Abstract
Background
The publication of a dissertation is an integral part of the four-year postgraduate degree of Master of Medicine (in clinical disciplines) within the School of Medicine at the University of Zambia. The governing research policy states that the subject matter of the dissertation is expected to cover a topic relevant to health care in the Zambian context, that it be conducted in a way that is consistent with international ethical guidelines for biomedical research involving human subjects, and that research outcomes should be maximally utilized. The aim of the study is to explore the characteristics of the Masters of Medicine research at the University of Zambia.
Methodology
This descriptive study explores the subject matter and research methodology by type of clinical specialty of all dissertations from 1986 to 2009.
Results
The 132 dissertations included 36 (27.3%) in Surgery, 35 (26.5%) in Paediatrics, 32 (24.2%) in Internal Medicine, 24 (18.2%) in Obstetrics and Gynaecology, and 5 (3.8%) in Orthopaedic Surgery. Only 7 (5.3%) were interventional/experimental studies (4 of which were randomized controlled trials). Cross-sectional studies were the predominant type of the 125 observational studies (n=112, 84.8%). Thirty-three dissertations (25.0%) predominantly addressed HIV (16 Internal Medicine, 10 Paediatrics, 6 Surgery and 1 Obstetrics and Gynaecology); and 18 (13.6%) predominantly addressed infections, excluding TB (11 in Paediatrics). Other subjects included malignancy (n=6), TB (n=5), and diabetes mellitus (n=4). Over half of the dissertations (76, 57.6%) addressed the determinants of the cause, risk and development of diseases; and a third dealt with management and evaluation of diseases (26 and 18, respectively).
Conclusions
Few dissertations were based on experimental designs and most addressed determinants of the cause of diseases through cross-sectional studies. HIV and infections predominate as diseases reflecting the prevailing disease patterns in Lusaka in particular, and Zambia in general.
Introduction
Doctors have graduated from the School of Medicine at the University of Zambia (UNZA) since 1973 with a joint degree of Bachelor of Medicine and Bachelor of Surgery (MB ChB). To meet the national demand for specialists in district and tertiary-level hospitals, a four-year programme leading to the award of the degree of Master of Medicine (M.Med) in the various clinical specialties commenced in 1981 with the first specialists graduating in 1986. The importance of being able to conduct and interpret research findings is included amongst the competencies and training outcomes of the programme. To that end, it is a requirement for M.Med candidates to develop and implement a research proposal that is published as a dissertation. The subject matter of the dissertation is expected to cover a topic relevant to health care in the Zambian context. The research is also expected to be conducted in a way that is consistent with international ethical guidelines for biomedical research involving human subjects, and research outcomes should be maximally utilized.1 This is consistent with the statements by the World Federation for Medical Education (WFME) relating to postgraduate education, which stress the need for formal training in research, as well as the need for settings with research facilities, activities and priorities.2 National health priorities and, by inference, health research priorities (for example, as listed in priority topics at the biennial National Health Research Conferences) cover issues that include but are not restricted to: HIV/AIDS/STIs, Malaria, Tuberculosis, Child Health, Maternal and Neonatal Health, and Adolescent Health. Various factors affect the choice of dissertation topics, study type and design in the M.Med programme and include: clinical specialty, prevalent disease patterns as presenting in Lusaka and at the tertiary-level University Teaching Hospital, availability of research supervision, data, laboratory and analysis facilities, and funding. The nature of M.Med dissertations have not been studied before. The aim of this enquiry, therefore, is to explore the breadth, depth and diversity of topics, and characteristics by study type of the research published as the dissertations for the M.Med at the University of Zambia.
Methods
We conducted a desk review of dissertations of graduates of the Master of Medicine programme at the University of Zambia. This consisted of a review of dissertations deposited in the special collections of the University of Zambia Library from the first graduates in 1986 through 2009. The list of masters dissertations held by the library was counter-checked against the list of all graduates through 2009. Each dissertation was assessed by 2 reviewers (YA and CWK) and information was abstracted for the year of graduation, and hence publication, of dissertation, clinical specialty of the M.Med, subject matter using MeSH subject headings, and the type of study design.3 As the subjects were varied, and included diseases, conditions, management, techniques, and evaluations, a coding system was used based on the UK Clinical Research Collaboration’s Health Research Classification System (HRCS), a system for classifying the full spectrum of biomedical and health research – from basic to applied – across all areas of health.4 In addition to the general subject headings, dissertations were identified in which a specific disease or condition formed the basis of the study. Based on whether a study was interventional (experimental and quasi-experimental) or non-interventional (non-experimental – i.e. exploratory, descriptive, or analytical); we identified the following 6 study designs: randomized controlled trials, quasi-experimental, cohort, case-control, cross-sectional, and ecologic. All data were entered on an Excel spreadsheet and the year and clinical specialty were double-checked before adding the variables for study design, main disease or condition, and HSRC code. We tabulated frequencies to describe the year, study design, main disease or condition, and HSRC coding, by dissertations from the different clinical specialties. As the numbers were small in different categories, we did not assess associations of clinical specialty with study design, main disease or condition, or HSRC coding.
Results
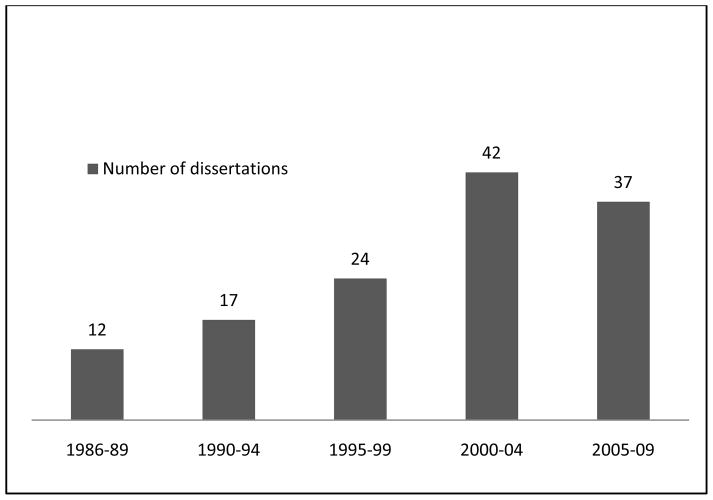
A total of 132 dissertations were reviewed, representing dissertations from all known graduates from 1986 to 2009. In 5-year intervals before 2009, the number of dissertations peaked at 42 in 2000–2004 (Figure 1) but declined to 37 in the next 5-year period of 2005–2009. The M.Med in Surgery programme had the highest number (n=36, 27.3%) while Obstetrics and Gynaecology had the fewest (24, 18.2%) (Table 1). The newer programme in Orthopaedic Surgery had its first graduate in 2000.
Figure 1.
Distributionof M.Med Dissertations at UNZA (1986–2009)
Table 1.
Distributionof dissertations by M.Med specialty and type of study
| Type of study
|
||||||||
|---|---|---|---|---|---|---|---|---|
| RCT | Other Exp. | Cohort | Case Control | CC and CS | Cross-Sectional n (%) | Ecologic | Total N (%) | |
| M.Med: | ||||||||
| Surgery | 2 | 1 | 3 | 1 | 3 | 26 (72.2) | 0 | 36 (27.3) |
| Paediatrics | 0 | 2 | 1 | 0 | 1 | 31 (88.6) | 0 | 35 (26.5) |
| Internal Medicine | 1 | 0 | 2 | 3 | 2 | 23 (71.9) | 1 | 32 (24.2) |
| Obstetrics and Gynaecology | 0 | 0 | 0 | 1 | 0 | 22 (91.7) | 1 | 24 (18.2) |
| Orthopaedic Surgery | 1 | 0 | 0 | 0 | 0 | 4 (80.0) | 0 | 5 (3.8) |
|
| ||||||||
| All M.Med n(%) | 4 (3.0) | 3 (2.3) | 6 (4.5) | 5 (3.8) | 6 (4.5) | 106 (80.3) | 2 (1.5) | 132 (100) |
Data are number (% of specific M.Med total) for cross-sectional study column; number (% of study total) for Total column.
RCT=Randomised Controlled Trial; Other Exp=Other Experimental; CC=Case Control; CS=Cross-sectional.
Only 7 of the 132 dissertations (5.3%) related to interventional studies and, of these, 4 were randomized controlled trials, 2 non-randomized clinical trials, and 1 (a dissertation in Paediatrics) had a quasi-experimental design (Table 1). Of the 125 non-interventional/observational studies, the majority were cross-sectional studies (n=106, 80.3%). The remaining 19 observational studies included 6 cohort (4.5%), 5 case-control (3.8%), 2 ecologic (1.5%) and a further 6 (5.5%) dissertations that had two components – one part cross-sectional and the other case-control. There were no systematic reviews or other types of dissertations. Though cross-sectional studies were the commonest type in all specialties, they ranged from 91.7% of all dissertations in Obstetrics and Gynaecology to 71.9% in Internal Medicine (Table 1). This difference was not statistically significant; OR=4.3, 95% CI (0.75–44.3), p=0.09.
The main disease or condition reported in the dissertations was varied but 5 topics that were common in more than one specialty accounted for 66 of the 132 dissertations (50%) (Table 2). Of the 66, the main subject matter in 33 cases (25% of all dissertations) related to HIV/AIDS. Almost half of HIV/AIDS-related dissertations were from Internal Medicine (16, 48.5%), and nearly a third from Paediatrics (10, 30.3%). Infections other than HIV or TB served as the primary subject matter in 18 dissertations and just under two-thirds were from Paediatrics (11, 61.1%). Other conditions included malignancy (6), TB (5), and diabetes mellitus (4). Malaria was the primary subject matter in 2 dissertations (1 in Internal Medicine and 1 in Paediatrics, which is counted in the “infection” group). Seven dissertations dealt with HIV/AIDS and an additional non-TB infection as co-subjects. We classified these studies in the HIV/AIDS category. Similarly, although 10 dissertations were related to TB, 5 had HIV/AIDS as a major subject matter and were classified as such. Though not shown in Table 2, there were 2 dissertations related to sickle cell (both in Paediatrics), and 2 on hepatitis (1 in Internal Medicine and 1 in Paediatrics).
Table 2.
Characteristics of dissertations by M.Med specialty and disease or condition
| Main disease or condition
|
|||||||
|---|---|---|---|---|---|---|---|
| HIV/AIDS n (%) | Infection n (%) | Malig. | TB | DM | Any Disease n (%) | Total N (%) | |
| M.Med: | |||||||
| Surgery | 6 (18.2) | 3 (16.7) | 5 | 0 | 0 | 14 (21.2) | 36 (27.3) |
| Paediatrics | 10 (30.3) | 11 (61.1) | 0 | 2 | 1 | 24 (36.4) | 35 (26.5) |
| Internal Medicine | 16 (48.5) | 2 (11.1) | 2 | 3 | 23 (34.8) | 32 (24.2) | |
| Obstetrics and Gynaecology | 1 (3.0) | 1 (5.6) | 1 | 0 | 0 | 3 (4.5) | 24 (18.2) |
| Orthopaedic Surgery | 0 (0) | 1 (5.6) | 0 | 1 | 0 | 2 (3.0) | 5 (3.8) |
|
| |||||||
| All M.Med | 33 (100) (25) | 18 (100) (13.6) | 6 (4.5) | 5 (3.8) | 4 (3.0) | 66 (100) (50) | 132 (100) (100) |
TB=Tuberculosis; Malig=Malignancy; DM=Diabetes Mellitus; *Percentage
Data are number (% of disease total) for disease column; number (% of study total) for Total column.
For All M.Med row (% by disease of Total). Note this applies to 66 (50%) of dissertations that studied a specific disease.
The classification of the 132 dissertations using the HRCS guidelines and codings is shown in Table 3. Over half of the dissertations (76, 57.6%) addressed aetiology through the determinants of the cause, risk and development of diseases or conditions; this was the commonest type in all specialties. The next commonest was related to management of diseases and conditions (26, 19.7%), and just under half of these were in Surgery (12, 46.2%). The third commonest category was on evaluation of treatments (18, 13.6%) and again Surgery had the highest proportion in this category (7, 38.9%). There was only one on prevention of a disease from Internal Medicine. Both Paediatrics (n=2) and Obstetrics and Gynaecology (n=1) had dissertations that were classified as Health Services Research.
Table 3.
Characteristics of dissertations by M.Med specialty and HRCS Classification
| HRCS Classification
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Aetiology n (%) | Mgmt. n (%) | Eval. n (%) | Diag. n (%) | HSR n (%) | Prev. n (%) | Total N (%) | ||
| M.Med: | ||||||||
| Surgery | 15 (19.7) | 12 (46.2) | 7 (38.9) | 2 (25) | 36 (27.3) | |||
| Paediatrics | 24 (31.6) | 4 (15.4) | 3 (16.7) | 2 (25) | 2 (66.7) | 35 (26.5) | ||
| Internal Medicine | 21 (27.6) | 2 (7.7) | 4 (22.2) | 4 (50) | 1 (100) | 32 (24.2) | ||
| Obstetrics and Gynaecology | 16 (21.1) | 5 (19.2) | 2 (11.1) | 1 (33.3) | 24 (18.2) | |||
| Orthopaedic Surgery | 3 (11.5) | 2 (11.1) | 5 (3.8) | |||||
|
| ||||||||
| All M.Med | 76 (100) (57.6) | 26 (100) (19.7) | 18 (100) (13.6) | 8 (100) (6.1) | 3 (100) (2.3) | 1 (100) (0.8) | 132 (100) (100) | |
Data are number (% of specific HRCS total) for cross-sectional study column; number (% of study total) for Total column. For All M.Med row (% by classification of Total).
HRCS: Health Research Classification System. Aetiology: determinants of the cause, risk and development of diseases or conditions. Mgmt: Management of diseases and conditions. Eval: Evaluation of Treatments and Therapeutic Interventions. Diag: Detection, screening and diagnosis. HSR: Health Services Research. Prev: Prevention of Disease and Conditions.
Discussion
The number of dissertations has increased over the years; between 4 and 6 candidates are currently enrolled in each specialty in each year of the 4-year programme. The newer programme in Orthopaedic Surgery has had 5 graduates and a recent programme in Urology only commenced in 2007. The 132 M.Med graduates in all specialties over the 24 years of the programme at the University of Zambia contrasts to the University of Nairobi, where 285 graduated between 1975 and 1999 from the postgraduate M.Med programme in the Department of Surgery alone.5 The Zambia Ministry of Health in the 2008 Health Statistical Bulletin reports there are 6 “third level” or specialist hospitals, 21 “second level” or general/provincial hospitals, and 72 “first level” or district hospitals.6 The number of specialists from the M.Med programme is still far from fulfilling national needs and places emphasis on increasing capacity and sustaining the programme.
The majority of dissertations (80.3%) were from cross-sectional studies (Table 1). A breakdown of those that were purely descriptive (assessed the nature, magnitude, and the management of a disease or condition) or analytic (assessed if certain factors were associations) was deferred for a future study that will assess the quality of dissertations. Cross-sectional studies are likely favoured due to the ease of conducting such research given the availability of clinical cases and conditions compared to interventional and cohort studies which require long-term follow-up and are costlier to conduct. The utility of the clinical research findings from the dissertations for the eventual goal of delivering optimal clinical care to patients has to be considered within the context of the strength and weight of the scientific evidence. In the absence of systematic reviews of randomized controlled trials, the highest level of evidence is from individual randomized controlled trials (level 1), followed by cohort studies (level 2), case-control studies (level 3), cross-sectional studies and case series (level 4), and expert opinion without critical appraisal (level 5).7 Most of the dissertations were cross-sectional studies providing low level of evidence regarding therapy, prevention, aetiology or harm. Nevertheless they provide useful pointers to manifestations of disease in the local context and generate hypotheses for further research.
The choice of subject matter, predominantly HIV/AIDS and infectious diseases, reflects prevalent disease patterns in Lusaka and Zambia. However, instead of a disease based classification, the Health Research Classification System (HRCS) allowed the research to be coded across a broader area of disease and health systems. Whereas most studies focused on determinants (n=76, Table 3), management (n=26) or evaluation of disease (n=18), there were few on diagnosis (n=8) and even fewer on health services research (n=3) and prevention (n=1). As with study type, this could be a reflection of the feasibility of conducting such research given the cases in the hospital or community setting. Rohrig et al (2009) point out that the choice of study type or design of medical studies are major determinants of a study’s scientific quality and clinical value but recognize they take into account available financial resources and practical feasibility, including staffing and number of patients.8
Since this was a desk review, challenges in dissertation supervision, ascertaining the number of years spent on the programme and completion or abandonment were not explored, nor were they the focus of this study. The M.Med programme provides for research methodology to be taught to candidates and the role of both the candidate and supervisor in completion of a successful dissertation is stressed by Isenberg and Salmon (2000).9 Adequate supervision is linked to completion of dissertations on time, and to their quality.10, 11 Biomedical journals increasingly mandate that authors follow set guidelines when reporting their studies to ensure accuracy and completeness.12, 13, 14, 15, 16 Reviewing these guidelines at the planning stage of the studies and before publication of the dissertations could be a useful adjunct to ensuring quality.
Whereas dissertations and theses are usually mandatory for students in postgraduate programmes, Gitanjali et al (1998) have questioned their role in teaching research methodology to postgraduates.17 Publications based on the M.Med dissertation findings could not be ascertained through this desk review, as they may have been published in non-indexed journals or by other authors. A further study would be required to determine the extent and characteristics of publications arising from the dissertations.
This study has identified the different types of studies undertaken for dissertations in the M.Med programme, including the preponderance of observational studies. Further research needs to be done on factors affecting the quality of dissertations and the extent to which research findings are published and translated in practice.
References
- 1.University of Zambia. Master of Medicine Handbook. School of Medicine Regulations and Practices. UNZA; 2009. [Google Scholar]
- 2.World Federation for Medical Education. Postgraduate Medical Education, WFME Global Standards for Quality Improvement. WFME Office: University of Copenhagen; Denmark: 2003. [Accessed 7 January 2010.]. http://www3.sund.ku.dk/Activities/WFME%20Postgraduate.pdf. [Google Scholar]
- 3. [Accessed 7 January 2010.];US National Library of Medicine, Mesh website. http://www.nlm.nih.gov/mesh.
- 4.The Health Research Classification System (HRCS) [Accessed 7 January 2010.];UK Clinical Research Collaboration. http://www.hrcsonline.net/rac/summary.
- 5.Magoha GA, Ngumi ZW. Training of surgeons in Kenya at the University of Nairobi teaching hospital. East Afr Med J. 1999 Aug;76(8):462–4. [PubMed] [Google Scholar]
- 6.Ministry of Health, Zambia. Annual Health Statistical Bulletin. 2008 [Google Scholar]
- 7. [Accessed 7 January 2010.];Centre for Evidence-Based Medicine, Oxford. http://www.essentialevidenceplus.com/product/ebm_loe.cfm?show=oxford.
- 8.Röhrig B, du Prel JB, Wachtlin D, Blettner M. Types of study in medical research: part 3 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2009 Apr;106(15):262–8. doi: 10.3238/arztebl.2009.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isenberg DA, Salmon M. How to supervise a thesis: best practice. Hosp Med. 2000 Jul;61(7):499–501. doi: 10.12968/hosp.2000.61.7.1382. [DOI] [PubMed] [Google Scholar]
- 10.Sciscione AC, Colmorgen GH, D’Alton ME. Factors affecting fellowship satisfaction, thesis completion, and career direction among maternal-fetal medicine fellows. Obstet Gynecol. 1998 Jun;91(6):1023–6. doi: 10.1016/s0029-7844(98)00076-3. [DOI] [PubMed] [Google Scholar]
- 11.Pugsley L, Brigley S, Allery L, MacDonald J. Making a difference: researching master’s and doctoral research programmes in medical education. Med Educ. 2008 Feb;42(2):157–63. doi: 10.1111/j.1365-2923.2007.02932.x. [DOI] [PubMed] [Google Scholar]
- 12.CONSORT guidelines (and extensions) [Accessed 7 January 2010.];The CONSORT Statement for randomized controlled trial (RCT), includes the trial’s design, conduct, analysis and interpretation, and to assess the validity of its results. http://www.consort-statement.org/consort-statement/
- 13.STROBE statement. [Accessed 7 January 2010.];STrengthening the Reporting of OBservational studies in Epidemiology (cohort, case–control, or cross-sectional studies) http://www.strobe-statement.org/
- 14.STARD guidelines. STAndards for the Reporting of Diagnostic accuracy studies. Accuracy and completeness of reporting of studies of diagnostic accuracy, to allow readers to assess the potential for bias in the study (internal validity) and to evaluate its generalisability (external validity) [Accessed 7 January 2010.];A checklist of 25 items and recommends the use of a flow diagram which describes the design of the study and the flow of patients. http://www.stard-statement.org/
- 15.STREGA guidelines. STrengthening the REporting of Genetic Association Studies. [Accessed 7 January 2010.];To improve the reporting of genetic association studies by proposing extensions to 12 of the 22 original items of the STROBE checklist. http://www.med.uottawa.ca/public-healthgenomics/web/assets/documents/Final-STREGA%20manuscript-Feb2%202009.pdf.
- 16.PRISMA guidelines. Preferred Reporting Items for Systematic Reviews and Meta-Analyses. [Accessed 7 January 2010.];An evidence-based minimum set of items for reporting in systematic reviews and meta-analyses. http://www.prisma-statement.org/
- 17.Gitanjali B, Raveendran R. Teaching research methodology to postgraduates: is dissertation the only method? Natl Med J India. 1998 Jan-Feb;11(1):23–5. [PubMed] [Google Scholar]