Abstract
Purpose.
Previously, we mapped the disease locus in the beagle model of autosomal recessive primary open angle glaucoma (POAG) to a 4-Mb interval on chromosome 20, and identified a Gly661Arg variant in ADAMTS10 as the candidate disease-causing variant. The purpose of this study was to test the hypothesis that the Gly661Arg variant of ADAMTS10 causes glaucoma by genotyping dogs of various breeds affected and unaffected by primary glaucoma.
Methods.
Dogs of various breeds, affected or unaffected with primary glaucoma, were genotyped for the Gly661Arg variant of ADAMTS10, as well as 7 other nonsynonymous single nucleotide polymorphisms (SNPs) in other genes in the beagle POAG locus that segregate with disease. Alternate allele frequencies were calculated with 95% confidence intervals and comparisons made to expected allele frequency relative to disease prevalence or between cases and controls.
Results.
For the nonsynonymous SNPs other than the ADAMTS10 variant, control dogs were identified that were homozygous for the alternative alleles, ruling out those variants as causative. None of the nonsynonymous SNPs were found associated with primary glaucoma in American cocker spaniels. The Gly661Arg variant of ADAMTS10 was the only variant with minor allele frequency consistent with the prevalence of primary glaucoma in the general beagle population. The only dog found homozygous for the Gly661Arg variant of ADAMTS10 was an affected beagle, unrelated to the POAG colony.
Conclusions.
These findings support the Gly661Arg mutation of ADAMTS10 as the likely cause of POAG in beagles.
Genotyping dogs of various breeds affected or unaffected by primary glaucoma support the Gly661Arg variant of ADAMTS10 as causative for primary open angle glaucoma in beagles.
Introduction
The genetic locus for POAG inherited as an autosomal recessive trait within a colony of beagles was previously identified on canine chromosome 20.1 Sequencing the entire locus revealed eight variants that segregated with disease and caused amino acid substitutions (Fig. 1), all of which were single nucleotide polymorphisms (SNPs). One of these variants found in ADAMTS10 results in the substitution in the ADAMTS10 protein (NCBI GI: 73986982) of an arginine for a highly conserved glycine residue at amino acid position 661 (Gly661Arg), a change predicted to have deleterious effects on protein function.1 Genotyping the eight nonsynonymous SNPs in 48 control beagles showed that the Gly661Arg variant of ADAMTS10 had the lowest minor allele frequency (MAF). The Gly661Arg variant of ADAMTS10 was identified as the likely cause of POAG in the beagle colony because it was the only rare nonsynonymous variant that caused a highly nonconservative amino acid substitution in a highly conserved region of a gene.
Figure 1.
Nonsynonymous SNPs segregating with disease in the beagle glaucoma colony. The locations of the eight nonsynonymous SNPs that segregate with disease in the POAG beagle colony are indicated by arrows labeled according to the gene in which the SNPs are located. The nonsynonymous SNPs are clustered in a region of approximately 1.2 Mb within the 4-Mb beagle glaucoma locus, represented by the gray bar, below which the location on canine chromosome 20 is indicated, based on the CanFam 2 reference canine genome. Two SNPs were found in GPCR108.
Extensive study of the POAG beagle colony has allowed for detailed characterization of the clinical course of canine POAG. In the POAG beagle colony, the initial manifestation of disease is reduced facility of aqueous humor outflow.2,3 Affected dogs develop elevated intraocular pressure (IOP) with open iridocorneal angles, as determined by gonioscopy and histology, and subsequently develop glaucomatous optic nerve damage.2,4,5 Following prolonged IOP elevation, the globes become enlarged and the lens zonules become weakened and disinserted, leading to luxation of the lens and narrowing of the iridocorneal angle.2 Since reduced facility of aqueous humor outflow precedes all other clinical signs in the POAG beagles, the causative locus is most likely involved in increased outflow resistance and the resultant elevation of IOP.
The hypothesis that the Gly661Arg variant of ADAMTS10 causes POAG in the beagle colony is supported by the pattern of tissue expression and the biological function of ADAMTS10. ADAMTS10 protein is expressed abundantly in the trabecular meshwork,1 consistent with a role for ADAMTS10 in aqueous humor outflow. Functionally, ADAMTS10 is a secreted matrix metalloproteinase which has been proposed to be involved in the structure and function of microfibrils.6–9 In addition to providing elastic support in tissues such as blood vessels and skin, microfibrils are the primary reservoir of latent transforming growth factor beta (TGF-β).10–12 In diseases associated with microfibril defects, such as Marfan syndrome, TGF-β signaling is hyperactivated and TGF-β concentration is elevated.13,14 Defective microfibrils could provide a mechanistic explanation for the well-established elevation of TGF-β concentration in the aqueous humor of human glaucoma patients.15,16 Discovery of the Gly661Arg variant of ADAMTS10 in beagle POAG led us to form the hypothesis that disruption of microfibril structure or function may be an underlying mechanism of increased resistance to aqueous humor outflow in POAG.1 Since the defective microfibrils hypothesis offers a fundamental mechanism of glaucoma pathogenesis, we sought further validation of the ADAMTS10 variant as causative for glaucoma in this study.
Many breeds of dogs are susceptible to primary glaucoma, defined as elevated IOP without other causative ocular disease. Although the course of glaucoma in affected beagles bred for research has been well established, in clinic settings nearly all dogs present at late stages of the disease and most often suffer from significant vision loss. The high prevalence of primary glaucoma in many breeds suggests a genetic component.17,18 To test our hypothesis that the Gly661Arg variant of ADAMTS10 causes glaucoma, we genotyped this SNP, and the other seven nonsynonymous SNPs that segregated with disease in the POAG colony, in dogs of various breeds, both those affected and those unaffected by primary glaucoma.
Materials and Methods
Animals and Samples
Subject dogs were examined by board certified veterinary ophthalmologists. Affected dogs were diagnosed with primary glaucoma, defined as elevated IOP in the absence of antecedent ocular disease. Control dogs were at least 2 years of age and were unaffected by glaucoma or other ocular disease, with the exception of cataracts. Blood samples from dogs were obtained by standard venipuncture, in adherence to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. DNA extractions from whole blood were performed on commercial equipment (Gentra Systems AutoPure robot; Qiagen, Inc., Valencia, CA) using a purification kit (Puregene; Qiagen, Inc.) by the DNA services core of the Vanderbilt Center for Human Genetics Research.
Genotyping
SNP genotyping was carried out by amplification of genomic sequence surrounding SNPs using PCR primers designed with Primer 3 software (available in the public domain, http://frodo.wi.mit.edu/) and based on the canine reference genome (canFam2) followed by Sanger sequencing by the Vanderbilt DNA Sequencing Facility using a 96-capillary ABI 3730xl DNA Analyzer (Life Technologies Corp., Carlsbad, CA). DNA Sequence data was analyzed using DNA sequencing software (Sequencher v. 4.8; Gene Codes Corp., Ann Arbor, MI).
Statistics
Statistical significance of MAF differences between affected and control American cocker spaniels was evaluated using a 2-tailed Fisher exact test calculated using software available online (in the public domain, http://www.langsrud.com/fisher.htm), with Bonferroni correction for multiple comparisons.
Results
To test the hypothesis that the Gly661Arg variant of ADAMTS10 causes primary glaucoma, we genotyped affected and unaffected dogs for the ADAMTS10 variant and for the other seven nonsynonymous SNPs that segregated with disease in the autosomal recessive beagle POAG locus (Fig. 1). Identification of control dogs homozygous for a variant allele would argue against that allele as causative. Additionally, the frequency of the alternative allele must be consistent with the disease prevalence. For beagles, with a prevalence of primary glaucoma of approximately 1%,17 the alternative allele frequency cannot be more than 10%, by Hardy-Weinberg equilibrium. Conversely, finding a dog affected with primary glaucoma that is homozygous for the alternative allele would support that allele as causative.
Beagles
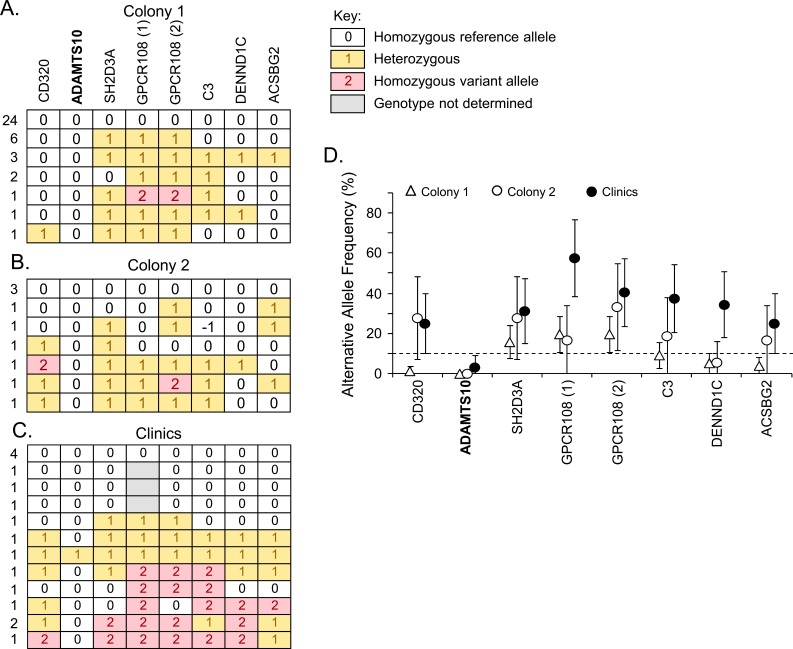
In our original study,1 we genotyped the eight nonsynonymous SNPs (Fig. 1) from the beagle POAG locus in a collection of 48 unaffected beagles, primarily obtained from two large colonies from animal supply companies (Figs. 2A, 2B). Within the two supplier colonies, one control dog was found homozygous for the alternative alleles in both SNPs in GPCR108 (Fig. 2A); one dog was found homozygous for one of the alternative alleles in a GPCR108 SNP (Fig. 2B); and another dog was found homozygous for the alternative allele in CD320 (Fig. 2B). Homozygosity of the alternative alleles in these control beagles argues against the GPCR108 and CD320 SNPs as causative. Minor allele frequencies in the supplier colony populations were consistent with GPCR108 and CD320 SNPs not being causative of primary glaucoma in beagles, though the other variants could not be ruled out, as the 95% confidence intervals (CI) for their minor allele frequencies fell below the upper threshold of 10% (Fig. 2D).
Figure 2.
Beagles genotyped for the nonsynonymous SNPs in the beagle glaucoma locus. Beagles not affected by glaucoma from two different animal supplier colonies (A, B) and from veterinary clinics (C) were genotyped for the eight nonsynonymous SNPs in the beagle glaucoma locus that segregated with disease in the beagle glaucoma colony. The key for genotype calls is shown (A). Each horizontal row represents the haplotype of one or more dogs, with number of dogs represented to the left of the row (A–C). The gene containing each variant is indicated above (A). In GPCR108, there are two nonsynonymous SNPs, GPCR108 (1) and GPCR108 (2). Minor allele frequencies for the variants are shown for the supplier colonies and clinic samples (D) with error bars representing +/− 95% CIs. The alternative allele frequency upper threshold of 10% for a putative disease allele in beagles is indicated by the dashed line (D).
To increase the genetic diversity of our control population, we genotyped an additional 15 older beagles from veterinary clinics that were also examined by veterinary ophthalmologists and found to be unaffected by glaucoma (Fig. 2C). For all variants except the one in ADAMTS10, examples of control dogs homozygous for the alternative allele were found in this general beagle population. This essentially rules out all variants as causative for primary glaucoma in beagles, except for the ADAMTS10 variant. The minor allele frequencies of the variants also rule out all variants other than ADAMTS10 (Fig. 2D). Only one of the control beagles was found heterozygous for the ADAMTS10 variant, giving a minor allele frequency in the clinic population of 3.1%, well below the upper limit of 10% for a causative allele.
Control Dogs of Various Breeds
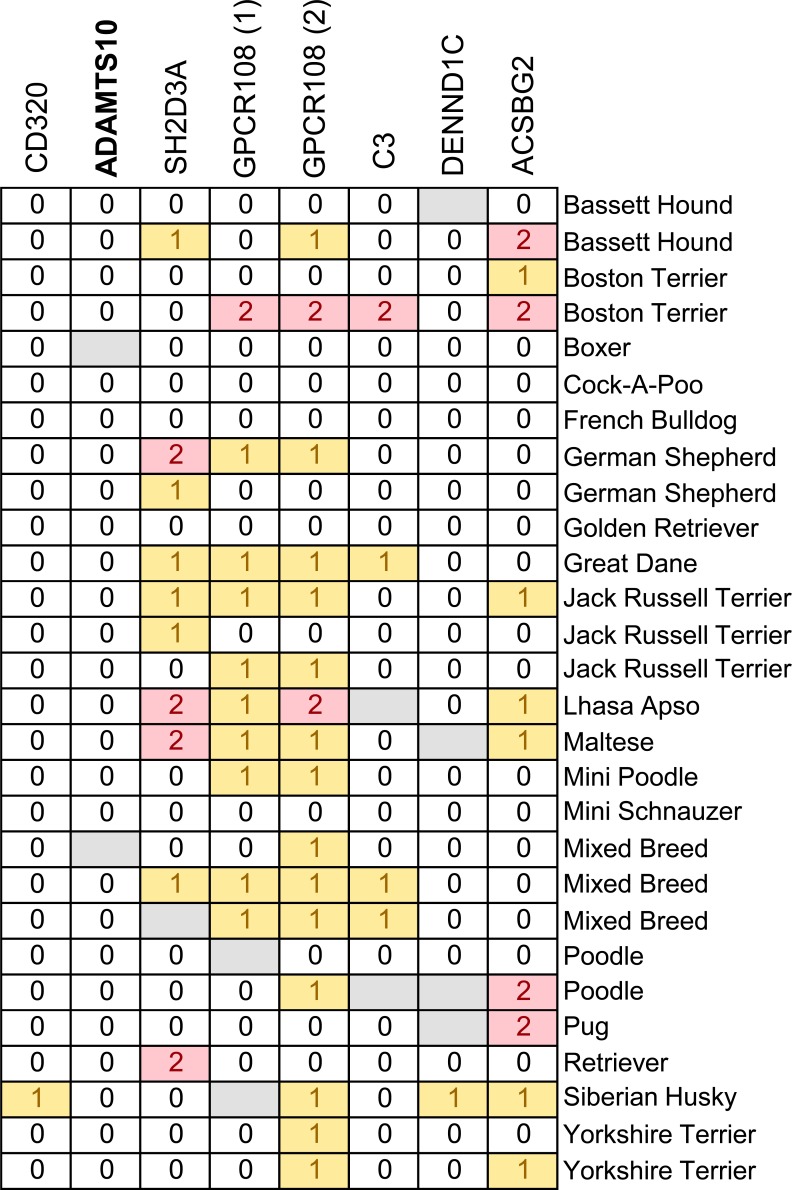
The eight nonsynonymous SNPs were also genotyped in a collection of 28 control dogs of 19 different breeds (Fig. 3). Dogs homozygous for the alternative alleles in SH2D3A, GPCR108, C3, and ACSBG2 were identified in this set, further ruling these out as causative. The alternative allele in ADAMTS10 was not ruled out since none of the 28 control dogs were found homozygous for the alternative allele.
Figure 3.
Control dogs of various breeds genotyped for the nonsynonymous SNPs in the beagle glaucoma locus. Dogs of various breeds, not affected by glaucoma, were genotyped for the eight nonsynonymous SNPs that segregated with disease in the beagle glaucoma colony. Each horizontal row represents the haplotype of an individual dog. The gene containing each variant is indicated above. Genotypes are displayed as in Figure 2.
American Cocker Spaniels
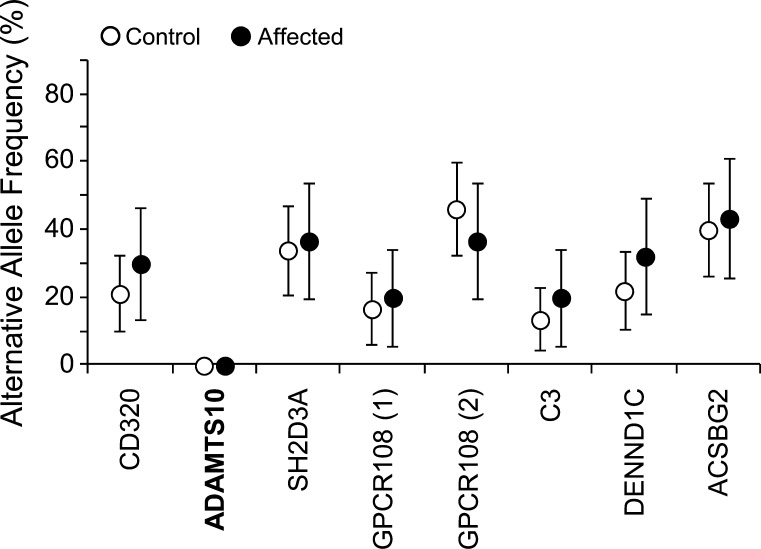
A collection of 16 affected and 26 control American cocker spaniels was genotyped for the eight nonsynonymous SNPs and minor allele frequencies determined (Fig. 4). The ADAMTS10 variant allele was not found in any of the American cocker spaniels, suggesting involvement of other genes. The alternative alleles for the other nonsynonymous SNPs were common, with no significant difference between affected and control groups, ruling out these SNPs as causative of primary glaucoma in American cocker spaniels.
Figure 4.
American cocker spaniels affected and unaffected by primary glaucoma genotyped for the nonsynonymous SNPs in the beagle glaucoma locus. Minor allele frequencies for the variants are shown for 26 unaffected (open symbols) and 16 affected (closed symbols) American cocker spaniels. Error bars represent +/− 95% CIs.
Various Breeds Affected with Primary Glaucoma
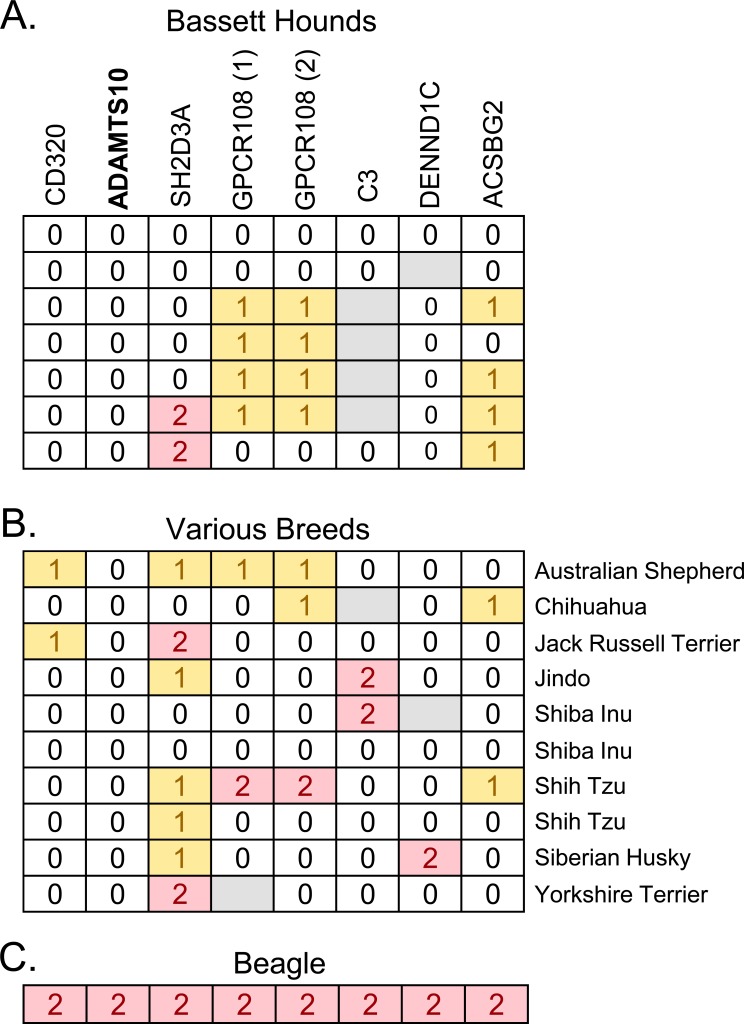
Since dog breeds are highly related genetically, we next investigated whether the ADAMTS10 variant might be found in dogs of various breeds affected with primary glaucoma. All eight nonsynonymous SNPs were genotyped in seven affected basset hounds (Fig. 5A) and a collection of 10 affected dogs from various breeds, including Shiba Inu, Shih Tzu, Chihuahua, Australian Cattle Dog, Jack Russell Terrier, Jindo, Siberian Husky, and Yorkshire Terrier (Fig. 5B). The ADAMTS10 variant was not found in any of these affected dogs, suggesting other genes are involved.
Figure 5.
Affected dogs of various breeds genotyped for the nonsynonymous SNPs in the beagle glaucoma locus. Bassett Hounds (A), dogs of various breeds (B), and a beagle (C), affected by primary glaucoma, were genotyped for the eight nonsynonymous SNPs in the beagle glaucoma locus. Each horizontal row represents the haplotype of an individual dog. The gene containing each variant is indicated above. Genotypes are displayed as in Figure 2.
A beagle not related to the POAG beagle colony was found with primary glaucoma. This beagle was the only dog identified as homozygous for the ADAMTS10 variant allele (Fig. 5C), strongly supporting this variant as causative for primary glaucoma in beagles. This affected beagle was also homozygous for the variant alleles across the 8-SNP locus formed by the nonsynonymous SNPs, suggesting a shared haplotype with the beagle glaucoma colony.
Discussion
Genotyping affected and unaffected dogs of various breeds proved to be an effective method for testing the hypothesis that the Gly661Arg variant of ADAMTS10 is the cause of inherited disease in the POAG beagle colony. The candidate disease allele had to satisfy the criterion that unaffected dogs could not be homozygous for the disease allele, since the disease is inherited as an autosomal recessive trait in the beagle POAG colony. The candidate disease allele also had to satisfy the criterion that its frequency in beagles was lower than the upper threshold of 10%, based on Hardy-Weinberg equilibrium, disease prevalence of 1% and autosomal recessive inheritance. The upper threshold is conservatively high because it assumes that a single genetic variant accounts for all beagle primary glaucoma, which is unlikely. Failure to satisfy the two criteria was strong evidence against a variant as being causative.
In addition to the ADAMTS10 variant, some of the nonsynonymous SNPs were functionally compelling but could not previously be ruled out on the basis of allele frequency. For example, complement factor C3 is functionally plausible, since there is evidence that complement could play a role in glaucoma.20 DENND1C is a GDP-GTP exchange factor for rab13,21 which coordinates assembly of tight junctions,22 which may play a role in aqueous humor outflow resistance.23 However, in the current study, complement factor C3, DENND1C and the other nonsynonymous SNPs other than that found in ADAMTS10 were ruled out because control beagles were found homozygous for the alternative alleles. Identification of control dogs of various breeds homozygous for nonsynonymous SNPs further argues against these variants as glaucoma-causing, though it is possible that breed-specific differences could result in variable penetrance of a disease allele. The Gly661Arg variant of ADAMTS10 was the only SNP that satisfied the criterion that no control dogs were found homozygous for the variant allele. ADAMTS10 was also the only variant with a minor allele frequency below the upper limit of 10% in beagles and therefore the only plausible candidate among the eight nonsynonymous SNPs. The criteria of no homozygosity in controls and sufficiently low allele frequency were effective at eliminating the presumably false-positive candidate SNPs and support the Gly661Arg variant of ADAMTS10 as causative for disease in the beagle POAG colony.
For breeds other than beagle, no evidence was found for involvement of ADAMTS10 in primary glaucoma. The ADAMTS10 variant was not found in a total of 43 affected dogs of breeds other than beagle, including 26 American Cocker Spaniels, seven Basset Hounds and 10 dogs of several other breeds. While the sample sizes are too small to rule out ADAMTS10 as causative, these data suggest that primary glaucoma in dogs is likely to be genetically complex, involving other loci in addition to ADAMTS10.
While a genetic basis for canine primary glaucoma is likely because of the high prevalence in many breeds, differences in age of onset, sex ratios, and inheritance patterns suggest distinct disease processes and involvement of multiple genes. Clinically, primary glaucoma in dogs most often presents with narrowed or closed angles, marked by dysplasia of the pectinate ligament structures, with complete angle closure and collapse of ciliary cleft typically seen after prolonged elevation of IOP. However, studies of POAG in the beagle have documented angle closure secondary to prolonged ocular hypertension, likely related to enlargement of the globe. Some dogs presenting to clinics at late stages of the disease, as is common with canine patients, may previously have had open angles with elevated IOP.
A colony of Basset Hounds with inherited glaucoma has been reported recently with the disease well-characterized as primary angle-closure glaucoma.24 Absence of the ADAMTS10 variant in the basset hounds affected with primary glaucoma would be consistent with a disease process different from POAG in beagles, a notion corroborated by the invariably dysgenic iridocorneal angles in these dogs. Although most American cocker spaniels present with severely dysgenic iridocorneal angles, primary glaucoma with open angles was reported in 1968 in this breed.25 In this study, three Cocker Spaniels bred from glaucomatous dogs were investigated. These Cocker Spaniels had POAG, with elevated IOP and reduced outflow facility, but normal-appearing and open angles by gonioscopy and postmortem histology. Lack of the Gly661Arg variant of ADAMTS10 in affected American cocker spaniels in the present study is consistent with either multiple genes involved in canine POAG or in most cases a distinct disease process.
A limitation of this study is that although seven of the eight variants have been effectively ruled out, it does not provide definitive evidence that the Gly661Arg variant is causal. Future studies either preventing glaucoma in dogs from the POAG beagle colony by expression of the normal form of the gene, or by inducing glaucoma by ablation of ADAMTS10 in mice would provide more conclusive data that the Gly661Arg variant of ADAMTS10 is causative.
The strongest evidence supporting the hypothesis that Gly661Arg variant of ADAMTS10 causes glaucoma is the identification of an affected beagle homozygous for the variant. At 5 years of age, this dog was carefully examined by a veterinary ophthalmologist (CEP) and found to have open iridocorneal angles and elevated IOP in both eyes. A total of 63 control beagles were sequenced, which is a relatively large number for dog breeds which are isolated populations with very limited genetic diversity relative to humans.26 A single control beagle was identified heterozygous for Gly661Arg, giving a MAF of 0.78%. By Hardy-Weinberg equilibrium, fewer than 1 in 1600 beagles would be homozygous for Gly661Arg. Though additional cases would strengthen our conclusions, our identification of a beagle that is both homozygous for this rare allele and affected by a disease with low prevalence supports the ADAMTS10 variant as causative. This support for the Gly661Arg variant of ADAMTS10 in beagle POAG is consistent with our more general hypothesis that disruption of microfibril structure or function may be an underlying mechanism of increased resistance to aqueous humor outflow in POAG.
Footnotes
Supported by NEI Grant EY020894 (RWK), a Departmental Unrestricted Award from Research to Prevent Blindness, Inc., and Vanderbilt Vision Research Center (P30EY008126).
Disclosure: J. Kuchtey, None; J. Kunkel, None; D. Esson, None; J.S. Sapienza, None; D.A. Ward, None; C.E. Plummer, None; K.N. Gelatt, None; R.W. Kuchtey, None
References
- 1. Kuchtey J, Olson LM, Rinkoski T, et al. Mapping of the disease locus and identification of ADAMTS10 as a candidate gene in a canine model of primary open angle glaucoma. PLoS Genet. 2011; 7: e1001306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gelatt KN, Peiffer RL Jr, Gwin RM, Gum GG, Williams LW. Clinical manifestations of inherited glaucoma in the beagle. Invest Ophthalmol Vis Sci. 1977; 16: 1135–1142 [PubMed] [Google Scholar]
- 3. Peiffer RL Jr, Gum GG, Grimson RC, Gelatt KN. Aqueous humor outflow in beagles with inherited glaucoma: constant pressure perfusion. Am J Vet Res. 1980; 41: 1808–1813 [PubMed] [Google Scholar]
- 4. Peiffer RL, Jr, Gelatt KN. Aqueous humor outflow in beagles with inherited glaucoma: gross and light microscopic observations of the iridocorneal angle. Am J Vet Res. 1980; 41: 861–867 [PubMed] [Google Scholar]
- 5. Samuelson DA, Gum GG, Gelatt KN. Ultrastructural changes in the aqueous outflow apparatus of beagles with inherited glaucoma. Invest Ophthalmol Vis Sci. 1989; 30: 550–561 [PubMed] [Google Scholar]
- 6. Somerville RP, Jungers KA, Apte SS. Discovery and characterization of a novel, widely expressed metalloprotease, ADAMTS10, and its proteolytic activation. J Biol Chem. 2004; 279: 51208–51217 [DOI] [PubMed] [Google Scholar]
- 7. Apte SS. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J Biol Chem. 2009; 284: 31493–31497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kutz WE, Wang LW, Bader HL, et al. ADAMTS10 protein interacts with fibrillin-1 and promotes its deposition in extracellular matrix of cultured fibroblasts. J Biol Chem. 2011; 286: 17156–17167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sengle G, Tsutsui K, Keene DR, et al. Microenvironmental regulation by fibrillin-1. PLoS Genet. 2012; 8: e1002425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chaudhry SS, Cain SA, Morgan A, Dallas SL, Shuttleworth CA, Kielty CM. Fibrillin-1 regulates the bioavailability of TGFbeta1. J Cell Biol. 2007; 176: 355–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramirez F, Rifkin DB. Extracellular microfibrils: contextual platforms for TGFbeta and BMP signaling. Curr Opin Cell Biol. 2009; 21: 616–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jensen SA, Robertson IB, Handford PA. Dissecting the fibrillin microfibril: structural insights into organization and function. Structure. 2012; 20: 215–225 [DOI] [PubMed] [Google Scholar]
- 13. Matt P, Schoenhoff F, Habashi J, et al. Circulating transforming growth factor-beta in Marfan syndrome. Circulation. 2009; 120: 526–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Neptune ER, Frischmeyer PA, Arking DE, et al. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003; 33: 407–411 [DOI] [PubMed] [Google Scholar]
- 15. Tripathi RC, Li J, Chan WF, Tripathi BJ. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp Eye Res. 1994; 59: 723–727 [DOI] [PubMed] [Google Scholar]
- 16. Fuchshofer R, Tamm ER. The role of TGF-beta in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 2012; 347: 279–290 [DOI] [PubMed] [Google Scholar]
- 17. Gelatt KN, MacKay EO. Prevalence of the breed-related glaucomas in pure-bred dogs in North America. Vet Ophthalmol. 2004; 7: 97–111 [DOI] [PubMed] [Google Scholar]
- 18. Strom AR, Hassig M, Iburg TM, Spiess BM. Epidemiology of canine glaucoma presented to University of Zurich from 1995 to 2009. Part 1: Congenital and primary glaucoma (4 and 123 cases). Vet Ophthalmol. 2011; 14: 121–126 [DOI] [PubMed] [Google Scholar]
- 19. Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S. eds Bioinformatics Methods and Protocols: Methods in Molecular Biology. Totowa, NJ: Humana Press; 2000: 365–386 [DOI] [PubMed] [Google Scholar]
- 20. Howell GR, Macalinao DG, Sousa GL, et al. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J Clin Invest. 2011; 121: 1429–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yoshimura S, Gerondopoulos A, Linford A, Rigden DJ, Barr FA. Family-wide characterization of the DENN domain Rab GDP-GTP exchange factors. J Cell Biol. 2010; 191: 367–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamamura R, Nishimura N, Nakatsuji H, Arase S, Sasaki T. The interaction of JRAB/MICAL-L2 with Rab8 and Rab13 coordinates the assembly of tight junctions and adherens junctions. Mol Biol Cell. 2008; 19: 971–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ye W, Gong H, Sit A, Johnson M, Freddo TF. Interendothelial junctions in normal human Schlemm's canal respond to changes in pressure. Invest Ophthalmol Vis Sci. 1997; 38: 2460–2468 [PubMed] [Google Scholar]
- 24. Grozdanic SD, Kecova H, Harper MM, Nilaweera W, Kuehn MH, Kardon RH. Functional and structural changes in a canine model of hereditary primary angle-closure glaucoma. Invest Ophthalmol Vis Sci. 2010; 51: 255–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lovekin L, Bellhorn RW. Clinicopathologic changes in primary glaucoma in the cocker spaniel. Am J Vet Res. 1968; 29: 379–385 [Google Scholar]
- 26. Lindblad-Toh K, Wade CM, Mikkelsen TS, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005; 438: 803–819 [DOI] [PubMed] [Google Scholar]