Abstract
Background
The airway epithelium can express factors that drive subepithelial airway remodeling. TGF-β2, vascular epithelial growth factor (VEGF), a disintegrin and metalloprotease 33 (ADAM33), and periostin are hypothesized to be involved in subepithelial remodeling and are overexpressed in adult asthmatic airways. Epidemiologic data suggest that lung function deficits in asthmatic patients are acquired in childhood.
Objectives
We sought to determine whether airway epithelial cells (AECs) from asthmatic children differentially express TGF-β2, VEGF, ADAM33, or periostin compared with cells from atopic nonasthmatic and healthy children intrinsically or in response to IL-4/IL-13 stimulation.
Methods
Bronchial and nasal epithelial cells were obtained from brushings from well-characterized asthmatic (n = 16), atopic nonasthmatic (n = 9), and healthy (n = 15) children after achievement of anesthesia for elective procedures. After differentiation at an air-liquid interface (ALI) for 3 weeks, conditioned media were sampled and RNA was extracted from unstimulated and IL-4/IL-13–stimulated cultures. TGF-β2 and VEGF levels were measured with ELISA. ADAM33 and periostin expression was assessed by using real-time PCR.
Results
TGF-β2 and VEGF production was significantly greater in bronchial and nasal ALI cultures from asthmatic children than in cultures from atopic nonasthmatic and healthy children. TGF-β2 levels increased significantly in asthmatic cultures after IL-4/IL-13 stimulation. Within-subject correlation between nasal and bronchial ALI production of TGF-β2 (r = 0.64, P = .001) and VEGF (r = 0.73, P < .001) was good. Periostin expression was 3.7-fold higher in bronchial cells (P < .001) and 3.9-fold higher in nasal cells (P < .004) from asthmatic children than in cells from atopic nonasthmatic or healthy children. ADAM33 was not differentially expressed by AECs from asthmatic patients compared with that from cells from atopic nonasthmatic or healthy children.
Conclusion
AECs from asthmatic children differentially express TGF-β2, VEGF, and periostin compared with cells from atopic nonasthmatic and healthy children. Nasal epithelial cells might be a suitable surrogate for bronchial cells that could facilitate investigation of the airway epithelium in future longitudinal pediatric studies.
Keywords: Asthma, children, airway remodeling, epithelial cells vascular endothelial growth factor, a disintegrin and metalloprotease 33, periostin, TGF-β2
Longitudinal studies following children with wheezing and asthma into the teenage years and adulthood have provided insight into the progression of asthma in children. Data from the Tucson Children’s Respiratory Study suggest that pulmonary function in children who ultimately have persistent asthma is normal at birth, with deficits acquired by as early as 6 years of age.1 Lung function data from a large cohort study in Melbourne, Australia, with follow-up from early childhood through age 35 years revealed that children with asthma had lower lung function during childhood and at age 35 years compared with that seen in cohort members without a history of childhood asthma.2 Interestingly, lung function of cohort members, including those with severe asthma, did not worsen after age 10 years, suggesting that lifelong lung function deficits among asthmatic patients were acquired by age 10 years. Endobronchial biopsy specimens from asthmatic children show features of airway remodeling.3,4 Together, these data suggest that structural changes in the asthmatic airway that might result in permanent lung function deficits occur early in the natural history of asthma.
In addition to serving as a passive barrier, the airway epithelium is the interface between the environment and the host and appears to play a critical role in orchestrating the host’s inflammatory response.5 There is an emerging consensus that the response of the asthmatic airway epithelium to environmental insults, infection, and inflammatory stimuli is dysfunctional, with resultant airway epithelial cell (AEC) expression of genes and factors that drive subepithelial airway remodeling.6 In vivo animal models of airway remodeling7-9 and descriptive data from human bronchial biopsy specimens10-12 implicate several epithelium-derived cytokines in the promotion of airway fibrosis and angiogenesis, including TGF-β2 and vascular endothelial growth factor (VEGF). AECs can secrete TGF-β2, and this growth factor is overexpressed in airway epithelium obtained during bronchial biopsy specimens from adult asthmatic patients.10 Vascular remodeling is another characteristic feature of the asthmatic airway.13,14 Data from animal models suggest that VEGF is an important mediator of angiogenesis, vascular permeability, and structural changes in the asthmatic airway.15,16 VEGF and VEGF receptor are reported to be overexpressed in adult asthmatic airways,17 and VEGF levels in sputum and lung tissue are increased in asthmatic patients and correlate with disease severity.11,18,19
A disintegrin and metalloprotease 33 (ADAM33) has been found in several diverse populations to be an asthma susceptibility gene.20 This transmembrane protein is believed to play a role in cell proliferation, differentiation, adhesion, signaling, and apoptosis among other functions.21 In a cohort of Dutch patients with asthma followed for 20 years, ADAM33 single nucleotide polymorphisms were found to be associated with an accelerated decrease in lung function.22 In a clinical study of Korean adults with asthma, ADAM33 protein levels in bronchoalveolar lavage fluid were inversely associated with lung function.23 In addition, ADAM33 expression was recently found to be higher in epithelial cells, submucosal cells, and smooth muscle from patients with moderate-to-severe asthma compared with that seen in patients with mild asthma and healthy control subjects.24
In a recent microarray study of gene expression in airway epithelial biopsy specimens, expression of the matricelluar protein periostin was found to be differentially upregulated in adults with asthma compared with that seen in healthy adults.25 Furthermore, periostin expression has been shown to be upregulated in bronchial epithelial cells from adults with asthma grown in vitro and stimulated with IL-13 and appears to play a role in activating TGF-β signaling pathways and promoting type 1 collagen production by airway fibroblasts.26
Using bronchial epithelial cells obtained from well-characterized atopic asthmatic children, atopic nonasthmatic children, and healthy children, one of our objectives was to determine whether lower AECs from asthmatic children differentiated at an air-liquid interface (ALI) intrinsically express growth factors and genes that have been associated with airway remodeling in animal models and human studies of adults with asthma, including TGF-β2, vascular endothelial growth factor (VEGF), periostin, and ADAM33. In addition, we sought to characterize expression of these factors by AECs in response to TH2 cytokine stimulation (IL-4 and IL-13). Our overall hypothesis was that AECs from children with asthma express a unique profile of factors that influence subepithelial mesenchymal remodeling. Finally, we compared the within-subject gene expression and cytokine production of bronchial and nasal epithelial cells to determine whether nasal cells can be used as a noninvasive proxy for bronchial cells in future longitudinal studies. Some of these results have been reported in abstract form.27
METHODS
Subjects
Atopic asthmatic, atopic nonasthmatic, and healthy children aged 6 to 16 years who underwent an elective surgical procedure requiring endotracheal intubation and general anesthesia were recruited for this study. A detailed medical history was obtained at enrollment to ensure participants met the following inclusion and exclusion criteria. Asthmatic children had a 1-year or greater history of physician-diagnosed asthma, had physician-documented wheezing in the 12 months before enrollment, used albuterol twice or more a month or were taking a daily inhaled corticosteroids (ICSs) or leukotriene receptor antagonists, and were born at 36 weeks’ gestation or later. Healthy and atopic nonasthmatic subjects were born at 36 weeks’ gestation or later and had no history of asthma, reactive airway disease, chronic daily cough, or physician-diagnosed obstructive lung disease, and no history of prior treatment with a systemic corticosteroid or ICS, albuterol, or oxygen. Children with asthma, as well as atopic nonasthmatic children, had a history of 1 or more of the following atopic features: positive skin prick test or RAST results for a common aeroallergen, increased serum IgE levels (>100 IU/mL), physician-diagnosed allergic rhinitis, and physician-diagnosed atopic dermatitis. Healthy subjects lacked a history of any of these atopic features.
Written consent was obtained from subjects’ parents, and assent was obtained for children 7 years or older. The study was approved by the Seattle Children’s Hospital Institutional Review Board.
Epithelial cell isolation
Immediately after the endotracheal tube was secured, 3 bronchial epithelial cell samples were obtained from subjects after achievement of general anesthesia with 4-mm Harrell unsheathed bronchoscope cytology brushes (ConMed Corp, Utica, NY). As described by Lane et al,28 the unprotected brush was inserted through an endotracheal tube, advanced until resistance was felt, and rubbed against the airway surface for 2 seconds. Simultaneous nasal brushings were also obtained in each subject. Cells were seeded onto T-25 cell culture flasks precoated with type I collagen. Cultures were maintained at 37°C in a 5% CO2 atmosphere in a humidified incubator. Cells were cultured in bronchial epithelial growth medium (Clonetics BEGM; Lonza, Basel, Switzerland) containing gentamicin and amphotericin B and further supplemented with penicillin-streptomycin (100 μg/mL; Invitrogen, Carlsbad, Calif). Fluconazole (25 μg/mL) was added to primary cultures for the first 96 hours, after which medium was aspirated and replaced with BEGM without fluconazole. BEGM was thereafter changed every 48 hours until the culture reached approximately 70% to 90% confluence. When P0 flasks became confluent, cells were passaged into 3 new passage1 (P1) flasks.
ALI epithelial cell cultures
For ALI cultures, Corning Costar 12-mm 0.4-μm Transwells (Corning Life Sciences, Corning, NY) were precoated with type I collagen and then seeded with P2 epithelial cells at a concentration of 100,000 cells per transwell. All seeded cells screened negatively for Mycoplasma species infection (Mycoprobe Mycoplasma Kit; R&D Systems, Inc, Minneapolis, Minn). Cells were grown submerged in BEGM until 100% confluent, at which time apical medium was removed and basolateral medium was replaced with ALI medium consisting of a 1:1 mixture of BEGM and Dulbecco modified Eagle medium supplemented with all-trans retinoic acid (30 ng/mL), human recombinant epidermal growth factor (EGF; 0.5 ng/mL), MgCl2 (0.6 mmol/L), CaCl2 (1 mmol/L), and penicillin-streptomycin (100 mg/mL). ALI medium in the basolateral compartment was changed every other day, and cells were differentiated at an ALI for 21 days before initiation of experiments (see Fig E1 in this article’s Online Repository at www.jacionline.org). Each experimental condition per cell line consisted of triplicate transwells. For transwells stimulated with IL-4 and IL-13, 50 ng/mL of each cytokine was added to basolateral medium. Sampling of the basolateral conditioned medium and RNA extraction was performed 48 hours after IL-4/IL-13 stimulation and 48 hours after a medium change for unstimulated transwells.
RNA extraction and real-time PCR
Three transwells from each experimental condition were harvested and pooled to isolate RNA by using the RNAqueous kit for total RNA purification from Ambion–Applied Biosystems (Austin, Tex). RNA concentration and integrity were determined by using the Agilent 2100 Bioanalyzer system and Agilent RNA 6000 Nano Chips (Agilent Technologies, Foster City, Calif). RNA samples (1 μg) with an RNA integrity number of 8 or greater were reverse transcribed with Moloney murine leukemia virus reverse transcriptase with a combination of random hexamers and oligo-dTs by using the SuperScript VILO cDNA Synthesis Kit from Invitrogen. Samples were diluted to a final volume of 100 μL (10 ng/μL). Semiquantitative real-time qPCR was performed by using the SensiMix II probe kit with SYBR Green (Bioline, London, United Kingdom) and in-house validated primers for periostin, ADAM33, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Additional details are provided in the Methods section in this article’s Online Repository at www.jacionline.org.
ELISA analyses
For each condition, sampled basolateral medium from triplicate transwells was pooled. Measurement of protein levels of TGF-β2 and VEGF in sampled conditioned media was completed by using Duoset ELISA Development Kits (R&D Systems, Inc), according to the manufacturer’s recommendations. The VEGF Duoset is specific for the VEGF-A isoform, without cross-reactivity to VEGF isoforms B, C, or D. All measurements were completed in duplicate.
Subjects’ clinical characterization
A blood sample was drawn and used to measure total serum IgE and RAST allergen-specific IgE levels to dust mite, cat epithelium, dog epithelium, Alternaria tenuis, Aspergillus fumigatus, and timothy grass. The fraction of exhaled nitric oxide (Feno) was measured according to American Thoracic Society guidelines with a NIOX chemiluminescent nitric oxide analyzer (Aerocrine, Solna, Sweden). Forced expiratory volumes and flows (forced vital capacity, FEV1, and forced expiratory flow between 25% and 75% of expiration [FEF25-75]) were measured according to American Thoracic Society guidelines with a VMAX series 2130 spirometer (VIASYS Healthcare, Hong Kong, China). Spirometry was repeated 15 minutes after administration of 2 puffs of albuterol in asthmatic children.
Statistical analysis
Protein levels are presented as means ± SDs. The Kolmogorov-Smirnov test was used to determine whether data were normally distributed. One-way ANOVA or the Kruskal-Wallis test for nonnormally distributed data was used to compare lung function parameters and Feno values among asthmatic, atopic nonasthmatic, and healthy children and to compare protein levels in ALI culture basolateral conditioned medium among cells from the 3 groups of subjects. The unpaired t test or the Mann-Whitney test for nonnormally distributed data was used to compare ALI culture protein levels between the asthmatic and healthy groups and between the asthmatic and atopic nonasthmatic groups. The paired t test or the Wilcoxon signed-rank test for nonnormally distributed data was used to compare protein levels in ALI cultures before and after IL-4/IL-13 stimulation. The Pearson coefficient was calculated to determine the intrasubject correlation of protein levels between ALI cultures from bronchial and nasal epithelial cells. Statistical analyses of clinical data and protein levels in ALI cultures were performed with Prism 5.0 software (GraphPad Software, Inc, San Diego, Calif). The relative expression of periostin and ADAM33 was standardized by using GAPDH as a nonregulated reference gene. We used the REST (Relative Expression Software Tool)–MCS software to compare gene expression.29,30 Final results were expressed as the fold change on a log2 scale relative to median expression of genes in healthy control samples. Additional details are provided in the Methods section in this article’s Online Repository.
RESULTS
Airway epithelial brushings were obtained from 21 asthmatic children, 11 atopic children without asthma, and 19 healthy children. ALI cultures were successfully established, and experiments were completed with cells from 16 asthmatic children, 9 atopic children without asthma, and 15 healthy children. Airway brushings were well tolerated by all subjects, with no adverse events. Baseline characteristics of subjects completing the study are presented in Table I. The mean age of children from the 3 groups was similar. Consistent with inclusion criteria, serum IgE levels were significantly higher in asthmatic and atopic nonasthmatic children than in healthy children. The FEV1/forced vital capacity ratio, FEV1, and FEF25-75 percent predicted values were significantly lower and the Feno value was significantly higher among children with asthma compared with atopic nonasthmatic and healthy children.
TABLE I.
Subjects’ characteristics
| Healthy control subjects (n = 15) | Atopic nonasthmatic patients (n = 9) | Asthmatic patients (n = 16) | P value | |
|---|---|---|---|---|
| Age (y [SD]) | 12.2 (4.6) | 10.8 (4.1) | 11.4 (3.9) | .6 |
| Female sex (%) | 47 | 44 | 38 | .8 |
| Current use of ICSs (yes; %) | NA | NA | 7 (44) | NA |
| IgE (IU/mL [SD]) | 40 (12) | 252 (519) | 341 (127) | .02 |
| FVC (% predicted [SD]) | 105 (8.5) | 110 (9.2) | 106 (14.5) | .4 |
| FEV1/FVC ratio (SD) | 0.84 (0.05) | 0.85 (0.1) | 0.77 (0.08) | .03 |
| FEV1 (% predicted [SD]) | 100 (10.5) | 109 (11) | 94 (14.7) | .03 |
| FEF25-75 (% predicted [SD]) | 102 (27.7) | 102 (23.3) | 72 (25.6) | .001 |
| BDR (yes; %) | NA | NA | 9 (56) | NA |
| Feno (ppb [SD]) | 10.3 (5.4) | 14.9 (15.5) | 36.0 (22.1) | .04 |
BDR, Bronchodilator responsiveness (defined as an increase of ≥12% in FEV1 or ≥25% in FEF25-75 after 2 puffs of albuterol through a metered-dose inhaler); FVC, forced vital capacity.
The concentration of TGF-β2 in ALI conditioned culture media of bronchial epithelial cells from children with asthma (Asthma BE group) was significantly higher than that in conditioned media of cells from atopic nonasthmatic (Atopic BE group) and healthy (Healthy BE group) children (Asthma BE group, 1018 ± 709 pg/mL; Atopic BE group, 456 ± 327 pg/mL; and Healthy BE group, 554 ± 372 pg/mL; P = .02; Fig 1). TGF-β2 production was also significantly higher in nasal epithelial cell ALI cultures from asthmatic (Asthma NE group) compared with atopic nonasthmatic (Atopic NE group) and healthy (Healthy NE group) children (Asthma NE group, 1067 ± 851 pg/mL; Atopic NE group, 449 ± 244 pg/mL; and Healthy NE group, 516 ± 293 pg/mL; P = .05; Fig 1). Stimulation of bronchial and nasal epithelial ALI cultures with IL-4 and IL-13 led to significant increases in TGF-β2 production in cells from asthmatic but not atopic nonasthmatic or healthy children (see Fig E2 in this article’s Online Repository at www.jacionline.org).
FIG 1.
TGF-β2 concentrations in ALI cultures from bronchial epithelial cells from healthy subjects (Healthy BE), bronchial epithelial cells from asthmatic patients (Asthma BE), bronchial epithelial cells from atopic nonasthmatic patients (Atopic BE), nasal epithelial cells from healthy subjects (Healthy NE), nasal epithelial cells from asthmatic patients (Asthma NE), and nasal epithelial cells from atopic nonasthmatic patients (Atopic NE). *Three-way group comparison of Asthma BE versus Healthy BE and Atopic BE: p = .02 (Kruskal-Wallis test). Comparison of Asthmatic BE with Healthy BE: p = .01 (Mann-Whitney test). Comparison of Asthmatic BE with Atopic BE: p = .02 (Mann-Whitney test). **Three-way group comparison of Asthma NE versus Healthy NE and Atopic NE: p = .05 (Kruskal-Wallis test). Comparison of Asthmatic NE with Healthy NE: p = .04 (Mann-Whitney test). Comparison of Asthmatic NE to Atopic NE: p = .04 (Mann-Whitney test).
VEGF production in ALI cultures of bronchial epithelial cells from children with asthma was significantly higher than that in cells from atopic nonasthmatic and healthy children (Asthma BE group, 1416 ± 864 pg/mL; Atopic BE group, 449 ± 290 pg/mL; and Healthy BE group, 636 ± 449 pg/mL; P <.001; Fig 2). VEGF production was also significantly higher in nasal epithelial cell ALI cultures from asthmatic compared with atopic nonasthmatic and healthy children (Asthma NE group, 1331 ± 1076 pg/mL; Atopic NE group, 358 ± 267 pg/mL; and Healthy NE group, 443 ± 289 pg/mL; P < .001; Fig 2). There was no change in VEGF production in bronchial or nasal epithelial cells from asthmatic, atopic nonasthmatic, or healthy children after stimulation with IL-4/IL-13 (see Fig E2). Production of TGF-β2 and VEGF in bronchial epithelial cells from asthmatic children who reported ICS use at the time of airway brushings was similar to production in cells from asthmatic children not using ICSs (see Fig E3 in this article’s Online Repository at www.jacionline.org).
FIG 2.
VEGF concentration in ALI cultures from bronchial epithelial cells from healthy subjects (Healthy BE), bronchial epithelial cells from asthmatic patients (Asthma BE), bronchial epithelial cells from atopic nonasthmatic patients (Atopic BE), nasal epithelial cells from healthy subjects (Healthy NE), nasal epithelial cells from asthmatic patients (Asthma NE), and nasal epithelial cells from atopic nonasthmatic patients (Atopic NE). *Three-way group comparison of Asthma BE versus Healthy BE and Atopic BE: P < .001 (1-way AN-OVA). Comparison of Asthmatic BE with Healthy BE: p = .003 (unpaired t test). Comparison of Asthmatic BE with Atopic BE: p = .004 (unpaired t test). **Three-way group comparison of Asthma NE versus Healthy NE and Atopic NE: P < .05 (Kruskal-Wallis test). Comparison of Asthmatic NE with Healthy NE: P < .001(Mann-Whitney test). Comparison of Asthmatic NE with Atopic NE: P = .001 (Mann-Whitney test).
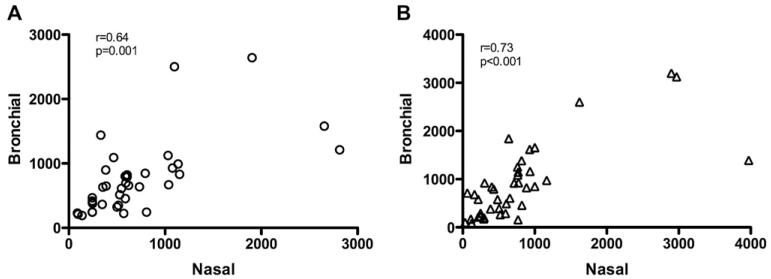
Production of TGF-β2 in nasal epithelial cells was significantly correlated with bronchial epithelial cell TGF-β2 production within subjects (r = 0.64, P = .001, Fig 3). Similarly, there was good within-subject correlation of nasal and bronchial epithelial cell production of VEGF (r = 0.73, P < .001, Fig 3).
FIG 3.
Correlation between bronchial and nasal epithelial cell ALI cultures for production of TGF-β2 (A) and VEGF (β).
In unstimulated bronchial epithelial cell ALI cultures, expression of periostin mRNAwas 3.7-fold higher in cells from children with asthma than in cells from healthy children (P = .0001, Fig 4), whereas periostin expression in atopic nonasthmatic cultures was similar to expression seen in healthy cells. Periostin expression was 3.9-fold higher in nasal epithelial cells from asthmatic children than in cells from healthy children (P = .003), whereas atopic and healthy nasal cell periostin expression was similar. Periostin expression was 2.4-fold higher in asthmatic bronchial epithelial cells stimulated with IL-4 and IL-13 compared with that in unstimulated asthmatic cells (P = .046, see Fig E4 in this article’s Online Repository at www.jacionline.org). There was no significant difference in periostin expression after IL-4/IL-13 stimulation in bronchial epithelial cells from healthy and atopic subjects. Periostin expression in asthmatic bronchial epithelial cells from children reporting use of ICSs at the time of airway brushings and from asthmatic children who were not using ICSs were both greater than that seen in cells from healthy children (see Fig E5 in this article’s Online Repository at www.jacionline.org).
FIG 4.
Periostin and ADAM33 mRNA expression in asthmatic bronchial (A) and nasal (B) epithelial cell ALI cultures compared with expression in cultures from atopic nonasthmatic and healthy children normalized to the housekeeping gene GAPDH. Fig 4, A: Periostin overall group differences, p = .001; asthmatic patients versus healthy subjects, p = .0001. ADAM33 showed no significant between-group differences. Fig 4, B: Periostin overall group differences, p = .05; asthmatic patients versus healthy subjects, p = .003. ADAM33 showed no significant differences between groups. C, Periostin, ADAM33, and GAPDH amplicons determined by means of gel electrophoresis.
ADAM33 was not differentially expressed in asthmatic AECs compared with cells from atopic nonasthmatic or healthy children (Fig 4). Furthermore, there was no change in ADAM33 expression in AECs from asthmatic, atopic nonasthmatic, or healthy children in response to IL-4 and IL-13 stimulation (see Fig E4).
DISCUSSION
In this in vitro study of differentiated AECs from well-characterized asthmatic, atopic nonasthmatic, and healthy children, we found that several factors believed to influence subepithelial airway remodeling were differentially expressed in AECs from asthmatic children. Specifically, production of TGF-β2 and VEGF was significantly higher in AECs from asthmatic children than in cells from atopic or healthy children. In AECs from asthmatic children, stimulation with IL-4 and IL-13 led to an increase in TGF-β2 production. We demonstrated good intrasubject correlation of in vitro bronchial and nasal epithelial production of both TGF-β2 and VEGF. Finally, we showed that expression of periostin was significantly higher in both bronchial and nasal epithelial cells from children with asthma than in cells from atopic or healthy children.
TGF-β2 expression in bronchial epithelial cells from adult asthmatic patients has been shown to be greater than expression in cells from healthy subjects.28 We have now demonstrated that TGF-β2 expression in the airway epithelium is also increased in children with asthma compared with that seen in atopic or healthy children. Chu et al31 found that epithelial TGF-β2 expression in bronchial mucosa from adult asthmatic patients was correlated with mucin expression, and in vitro they showed that IL-13 stimulation of bronchial epithelial cells significantly increased TGF-β2 expression. Furthermore, they found that TGF-β2 stimulation of bronchial epithelial cells grown in vitro directly increased mucin expression. Our data are consistent with the findings of Chu et al31; however, in our experiments cells were stimulated with a combination of IL-4 and IL-13. Using normal bronchial epithelial cells differentiated at an ALI and cocultured with normal human fibroblasts, Thompson et al32 showed that an epithelial scrape injury led to a 2- to 3-fold increase in TGF-β2 production, which subsequently enhanced α-smooth muscle actin expression in the underlying collagen-embedded fibroblasts and secretion of tenascin-C into the matrix. By using a similar model system, Malavia et al33 demonstrated that IL-13 stimulation also resulted in an increase in TGF-β2 release by bronchial epithelial cells and that TGF-β2 stimulation of AECs led to collagen secretion in the subepithelial matrix. These findings, together with our data, suggest that differential expression of TGF-β2 by the asthmatic airway epithelium might play a role in the development of subepithelial airway remodeling in asthmatic children.
Vascular remodeling is another characteristic feature of the asthmatic airway.13,14 Data from animal models suggest that VEGF is an important mediator of angiogenesis, vascular permeability, and structural changes in the asthmatic airway.15 VEGF and VEGF receptor are reported to be overexpressed in adult human asthmatic airways,18 VEGF levels are increased in sputum from asthmatic children,34 and VEGF expression is increased in lung tissue from adult asthmatic patients and correlated with disease severity.11,18,19 Recently, Sharma et al35 found that VEGF polymorphisms were associated with childhood asthma, lung function, and airway responsiveness. To our knowledge, we now demonstrate, for the first time, using bronchial epithelial cells grown and differentiated in vitro from well-characterized children that VEGF expression is significantly greater in AECs from asthmatic children than in cells from atopic or healthy children. Our finding supports the hypothesis that epithelium-derived VEGF might contribute to airway remodeling early in the natural history of asthma.
In this study we have demonstrated significantly greater expression of periostin in bronchial and nasal epithelial cells from children with asthma than in cells from atopic or healthy children, with increased expression in cells from asthmatic patients that are stimulated with IL-4 and IL-13. Periostin is a 90-kd disulfide-linked matricellular protein expressed by connective tissues, bone, myocardium, heart valves, and skin36,37 that is believed to interact with multiple integrins and be involved in cell proliferation and regeneration, epithelial-mesenchymal transformation, fibrosis, and angiogenesis.38-40 In 2006, Takayama et al reported that periostin expression was higher in asthmatic than normal airways.41 Genome-wide profiling of airway epithelium from adult asthmatic patients and healthy control subjects was performed by Woodruff et al,25 who reported that periostin was differentially upregulated in epithelial tissue from adult asthmatic patients compared with that from healthy subjects and smokers. Sidhu et al26 recently demonstrated in epithelial ALI cultures that periostin is basally secreted and that its production is enhanced by IL-13 stimulation. Furthermore, they showed that AEC stimulation by periostin led to increased expression of type I collagen and evidence of epithelial to mesenchymal transition. Our finding of differential expression of periostin in AECs from pediatric asthmatic patients is consistent with the findings of Woodruff et al25 and Sidhu et al26 and provide additional evidence that periostin might be associated with subepithelial changes in asthma, even at a young age.
Foley et al24 reported that ADAM33 expression was higher in endobronchial biopsy samples from adults with moderate-to-severe asthma compared with that seen in patients with mild asthma and healthy subjects and that ADAM33 immunostaining was increased in the epithelium, submucosal glands, and smooth muscle in biopsy specimens from patients with severe asthma. In contrast, Yang et al42 found that ADAM33 was silenced due to DNA methylation in primary bronchial epithelial cells from asthmatic and healthy adults. In this study we were able to detect AEC ADAM33 expression by using PCR, which we confirmed with gel electrophoresis. However, we found no difference in ADAM33 expression among AECs from asthmatic patients, atopic patients, and healthy subjects. Possible explanations for the apparent discrepancy between our findings and those of Foley et al24 include that our asthmatic patients had mild disease, whereas Foley et al reported increased ADAM33 expression in patients with moderate and severe asthma, and that our investigation consisted of in vitro experiments using P2 AECs as opposed to an analysis of bronchial biopsy specimens, which likely reflects interactions between multiple cell types, including immune cells.
An important finding of this study was good correlation between bronchial and nasal epithelial expression of proremodeling factors. This suggests that nasal epithelial cells might serve as an acceptable and less invasive proxy for lower AEC numbers in future longitudinal studies and ultimately as a potential adjunctive diagnostic strategy for asthma. Specific noninvasive methods to identify infants and young children with persistent asthma or children with asthma at risk for progressive airway remodeling would be useful because clinicians are generally unable to distinguish young children with recurrent transient wheezing from those with early persistent asthma or to predict which patients are at risk for progressive decreases in lung function.
Some data exist to suggest that higher doses of ICSs can reduce reticular basement membrane thickening in adult asthmatic patients.43,44 In our study differential expression of TGF-β2, VEGF, and periostin was not affected by a history of ICS use at the time of bronchial epithelial sampling, although the small numbers of subjects included in these subset analyses preclude detection of subtle differences. However, given that our in vitro experiments were performed with cells several passages removed from exposure, we did not expect to see effects on expression of proremodeling factors by a history of ICS treatment.
There are several limitations to this study. First, cells used in our experiments were several passages removed from the host and interactions with other cell types that occur in vivo. However, differential expression of factors by P2 AECs that are associated with asthma or airway remodeling support an inherent epithelial cell role in the pathogenesis of asthma.
Second, in our TH2 cytokine stimulation experiments, cells were stimulated with a combination of IL-4 and IL-13. Therefore we were unable to determine which of these cytokines was directly responsible for the observed increase in TGF-β2 expression. However, in vivo AECs are likely exposed to both cytokines simultaneously in asthmatic patients.
Another limitation of our study was that our asthmatic patients had relatively mild disease. Our finding of differential expression of TGF-β2, VEGF, and periostin by AECs from children with mild asthma suggest that these factors play important roles in the disease during childhood. Our observed lack of differential expression of these factors by AECs from atopic nonasthmatic children contrasts with the findings of several groups demonstrating changes in the basement membrane of atopic nonasthmatic adults.45,46 However, we did not directly evaluate the in vivo airway structure of children in this study, and therefore we cannot conclude that lower airway basement membrane changes do not occur in atopic nonasthmatic children.
In conclusion, we have demonstrated that AECs from well-characterized children with asthma grown in vitro differentially express several sentinel factors believed to influence subepithelial airway remodeling, namely TGF-β2, VEGF, and periostin. Stimulation of AECs with IL-4 and IL-13 led to enhanced TGF-β2 production by cells from asthmatic patients. We observed good correlation between bronchial and nasal epithelial expression of these proremodeling factors, suggesting that nasal epithelial cells might be useful as surrogates for lower AEC numbers and potentially allowing for serial sampling in future longitudinal pediatric studies. Our findings of differential expression of TGF-β2, VEGF, and periostin in epithelial cells from pediatric asthmatic patients are consistent with the global hypothesis by Holgate et al6 and others, suggesting an intrinsic dysregulation of the epithelial-mesenchymal trophic unit in patients with asthma.47
Supplementary Material
Acknowledgments
Supported by American Lung Association Biomedical grant RG-71839-N (PI: J.S.D.) and National Heart, Lung, and Blood Institute grant K23HL077626 (PI: J.S.D.).
Abbreviations used
- ADAM33
A disintegrin and metalloprotease 33
- AEC
Airway epithelial cell
- ALI
Air-liquid interface
- ATS
American Thoracic Society
- BEGM
Bronchial epithelial growth medium
- Ct
Cycle threshold
- FEF25-75
Forced expiratory flow between 25% and 75% of expiration
- Feno
Fraction of exhaled nitric oxide
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- ICS
Inhaled corticosteroid
- VEGF
Vascular epithelial growth factor
Footnotes
Disclosure of potential conflict of interest: J. S. Debley receives research support from the American Lung Association and the National Institutes of Health/National Heart, Lung, and Blood Institute. The rest of the authors declare that they have no relevant conflicts of interest.
Clinical implications: AECs from children with mild asthma exhibit greater expression of factors associated with subepithelial airway remodeling than cells from atopic and healthy children.
REFERENCES
- 1.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 2.Kelly WJ, Hudson I, Raven J, Phelan PD, Pain MC, Olinsky A. Childhood asthma and adult lung function. Am Rev Respir Dis. 1988;138:26–30. doi: 10.1164/ajrccm/138.1.26. [DOI] [PubMed] [Google Scholar]
- 3.Payne DN, Rogers AV, Adelroth E, Bandi V, Guntupalli KK, Bush A, et al. Early thickening of the reticular basement membrane in children with difficult asthma. Am J Respir Crit Care Med. 2003;167:78–82. doi: 10.1164/rccm.200205-414OC. [DOI] [PubMed] [Google Scholar]
- 4.Barbato A, Turato G, Baraldo S, Bazzan E, Calabrese F, Panizzolo C, et al. Epithelial damage and angiogenesis in the airways of children with asthma. Am J Respir Crit Care Med. 2006;174:975–81. doi: 10.1164/rccm.200602-189OC. [DOI] [PubMed] [Google Scholar]
- 5.Kato A, Schleimer RP. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr Opin Immunol. 2007;19:711–20. doi: 10.1016/j.coi.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holgate ST, Roberts G, Arshad HS, Howarth PH, Davies DE. The role of the airway epithelium and its interaction with environmental factors in asthma pathogenesis. Proc Am Thorac Soc. 2009;6:655–9. doi: 10.1513/pats.200907-072DP. [DOI] [PubMed] [Google Scholar]
- 7.Kelly MM, Leigh R, Bonniaud P, Ellis R, Wattie J, Smith MJ, et al. Epithelial expression of profibrotic mediators in a model of allergen-induced airway remodeling. Am J Respir Cell Mol Biol. 2005;32:99–107. doi: 10.1165/rcmb.2004-0190OC. [DOI] [PubMed] [Google Scholar]
- 8.Kumar RK, Herbert C, Foster PS. Expression of growth factors by airway epithelial cells in a model of chronic asthma: regulation and relationship to subepithelial fibrosis. Clin Exp Allergy. 2004;34:567–75. doi: 10.1111/j.1365-2222.2004.1917.x. [DOI] [PubMed] [Google Scholar]
- 9.Baluk P, Lee CG, Link H, Ator E, Haskell A, Elias JA, et al. Regulated angiogenesis and vascular regression in mice overexpressing vascular endothelial growth factor in airways. Am J Pathol. 2004;165:1071–85. doi: 10.1016/S0002-9440(10)63369-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torrego A, Hew M, Oates T, Sukkar M, Fan Chung K. Expression and activation of TGF-beta isoforms in acute allergen-induced remodelling in asthma. Thorax. 2007;62:307–13. doi: 10.1136/thx.2006.063487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chetta A, Zanini A, Foresi A, D’Ippolito R, Tipa A, Castagnaro A, et al. Vascular endothelial growth factor up-regulation and bronchial wall remodelling in asthma. Clin Exp Allergy. 2005;35:1437–42. doi: 10.1111/j.1365-2222.2005.02360.x. [DOI] [PubMed] [Google Scholar]
- 12.Amishima M, Munakata M, Nasuhara Y, Sato A, Takahashi T, Homma Y, et al. Expression of epidermal growth factor and epidermal growth factor receptor immunoreactivity in the asthmatic human airway. Am J Respir Crit Care Med. 1998;157:1907–12. doi: 10.1164/ajrccm.157.6.9609040. [DOI] [PubMed] [Google Scholar]
- 13.Vrugt B, Wilson S, Bron A, Holgate ST, Djukanovic R, Aalbers R. Bronchial angiogenesis in severe glucocorticoid-dependent asthma. Eur Respir J. 2000;15:1014–21. doi: 10.1034/j.1399-3003.2000.01507.x. [DOI] [PubMed] [Google Scholar]
- 14.Salvato G. Quantitative and morphological analysis of the vascular bed in bronchial biopsy specimens from asthmatic and non-asthmatic subjects. Thorax. 2001;56:902–6. doi: 10.1136/thorax.56.12.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhandari V, Choo-Wing R, Chapoval SP, Lee CG, Tang C, Kim YK, et al. Essential role of nitric oxide in VEGF-induced, asthma-like angiogenic, inflammatory, mucus, and physiologic responses in the lung. Proc Natl Acad Sci U S A. 2006;103:11021–6. doi: 10.1073/pnas.0601057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004;10:1095–103. doi: 10.1038/nm1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoshino M, Nakamura Y, Hamid QA. Gene expression of vascular endothelial growth factor and its receptors and angiogenesis in bronchial asthma. J Allergy Clin Immunol. 2001;107:1034–8. doi: 10.1067/mai.2001.115626. [DOI] [PubMed] [Google Scholar]
- 18.Asai K, Kanazawa H, Kamoi H, Shiraishi S, Hirata K, Yoshikawa J. Increased levels of vascular endothelial growth factor in induced sputum in asthmatic patients. Clin Exp Allergy. 2003;33:595–9. doi: 10.1046/j.1365-2222.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- 19.Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol. 2006;290:L209–21. doi: 10.1152/ajplung.00185.2005. [DOI] [PubMed] [Google Scholar]
- 20.Van Eerdewegh P, Little RD, Dupuis J, Del Mastro RG, Falls K, Simon J, et al. Association of the ADAM33 gene with asthma and bronchial hyperresponsiveness. Nature. 2002;418:426–30. doi: 10.1038/nature00878. [DOI] [PubMed] [Google Scholar]
- 21.Wolfsberg TG, Primakoff P, Myles DG, White JM. ADAM, a novel family of membrane proteins containing a disintegrin and metalloprotease domain: multipotential functions in cell-cell and cell-matrix interactions. J Cell Biol. 1995;131:275–8. doi: 10.1083/jcb.131.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jongepier H, Boezen HM, Dijkstra A, Howard TD, Vonk JM, Koppelman GH, et al. Polymorphisms of the ADAM33 gene are associated with accelerated lung function decline in asthma. Clin Exp Allergy. 2004;34:757–60. doi: 10.1111/j.1365-2222.2004.1938.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee JY, Park SW, Chang HK, Kim HY, Rhim T, Lee JH, et al. A disintegrin and metalloproteinase 33 protein in patients with asthma: relevance to airflow limitation. Am J Respir Crit Care Med. 2006;173:729–35. doi: 10.1164/rccm.200409-1175OC. [DOI] [PubMed] [Google Scholar]
- 24.Foley SC, Mogas AK, Olivenstein R, Fiset PO, Chakir J, Bourbeau J, et al. Increased expression of ADAM33 and ADAM8 with disease progression in asthma. J Allergy Clin Immunol. 2007;119:863–71. doi: 10.1016/j.jaci.2006.12.665. [DOI] [PubMed] [Google Scholar]
- 25.Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A. 2007;104:15858–63. doi: 10.1073/pnas.0707413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L, et al. Roles of epithelial cell-derived periostin in TGF-beta activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci U S A. 2010;107:14170–5. doi: 10.1073/pnas.1009426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Debley JCE, Ohanian A, Ziegler S, Redding G. Pro-remodeling and immunoregulatory-associated cytokine production by airway epithelial cells from asthmatic children. Am J Respir Crit Care Med. 2010;181:A1074. [Google Scholar]
- 28.Lane C, Burgess S, Kicic A, Knight D, Stick S. The use of non-bronchoscopic brushings to study the paediatric airway. Respir Res. 2005;6:53. doi: 10.1186/1465-9921-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. [Accessed December 14, 2011];REST® (Relative Expression Software Tool) Available atL http://rest.gene-quantification.info/
- 30.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chu HW, Balzar S, Seedorf GJ, Westcott JY, Trudeau JB, Silkoff P, et al. Transforming growth factor-beta2 induces bronchial epithelial mucin expression in asthma. Am J Pathol. 2004;165:1097–106. doi: 10.1016/s0002-9440(10)63371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson HG, Mih JD, Krasieva TB, Tromberg BJ, George SC. Epithelial-derived TGF-beta2 modulates basal and wound-healing subepithelial matrix homeostasis. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1277–85. doi: 10.1152/ajplung.00057.2006. [DOI] [PubMed] [Google Scholar]
- 33.Malavia NK, Mih JD, Raub CB, Dinh BT, George SC. IL-13 induces a bronchial epithelial phenotype that is profibrotic. Respir Res. 2008;9:27. doi: 10.1186/1465-9921-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hossny E, El-Awady H, Bakr S, Labib A. Vascular endothelial growth factor over-expression in induced sputum of children with bronchial asthma. Pediatr Allergy Immunol. 2009;20:89–96. doi: 10.1111/j.1399-3038.2008.00730.x. [DOI] [PubMed] [Google Scholar]
- 35.Sharma S, Murphy AJ, Soto-Quiros ME, Avila L, Klanderman BJ, Sylvia JS, et al. Association of VEGF polymorphisms with childhood asthma, lung function and airway responsiveness. Eur Respir J. 2009;33:1287–94. doi: 10.1183/09031936.00113008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kii I, Amizuka N, Minqi L, Kitajima S, Saga Y, Kudo A. Periostin is an extracellular matrix protein required for eruption of incisors in mice. Biochem Biophys Res Commun. 2006;342:766–72. doi: 10.1016/j.bbrc.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 37.Kikuchi Y, Kashima TG, Nishiyama T, Shimazu K, Morishita Y, Shimazaki M, et al. Periostin is expressed in pericryptal fibroblasts and cancer-associated fibroblasts in the colon. J Histochem Cytochem. 2008;56:753–64. doi: 10.1369/jhc.2008.951061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dorn GW., 2nd Periostin and myocardial repair, regeneration, and recovery. N Engl J Med. 2007;357:1552–4. doi: 10.1056/NEJMcibr074816. [DOI] [PubMed] [Google Scholar]
- 39.Butcher JT, Norris RA, Hoffman S, Mjaatvedt CH, Markwald RR. Periostin promotes atrioventricular mesenchyme matrix invasion and remodeling mediated by integrin signaling through Rho/PI 3-kinase. Dev Biol. 2007;302:256–66. doi: 10.1016/j.ydbio.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res. 2002;62:5358–64. [PubMed] [Google Scholar]
- 41.Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, et al. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118:98–104. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y, Haitchi HM, Cakebread J, Sammut D, Harvey A, Powell RM, et al. Epigenetic mechanisms silence a disintegrin and metalloprotease 33 expression in bronchial epithelial cells. J Allergy Clin Immunol. 2008;121:1393–9. e1–14. doi: 10.1016/j.jaci.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 43.Sont JK, Williams LA, Bel EH, van Krieken J, Vandenbroucke JP, Sterk PJ. Clinical control and histopathologic outcome of asthma when using airway hyperresponsiveness as an additional guide to long-term treatment. Am J Respir Crit Care Med. 1999;159:1043–51. doi: 10.1164/ajrccm.159.4.9806052. [DOI] [PubMed] [Google Scholar]
- 44.Ward C, Pais M, Bish R, Reid D, Feltis B, Johns D, Walters EH. Airway inflammation, basement membrane thickening and bronchial hyperresponsiveness in asthma. Thorax. 2002;57:309–16. doi: 10.1136/thorax.57.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chakir J, Laviolette M, Boutet M, Laliberté R, Dubé J, Boulet LP. Lower airways remodeling in nonasthmatic subjects with allergic rhinitis. Lab Invest. 1996;75:735–44. [PubMed] [Google Scholar]
- 46.Braunstahl GJ, Fokkens WJ, Overbeek SE, KleinJan A, Hoogsteden HG, Prins JB. Mucosal and systemic inflammatory changes in allergic rhinitis and asthma: a comparison between upper and lower airways. Clin Exp Allergy. 2003;33:579–87. doi: 10.1046/j.1365-2222.2003.01652.x. [DOI] [PubMed] [Google Scholar]
- 47.Hackett TL, Knight DA. The role of epithelial injury and repair in the origins of asthma. Curr Opin Allergy Clin Immunol. 2007;7:63–8. doi: 10.1097/ACI.0b013e328013d61b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.