Abstract
Earlier diagnosis is a key aim in achieving improved outcomes for patients with cancer. Bone and soft tissue sarcomas represent approximately 1% of all malignant tumours. Delays in diagnosis are frequent both because of their rarity and because the clinical features are easily confused with other conditions. In 2000 advice on earlier diagnosis was widely publicised. This study investigates how two factors that may act as a proxy for delay in diagnosis have varied over a 25-year period and whether there is evidence of improvement. Data on symptom duration and tumour size were collected prospectively on all new sarcoma patients referred to an orthopaedic oncology unit over 25 years.
Data were available for 2,568 patients with primary bone sarcomas and 2,366 with soft tissue sarcomas. The mean sarcoma size at diagnosis was 10.7cm and 9.9cm respectively. The size of bone sarcomas had not changed over time but there had been a slight decrease in the size of soft tissue sarcomas (10.3cm before 2000 vs 9.6cm after 2000, p=0.03). The duration of symptoms reported by patients varied widely with a median of 16 weeks for bone sarcomas and 26 weeks for soft tissue sarcomas. The median duration of symptoms for bone sarcomas had actually increased since 2000 (16 weeks before vs 20 weeks after 2000, p<0.01). It remained unchanged for soft tissue sarcomas. These data show there is huge room for improvement in diagnosing bone and soft tissue sarcomas. New strategies are needed urgently.
Keywords: Sarcoma, Delayed diagnosis
Early diagnosis of cancer has been topical for many years and has recently become a political imperative.1,2 The reason for this is that earlier diagnosis should improve outcomes both in terms of local control and overall survival.3
Sarcomas are a relatively rare group of tumours that can be broadly categorised into soft tissue sarcomas, comprising approximately 1% of tumours in the UK and 2% of cancer deaths,4 and bone sarcomas, which, according to the figures from the Office of National Statistics, comprise 0.18% of all cancers and 0.21% of all cancer deaths in 2006.5,6 Although there are many histological subtypes of bone and soft tissue sarcomas, the general principles of treatment remain the same, with surgical excision accompanied sometimes by adjuvant or neoadjuvant chemotherapy and sometimes radiotherapy.
Surgical excision for sarcomas is usually attempted with a limb salvage technique, which is easier when local invasion is minimal and the tumour size is small.7–9 Patients who have small tumours or who are free of metastasis at diagnosis have an increased survival rate and the chance of survival is increased with more rapid diagnosis.10 The prognosis for any one individual is determined by a combination of many factors including the effectiveness of treatment and response to chemotherapy when it is used. Other prognostic factors include the grade, site and size of the tumour along with the age of the patient.11
Of all of these factors, size is the only one that can be varied significantly by earlier diagnosis. Earlier diagnosis should lead to smaller tumours at diagnosis that in turn should result in better prognosis and easier treatment.12
Guidelines designed to lead to earlier diagnosis of the most common cancers were produced in 2000 by the Department of Health (DH) and included simple guidance to try to lead to earlier diagnosis of bone and soft tissue sarcomas.13 This guidance was updated by the National Institute of Health and Clinical Excellence (NICE) in 2005 but with virtually identical advice for early diagnosis of bone and soft tissue sarcomas.14 In essence, both sets of guidance highlighted the need to refer anyone with non-mechanical bone pain or an x-ray suggestive of a bone tumour to a specialist centre. It was also recommended that any patient with a soft tissue lump that was either larger than 5cm, increasing in size, painful or deep to the fascia should be referred for further investigation to rule out malignancy.14
Previous studies into the management of sarcomas have examined both the average size and the duration of symptoms for soft tissue sarcomas as well as the duration of symptoms alone for bone sarcomas.15–18 However, these studies have not investigated what factors influence either the size of these tumours at presentation or whether there has been a change with time. We also wanted to investigate whether the DH guidance had led to any reduction in the size of the tumours or symptom duration of patients by the time of diagnosis.
Methods
A database was established in 1985 and since then data have been collected prospectively about patients with bone and soft tissue sarcomas, and, in addition, tumour and treatment factors for these patients. Data were analysed concerning the patients’ sex and age at diagnosis along with the duration of symptoms, tumour size at diagnosis and date of diagnosis as well as the final histological diagnosis and, specifically, whether the tumour was a bone or soft tissue sarcoma.
The tumour size was measured using computed tomography or magnetic resonance imaging at the time of diagnosis prior to treatment with chemotherapy, with the maximum dimension of the tumour in any one plane being used as the measurement. For patients who had undergone a previous inadvertent excision of a lump that was subsequently found to be a soft tissue sarcoma, the size of the tumour identified by the initial pathology report was used. Symptom duration was defined as the length of time the patient reported to have experienced symptoms prior to the date when he or she was first seen at our unit.
Data were collected for all patients with newly diagnosed bone and soft tissue sarcomas from 1985 to 2009. Patients were excluded if they presented with a local recurrence, if they had previously undergone definitive treatment elsewhere, if they had been referred for second opinions or if they did not have a sarcoma. The pathological diagnosis made at the time was used when subdividing different tumour categories. This means that some entities, such as malignant fibrous histiocytoma (MFH), are no longer ‘fashionable’.
The tumour size at diagnosis was found to follow a normal distribution so the arithmetic mean size was taken and a t-test was used to identify differences between different groups. Symptom duration prior to diagnosis was found to have a skewed distribution so median values and the interquartile range (IQR) were reported with a Mann—Whitney U test used for comparing groups. We compared tumour size and symptom duration before and after 1 January 2000 (around the time that the DH guidelines were produced), using that as a transition date. Other factors were then investigated to see how they affected both the size of the tumour at diagnosis and the duration of symptoms.
Results
Patients
A total of 4,934 patients were included in the analysis, of which 2,568 (52.0%) had bone sarcomas and 2,366 (48.0%) had soft tissue sarcomas. In the bone sarcoma group, 1,054 patients (41.0%) were female and the median age was 25, with 1,522 (59.3%) being seen before the transition date (1 January 2000) and 1,046 (40.7%) seen after. In the soft tissue sarcoma group, 1,009 (42.6%) were female and the median age was 57, with 976 (41.3%) seen before the transition date and 1,390 (58.7%) seen after. Overall, 3,475 patients (70.4%) had complete tumour size data and 4,099 (83.1%) had complete symptoms duration data, with 2,640 (53.5%) having both tumour size and symptoms duration recorded.
Tumour size
The mean tumour size for bone sarcomas diagnosed was 10.65cm (standard deviation [SD]: 5.47cm, range: 0.8—47.0cm, 25% of cases >13.5cm) and for soft tissue sarcomas it was 9.87cm (SD: 6.50, range: 0.3—54.0cm, 25% of cases >13cm) (t=-3.830, p=0.0002) (Figs 1 and 2). For bone sarcomas, there was no difference in the mean tumour size before and after the transition date in 2000 (10.65cm vs 10.66cm respectively, t=-0.017, p=0.99) (Fig 3). For soft tissue sarcomas, there was a small but statistically significant difference in the mean tumour size before and after the transition date (10.27cm vs 9.62cm respectively, t=2.163, p=0.03) (Fig 3).
Figure 1.
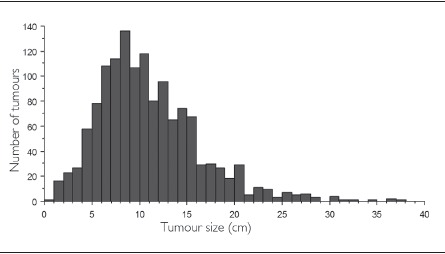
Distribution of tumour size at diagnosis for bone sarcomas
Figure 2.

Distribution of tumour size at diagnosis for soft tissue sarcomas
Figure 3.
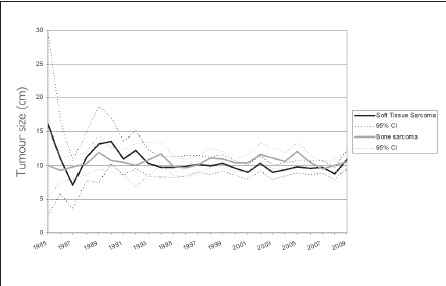
Mean tumour size at diagnosis for both bone and soft tissue sarcomas
Symptom duration
Symptom duration was found to be highly (left) skewed for both bone and soft tissue sarcomas. For bone sarcomas, the median symptom duration experienced by patients was 16 weeks (IQR: 32 weeks, 25% of patients >32 weeks). There was a significant increase in symptom duration before and after the transition date (p=0.01) with observed data showing an increase in median value (16 weeks; IQR: 28 weeks) to 20 weeks (IQR: 44 weeks) (Fig 4). For patients diagnosed since the transition date, 25% had a symptom duration of more than 52 weeks. The median symptom duration experienced for soft tissue sarcomas before and after the transition date was unchanged at 26 weeks (IQR: 40 weeks) (Fig 4). A quarter of patients (25%) diagnosed since the transition date had a symptom duration of more than 61 weeks.
Figure 4.
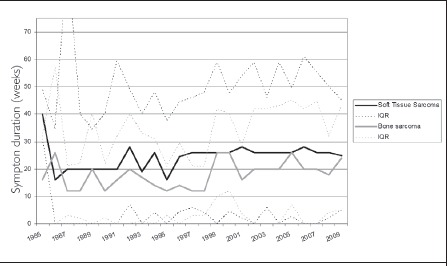
Median duration of symptoms experienced by patients with bone and soft tissue sarcomas
Effect of other factors
Sex: There was no difference in the symptom duration reported by men and women for either bone (p=0.154) or soft tissue sarcomas (p=0.416). There was, however, a slight difference in the size of sarcomas at presentation: women presented with tumours that were on average 0.7cm smaller than men (9.79cm vs 10.47cm, t=3.210, p=0.001) and this was true both for bone sarcomas (women 10.20cm, men 10.99cm, t=2.640, p=0.008) and soft tissue sarcomas (women 9.53cm, men 10.13cm, t=2.070, p=0.038).
Site: Patients with superficial soft tissue sarcomas (n=615, 26%) had considerably smaller tumours at diagnosis (6cm) than deeper ones (11.2cm) (p<0.0001) but had a longer median duration of symptoms (45 weeks vs 26 weeks respectively, p<0.0001).
Previous inadvertent excision: 589 patients (24.9%) with soft tissue sarcomas had a previous inadvertent excision. The mean tumour size was 5.8cm compared with 11.2cm for patients whose primary treatment was at our unit (t=20.920, p<0.0001). The median symptom duration in patients with a previous inadvertent excision was 52 weeks compared to 26 weeks for tumours treated primarily at our unit (p<0.0001).
When this was analysed further, it was revealed that, for the 615 patients with a superficial tumour, the mean tumour size of the 338 patients who had a previous inadvertent excision (55%) was 5cm compared to 7.9cm in the 277 patients (45%) who were treated primarily at our unit (p<0.0001). The median symptom duration in these patients was 52 weeks compared to 32 weeks for tumours treated primarily at our unit (p<0.0001).
For the 1,751 patients with a deep sarcoma at the time of diagnosis, only 262 (15%) had a previous inadvertent excision. The mean size of their tumours was 7cm compared with 12cm in the 1,481 patients (85%) who were treated primarily at our unit (p<0.0001). The median symptom duration in these patients was 38 weeks compared with 20 weeks for patients with deep tumours treated primarily at our unit (p<0.0001).
With the exception of apparently random perturbations in the data in 1987 and 2002, the proportion of patients referred to our unit who had had a previous inadvertent excision remained relatively constant over the 25 years of the study, averaging 25%. Similarly, there was no significant change over time in the mean size of the lumps that were removed before they were discovered to be sarcomas (Fig 5).
Figure 5.
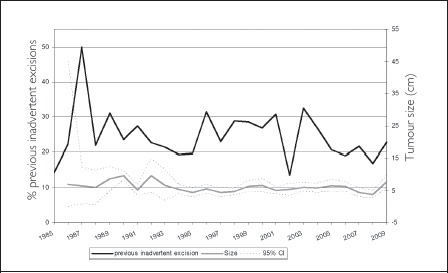
Previous inadvertent excisions (proportion of workload and mean size) over time
Diagnosis: For bone tumours, the mean size of the four most common tumours was: osteosarcoma 10.7cm (SD: 5.19), chondrosarcoma 11.3cm (SD: 6.44), Ewing sarcoma 9.9cm (SD: 5.12), spindle cell sarcoma 10.4cm (SD: 4.7). For soft tissue sarcomas the mean size of the five most common tumours were: liposarcoma 13.3cm (SD: 7.38), leiomyosarcoma 7.7cm (SD: 5.63), MFH 10.3cm (SD: 6.84), synovial sarcoma 6.9cm (SD: 4.8), malignant peripheral nerve sheath tumour 10.5cm (SD: 6.17).
The median duration of symptoms also varied with diagnosis: osteosarcoma 12 weeks, chondrosarcoma 52 weeks, Ewing sarcoma 20 weeks, spindle cell sarcoma 20 weeks. For soft tissue sarcomas, the median duration of symptoms was: liposarcoma 28 weeks, leiomyosarcoma 26 weeks, MFH 20 weeks, synovial sarcoma 52 weeks, malignant peripheral nerve sheath tumour 26 weeks. The most common diagnosis was osteosarcoma (n=1,100). There was no identifiable difference in either size at diagnosis or duration of symptoms for osteosarcomas when comparing the groups diagnosed before and after the transition date: the mean size at diagnosis of the osteosarcomas changed from 10.5cm to 11.1cm (p=0.15) and the median symptom duration changed from 10 to 12 weeks (p=0.1) respectively.
Location: The location of the tumours was split into four categories (upper limb, lower limb, trunk and pelvis) for both bone and soft tissue sarcomas and the mean size and median duration of symptoms are shown in Table 1.
Table 1.
Mean size and median symptom duration split by site and tumour category
| Upper limb | Lower limb | Trunk | Pelvis | |
| Size | ||||
| Soft tissue sarcomas | 7.7cm | 10.5cm | 8.4cm | 11.3cm |
| Bone sarcomas | 10.5cm | 10.6cm | 9.0cm | 11.5cm |
| Symptom duration | ||||
| Soft tissue sarcomas | 26 weeks | 26 weeks | 20 weeks | 28 weeks |
| Bone sarcomas | 12 weeks | 12 weeks | 26 weeks | 29 weeks |
Age: For soft tissue sarcomas, there was a steady increase in the size of the tumours with increasing age at diagnosis but this was less apparent for bone sarcomas (Table 2). The median duration of symptoms prior to diagnosis was least for both bone and soft tissue sarcomas in children, followed by teenagers (Table 2). The longest median delay to diagnosis was in middle age but became less in the older patient groups. There was no correlation identified at all between symptom duration and size of tumour at diagnosis for any diagnostic group or age group.
Table 2.
Mean size and median symptom duration split by age group
| Age group (years) | <10 | 10—19 | 20—29 | 30—39 | 40—49 | 50—59 | 60—69 | 70—79 | 80+ |
| Size | |||||||||
| Soft tissue sarcomas | 4.2cm | 7.4cm | 8.2cm | 8.9cm | 10.0cm | 10.5cm | 10.3cm | 10.3cm | 11.1cm |
| Bone sarcomas | 9.8cm | 10.5cm | 10.1cm | 10.1cm | 9.6cm | 11.6cm | 10.6cm | 11.9cm | 11.6cm |
| Symptom duration | |||||||||
| Soft tissue sarcomas | 8 weeks | 16 weeks | 26 weeks | 26 weeks | 26 weeks | 26 weeks | 26 weeks | 20 weeks | 26 weeks |
| Bone sarcomas | 7 weeks | 12 weeks | 20 weeks | 26 weeks | 36 weeks | 36 weeks | 32 weeks | 28 weeks | 26 weeks |
Discussion
The data analysed here represent the largest collection of data on both size and symptom duration for bone and soft tissue sarcomas that has been reported to date. The data were collected prospectively from 1985 onwards for all new patients referred to our unit. The data on size have been recorded consistently for the past 25 years as being the largest diameter of the tumour as measured either at the time of resection or, if that was not available or the patient had undergone neoadjuvant therapy, from imaging at the time of diagnosis. The data for recorded tumour size are likely to be as reliable as can be achieved.
Missing data mostly related to cases where there had been a previous inadvertent excision and the size of the tumour had not been recorded. In those cases (about 15%) we used the size reported by the pathologist, which is likely, if anything, to be an underestimate of the size as many excisions were incomplete.
The data on symptom duration are very much reliant on patient recall and based on patients’ responses to the query: ‘How long have you had any symptoms from this tumour?’ The responses ranged from one day (eg a patient admitted with a pathological fracture through an osteosarcoma and no preceding symptoms) to twenty years (a patient who had been aware of a lump on her shin that eventually started to itch and she requested its removal; the lump turned out to be a synovial sarcoma). In this study we have made no attempt to differentiate between causes of the long duration of symptoms in some patients or to investigate patient or doctor delay prior to diagnosis as this has been done previously.19
It is possible that the slight increase in symptom duration for patients with bone tumours is due to a change in reporting behaviours by patients, be it exaggerating symptom duration now or underestimating in the past. A limitation of using symptom duration as a measure of changing medical practice is that it is subjective and vulnerable to significant recall bias, an effect that is amplified as length of history increases. However, a previous study investigating delays in referral of soft tissue sarcomas showed that patient recall of their journey to referral when compared with dates of clinical notes showed no significant difference.19
There was remarkable homogeneity of tumour size across this group of sarcomas, with bone sarcomas being slightly larger than soft tissue sarcomas. There was, however, a significant difference in size between different sarcoma types, especially for soft tissue sarcomas. The largest tumours were liposarcomas (mean: 13.3cm) while the smallest were epithelioid sarcomas (mean: 3cm). The reasons for these differences in size are multiple but in general the larger tumours tended to be the slower growing, deep ones (eg liposarcomas and chondrosarcomas) while the smaller tumours tended to be those that were likely to be near the surface (eg epithelioid sarcomas typically arise in the hand or foot in a superficial location).
It appears that, for bone sarcomas, the publication of guidelines13,14 has neither reduced the tumour size nor the duration of symptoms at diagnosis. The mean size of soft tissue sarcomas does seem to have slightly decreased although the median symptom duration has increased. The reasons for this are not clear but a previous study suggests delays at every level of referral from initial symptoms to sarcoma centre, part of this delay being due to patient factors, part being due to general practitioners (GPs) and part at referring hospitals.19 This study also showed that obesity did not correlate with larger size at diagnosis.
It is also probable that some of this decrease in size may have been due to changes in referral practice as the number of soft tissue sarcomas referred directly to the unit has increased with time. Since 2000 all patients with a potential cancer could be referred urgently to hospital under a two-week wait rule to expedite diagnosis and treatment.20 Three papers have now reviewed experience of this for patients with sarcomas and showed disappointingly that this does not seem to either speed up the process of diagnosis or reduce the size of the tumours at diagnosis.21–23 The fact that the proportion of patients seen at the unit who had undergone a previous inadvertent excision has not changed is also depressing, as is the fact that the average size of these tumours has reduced. Surgeons are therefore still removing lumps without knowing what they are even though half of them are bigger than 5cm.
As a result of NICE guidance in 2006, which recommended that all patients with a sarcoma should be referred to a specialist sarcoma unit,24 many small soft tissue sarcomas were referred that previously may have been treated at local centres. This may be an explanation for the decrease in size since that time.
This study of almost 5,000 patients does not show a convincing improvement either in the tumour size or the duration of symptoms reported over a 25-year period. This is despite moves designed especially to encourage earlier diagnosis of patients with cancer and specific guidance for patients with potential sarcomas. The problem, of course, is that sarcomas are rare and most GPs will not see a single bone sarcoma in their working lifetime although most will see at least one soft tissue sarcoma. Identifying the potentially malignant soft tissue lump compared with the large number of benign ones is a continuing problem and one that does not seem to have been addressed successfully by either the guidance from 2000 or 2005.13,14
Other countries do, however, seem to have been more successful in diagnosing tumours earlier. In Scandinavia the average size of soft tissue sarcomas at diagnosis has decreased to 7cm and in Italy it is 6cm.25,26 These figures come from similar specialist centres to our own and it is likely that there will have been similar referral patterns.
Conclusions
This study has confirmed some of the factors that are associated with the size of bone and soft tissue sarcomas at diagnosis and it has also highlighted the long duration of symptoms experienced by many patients. It has shown that while there has been a small decrease in size for soft tissue sarcomas with time, this has not been found for bone tumours.
Future work should try to identify more clearly what factors led to delays in diagnosis and clarify what symptoms patients experience early in their disease. It should also explore the possibility of both patient and doctor education programmes and awareness campaigns to attempt to improve the delays documented in this study. The senior author has previously suggested that the size of a golf ball (42mm) should be used as a reference to raise suspicion of a possible sarcoma.10 We suggest the following motto, which should be more widely advertised: Any lump bigger than a golf ball must have a diagnosis (Fig 6).
Figure 6.
A golf ball (42mm) is suggested as a suitable object to raise awareness of a possible soft tissue sarcoma
References
- 1.Department of Health. The NHS Cancer Plan. London: DH; 2000. [Google Scholar]
- 2. David Cameron claims cancer measures could save 3,000 lives a year. The Guardian. 2010 October 3.
- 3. Why is early diagnosis important? Cancer Research UK. http://info.cancerresearchuk.org/spotcancerearly/cancersignandsymptoms/whyisearlydiagnosisimportant (cited June 2011).
- 4.Tukiainen E, Böhling T, Huuhtanen R. Soft tissue sarcoma of the trunk and extremities. Scand J Surg. 2003;92:257–263. doi: 10.1177/145749690309200404. [DOI] [PubMed] [Google Scholar]
- 5.Office for National Statistics. Cancer Registrations in England, 2006. Newport: ONS; 2008. [Google Scholar]
- 6.Office for National Statistics. Mortality Statistics: Deaths Registered in 2006. Newport: ONS; 2008. [Google Scholar]
- 7.Widhe B, Widhe T. Initial symptoms and clinical features in osteosarcoma and Ewing sarcoma. J Bone Joint Surg Am. 2000;82:667–674. doi: 10.2106/00004623-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 9.Wafa H, Grimer RJ. Surgical options and outcomes in bone sarcoma. Expert Rev Anticancer Ther. 2006;6:239–248. doi: 10.1586/14737140.6.2.239. [DOI] [PubMed] [Google Scholar]
- 10.Grimer RJ. Size matters for sarcomas! Ann R Coll Surg Engl. 2006;88:519–524. doi: 10.1308/003588406X130651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pisters PW, Leung DH, Woodruff J, et al. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1,679–1,689. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 12.Grimer RJ, Briggs TW. Earlier diagnosis of bone and soft-tissue tumours. J Bone Joint Surg Br. 2010;92:1,489–1,492. doi: 10.1302/0301-620X.92B11.24326. [DOI] [PubMed] [Google Scholar]
- 13.Department of Health. Referral Guidelines for Suspected Cancer. London: DH; 2000. [Google Scholar]
- 14.National Institute for Health and Clinical Excellence. Referral Guidelines for Suspected Cancer. London: NICE; 2005. [Google Scholar]
- 15.Brouns F, Stas M, De Wever I. Delay in diagnosis of soft tissue sarcomas. Eur J Surg Oncol. 2003;29:440–445. doi: 10.1016/s0748-7983(03)00006-4. [DOI] [PubMed] [Google Scholar]
- 16.Clark MA, Thomas JM. Delay in referral to a specialist soft-tissue sarcoma unit. Eur J Surg Oncol. 2005;31:443–448. doi: 10.1016/j.ejso.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Hussein R, Smith MA. Soft tissue sarcomas: are current referral guidelines sufficient? Ann R Coll Surg Engl. 2005;87:171–173. doi: 10.1308/1478708051658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glencross J, Balasubramanian SP, Bacon J, et al. An audit of the management of soft tissue sarcoma within a health region in the UK. Eur J Surg Oncol. 2003;29:670–675. doi: 10.1016/s0748-7983(03)00134-3. [DOI] [PubMed] [Google Scholar]
- 19.Johnson GD, Smith G, Dramis A, Grimer RJ. Delays in referral of soft tissue sarcomas. Sarcoma. 2008 doi: 10.1155/2008/378574. 378574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Department of Health. The New NHS: Modern, Dependable. London: DH; 1997. [Google Scholar]
- 21.Collin T, Blackburn AV, Milner RH, et al. Sarcomas in the groin and inguinal canal — often missed and difficult to manage. Ann R Coll Surg Engl. 2010;92:326–329. doi: 10.1308/003588410X12628812460056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pencavel TD, Strauss DC, Thomas GP, et al. Does the two-week rule pathway improve the diagnosis of soft tissue sarcoma? A retrospective review of referral patterns and outcomes over five years in a regional sarcoma centre. Ann R Coll Surg Engl. 2010;92:417–421. doi: 10.1308/003588410X12664192075972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor WS, Grimer RJ, Carter SR, et al. ‘Two week waits’ — are they leading to earlier diagnosis of soft-tissue sarcomas? Sarcoma. 2010 doi: 10.1155/2010/312648. 312648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Institute for Health and Clinical Excellence. Improving Outcomes for Patients with Sarcomas. London: NICE; 2006. [Google Scholar]
- 25.Bauer HC, Trovik CS, Alvegård TA, et al. Monitoring referral and treatment in soft tissue sarcoma: study based on 1,851 patients from the Scandinavian Sarcoma Group Register. Acta Orthop Scand. 2001;72:150–159. doi: 10.1080/000164701317323408. [DOI] [PubMed] [Google Scholar]
- 26.Gronchi A, Lo Vullo S, Colombo C, et al. Extremity soft tissue sarcoma in a series of patients treated at a single institution: local control directly impacts survival. Ann Surg. 2010;251:506–511. doi: 10.1097/SLA.0b013e3181cf87fa. [DOI] [PubMed] [Google Scholar]


