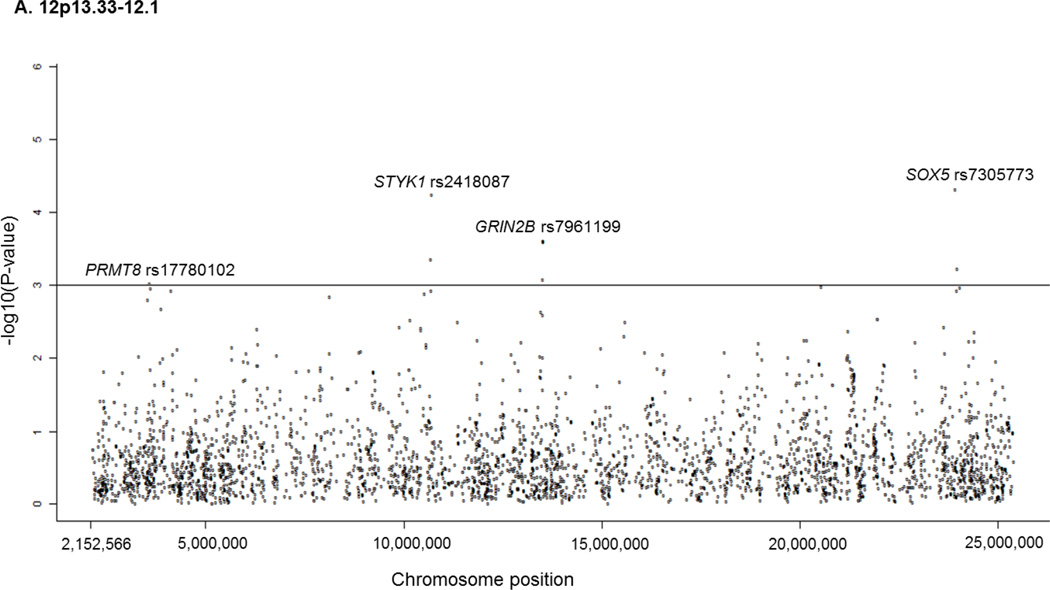
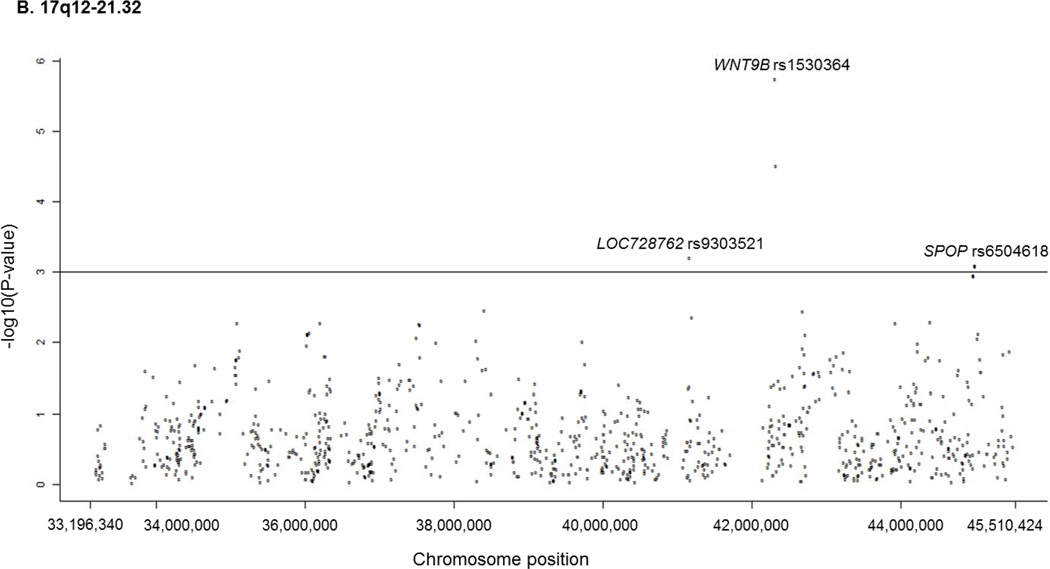
Yanhong Liu
Yanhong Liu
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Beatrice S Melin
Beatrice S Melin
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Preetha Rajaraman
Preetha Rajaraman
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Zhaoming Wang
Zhaoming Wang
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Martha Linet
Martha Linet
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Sanjay Shete
Sanjay Shete
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Christopher I Amos
Christopher I Amos
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Ching C Lau
Ching C Lau
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Michael E Scheurer
Michael E Scheurer
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Spiridon Tsavachidis
Spiridon Tsavachidis
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Georgina N Armstrong
Georgina N Armstrong
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Richard S Houlston
Richard S Houlston
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Fay J Hosking
Fay J Hosking
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Elizabeth B Claus
Elizabeth B Claus
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Jill Barnholtz-Sloan
Jill Barnholtz-Sloan
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Rose Lai
Rose Lai
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Dora Il’yasova
Dora Il’yasova
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Joellen Schildkraut
Joellen Schildkraut
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Siegal Sadetzki
Siegal Sadetzki
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Christoffer Johansen
Christoffer Johansen
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Jonine L Bernstein
Jonine L Bernstein
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Sara H Olson
Sara H Olson
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Robert B Jenkins
Robert B Jenkins
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Daniel LaChance
Daniel LaChance
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Nicholas A Vick
Nicholas A Vick
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Margaret Wrensch
Margaret Wrensch
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Faith Davis
Faith Davis
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Bridget J McCarthy
Bridget J McCarthy
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Ulrika Andersson
Ulrika Andersson
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Patricia A Thompson
Patricia A Thompson
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,
Stephen Chanock
Stephen Chanock
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1;
The Gliogene Consortium1,
Melissa L, Bondy
Melissa L, Bondy
1Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (Yanhong Liu, Michael E Scheurer, Spiridon Tsavachidis, Georgina N Armstrong, Ching C Lau, Melissa L Bondy); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department Health and Human Services, Bethesda, Maryland (Preetha Rajaraman, Zhaoming Wang, Martha Linet, Stephen Chanock); Departments of Biostatistics (Sanjay Shete), Genetics (Christopher I. Amos), The University of Texas MD Anderson Cancer Center, Houston, Texas; Division of Genetics and Epidemiology, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Fay J Hosking); Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio (Jill Barnholtz-Sloan); The Neurological Institute of Columbia University, New York, New York (Rose Lai); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il’yasova, Joellen Schildkraut); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); and Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Danish Cancer Society, Department of Neurology, Copenhagen, Denmark (Christoffer Johansen); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Jonine L. Bernstein, Sara H. Olson); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins, Daniel LaChance); Evanston Kellogg Cancer Care Center NorthShore University HealthSystem, Evanston, Illinois (Nicholas A Vick); Department of Neurological Surgery, University of California, San Francisco, San Francisco, California (Margaret Wrensch); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, Chicago, Illinois (Bridget J McCarthy, Faith Davis); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (Beatrice S. Melin, Ulrika Andersson); Arizona Cancer Center, University of Arizona, Tucson, Arizona (Patricia A. Thompson)
1,*