Abstract
Background
It has been demonstrated that N-ethyl-lidocaine (QX-314) can target the transient receptor protein vanilloid 1 (TRPV1) nociceptors when coadministered with capsaicin, resulting in a selective block of the nociceptors. Capsaicin is problematic in therapeutic use because it induces firing of nociceptors. The present study aimed to search for substitutes for capsaicin. We also examined the transportability of QX-314 into nociceptive neurons, through the pores of transient receptor potential ankyrin 1 (TRPA1), transient receptor potential melastatin-8 (TRPM8), and TRPV1.
Methods
To investigate the effect on TRPA1, injections of a vehicle, allyl isothiocyanate (AITC), QX-314, or AITC/QX-314 were made into the hind paws of rats. The effects of menthol and capsaicin on the opening of TRPM8 and TRPV1 were also examined and compared with the potency of QX-314. To examine inhibition of the antinociceptive effect by capsaicin/ QX-314, capsazepine (50 μg/mL; 10 μL) was injected 30 minutes prior to capsaicin/QX-314 (10 μL) injection. Thermal sensitivity was investigated by the Hargreaves method. 5(6)-carboxyfluorescein (FAM)-conjugated QX-314 was used as a tracer to examine how many and which kind of dorsal root ganglia accumulate this molecule. QX-314-FAM, capsaicin/QX-314-FAM, AITC/QX-314-FAM, and menthol/QX-314-FAM were injected into the paw. Two weeks after injections, dorsal root ganglia were removed and sectioned with a cryostat.
Results
The capsaicin/QX-314 group induced longer withdrawal-response latency at 60 to 300 minutes after injection than the control. Both menthol only and menthol/QX-314 injections showed analgesia 10 to 60 minutes after injection. No significant difference was seen between the capsazepine/capsaicin/QX-314 group and the vehicle group. The fluorescence in small- and medium-sized neurons was conspicuous in only the dorsal root ganglia injected with capsaicin/ QX-314-FAM.
Conclusion
These results indicate that TRPA1 and TRPM8 are ineffective in the transport of QX-314 compared with TRPV1.
Keywords: anesthetics, capsaicin, AITC, menthol, capsazepine, behavioral tests
Introduction
Local anesthetics are drugs that produce reversible inhibition of nerve conduction when applied to the peripheral nerve fiber. These work by blocking the voltage-gated sodium channel, which results in loss of pain or hypoalgesia in the applied area.1 Lidocaine, an anesthetic widely used clinically, is a tertiary amine that exists in a mixture of protonated and uncharged base forms under physiological conditions.2 The uncharged hydrophobic form of lidocaine can penetrate the membrane of all neurons so that in addition to blocking pain signals, it produces numbness due to blocking of low-threshold sensory nerves, deficits in motor function, and blocking of autonomic nerves.
Binshtok et al3 devised a new application for permanently charged sodium channel blockers such as N-ethyl-lidocaine (QX-314), a lipophobic lidocaine derivative. QX-314 was found to block the sodium channel when introduced directly into the cytoplasm of the cell but not when applied extracellularly in a standard local anesthetic method4,5 because it failed to penetrate the membrane (owing to its lipophobic nature). Subsequently, it was shown that QX-314 could selectively hit the nociceptor target when coadministered with capsaicin, leading to the preferential block of the sodium channels associated with the inhibition of excitability in nociceptors.3,6 The pore size of the transient receptor protein vanilloid 1 (TRPV1) channels that were opened by an agonist, capsaicin, permitted delivery of QX-314 into nociceptive neurons. As a consequence, the new method produced a selective nociception block, with loss of numbness and motor paralysis. However, capsaicin evoked pain when coinjected with Qx-314, therefore, TRP channel agonists with less pungent or burning characteristics would be better to use with QX-314. In Binshtok et al and Roberson et al, it was reported that lidocaine could substitute for capsaicin to introduce QX-314 into nociceptors through TRPV1 channels because TRPV1 channels are also activated by lidocaine.7,8 However, a fault with lidocaine is that it blocks the excitability of all neurons, not just the sensory neurons.
This study aimed first to find substances apart from lidocaine, already reported by Binshtok,3 to use in place of capsaicin to adapt drug delivery in the clinical setting. Then, we examined the ability of allyl isothiocyanate (AITC) and menthol to deliver QX-314 into nociceptive neurons through the pores of both transient receptor potential ankyrin 1 (TRPA1) and transient receptor potential melastatin-8 (TRPM8), and examined whether they produced an antinociceptive effect or not.
Materials and methods
Animals
Fifty-four male Wistar rats (120–350 g) were used for behavioral studies, and eight rats were used for histochemical studies. The animals were housed and given rodent feed and water ad libitum. Behavioral studies were conducted at approximately the same time each day to reduce circadian effects. The study protocol was approved by the Tokushima University Care and Use of Animals Committee.
Application of chemicals
Drugs
Capsaicin (Nacalai Tesque Inc, Kyoto, Japan), AITC ([a component of mustard oil] Polysciences Inc, Warrington, PA, USA), menthol ([a mint essential oil] Enzo Life Sciences Inc, Farmingdale, NY, USA), and capsazepine (Cayman Chemical Co, Ann Arbor, MI, USA) were freshly prepared with a vehicle comprised of 10% ethanol, 10% Tween® 80, and 80% normal saline. QX-314 (Enzo Life Sciences Inc) was dissolved in physiological saline. QX-314 coupled with 5(6)-carboxyfluorescein (FAM) was used for histochemical methods (Figure 1).
Figure 1.
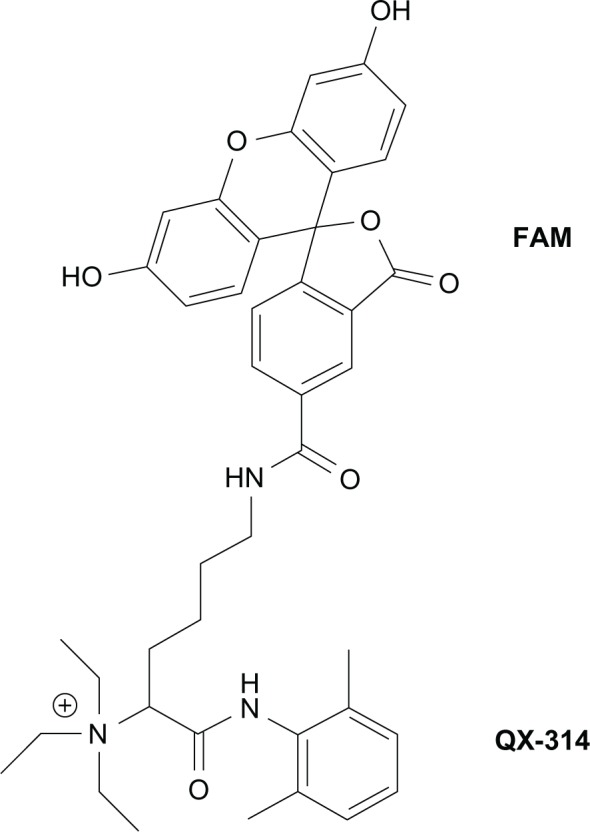
Chemical structure of FAM-conjugated QX-314 (molecular mass, 692 Da).
Abbreviations: FAM, 5(6)-carboxyfluorescein; QX-314, N-ethyl-lidocaine.
Intraplantar injection with agonist (behavioral tests) To detect the effect on the TRPA1 channel, injections (10 μL in each group) of 5% AITC only (AITC group), 2% QX-314 only (QX-314 group), a mixture of AITC and QX-314 (AITC/QX-314 group), and the vehicle alone (vehicle group), were made into the rat right plantar hind paws (TRPA1 experiment). Similarly, 10 μL of 5% menthol and 0.1% capsaicin were injected into right plantar hind paws, to detect their effect on TRPM8 and TRPV1 (TRPM8 and TRPV1 experiments). For each experimental group, six animals were used. The data from the QX-314 and vehicle groups were used in all the experiments. The concentration of drugs used in our study was based on the Binshtok et al3 or Chen protcols.9
Intraplantar injection with TRPV1 antagonist (behavioral tests)
Capsazepine is a specific, competitive capsaicin receptor antagonist.10 To examine the inhibition of the antinociceptive effect of capsaicin/QX-314, capsazepine (50 μg/mL; 10 μL) was injected 30 minutes prior to capsaicin/QX-314 (10 μL) injection (capsazepine/capsaicin/QX-314 group) in the right plantar hind paw. The data of the capsaicin/QX-314 group from the TRPV1 experiment were used as the comparison. The concentration of capsazepine and time of administration used in this study was based on Kwak et al’s experiment.11
Intraplantar injection (histochemical studies)
FAM-conjugated QX-314 (QX-314-FAM) (Toray Research Center Inc, Tokyo, Japan) was used as the tracer to examine whether QX-314 could pass through TRP channels. A mixture of either 5% AITC and 0.5% QX-314-FAM (50 μL), a mixture of menthol (5%) and 0.5% QX-314-FAM (50 μL), or a mixture of capsaicin (0.1%) and 0.5% QX-314-FAM (50 μL) were injected into rat right plantar hind paws to confirm the opening of TRPA1, TRPM8, and TRPV1 channels, respectively. As a control, an injection of 0.5% QX-314-FAM (50 μL) only was made into rat right plantar hind paws. The dose of 50 μL was injected for the histochemical study for assurance of sufficient amount of the tracer for detection. We qualitatively examined the number and size of cells that took up the tracer.
Behavioral tests
Hind paw withdrawal to noxious heat (Hargreaves method)
Thermal sensitivity was investigated by exposing the hind paws to a defined radiant heat stimulus through a transparent perspex surface (Plantar Test; Ugo Basile Srl, Comerio, Italy). The paw-withdrawal latencies were recorded.12 The intensity of the thermal stimulation was adjusted to 50, according to our previous study.13 A cut-off time of 20 seconds was set, to avoid tissue damage. Each rat received two consecutive stimuli, and the interstimulus interval for each trial was at least 2 minutes Before the drug injection, baseline withdrawal-response latencies were determined for all of the animals. The withdrawal-response latencies were measured at 10, 30, 60, 120, 180, 240, and 300 minutes after the drug injections. The experimenter was blind to the treatment group, in all behavioral tests.
Data analysis
The experimental data were analyzed with repeated measures analysis of variance (ANOVA), followed by the least significant difference (LSD) post hoc test. Results were expressed as the mean + standard error (SE). P < 0.05 was considered to be significantly different.
Histochemical methods
After injection with a either a mixture of AITC and QX-314-FAM, a mixture of menthol and QX-314-FAM, a mixture of capsaicin and QX-314-FAM, or only QX-314-FAM, the rats were housed in their cages for 2 weeks to allow an ample labeling of dorsal root ganglion (DRG) neurons by QX-314-FAM. Then, the rats were anesthetized and perfused transcardially with saline followed by 4% paraformaldehyde in phosphate-buffered saline (PBS) (pH 7.4). After perfusion, right dorsal root ganglia (L4-6) were collected postfixed in the same fixative solution (4°C, 2 hours), and immersed overnight in PBS containing 30% sucrose. The ganglia were cut into 30 μm serial sections on a cryostat and embedded on glass slides with 1% propyl gallate. DRG cells in every frozen section were observed under a fluorescent microscope. Those cells entirely showing a marked green fluorescence were confirmed to have incorporated the tracer. The fluorescently-labeled neurons were counted and the diameter of the neurons with nuclei were measured to know their size distribution.
Results
Behavioral tests
Effect on TRPA1 channel opening
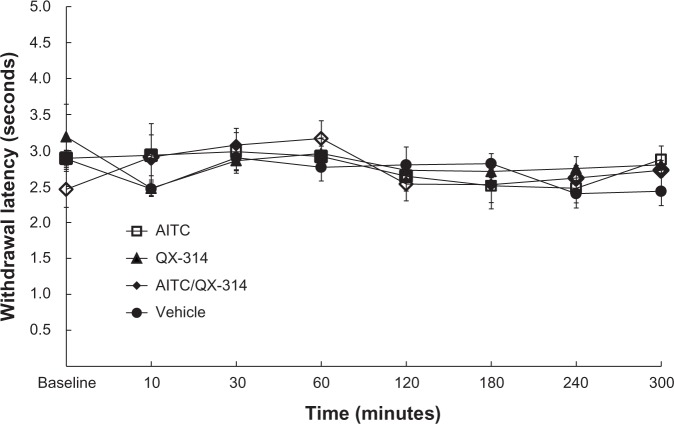
The experimental groups showed no significant difference compared with the vehicle group (Figure 2).
Figure 2.
Effect on thermal sensitivity in TRPA1 channels opening.
Notes: injections (10 μL in each group) of only 5% AITC (AITC group), only 2% QX-314 (QX-314 group), a mixture of AITC and QX-314 (AITC/QX-314 group), or the vehicle (vehicle group) were made into rat right plantar hind paws. The experimental groups were not significantly different from the vehicle group.
Abbreviations: AITC, allyl isothiocyanate; QX-314, N-ethyl-lidocaine; TRPAI, transient receptor potential ankyrin I.
Effect on TRPM8 channel opening
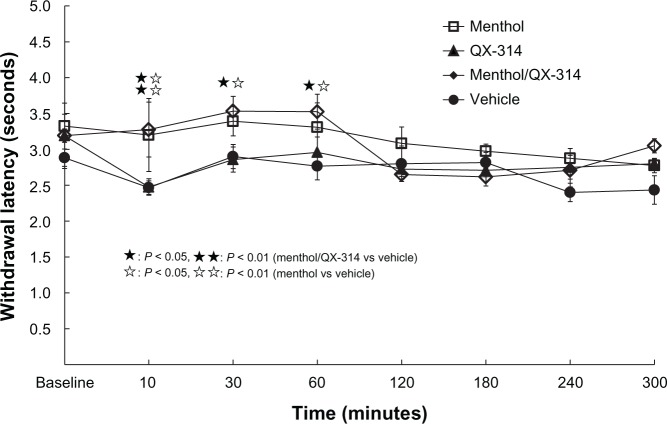
The menthol/QX-314 group showed analgesia compared with vehicle group at 10 to 60 minutes after injection (P < 0.01). Similarly, the menthol group showed analgesia compared with vehicle group, at 10 to 60 minutes after injection (P < 0.01). No significant difference was seen between the menthol group and menthol/QX-314 group (Figure 3).
Figure 3.
Effect on thermal sensitivity in TRPM8 channels opening.
Notes: Injections (10 μL in each group) of only 5% menthol (menthol group), only 2% QX-314 (QX-314 group), a mixture of menthol and QX-314 (menthol/QX-314 group), or the vehicle (vehicle group), were made into rat right plantar hind paws. The menthol/QX-314 group showed more analgesia than the vehicle group at 10 to 60 minutes (P < 0.01). The menthol group showed more analgesia than the vehicle group at 10 to 60 minutes (P < 0.01). No significant difference was seen between the menthol group and the menthol/QX-314 group. The results indicate the independent analgesic effect of menthol.
Abbreviations: QX-314, N-ethyl-lidocaine; TRPM8, transient receptor potential melastatin-8.
Effects on TRPV1 channel opening
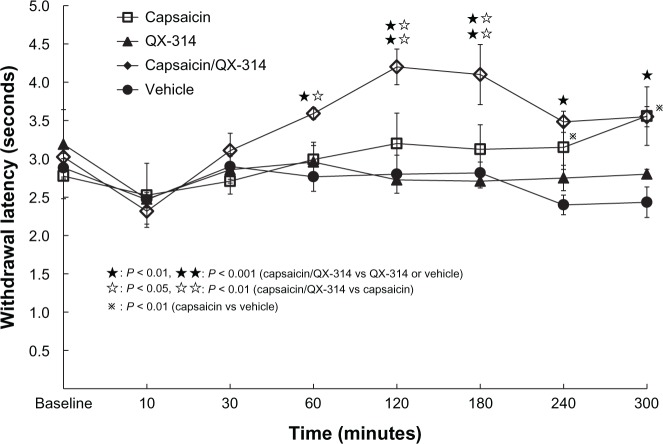
The capsaicin/QX-314 group showed a longer withdrawal latency (analgesia) than either the QX-314 group or vehicle group at 60 to 300 minutes after injection (P < 0.01 and P < 0.001, respectively). The capsaicin/QX-314 group showed analgesia compared with the capsaicin group, at 60 to 180 minutes after injection (P < 0.05, P < 0.01). Capsaicin group also showed analgesia compared with QX-314 group or vehicle group, at 240 to 300 minutes after injection (P < 0.01). No significant difference can be seen between QX-314 group and vehicle group (Figure 4).
Figure 4.
Effect on thermal sensitivity in TRPV1 channels opening.
Notes: Injections (10 μL in each group) of only 0.1% capsaicin (capsaicin group), only 2% QX-314 (QX-314 group), a mixture of capsaicin and QX-314 (capsaicin/QX-314 group), or the vehicle (vehicle group), were made into rat right plantar hind paws. The capsaicin/QX-314 group showed longer withdrawal latency than the QX-314 group or the vehicle group at 60 to 300 minutes (P < 0.01, and P < 0.001, respectively). The capsaicin/QX-314 group showed analgesia compared with the capsaicin group at 60 to 180 minutes (P < 0.05, and P < 0.01, respectively). The capsaicin group showed analgesia compared with vehicle group at 240 to 300 minutes (P < 0.01). The QX-314 group did not differ significantly from the vehicle group.
Abbreviations: QX-314, N-ethyl-lidocaine; TRPV1, transient receptor potential ankyrin.
Inhibition of the antinociceptive effect of capsaicin/QX-314 by capsazepine
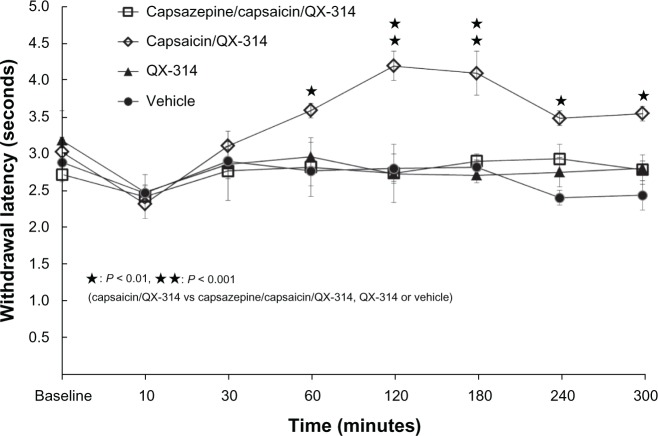
Capsaicin/QX-314 group showed analgesia compared with the capsazepine/capsaicin/QX-314 group, 60 to 300 minutes after injection (P < 0.01 and P < 0.001, respectively). No significant difference was seen between the capsazepine/ capsaicin/QX-314 group and the vehicle group (Figure 5).
Figure 5.
Inhibition of the antinociceptive effect of capsaicin/QX-314 by capsazepine.
Notes: Capsazepine (50 μg/mL; 10 μL) was injected 30 minutes prior to the capsaicin/QX-314 (10 μL) injection (capsazepine/capsaicin/QX-314 group) into rat right plantar hind paws. The capsaicin/QX-314 group showed a longer withdrawal latency than the capsazepine/capsaicin/QX-314 group, the QX-314 group, or the vehicle group at 60 to 300 minutes (P < 0.01, P < 0.001). The capsazepine/capsaicin/QX-314 group did not differ significantly from the vehicle group.
Abbreviation: QX-314, N-ethyl-lidocaine.
Effects on TRPA1, TRPM8, and TRPV1 channel opening (histochemical studies)
The cell sizes of DRG were divided into three diameter ranges: small (<30 μm), medium (30–50 μm), and large (>50 μm).14 The fluorescence in the small- and middle-sized neurons was conspicuous in the DRGs injected with capsaicin/QX-314-FAM, accounting for 40.7% and 54.2% of all labeled neurons, respectively (Figure 6A and Table 1). Fluorescence was not seen in DRGs injected with only QX-314-FAM, AITC/QX-314-FAM, or menthol/QX-314-FAM (Figure 6B–D).
Figure 6.
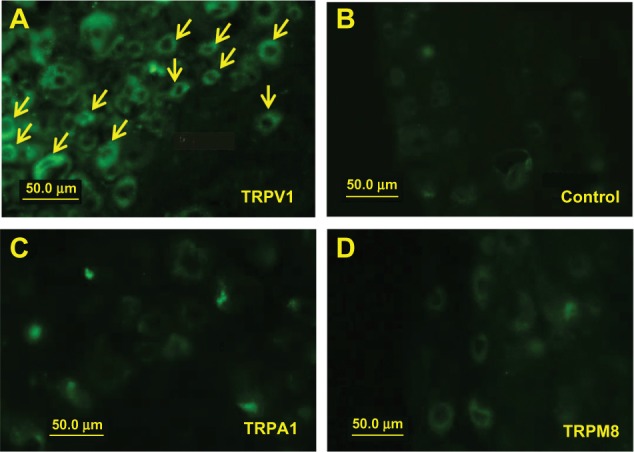
Effect on uptake of the fluorescence tracer (FAM). The fluorescence in small neurons (arrows) was conspicuous in DRGs injected with capsaicin and QX-314-FAM (A). Fluorescence was not detected in DRGs injected with only QX-314-FAM (B), AITC/QX-314-FAM (C), and menthol/QX-314-FAM (D).
Abbreviations: AIT, allyl isothiocyanate; DRG, dorsal root ganglia; FAM, 5(6)-carboxyfluorescein; QX-314, N-ethyl-lidocaine; TRPA1, transient receptor potential ankyrin 1; TRPM8, transient receptor potential melastatin-8; TRPV1, transient receptor protein vanilloid 1.
Table 1.
The size distribution of fluorescently labeled neurons in DRGs injected with capsaicin and fluorescent QX-314
| Size | Diameter (μm) | Number | % |
|---|---|---|---|
| Small | 16–20 | 7 | 40.7 |
| 21–25 | 6 | ||
| 26–30 | 11 | ||
| Medium | 31–35 | 10 | 54.2 |
| 36–40 | 6 | ||
| 41–45 | 8 | ||
| 46–50 | 8 | ||
| Large | 51–55 | 1 | 5.1 |
| 56–60 | 2 |
Abbreviations: DRG, dorsal root ganglia; QX-314, N-ethyl-lidocaine.
Discussion
Behavioral tests
Effect on TRPA1 channel opening
TRPA1 is also known to be expressed in a subset of sensory neurons and to be activated by noxious cold, AITC, cinnamon, and icilin (a synthetic cooling compound).15 We used AITC which is known as the most potent TRPA1 agonist.9 The experimental groups were not significantly different from those in the vehicle group. Chen et al9 showed that AITC-induced YO-PRO® (a fluorescent dye that binds to nucleic acids; Life Technologies, Carlsbad, CA, USA) is taken up into TRPA1-positive cells. Accordingly, TRPA1 pores could be expected to mediate the entry of QX-314 into TRPA1-positive neurons. There are two possible reasons why TRPA1 could not induce analgesia in our experiment. Park et al16 reported that 75% of primary afferent dental neurons (<24 μm) expressed TRPV1, 20% expressed TRPA1, and 20% coexpressed both TRPV1 and TRPA1; probably, a lower level of TRPA1 expression in the noxious heat-sensitive polymodal nociceptors did not allow sufficient entry of QX-314 into the nociceptive neurons. The other possible reason is that we used noxious thermal stimuli, although TRPA1 is activated by noxious cold; TRPA1 is probably insensitive to noxious heat. Further study will be necessary to confirm whether the coapplication of QX-314 and AITC can induce analgesia under noxious cold stimulation.
Effect on TRPM8 channel opening
TRPM8 channels are activated by low temperatures (threshold: 25°C) and by exposure to cooling compounds, such as menthol.17,18 These channels are expressed selectively in sensory neurons of the DRG and the trigeminal ganglion.19,20 In the present study, we used menthol as the most convenient agonist of TRPM8. The menthol/QX-314, as well as the menthol group, showed a greater degree of analgesia than did the vehicle group; on the other hand, no significant difference was seen between the menthol only group and the menthol/QX-314 group. These results indicate that menthol could independently induce analgesia. This is consistent with previous reports that showed thermal-pain suppression by menthol.21–26 In addition, the present results indicated that QX-314 cannot pass through the pores of TRPM8. This may be consistent with the results of Chen et al’s study,9 which showed that YO-PRO could not be taken up through TRPM8 following activation by menthol only.
Effect on TRPV1 channel opening
TRPV1 is expressed in a subset of sensory neurons and activated by noxious heat, capsaicin, and protons.27–29 In our experiments, the thermal latencies in the capsaicin/QX-314 group were significantly different from those in the capsaicin group, QX-314 group, and vehicle group. These results are consistent with the Binshtok et al report.3 QX-314 seemed to penetrate directly through TRPV1 channel pores when it was administered with capsaicin. The TRPV1 channel pores appear to be large enough to allow the passage of compounds smaller than styryl pyridinium dye (FM1-43, molecular mass: 452Da).30,31 Accordingly, QX-314 (molecular mass: 263Da) is thought to easily pass through the pores of TRPV1. However, the onset time of local anesthesia induced by capsaicin/QX-314 was delayed because the diffusion of QX-314 into the sensory neurons was delayed.32 The result in the QX-314 group was not significantly different from that in the vehicle group, consistent with the results reported by Binshtok et al.3 In other words, QX-314 could not penetrate the membrane due to its lipophobic characteristics.33,34
If capsaicin was sufficient to activate TRPV1, the animals should have exhibited a hyperalgesic response. However, the response to noxious heat was not significantly different between capsaicin group and the vehicle group at 10 to 30 minutes. The reason why the animals exhibited no hyperalgesic response is that TRPV1 is typically activated under inflammatory conditions,35 and inflammation was not induced in the animals in this study. On the other hand, a significantly greater degree of analgesia was observed in the capsaicin group compared with the vehicle group, at 240 to 300 minutes after the injection. The local nociceptor-desensitization action of capsaicin is considered to be the underlying mechanism for the hypoalgesia.36–38 However, no significant differences were reported between the capsaicin group and the vehicle group in the Binshtok et al study.3 The reason for the difference in results between our experiments and those reported by Binshtok et al3 remains unclear.
Inhibition of the antinociceptive effect of capsaicin/QX-314 by capsazepine
To examine inhibition of the antinociceptive effect of capsaicin/QX-314, capsazepine (50 μg/mL; 10 μL) was injected into the plantar aspect of the right hind paw 30 minutes prior to the injection of capsaicin/QX-314 (10 μL) (capsazepine group). The capsaicin/QX-314 group exhibited a greater degree of analgesia compared with the capsazepine group, at 10 to 60 minutes after the injection; on the other hand, no significant difference was observed between the capsazepine group and the vehicle group. This result indicates that capsazepine inhibited QX-314 entry into the nociceptive neurons through the pores of the TRPV1 channels.
Histochemical studies
The fluorescence in small- and medium-sized neurons was marked in only the ipsilateral DRG injected with capsaicin and showing fluorescence of QX-314. This result is in agreement with the previous finding that TRPV1 is expressed in small-to medium-sized primary afferent neurons.14 Furthermore, the results indicate that QX-314 can be transported through TRPV1 channels but cannot pass through the pores of TRPA1 or TRPM8. Thus, the histochemical results support the behavioral results in the animals injected with capsaicin/QX-314 (Binshtok et al3 and the present study), AITC/QX-314 (present study), and menthol/QX-314 (present study).
Conclusion
The results of this study indicate that TRPA1 and TRPM8 channels are ineffective for the transport of QX-314 compared with the TRPV1 channel. We would like to develop a new method, using a different substance from capsaicin to eliminate the action-potential firing in nociceptors by capsaicin. The actual transport of QX-314 through TRPV1 channels was verified visually.
Acknowledgment
This work was supported by the Japan Society for the Promotion of Science; KAKENHI, grant number 22592289.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Butterworth J, Oxford GS. Local anesthestics: A new hydrophilic pathway for the drug-receptor reaction. Anesthesiology. 2009;111(1):12–14. doi: 10.1097/ALN.0b013e3181a91624. [DOI] [PubMed] [Google Scholar]
- 2.Butterworth JF, 4th, Strichartz GR. Molecular mechanisms of local anesthesia: a review. Anesthesiology. 1990;72(4):711–734. doi: 10.1097/00000542-199004000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Binshtok AM, Bean BP, Woolf CJ. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature. 2007;449(7162):607–610. doi: 10.1038/nature06191. [DOI] [PubMed] [Google Scholar]
- 4.Omana-Zapata I, Khabbaz MA, Hunter JC, Bley KR. QX-314 inhibits ectopic nerve activity associated with neuropathic pain. Brain Res. 1997;771(2):228–237. doi: 10.1016/s0006-8993(97)00770-1. [DOI] [PubMed] [Google Scholar]
- 5.Ries CR, Pillai R, Chung CC, Wang JT, MacLeod BA, Schwarz SK. QX-314 produces long-lasting local anesthesia modulated by transient receptor potential vanilloid receptors in mice. Anesthesiology. 2009;111(1):122–126. doi: 10.1097/ALN.0b013e3181a9160e. [DOI] [PubMed] [Google Scholar]
- 6.Kim HY, Kim K, Li HY, et al. Selectively targeting pain in the trigeminal system. Pain. 2010;150(1):29–40. doi: 10.1016/j.pain.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binshtok AM, Gerner P, Oh SB, et al. Co-application of lidocaine and the permanently charged sodium channel blocker QX-314 produces a long-lasting nociceptive blockade in rodents. Anesthesiology. 2009;111(1):127–137. doi: 10.1097/ALN.0b013e3181a915e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberson DP, Binshtok AM, Blasl F, Bean BP, Woolf CJ. Targeting of sodium channel blockers into nociceptors to produce long-duration analgesia: a systematic study and review. Br J Pharmacol. 2011;164(1):48–58. doi: 10.1111/j.1476-5381.2011.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Ki D, Bianchi BR, et al. Pore dilation occurs in TRPA1 but not in TRPM8 channels. Mol Pain. 2009;5:3. doi: 10.1186/1744-8069-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bevan S, Hothi S, Hughes G, et al. Capsazepine: a competitive antagonist of the sensory neuron excitant capsaicin. Br J Pharmacol. 1992;107(2):544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwak JY, Jung JY, Hwang SW, Lee WT, Oh U. A capsaicin-receptor antagonist, capsazepine, reduces inflammation-induced hyperalgesic responses in the rat: evidence for an endogenous capsaicin-like substance. Neuroscience. 1998;86(2):619–626. doi: 10.1016/s0306-4522(98)00012-8. [DOI] [PubMed] [Google Scholar]
- 12.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 13.Hiura A, Nakagawa H, Koshigae Y, Yoshizako A, Kubo Y, Ishizuka H. Age-related changes in the response to thermal noxious heat and reduction of C-fibers by neonatal treatment with capsaicin. Somatosens Mot Res. 1999;16(2):115–121. doi: 10.1080/08990229970555. [DOI] [PubMed] [Google Scholar]
- 14.Caffrey JM, Eng DL, Black JA, Waxman SG, Kocsis JD. Three types of sodium channels in adult rat dorsal root ganglion neurons. Brain Res. 1992;592(1–2):283–297. doi: 10.1016/0006-8993(92)91687-a. [DOI] [PubMed] [Google Scholar]
- 15.Story GM, Peier AM, Reeve AJ, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112(6):819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 16.Park CK, Kim MS, Fang Z, et al. Functional expression of thermo-transient receptor potential channels in dental primary afferent neurons: implication for tooth pain. J Biol Chem. 2006;281(25):17304–17311. doi: 10.1074/jbc.M511072200. [DOI] [PubMed] [Google Scholar]
- 17.Madrid R, Donovan-Rodríguez T, Meseguer V, Acosta MC, Belmonte C, Viana F. Contribution of TRPM8 channels to cold transduction in primary sensory neurons and peripheral nerve terminals. J Neurosci. 2006;26(48):12512–12525. doi: 10.1523/JNEUROSCI.3752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reid G, Babes A, Pluteanu F. A cold- and menthol-activated current in rat dorsal root ganglion neurones: properties and role in cold transduction. J Physiol. 2002;545(Pt 2):595–614. doi: 10.1113/jphysiol.2002.024331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416(6876):52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 20.Peier AM, Moqrich A, Hergarden AC, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108(5):705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 21.Albin KC, Carstens MI, Carstens E. Modulation of oral heat and cold pain by irritant chemicals. Chem Senses. 2008;33(1):3–15. doi: 10.1093/chemse/bjm056. [DOI] [PubMed] [Google Scholar]
- 22.Galeotti N, Ghelardini C, Mannelli L, Mazzanti G, Baghiroli L, Bartolini A. Local anaesthetic activity of (+)- and (−)-menthol. Planta Med. 2001;67(2):174–176. doi: 10.1055/s-2001-11515. [DOI] [PubMed] [Google Scholar]
- 23.Galeotti N, Di Cesare Mannelli L, Mazzanti G, Bartolini A, Ghelardini C. Menthol: a natural analgesic compound. Neurosci Lett. 2002;322(3):145–148. doi: 10.1016/s0304-3940(01)02527-7. [DOI] [PubMed] [Google Scholar]
- 24.Green BG. Menthol inhibits the perception of warmth. Physiol Behav. 1986;38(6):833–838. doi: 10.1016/0031-9384(86)90050-8. [DOI] [PubMed] [Google Scholar]
- 25.Green BG. Lingual heat and cold sensitivity following exposure to capsaicin or menthol. Chem Senses. 2005;30(Suppl 1):S201–S202. doi: 10.1093/chemse/bjh184. [DOI] [PubMed] [Google Scholar]
- 26.Klein AH, Sawyer CM, Carstens MI, Tsagareli MG, Tsiklauri N, Carstens E. Topical application of L-menthol induces heat analgesia, mechanical allodynia, and a biphasic effect on cold sensitivity in rats. Behav Brain Res. 2010;212(2):179–186. doi: 10.1016/j.bbr.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Woolf CJ. Pain TRPs. Neuron. 2005;46(1):9–12. doi: 10.1016/j.neuron.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 29.Nakagawa H, Hiura A. Capsaicin, transient receptor potential (TRP) protein subfamilies and the particular relationship between capsaicin receptors and small primary sensory neurons. Anat Sci Int. 2006;81(3):135–155. doi: 10.1111/j.1447-073X.2006.00141.x. [DOI] [PubMed] [Google Scholar]
- 30.Meyers JR, MacDonald RB, Duggan A, et al. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J Neurosci. 2003;23(10):4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of TRP channels. Annu Rev Physiol. 2006;68:685–717. doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- 32.Lee RH, Heckman CJ. Paradoxical effect of QX-314 on persistent inward currents and bistable behavior in spinal motoneurons in vivo. J Neurophysiol. 1999;82(5):2518–2527. doi: 10.1152/jn.1999.82.5.2518. [DOI] [PubMed] [Google Scholar]
- 33.Strichartz GR. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J Gen Physiol. 1973;62(1):37–57. doi: 10.1085/jgp.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeh JZ. Sodium inactivation mechanism modulates QX-314 block of sodium channels in squid axons. Biophys J. 1978;24(2):569–574. doi: 10.1016/S0006-3495(78)85403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hiura A, Nakagawa H. An overview of actions of capsaicin and its receptor, TRPV1, and their relations to small primary sensory neurons. Curr Med Chem Anti Inflamm Anti Allergy Agents. 2011;10(1):2–9. [Google Scholar]
- 36.Baranowski R, Lynn B, Pini A. The effects of locally applied capsaicin on conduction in cutaneous nerves in four mammalian species. Br J Pharmacol. 1986;89(2):267–276. doi: 10.1111/j.1476-5381.1986.tb10256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung JM, Lee KH, Hori Y, Willis WD. Effects of capsaicin applied to a peripheral nerve on the responses of primate spinothalamic tract cells. Brain Res. 1985;329(1–2):27–38. doi: 10.1016/0006-8993(85)90509-8. [DOI] [PubMed] [Google Scholar]
- 38.Petsche U, Fleischer E, Lembeck F, Handwerker HO. The effect of capsaicin application to a peripheral nerve on impulse conduction in functionally identified afferent nerve fibres. Brain Res. 1983;265(2):233–240. doi: 10.1016/0006-8993(83)90337-2. [DOI] [PubMed] [Google Scholar]