Abstract
Purpose.
To determine whether fixation instability contributes to reduced visual acuity in amblyopia, we compared fixation instability, quantified by the Nidek MP-1 microperimeter, in amblyopic and nonamblyopic children.
Methods.
Participants were 89 children (5–17 years old) with strabismus (n = 31), anisometropia (n = 29), or both conditions (n = 29). Fixation instability was measured using the Nidek MP-1 microperimeter, which calculated horizontal and vertical eye position at 25 Hz as the child attempted steady fixation for 30 seconds. Fixation instability was quantified as the 95% bivariate contour ellipse area (95% BCEA), the best-fit ellipse within which 95% of fixation occurred during the 30-second test. BCEA was normalized by log transformation.
Results.
Children with amblyopia had significantly larger BCEAs for amblyopic eyes (mean = 0.56 log deg2) than fellow eyes (mean = 0.2 log deg2, P < 0.01) and right eyes of normal controls (mean = 0.12 log deg2, P ≤ 0.01). Fixation instability was significantly greater along the horizontal axis of the ellipse for amblyopic (mean = 3.53°) than fellow (mean = 1.98°, P = 0.008), and control (mean = 1.62°, P < 0.001) eyes.
Conclusions.
Fixation instability in amblyopic eyes of children with strabismus and/or anisometropia, and the associated poor stereoacuity probably is the consequence of decorrelated binocular experience early in life. Longer duration of decorrelated visual experience is associated with increased fixation instability, poorer stereoacuity, and more severe amblyopia. Treatments that minimize the duration of decorrelated visual experience may improve stereoacuity and decrease fixation instability.
Fixation stability, measured using the Nidek MP-1 microperimeter, was compared in amblyopic, nonamblyopic, and normal vision children. We demonstrate that fixation instability in amblyopia is poorer and of larger amplitude than fixation instability in nonamblyopic children and controls.
Introduction
The ability to hold steady fixation is a fundamental aspect of good visual function. During normal fixation, the eye constantly makes physiologic involuntary micro-eye movements, including small amplitude drifts, micro-saccades, and tremor, which may have a role in maintaining visibility and visual attention. Retinal diseases that affect central vision and reduce visual acuity, like age-related macular degeneration (AMD), can cause fixation instability.1,2 In AMD, fixation instability is associated positively with the magnitude of visual acuity deficit, declines with disease progression, and improves following treatment.1–3 Hence, in AMD, fixation instability is likely to be secondary to visual acuity loss.
Fixation instability also has been noted in amblyopia. However, the relationship between fixation instability and amblyopic visual acuity deficits is not well understood. Regan et al. used repeat letter cards in an attempt to compensate for gaze instability in 30 amblyopic children and adults, and found improved visual acuity compared to the more conventional linear letter format in approximately 1/3 of patients.4 Carpineto et al. categorized fixation as stable, relatively unstable, or unstable using a Nidek MP-1 microperimeter (Navis software version 3.6; Nidek Technologies Srl, Padova, Italy) for 33 micostrabismic children who had been treated for amblyopia.5 They reported that fixation instability was associated with pretreatment visual acuity and duration of amblyopia treatment. More recently, Gonzalez et al. quantified fixation instability of 13 adults with strabismic or combined mechanism amblyopia as the area within which fixations occurred while steady fixation was attempted for 15 seconds.6 They reported significantly larger fixation areas (more instability) in amblyopic eyes compared to fellow eyes and control eyes.
In our study, we evaluated the association between fixation instability and amblyopia in children. The primary aim was to quantify fixation instability in children with amblyopia and to compare their fixation instability to that of nonamblyopic children and normal controls. Secondary aims were to determine whether depth of amblyopia (visual acuity) was associated with the degree of fixation instability in amblyopia, and to explore associations between clinical factors (type of amblyopia, age of onset, history of surgery, duration of amblyopia treatment, stereoacuity) and fixation instability.
Methods
Participants were 89 children between the ages of 5 and 17 years with strabismus (n = 31), anisometropia (n = 29), or both conditions (n = 29). They were referred to the study by 18 Dallas-Fort Worth pediatric ophthalmologists. They were grouped as amblyopic or nonamblyopic based on clinical diagnosis, confirmed in the laboratory by visual acuity testing with EVA HOTV7,8 or E-ETDRS9 in the research laboratory. Amblyopia was defined as best-corrected letter optotype visual acuity of ≥0.2 logMAR (20/32 or worse) and ≥0.2 logMAR interocular difference (≥2 lines). The amblyopic group was composed of 52 children with strabismus (n = 7), anisometropia (n = 21), or both conditions (n = 24). The nonamblyopic group was composed of 37 children with strabismus (n = 24), anisometropia (n = 8), or strabismus plus anisometropia (n = 5). For the 37 nonamblyopic participants, visual acuity ranged from −0.09 to 0.3 logMAR. In addition, 40 normal controls with visual acuity of 0.1 logMAR or better in each eye and 40 arcsec or better stereoacuity were included as a comparison group. See the Table for additional details about participants. None of the children had known developmental delay, or concurrent ophthalmic or systemic diseases. None of the children was born preterm (≤36 weeks).
Table.
Clinical Characteristics of the Participants
|
|
Amblyopic Children |
Nonamblyopic Children |
Normal Controls |
||||
|
Strabismic |
Anisometropic |
Combined |
Strabismic |
Anisometropic |
Combined |
||
| Participants | n = 7 | n = 21 | n = 24 | n = 24 | n = 8 | n = 5 | n = 40 |
| Age, y Mean ± SD Range | 8.0 ± 1.8 | 9.1 ± 2.4 | 7.5 ± 1.5 | 9.4 ± 3.2 | 8.2 ± 2.8 | 7.1 ± 1.9 | 9.5 ± 2.5 |
| 6.3 to 11.5 | 5.5 to 13.3 | 5.3 to 10.6 | 5.3 to 17.3 | 5.2 to 12.1 | 5.2 to 10.2 | 5.6 to 17.3 | |
| Condition | Acc ET (n = 6), ANAET (n = 1) |
Hyperopic (n = 19), Myopic (n = 1), Astigmatic (n = 1) | Hyp. Aniso+ET (n = 18), Hyp. Aniso + E(T) (n = 4), Hyp. Aniso + X(T) (n = 1), My. Aniso + XT (n = 1) |
Acc ET (n = 12), ANAET (n = 1), Inf ET (n = 10), X(T) (n = 1) | Hyperopic (n = 5), Myopic (n = 1), Astigmatic (n = 2) | Hyp. Aniso + ET (n = 2), Hyp. Aniso + E(T) (n = 3) | – |
| Visual acuity amblyopic eye, logMAR, range | 0.4 to 0.8 | 0.2 to 0.9 | 0.2 to 1.3 | – | – | – | – |
| Visual acuity fellow/right eye, logMAR, range | 0.0 to 0.3 | −0.18 to 0.3 | −0.22 to 0.2 | −0.09 to 0.3 | −0.09 to 0.3 | 0.09 to 0.3 | −0.22 to 0.2 |
| Stereoacuity, arcsec, range | nil to 3000 | nil to 40 | nil to 400 | nil to 20 | 200 to 30 | nil to 700 | 40 to 20 |
| Pretreatment visual acuity amblyopic eye, logMAR, range | 0.09 to 1.0 | 0.17 to 0.8 | 0.3 to 0.7 | – | – | – | – |
| Onset | Infantile (n = 2), late (n = 5) | Infantile (n = 2), late (n = 19) | Infantile (n = 7), late (n = 17) | Infantile (n = 17), late (n = 7) | Infantile (n = 1), late (n = 7) | Infantile (n = 2), late (n = 3) | – |
| Surgery | Yes = 4, no = 3 | Yes = 1,* no = 20 | Yes = 14, no = 10 | Yes = 15, no = 9 | Yes = 0, no = 8 | Yes = 1, no = 4 | – |
| Duration of patching† | 0 to 1 y (n = 2) | 0 to 1 y (n = 9) | 0 to 1 y (n = 5) | – | – | – | – |
| 13 mo to 2 y (n = 1) | 13 mo to 2 y (n = 3) | 13 mo to 2 y (n = 4) | |||||
| >2 y (n = 3) | >2 y (n = 6) | >2 y (n = 14) | |||||
ET, esotropia; Acc ET, accommodative esotropia; ANAET, acquired nonaccommodative esotropia; Hyp. Aniso, hyperopic anisometropia; My. Aniso, myopic anisometropia; E(T), intermittent esotropia; X(T), intermittent exotropia; XT, exotropia.
This anisometropic child had surgery for DVD at age 7 years.
Only individuals with detailed history available were included in the analysis of duration.
Informed consent was obtained from parents, as the children were younger than 18 years, before participation in the study. This research protocol observed the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center. The Retina Foundation of the Southwest is an HIPAA exempt institution.
Procedure
Fixation instability was measured using the Nidek MP-1 microperimeter (Navis software version 3.6; Nidek Technologies Srl). The Nidek MP-1 combines a nonmydriatic infrared (IR) fundus camera to obtain real-time retinal images and a liquid crystal display (LCD) to present a fixation target. The fixation target was a 1° radius red circular ring, displayed in the center of the LCD screen, and children were instructed to fixate steadily at the center of the ring. Measurements were monocular. For each eye, assessment of fixation instability began with capture of a reference fundus image with 1 pixel (0.1°) resolution, identification of a high contrast retinal landmark in the frozen image by the examiner. During the 30-second test period, software (Navis software version 3.6; Nidek Technologies Srl) calculated the shift between the reference image and the real-time fundus image at 25 Hz, acquiring 750 X and Y coordinates for fixation.
At the conclusion of testing, a scatter graph of fixation points was displayed and fixation instability was quantified as the area of the 95% bivariate contour ellipse (BCEA); that is an ellipse that provided the best fit to the boundary of 95% of the 750 fixation points acquired during the 30-second test interval10:
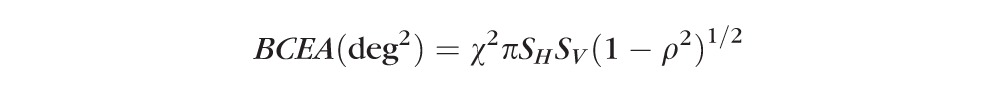 |
where χ2 corresponds to the χ2 with P = 0.95 (2 SD), SH and SV are SDs of horizontal and vertical eye movements, and ρ is the Pearson correlation coefficient of the horizontal and vertical eye movements.
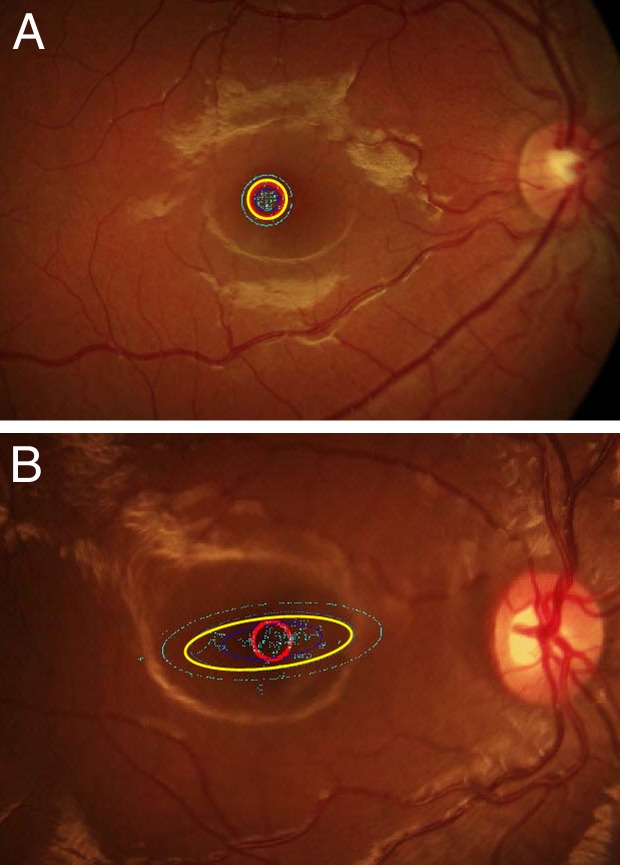
A smaller BCEA indicates more stable fixation. Figure 1 provides examples of fixation data and BCEAs for a normal control and for an amblyopic child.
Figure 1.
Fixation patterns of a normal control and a child with amblyopia. The red circle is the fixation target. The 95% BCEA is indicated by the yellow ellipse. The 68% and 99% BCEAs are indicated by the inner blue ellipse and the outer turquoise ellipse, respectively. (A) Fixation pattern from the right eye of a 9-year-old normal control. Visual acuity 20/16, BCEA = 0.49 log deg2, major axis diameter = 2.01°, minor axis diameter = 1.99°. (B) Fixation pattern from the right eye of a 7-year-old with esotropia, hyperopic anisometropia, and amblyopia. Visual acuity 20/250, BCEA = 1.3 log deg2, major axis diameter = 8.67°, minor axis diameter = 2.95°.
Stereoacuity was measured using the Randot Preschool Stereoacuity Test, Randot Stereo Butterfly, and the Titmus Fly. The latter two were a measure of coarse stereopsis for those unable to respond to the Randot Preschool Stereoacuity Test. The Randot Preschool Stereoacuity Test tested stereoacuity in the range of 1.6 to 2.9 log arcsecs (40–800 arcsecs) in 0.2 to 0.3 log steps. The Randot Stereo Butterfly measured stereoacuity of 2.8, 3.0, and 3.3 log arcsecs (700, 1150, 2000), and the Titmus Fly provided an additional step of 3.5 log arcsecs (3000 arcsecs). Children with nil stereoacuity were assigned a value of 4.0 log arcsecs (an additional 0.5 log step beyond the largest measurable stereoacuity).
Data Analysis
BCEAs were log10 transformed to normalize the data; raw BCEA values were not distributed normally using the Shapiro-Wilk test. Initially, because BCEAs of the two eyes may be correlated, repeated measures ANOVA was performed to compare BCEAs of amblyopic (amblyopic eye and fellow eye), nonamblyopic (right eye and left eye), and normal control (right eye and left eye) children.11 Because no significant differences were found between right and left eyes of normal controls or nonamblyopic children, all subsequent analyses compared amblyopic eyes and fellow eyes with normal control right eyes and right eyes of nonamblyopic children. All pairwise analyses were Bonferroni corrected for multiple comparisons.
Comparisons of the spread of fixation points along the BCEA axes between the different patient groups were done using nonparametric tests as the lengths of the major and minor axes of the BCEAs were not distributed normally. The spread of fixation points along the BCEA axes for amblyopic eyes and fellow eyes was compared to normal control right eyes and right eyes of nonamblyopic children using Kruskal-Wallis ANOVA and Mann-Whitney U tests. Pearson correlation coefficient was calculated to examine the association between visual acuity and fixation instability of amblyopic eyes, and between stereoacuity and fixation instability.
Results
Fixation Instability in Amblyopic Children
Figure 2 illustrates mean 95% BCEAs for amblyopic, nonamblyopic, and normal control children. Mean BCEA for amblyopic children was significantly larger than for normal control and nonamblyopic children (F[2,123] = 5.4, P = 0.005). Bonferroni post hoc analysis showed that amblyopic eyes had significantly more fixation instability (mean ± SEM 0.56 ± 0.07 log deg2) than fellow eyes (0.2 ± 0.06 log deg2, P < 0.001), nonamblyopic right eyes (0.28 ± 0.04 log deg2, t = 2.85, P = 0.005), and normal controls' right eyes (0.12 ± 0.04 log deg2, P ≤ 0.01). Fixation instability of fellow eyes did not differ significantly from right eyes of normal controls. Mean BCEA for nonamblyopic right eyes (0.28 ± 0.04 log deg2) was significantly larger than normal controls' right eyes (0.12 ± 0.04 log deg2, P = 0.02). Normal control right eyes did not differ significantly from normal control left eyes.
Figure 2.
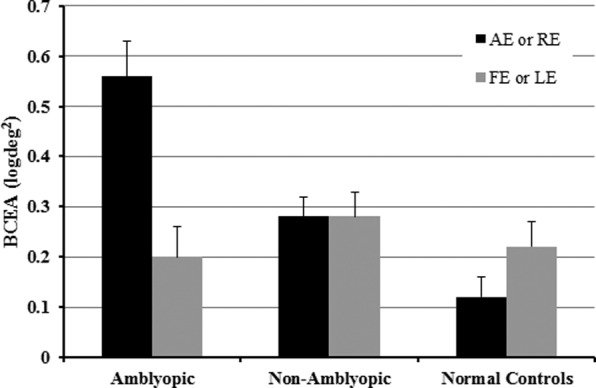
Comparison of mean (± SEM) log10 95% BCEAs for amblyopic eyes (AE) and fellow eyes (FE) of amblyopic children, and right and left eyes (RE, LE) of nonamblyopic children and normal controls.
The major axis of the BCEA for amblyopic eyes was located at −9.3 ± 35.6°, that is the spread of fixation points was primarily horizontal. Amblyopic eyes had significantly greater major/minor axes BCEA ratios (mean ± SEM 1.8 ± 0.09) than normal control right eyes (1.5 ± 0.06, Mann-Whitney U = 754, P = 0.003). In other words, the distribution of fixation points was more elliptical in the BCEAs of amblyopic eyes than in right eyes of normal controls.
The spread of the fixation points (length of the horizontal axis of the BCEA) was significantly different for amblyopic, nonamblyopic, and normal control children (Kruskal-Wallis H = 24.58, P < 0.001). Amblyopic eyes had more instability along the horizontal axis (mean ± SEM 3.53 ± 0.37°) than fellow eyes (1.98 ± 0.14°, P = 0.008) and normal control right eyes (1.62 ± 0.08°, P < 0.001). However, only a trend for more instability along the horizontal axis for amblyopic eyes compared to nonamblyopic right eyes was observed (2.09 ± 0.11°, P = 0.057). Although there was less vertical instability than horizontal instability, there were significant differences among amblyopic and normal control children (H = 13.71, P = 0.001). Amblyopic eyes (1.99 ± 0.22°) had significantly wider spread of fixation along the vertical axis than fellow eyes (1.28 ± 0.08°, P = 0.003) and normal control right eyes (1.12 ± 0.05°, P < 0.001). However, no difference between amblyopic eyes and nonamblyopic right eyes (1.38 ± 0.09°, P = 0.18) was observed.
Fixation Instability and Visual Acuity
Overall, there was a statistically significant positive correlation between visual acuity and BCEA (r = 0.60, P < 0.001) for amblyopic eyes (Fig. 3). Looking at subgroups by mechanism, visual acuity was correlated positively with log10BCEA for amblyopic eyes in the strabismic (r = 0.94, P = 0.002, n = 7) and combined mechanism (r = 0.42, P = 0.04, n = 24) groups. However, no significant correlation between visual acuity and logBCEA for amblyopic eyes was observed in the anisometropic group (r = 0.26, P = 0.26, n = 20). Fixation instability was not associated with pretreatment visual acuity (n = 21, r = 0.14, P = 0.53).
Figure 3.
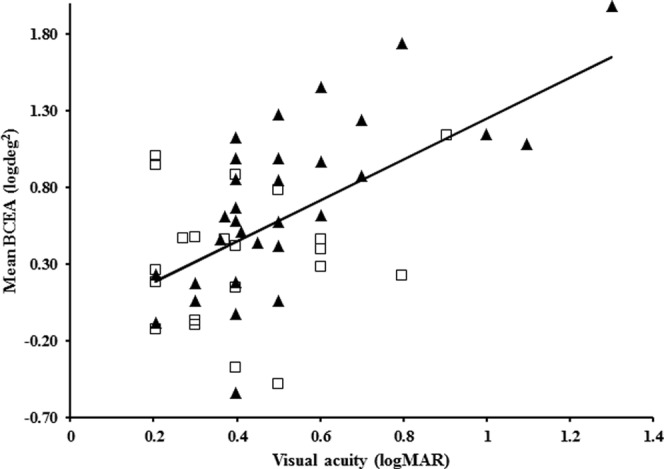
Correlation between BCEA and visual acuity for strabismic (solid triangle) and anisometropic (open square) amblyopic eyes. Best-fit line, y = 1.3214x − 0.0746.
Fixation Instability and Clinical Variables
Fixation instability was not associated with the type of amblyopia (strabismic, anisometropic, or combined mechanism; F[2,49] = 2.74, P = 0.07) or its age of onset (infantile versus late onset; F[1,49] < 0.001, P = 0.99). No significant difference in BCEA was found between children who underwent strabismus surgery compared to those who did not in either the amblyopic group (F[1,50] = 3.64, P = 0.06) or nonamblyopic group (F[1,35] = 0.49, P = 0.48). There was no significant difference in the magnitude of fixation instability between children who had an amblyopia treatment duration of 0 to 1 year, 13 months to 2 years, and >2 years of treatment (F[2,44] = 1.17, P = 0.319). There was a statistically significant positive correlation between log stereoacuity and BCEA (r = 0.27, P = 0.05) for amblyopic eyes (Fig. 4). Analysis of covariance showed that the association between log stereoacuity and BCEA was statistically significant (F[8,42] = 2.16, P = 0.05), even after adjusting for visual acuity.
Figure 4.
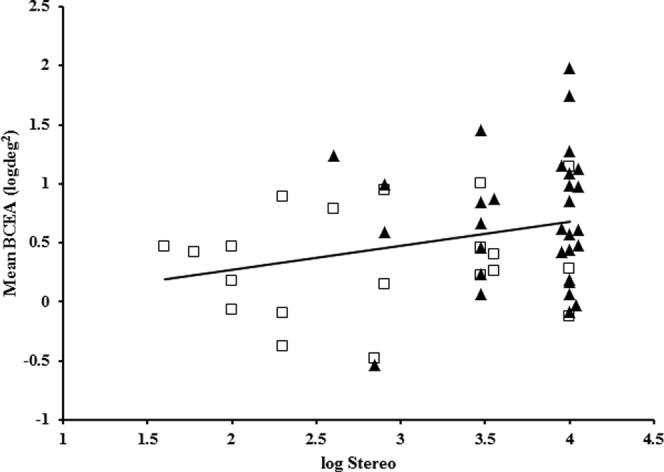
Correlation between BCEA and stereoacuity for strabismic (solid triangle) and anisometropic (open square) amblyopic eyes. Nil stereoacuity has been assigned a value of 4.0 log arcsec. Best-fit line, y = 0.2028x − 0.1307.
Discussion
During attempted steady fixation with their amblyopic eyes, children had 3 times greater fixation instability (larger BCEA areas) than when fixing with their fellow eyes and compared to normal controls. These results agree with the Nidek MP-1 categoric fixation instability data reported by Carpineto et al.5 for children with microstrabimus; that is, amblyopic eyes were classified commonly as relatively unstable or unstable by Nidek MP-1 software (Navis software version 3.6; Nidek Technologies Srl). Our results also agreed well with those of Gonzalez et al.6 However, the magnitude of fixation instability (BCEAs) reported here is larger by a factor of 6 than reported by Gonzalez et al.6 Most of the discrepancy is due to our report of the 95% BCEA and the report of Gonzalez et al. of the 68.2% BCEA.6 However, even the 68.2% BCEAs for the children in our study is approximately twice as large as that reported by Gonzalez et al.6 Whether the remaining, unexplained difference between their amblyopic adult data and our data from children is related to age of the participants or to differences in the devices used to record eye position (Nidek MP-1 versus EyeLink) is unknown. However, we have shown previously good agreement between Nidek MP-1 and EyeLink data obtained from amblyopic children (Birch EE, et al. IOVS 2011;52:ARVO E-Abstract 4691). Despite the differences in absolute magnitude of the BCEAs, there is agreement between the two studies in finding that amblyopic subjects have approximately 3 times greater fixation instability (BCEA area) than normal controls.
Because the visual system of the infant is plastic, development of functional connections can be disrupted by decorrelated binocular experience. Maturation of binocular fusion and stereoacuity during early development requires correlated activity between right and left eye inputs to the visual cortex.12,13 Consequences of the maldevelopment that result from early decorrelated visual experience include abnormal or nil stereoacuity. and fixation instability in the form of fusion maldevelopment nystagmus.14 Thus, fixation instability has been reported to be associated with strabismus.15–17 The current data support this association. Stereoacuity was correlated with BCEA, that is children with amblyopia who showed poorer stereoacuity also had greater fixation instability. Moreover, amblyopic eyes had highly elliptical BCEAs, with a mean major/minor axis ratio of 1.8. Fixation instability along the horizontal and vertical axes was approximately 8 times larger for amblyopic eyes than normal control eyes, consistent with the slow nasal-ward drift and temporal-ward refixation that characterizes fusion maldevelopment nystagmus syndrome (FMNS) (Birch EE, et al. IOVS 2011;52:ARVO E-Abstract 4691; Felius J, et al. IOVS 2011;52:ARVO E-Abstract 4960; Felius J, et al. IOVS 2011;53:ARVO E-Abstract 3898; and Ref. 18).
Visual acuity was correlated with BCEA. That is, amblyopic eyes with poorer visual acuity had greater fixation instability and, thus, larger and wider bivariate contour ellipse areas. Categorical severity of fixation instability has been reported previously to be associated with depth of amblyopia.5 One possible cause of the additional fixation instability associated with more severe amblyopia is that the monocular acuity deficit has a direct effect on fixation instability in addition to the effect of decorrelated visual experience. This hypothesis is supported by the finding that right eyes of nonamblyopic children with strabismus and/or anisometropia exhibited more fixation instability than normal controls, but less than amblyopic children. Decorrelation was sufficient to cause fixation instability, but decorrelation and amblyopia together caused greater fixation instability. The extent to which decorrelation and amblyopia contributed to fixation instability may have been possible to determine by comparing fogging versus occlusion, but this was not done in our study. Longer durations of abnormal binocular experience may result in more severe amblyopia and more severe disruption of fixation control. It is known that longer duration of decorrelation is associated with increased intensity of FMNS, the most common fixation instability associated with strabismus and anisometropia.19 However, to our knowledge there is no evidence as yet that longer duration of decorrelation results in more severe amblyopia.
Fixation instability was not found to vary by age of onset. The lack of an association with age of onset was somewhat surprising. We had expected to find a larger fixation instability in infantile onset, because decorrelation of binocular inputs during an early critical period has been shown to disrupt gaze stability due to fusion maldevelopment.14,20,21 The late-onset group enjoyed a normal binocular experience with correlated binocular inputs during the first 18 to 48 months of life. However, there is evidence that the critical period for fusion development may extend well beyond the first year of life,22 and that periods of decorrelated visual experience during the preschool years that last longer than 3 months can disrupt fusion and stereopsis permanently.23–25
There is evidence that extraocular muscle surgery can decrease fixation instability in children with infantile nystagmus.26 It has been hypothesized that surgical effects on extraocular muscle or tendon proprioception has an influence on central ocular motor pathways, resulting in less fixation instability.27 In our study, we found that fixation instability was not significantly different between children with and without a history of strabismus surgery. However, note that the extraocular muscle surgery performed for infantile nystagmus and/or anomalous head posture typically includes tenotomy, not solely the bimedial recessions that were performed for esotropia in our cohort.28
Taken together, these results suggested that minimizing the duration of decorrelated visual experience not only will improve stereoacuity outcomes,23–25 but also will result in more stable fixation.
Footnotes
Supported by EY022313.
Disclosure: V. Subramanian, None; R.M. Jost, None; E.E. Birch, None
References
- 1. Tarita-Nistor L, Gonzalez EG, Markowitz SN, Steinbach MJ. Fixation characteristics of patients with macular degeneration recorded with the mp-1 microperimeter. Retina. 2008; 28: 125–133 [DOI] [PubMed] [Google Scholar]
- 2. Tarita-Nistor L, Gonzalez EG, Mandelcorn MS, Lillakas L, Steinbach MJ. Fixation stability, fixation location, and visual acuity after successful macular hole surgery. Invest Ophthalmol Vis Sci. 2009; 50: 84–89 [DOI] [PubMed] [Google Scholar]
- 3. Gonzalez EG, Tarita-Nistor L, Mandelcorn ED, Mandelcorn M, Steinbach MJ. Fixation control before and after treatment for neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011; 52: 4208–4213 [DOI] [PubMed] [Google Scholar]
- 4. Regan D, Giaschi DE, Kraft SP, Kothe AC. Method for identifying amblyopes whose reduced line acuity is caused by defective selection and/or control of gaze. Ophthalmic Physiolog Opt. 1992; 12: 425–432 [PubMed] [Google Scholar]
- 5. Carpineto P, Ciancaglini M, Nubile M, et al. Fixation patterns evaluation by means of MP-1 microperimeter in microstrabismic children treated for unilateral amblyopia. Eur J Ophthalmol. 2007; 17: 885–890 [DOI] [PubMed] [Google Scholar]
- 6. Gonzalez EG, Wong AM, Niechwiej-Szwedo E, Tarita-Nistor L, Steinbach MJ. Eye position stability in amblyopia and in normal binocular vision. Invest Ophthalmol Vis Sci. 2012; 53: 5386–5394 [DOI] [PubMed] [Google Scholar]
- 7. Moke PS, Turpin AH, Beck RW, et al. Computerized method of visual acuity testing: adaptation of the amblyopia treatment study visual acuity testing protocol. Amer J Ophthalmol. 2001; 132: 903–909 [DOI] [PubMed] [Google Scholar]
- 8. Holmes JM, Beck RW, Repka MX, et al. The amblyopia treatment study visual acuity testing protocol. Arch Ophthalmol. 2001; 119: 1345–1353 [DOI] [PubMed] [Google Scholar]
- 9. Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Amer J Ophthalmol. 2003; 135: 194–205 [DOI] [PubMed] [Google Scholar]
- 10. Crossland MD, Dunbar HM, Rubin GS. Fixation stability measurement using the MP1 microperimeter. Retina. 2009; 29: 651–656 [DOI] [PubMed] [Google Scholar]
- 11. Sainani K. The importance of accounting for correlated observations. PM R. 2010; 2: 858–861 [DOI] [PubMed] [Google Scholar]
- 12. Chino YM, Smith EL III, Hatta S, Cheng H. Postnatal development of binocular disparity sensitivity in neurons of the primate visual cortex. J Neurosc. 1997; 17: 296–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Endo M, Kaas JH, Jain N, Smith EL III, Chino Y. Binocular cross-orientation suppression in the primary visual cortex (V1) of infant rhesus monkeys. Invest Ophthalmol Vis Sci. 2000; 41: 4022–4031 [PubMed] [Google Scholar]
- 14. Tychsen L, Richards M, Wong A, Foeller P, Bradley D, Burkhalter A. The neural mechanism for latent (fusion maldevelopment) nystagmus. J Neuroophthalmol. 2010; 30: 276–283 [DOI] [PubMed] [Google Scholar]
- 15. Bedell HE, Yap YL, Flom MC. Fixational drift and nasal-temporal pursuit asymmetries in strabismic amblyopes. Invest Ophthalmol Vis Sci. 1990; 31: 968–976 [PubMed] [Google Scholar]
- 16. Zhang B, Stevenson SS, Cheng H, et al. Effects of fixation instability on multifocal VEP (mfVEP) responses in amblyopes. J Vis. 2008; 8: 16: 11–14 [DOI] [PubMed] [Google Scholar]
- 17. Westall CA, Schor CM. Asymmetries of optokinetic nystagmus in amblyopia: the effect of selected retinal stimulation. Vision Res. 1985; 25: 1431–1438 [DOI] [PubMed] [Google Scholar]
- 18. Birch EE, Subramanian V, Weakley DRJ. Is the “flick” in anisometropia a sign of microstrabismus or fixation instability? (E-Abstract e2). J AAPOS. 2012; 16 [Google Scholar]
- 19. Tychsen L, Richards M, Wong AM, et al. Decorrelation of cerebral visual inputs as the sufficient cause of infantile esotropia. Am Orthopt J. 2008; 58: 60–69 [DOI] [PubMed] [Google Scholar]
- 20. Wong AM, Foeller P, Bradley D, Burkhalter A, Tychsen L. Early versus delayed repair of infantile strabismus in macaque monkeys: I. ocular motor effects. J AAPOS. 2003; 7: 200–209 [DOI] [PubMed] [Google Scholar]
- 21. Richards M, Wong A, Foeller P, Bradley D, Tychsen L. Duration of binocular decorrelation predicts the severity of latent (fusion maldevelopment) nystagmus in strabismic macaque monkeys. Invest Ophthalmol Vis Sci. 2008; 49: 1872–1878 [DOI] [PubMed] [Google Scholar]
- 22. Fawcett SL, Wang YZ, Birch EE. The critical period for susceptibility of human stereopsis. Invest Ophthalmol Vis Sci. 2005; 46: 521–525 [DOI] [PubMed] [Google Scholar]
- 23. Fawcett S, Leffler J, Birch EE. Factors influencing stereoacuity in accommodative esotropia. J AAPOS. 2000; 4: 15–20 [DOI] [PubMed] [Google Scholar]
- 24. Fawcett SL, Birch EE. Risk factors for abnormal binocular vision after successful alignment of accommodative esotropia. J AAPOS. 2003; 7: 256–262 [DOI] [PubMed] [Google Scholar]
- 25. Birch EE. Marshall Parks lecture. Binocular sensory outcomes in accommodative ET. J AAPOS. 2003; 7: 369–373 [DOI] [PubMed] [Google Scholar]
- 26. Hertle RW, Felius J, Yang D, Kaufman M. Eye muscle surgery for infantile nystagmus syndrome in the first two years of life. Clin Ophthalmol. 2009; 3: 615–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hertle RW. Does eye muscle surgery improve vision in patients with infantile nystagmus syndrome? Ophthalmology. 2009; 116: 1837–1838 [DOI] [PubMed] [Google Scholar]
- 28. Hertle RW. Nystagmus in infancy and childhood: characteristics and evidence for treatment. Am Orthoptic J. 2010; 60: 48–58 [DOI] [PubMed] [Google Scholar]