and promote health via the stress-buffering model, where greater amounts of social support are linked with lower stress and subsequently lessened activation of biological systems linked with illness. This author also suggests that social support may boost health positive behaviors, including greater amounts of exercise, nutrition, and rest. Further research on this topic is warranted; as investigations have yet to determine which social support interventions are most effective in the promotion of health and well-being.
Several limitations of this study should be mentioned. First, we did not examine the possible negative effects of social engagement on health-related quality of life and physical function. That is, social relations are not always beneficial and may have negative consequences for the individual in some cases (for reviews, see Burg & Seeman, 1994; Rook, 1990). For instance, prior findings have shown that negative social exchanges with family, friends and neighbors may have a detrimental effect on emotional health (e.g., Rook, 2001), psychological well-being (e.g., Finch et al., 1989; Silverstein, Chen & Heller, 1996) and physical health (Newsome, Mahan, Rook, & Krause, 2008). Addressing the possible negative effects of social support on health in very old adults represents an important direction for future research. Second, only two variables, alcohol use and tobacco use, were used to represent positive health behaviors. Performance-based measures, such as exercise and/or physical activity indices, would permit a more definitive analysis of the contribution of positive health behaviors to health status and physical functioning across the life span. Additional measures of positive health behaviors with greater sensitivity and specificity than the current measures may also permit an analysis of the mediational role of health behaviors as a possible pathway through which social network involvement affects health (Tucker et al., 2004). Third, the study utilized a cross-sectional design so the potential bias of age group and birth cohort should be considered in the interpretation of age effects. Finally, the study design does not permit a determination of the causal direction of the observed relationships. Individuals may be more socially engaged because their physical health allows them to or, conversely, being more socially engaged may contribute to better physical health. Future research that incorporates longitudinal assessments would be desirable to provide insight into causal relationships among these variables over time. Future research incorporating a more refined assessment of social engagement and health variables would also be desirable for the development and evaluation of a social support intervention geared toward very old adults.
In closing, the growing numbers of older persons in society today have sparked scientific interest in longevity and steps to promote healthy aging (Winerman, 2006). Given the current trends of increasing likelihood of survival into very old age, gaining new insights into variables that contribute to health and well-being is a timely and critical challenge. The unique contribution of the present study is that social engagement was associated with self-reported health and objective health status in a life span sample of adults who ranged from 20 to over 90 years of age. Our findings, among others, confirm the importance of social engagement as an important component of physical health in later life. Further research to examine the generality of these findings seems warranted.
Acknowledgments
Portions of study were conducted in partial fulfillment of the upper division Honors Distinction in Psychology at LSU by the second author. We thank thesis committee members, Drs. Janet McDonald and Robin Roberts for their valuable comments on this research.
This research was supported by grants from the Louisiana Board of Regents through the Millennium Trust Health Excellence Fund [HEF(2001-06)-02] and the National Institute on Aging (P01 AG022064). This support is gratefully acknowledged.
Footnotes
The all possible submodels selection method is a variable selection procedure conducted using multiple regression, where the best model is selected based on maximal R2 and minimal MSE values. In each model, for each level of a factor, a dummy variable was created and the groups of dummies corresponding to a factor were kept together when kept in or left out from the model. In this type of analysis we considered only additive main effects. Among all 1023 models, for each fixed number of factors in a model, the model with the highest R2 value was chosen. Among selected 10 models, the one with minimal MSE was picked as best predictive additive models. All statistical analyses were conducted with SAS Version 9.1.3 statistical software package, using a regression multifactor analysis macro that called Proc GLM for each of the 210−1 = 1023 models.
Previous research documents the concurrent validity of the GDS short form with the Beck Depression Inventory (r = 0.84), confirming the usefulness of the GDS as an effective screening instrument for probable depression in college student samples (see Ferraro & Chelminski, 1996).
In order to verify that the additive model is satisfactory, we tested this additive model against a model with these same six factors with all possible two-way interactions included. The F-test comparing the two models yielded F(94, 253) = 0.78, p = 0.91.
Contributor Information
Katie E. Cherry, Louisiana State University
Erin Jackson Walker, Louisiana State University.
Jennifer Silva Brown, Drury University.
Julia Volaufova, Louisiana State University Health Sciences Center.
Lynn R. LaMotte, Louisiana State University Health Sciences Center
David A. Welsh, Louisiana State University Health Sciences Center
L. Joseph Su, Louisiana State University Health Sciences Center.
S. Michal Jazwinski, Tulane University School of Medicine.
Rebecca Ellis, Georgia State University.
Robert H. Wood, Husson College
Madlyn I. Frisard, Virginia Polytechnic Institute and State University
References
- Anderson RN, Smith BL. National Vital Statistics Reports. 17. Vol. 53. Hyattsville, Maryland: National Center for Health Statistics; 2005. Deaths: Leading causes for 2002. Retrieved November 20, 2007 from http://www.cdc.gov/nchs/data/nvsr/nvsr53/nvsr53_17.pdf. [PubMed] [Google Scholar]
- Antonucci TC. Social relations: An examination of social networks, social support, and sense of control. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. 5. San Diego, CA: Academic Press; 2001. pp. 427–453. [Google Scholar]
- Antonucci TC, Ajrouch KJ, Birditt K. Social relations in the third age: Assessing strengths and challenges using the convoy model. Annual Review of Gerontology and Geriatrics. 2006;26:193–209. [Google Scholar]
- Antonucci TC, Akiyama H. Convoys of social relations: Family and friendships within a life span context. In: Blieszner R, Bedford VH, editors. Handbook of aging and the family. Westport, Connecticut: Greenwood Press; 1995. pp. 355–371. [Google Scholar]
- Antonucci TC, Jackson JS. Social support, interpersonal efficacy, and health. In: Carstensen LL, Edelstein BA, editors. Handbook of clinical gerontology. New York: Pergamon Press; 1987. pp. 291–311. [Google Scholar]
- Berkman LF. The role of social relations in health promotion. Psychosomatic Medicine. 1995;57:245–254. doi: 10.1097/00006842-199505000-00006. [DOI] [PubMed] [Google Scholar]
- Bisschop MI, Kriegsman DMW, van Tilburg TG, Penninx BWHJ, van Eijk JTM, Deeg DJH. The influence of differing social ties on decline in physical functioning among older people with and without chronic diseases: The longitudinal aging study Amsterdam. Aging Clinical and Experimental Research. 2003;15:164–173. doi: 10.1007/BF03324496. [DOI] [PubMed] [Google Scholar]
- Burg MM, Seeman TL. Families and health: The negative side of social ties. Annals of Behavioral Medicine. 1994;16:109–115. [Google Scholar]
- Carstensen LL. Socioemotional selectivity theory: Social activity in life-span context. Annual Review of Gerontology and Geriatrics. 1991;11:195–217. [Google Scholar]
- Carstensen LL. Social and emotional patterns in adulthood: Support for socioemotional selectivity theory. Psychology and Aging. 1992;7:331–338. doi: 10.1037//0882-7974.7.3.331. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. The state of aging and health in America. 2007 Retrieved October 10, 2009 from http://www.cdc.gov/aging/saha.htm.
- Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. The New England Journal of Medicine. 2007;357:370–379. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- Cohen S. Social relationships and health. American Psychologist. 2004;59:676–684. doi: 10.1037/0003-066X.59.8.676. [DOI] [PubMed] [Google Scholar]
- Duke University Center for the Study of Aging and Human Development. OARS: Multidimensional Functional Assessment Questionnaire. Durham, NC: Author; 1975. [Google Scholar]
- Fabre JM, Wood RH, Cherry KE, Su LJ, Cress ME, King CM, deVeer MJ, Ellis R, Jazwinski SM. Age-related deterioration in flexibility is associated with health-related quality of life in nonagenarians. Journal of Geriatric Physical Therapy. 2007;30:16–22. doi: 10.1519/00139143-200704000-00004. [DOI] [PubMed] [Google Scholar]
- Ferraro FR, Chelminski I. Preliminary normative data on the Geriatric Depression Scale-Short Form (GDS-SF) in a young adult sample. Journal of Clinical Psychology. 1996;52:443–447. doi: 10.1002/(SICI)1097-4679(199607)52:4<443::AID-JCLP9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Finch JF, Okun MA, Barrera M, Zautra AJ, Reich JW. Positive and negative social ties among older adults: Measurement models and the prediction of psychological distress and well-being. American Journal of Community Psychology. 1989;17:585–605. doi: 10.1007/BF00922637. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gale EA, Gillespie KM. Diabetes and gender. Diabetologia. 2001;44(1):3–15. doi: 10.1007/s001250051573. [DOI] [PubMed] [Google Scholar]
- Hogan BE, Linden W, Najarian B. Social support interventions: Do they work? Clinical Psychology Review. 2002;22:381–440. doi: 10.1016/s0272-7358(01)00102-7. [DOI] [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Jastak J, Jastak S. Short forms of the WAIS vocabulary subtest. Journal of Clinical Psychology. 1965;20:167–199. doi: 10.1002/1097-4679(196404)20:2<167::aid-jclp2270200202>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Kahn RL, Antonucci TC. Conoys over the life course: Attachment, roles and social support. In: Baltes PB, Brim O, editors. Life-span development and behavior. Vol. 3. New York: Academic Press; 1980. pp. 253–286. [Google Scholar]
- Kaplan RM, Anderson JP, Wingard DL. Gender differences in health-related quality of life. Health Psychology. 1991;10:86–93. doi: 10.1037//0278-6133.10.2.86. [DOI] [PubMed] [Google Scholar]
- Krause N. Exploring age difference in the stress-buffering functions of social support. Psychology and Aging. 2005;20:714–717. doi: 10.1037/0882-7974.20.4.714. [DOI] [PubMed] [Google Scholar]
- Levitt MJ, Weber RA, Guacci N. Convoys of social support: An intergenerational analysis. Psychology and Aging. 1993;8:323–326. doi: 10.1037//0882-7974.8.3.323. [DOI] [PubMed] [Google Scholar]
- Menec VH. The relation between everyday activities and successful aging: A 6-year longitudinal study. Journal of Gerontology: Social Sciences. 2003;58:S74–S82. doi: 10.1093/geronb/58.2.s74. [DOI] [PubMed] [Google Scholar]
- Merrill SS, Seeman TE, Kasl SV, Berkman LF. Gender differences in the comparison of self-reported disability and performance measures. Journal of Gerontology: Medical Sciences. 1997;52:M19–M26. doi: 10.1093/gerona/52a.1.m19. [DOI] [PubMed] [Google Scholar]
- Michael YL, Colditz GA, Coakley E, Kawachi I. Health behaviors, social networks, and healthy aging: cross-sectional evidence from the nurses’ health study. Quality of Life Research. 2000;8:711–722. doi: 10.1023/a:1008949428041. [DOI] [PubMed] [Google Scholar]
- Newsome JT, Mahan TL, Rook KS, Krause N. Stable negative social exchanges and health. Health Psychology. 2008;27:78–86. doi: 10.1037/0278-6133.27.1.78. [DOI] [PubMed] [Google Scholar]
- Ostir GV, Simonsick E, Kasper JD, Guralnik JM. Satisfaction with support given and its association with subsequent health status. Journal of Aging and Health. 2002;14:355–369. doi: 10.1177/08964302014003003. [DOI] [PubMed] [Google Scholar]
- Reynolds SL, Saito Y, Crimmins EM. The impact of obesity on active life expectancy in older American men and women. The Gerontologist. 2005;45:438–444. doi: 10.1093/geront/45.4.438. [DOI] [PubMed] [Google Scholar]
- Rowe JW, Kahn RL. Successful aging. The Gerontologist. 1997;37:433–440. doi: 10.1093/geront/37.4.433. [DOI] [PubMed] [Google Scholar]
- Rook KS. Stressful aspects of older adults’ social relationships: Current theory and research. In: Parris Stephens MA, Crowther JH, Hobfoll SE, Tennenbaum DL, editors. Stress and coping in later-life families. New York: Hemisphere Publishing Corporation; 1990. pp. 173–192. [Google Scholar]
- Rook KS. Emotional health and positive versus negative social exchanges: A daily diary analysis. Applied Developmental Science. 2001;5:86–97. [Google Scholar]
- Seeman TE. Social ties and health: The benefits of social integration. Annals of Epidemiology. 1996;6:442–451. doi: 10.1016/s1047-2797(96)00095-6. [DOI] [PubMed] [Google Scholar]
- Sheikh JI, Yesavage JA. Geriatric depression scale (GDS): Recent evidence and development of a shorter version. In: Brink TL, editor. Clinical Gerontology. NY: Haworth Press; 1986. pp. 165–173. [Google Scholar]
- Silverstein M, Chen X, Heller K. Too much of a good thing? Intergenerational social support and the psychological well-being of older parents. Journal of Marriage and the Family. 1996;58:970–982. [Google Scholar]
- Steinhagen-Thiessen E, Borchelt M. Morbidity, medication, and functional limitations in very old age. In: Baltes PB, Mayer KU, editors. The Berlin aging study: Aging from 70 to 100. New York: Cambridge University Press; 1999. pp. 131–166. [Google Scholar]
- Tucker JS, Klein DJ, Elliott MN. Social control of health behaviors: A comparison of young, middle-aged and older adults. Journal of Gerontology: Psychological Sciences. 2004;59B:P147–150. doi: 10.1093/geronb/59.4.p147. [DOI] [PubMed] [Google Scholar]
- Uchino BN. Understanding the links between social support and physical health: A life-span perspective with emphasis on the separability of perceived and received support. Perspectives on Psychological Science. 2009;4:236–255. doi: 10.1111/j.1745-6924.2009.01122.x. [DOI] [PubMed] [Google Scholar]
- Unger JB, McAvay G, Bruce ML, Berkman L, Seeman T. Variation in the impact of social network characteristics on physical functioning in elderly persons: MacArthur studies of successful aging. Journal of Gerontology: Social Sciences. 1999;54B:S245–S251. doi: 10.1093/geronb/54b.5.s245. [DOI] [PubMed] [Google Scholar]
- U. S. Department of Health and Human Services. Physical activity and health: A report of the surgeon general. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996. Retrieved January 30, 2007 from http://www.cdc.gov/nccdphp/sgr/intro.htm. [Google Scholar]
- Vaillant GE, Meyer SE, Mukamal K, Soldz S. Are social supports in late midlife a cause or a result of successful aging? Psychological Medicine. 1998;28:1159–1168. doi: 10.1017/s0033291798007211. [DOI] [PubMed] [Google Scholar]
- Vaupel JW, Carey JR, Christensen K, Johnson TE, Yashin AI, Iachine IA, Kannisto V, Khazaeli AA, Liedo P, Longo VD, Zeng Y, Manton KG, Curtsinger JW. Biodemographic trajectories of longevity. Science. 1998 May 8;280:855–860. doi: 10.1126/science.280.5365.855. [DOI] [PubMed] [Google Scholar]
- VonDras DD, Madey SF. The attainment of important health goals throughout adulthood: An integration of the theory of planned behavior and aspects of social support. International Journal of Aging and Human Development. 2004;59:205–234. doi: 10.2190/78UQ-5NMW-7YLD-TFWV. [DOI] [PubMed] [Google Scholar]
- Ware JE. SF-36 health survey update. Spine. 2000;25:3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Keller SD. SF-36: Physical and mental health summary scales: A user’s manual. Boston, MA: The Health Institute, New England Medical Center; 1994. [Google Scholar]
- Ware JE, Sherbourne CD. The MOS 36 item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical Care. 1992;30:473–483. [PubMed] [Google Scholar]
- Walter-Ginzburg A, Blumstein T, Modan B. Social factors and mortality in the old-old in Israel: The CALAS study. Journal of Gerontology: Social Sciences. 2002;57B:S308–S318. doi: 10.1093/geronb/57.5.s308. [DOI] [PubMed] [Google Scholar]
- Winerman L. Keys to longevity and well-being. American Psychological Association Monitor; Nov, 2006. pp. 42–44. [Google Scholar]
- Wood RH, Gardner RE, Ferachi KA, King C, Ermolao A, Cherry KE, Cress ME, Jazwinski SM. Physical function and quality of life in older adults: Sex differences. Southern Medical Journal. 2005;98:504–512. doi: 10.1097/01.SMJ.0000157534.08859.4B. [DOI] [PubMed] [Google Scholar]