Abstract
We have examined influence of hypocapnia, mild hypercapnia and hypoxia on the durations of fictive apnea and respiratory disruption elicited by injection of 0.1 ml of water into the laryngeal lumen—the laryngeal chemoreflex (LCR)—in 20 unanesthetized, decerebrate, vagotomized piglets aged 4 to 10 days that were paralyzed and ventilated with a constant frequency and tidal volume. The LCR was enhanced by hypocapnia and attenuated by hypercapnia as reported by others. The responses to laryngeal stimulation during hypoxia were varied and complex: some animals showed abbreviated responses during the tachypnea of early hypoxia, followed after 10-15 min by more prolonged apnea and respiratory disruption accompanying the reduction in ventilatory activity that commonly occurs during sustained hypoxia in neonates. We speculate that this later hypoxic enhancement of the LCR may be due to accumulation of adenosine in the brain stem.
Keywords: Laryngeal chemoreflex, Sudden Infant Death Syndrome, Hypocapnia, Hypercapnia, Hypoxia, Piglet
1. Introduction
The larynx is not only the principal organ of speech and song, but also an important valve that both regulates the flow of respiratory gases and protects the lower respiratory tract from aspiration of harmful materials (Bartlett, 1989; Thach, 2010). In accordance with the latter function, the laryngeal mucosa is richly endowed with sensory nerve endings (Sant'Ambrogio et al., 1985), which, when stimulated chemically or mechanically, give rise to rapid and profound protective responses (Sant'Ambrogio and Mathew, 1986). Most laryngeal afferents travel ipsilaterally in the superior laryngeal nerve (SLN) through the nodose ganglion and enter the brain stem in the nucleus of the solitary tract (NTS); there they are distributed via interneurons to higher brain centers and to autonomic and motor nuclei that reflexly influence laryngeal, cardiovascular and respiratory control systems (Miflin, 1993).
The reflex responses to laryngeal stimulation undergo a major developmental shift during early postnatal life (Thach, 2001). An intralaryngeal drop of water or many other fluids in adults generally elicits coughing and only a brief interruption of breathing. The same stimulus in neonates usually produces little or no coughing, but instead apnea, often accompanied by bradycardia (Lee et al., 1977), and these responses may last for many seconds, leading to dangerous levels of arterial and tissue hypoxia (Downing and Lee, 1975). The long suspicion that the LCR may play a role in some cases of the Sudden Infant Death Syndrome (SIDS) (Downing and Lee, 1975; Johnson et al., 1975) is reinforced by the observation that it may elicit fatal apnea in anesthetized animals (Richardson and Adams, 2005). Factors that influence the reflex in neonatal animals have therefore been examined fairly extensively.
To result in apnea, an inhibitory reflex must overcome the individual's underlying drive to breathe. Thus it is not surprising that when that drive is reduced by hypocapnia (Lawson, 1982) or general anesthesia (Lee et al., 1977; Richardson and Adams, 2005), laryngeal reflex apnea may be profound, long-lasting and occasionally fatal. At the other end of the “drive” scale, animals that have their breathing stimulated by hypercapnia (Litmanovitz et al, 1994; Sasaki et al., 2009), exercise (Haouzi et al., 1997) or aminophylline (Lee et al., 1977) are relatively resistant to reflex respiratory inhibition: laryngeal stimulation only results in a brief period of apnea before breathing resumes.
The respiratory consequences of hypoxia are more complicated, reflecting the enhancement of breathing by stimulation of the peripheral chemoreceptors (Kumar, 2009) and the depression of brain function by the direct action of hypoxia centrally (Bissonnette, 2000). A further complication is the time dependent, biphasic respiratory response to sustained hypoxia, which is particularly prominent in neonates, including human infants (Cohen et al., 1997). The initial increase in breathing induced at the onset of hypoxia is followed within seconds or a few minutes by a reduction in ventilation, sometimes to levels below the normoxic baseline. This ventilatory “roll-off” is due—at least in part—to the central accumulation of adenosine (Darnall and Bruce, 1987; Koos, 2011). A final complexity of the interaction of hypoxia and the LCR is that hypoxia becomes progressively severe during the course of an apneic period; by contrast, the arterial PCO2 only rises to approach the value in mixed venous blood (Sasse et al., 1996). After one circulation time, the mixed venous value begins to rise, but only slowly because of the large capacity for CO2 storage (Eger and Severinghaus, 1961).
Beyond the multiple ways in which acute hypoxia can influence breathing, as outlined above, recent observations indicate that neonatal animals that have been exposed to chronic intermittent hypoxia in utero or in early postnatal life have altered responses to respiratory challenges (Reeves and Gozal, 2005). This finding is of uncertain consequence for the LCR and is not addressed in this study, but the question is of potential importance with regard to SIDS as some SIDS infants are known to experience episodes of apnea, bradycardia and hypoxia days or weeks before their demise (Meny et al.,1994).
We have examined the influence of hypocapnia, mild hypercapnia and hypoxia on the LCR in neonatal animals under conditions that control or minimize the influence of some of the variables discussed above. Thus, to avoid the effects of general anesthesia, time-varying blood gases and changes in mechanical ventilation, we studied unanesthetized, decerebrate piglets that were paralyzed and artificially ventilated at a constant frequency and tidal volume with controlled gas mixtures. We monitored phrenic nerve activity, and the animals were vagotomized to avoid reflex effects of lung inflation by the ventilatory pump.
We examined the influence of hypocapnia and hypercapnia to test whether the findings reported previously (Lawson, 1982; Litmanovitz et al., 1994) were directly related to respiratory drive rather than to secondary reflex effects of changes in ventilation and to determine whether the effects of CO2 on the LCR could be shown in our reduced preparation. These hypotheses were confirmed.
The hypoxia study was carried out to test the hypothesis that LCR-induced apnea would be shortened during the early hyperpnea of sustained hypoxia, but would become prolonged during the time-dependent reduction in ventilatory activity during continued hypoxia in a way that might be important for the pathogenesis of SIDS. This hypothesis was confirmed for some animals, but the response pattern for the group of piglets was too variable for this finding to be statistically significant.
2. Methods
We studied 20 piglets (8 male, 12 female), ranging from 4 to 10 days in age and from 1.8 to 3.3 kg in body weight. The Institutional Animal Care and Use Committee of Dartmouth College approved all procedures.
2.1 Surgical preparation
Each piglet was anesthetized with 2-4% halothane in O2. Femoral arterial and venous catheters were placed, and arterial pressure was monitored with a transducer. Rectal temperature was maintained at 38 ± 1° C. by means of a thermostatically controlled heating pad. A cannula was inserted through a low cervical tracheotomy, and the animal was ventilated with O2 at a rate and depth to maintain the end-tidal CO2 concentration, monitored by an infrared analyzer, at ∼5%. After surgical exposure of the carotid sinus regions, the internal and external carotid arteries were ligated to reduce blood loss during decerebration. The animal was placed in the prone position, with the head secured in a stereotaxic head holder. The scalp was incised, the skull was opened, and the brain stem was transected at the level of the superior colliculi; arterial bleeders were clipped, and all brain tissue rostral to the section was removed by suction. After decerebration, halothane was discontinued, and the animal was paralyzed with pancuronium bromide 1 mg/kg iv, supplemented as needed. A phrenic nerve was isolated and sectioned low in the neck; the central cut end was desheathed and positioned under viscous paraffin on a bipolar wire recording electrode to monitor respiratory activity. The phrenic signal was filtered (0.03 – 10 kHz), amplified and integrated as a moving time average with a time constant of 100 msec. The integrated phrenic signal, rectal temperature, end-tidal CO2 concentration and blood pressure were recorded on a laboratory computer for later analysis.
A pharyngeal catheter (PE-90) was inserted through a nostril and positioned with its tip at the rostral border of the larynx. The catheter was filled with water, of which 0.1 ml was injected into the laryngeal lumen during phrenic expiration each time the LCR was elicited. As the volume of the catheter exceeded that of the injectate, the water reaching the larynx was approximately at body temperature. Previous experiments indicate that the laryngeal receptors responding to water are not appreciably temperature sensitive in the range we studied (Xia et al., 2006). After these preparations, the animal was left undisturbed for a period of at least 30 min.
2.2 Experiments with hypercapnia and hypocapnia
In 11 piglets, the inspired gas mixture was changed to ∼2% CO2 in O2, and the ventilatory pump was readjusted to return the end-tidal CO2 concentration to ∼5%. Once a stable state was reached (3-5 min), The LCR was tested 3 times, separated by at least 5 min. Then, without changing the respiratory frequency or tidal volume provided by the ventilator, enough CO2 was removed from the inspired gas to lower the end-tidal CO2 concentration to ∼4%, and after 5 min another 3 tests of the LCR were performed. Finally, the end-tidal CO2 concentration was raised to ∼6% by adding CO2 to the inspired mixture, and the testing of the LCR was repeated. These maneuvers enabled us to assess the influence of CO2 on the LCR without any confounding changes in mechanical stimulation by the pump.
2.3 Experiments with hypoxia
Nineteen piglets (10 of which were previously used in the hypercapnia/hypocapnia experiments) were studied during acute, sustained exposure to either 10% or 12% O2 in the inspired air for 10 -20 min. The initial experiments were with 10% O2, but as this was associated with high mortality, we changed to 12% O2. Ventilation was maintained at the control rate, and no significant change occurred in end-tidal CO2 concentration. The LCR was elicited 3 times at 5-min intervals before the hypoxic exposure and was tested at intervals during the hypoxic period.
2.4 Analysis
We evaluated the LCR responses based entirely on time-related events, as we have done before (Curran et al., 2005; Duy et al., 2010). We defined the duration of the LCR as the time from the beginning of water-induced phrenic apnea until the onset of five regular fictive breaths; these breaths did not have to be identical in frequency or amplitude to those occurring before the reflex was elicited, but had to occur at regular intervals (variation < 20%). We also measured the longest fictive apnea duration occurring with each LCR trial; this response measure was more conservative and less subjective than the duration of the LCR. In order to compare the responses of different animals studied under similar conditions, we expressed each response of each piglet as a percentage of the average baseline value for that piglet. The data were not normally distributed, and we employed non-parametric statistical testing (Wilcoxon signed rank test) with P > 0.05 as the criterion to assess the significance of response changes with conditions. Because we sometimes had to reposition the phrenic nerve on the electrode during the experiment, we have not done a formal analysis of the peak amplitude of the integrated phrenic activity per breath.
3. Results
3.1 Experiments with hypercapnia and hypocapnia
Hypercapnia generally increased the peak amplitude of integrated phrenic nerve bursts and consistently slowed respiratory frequency in these vagotomized piglets. As illustrated in Fig. 1, eliciting the LCR under mildly hypercapnic conditions (end-tidal CO2 = ∼ 6%) disrupted respiratory activity less than when the same animal was eucapnic or hypocapnic. The results for all 11 piglets are summarized in Table 1. The duration of the LCR was significantly shorter during hypercapnia than during hypocapnia. The duration of the longest apnea showed a similar trend, but the difference was not significant. The mean arterial blood pressure did not change significantly during these experiments.
Figure 1.
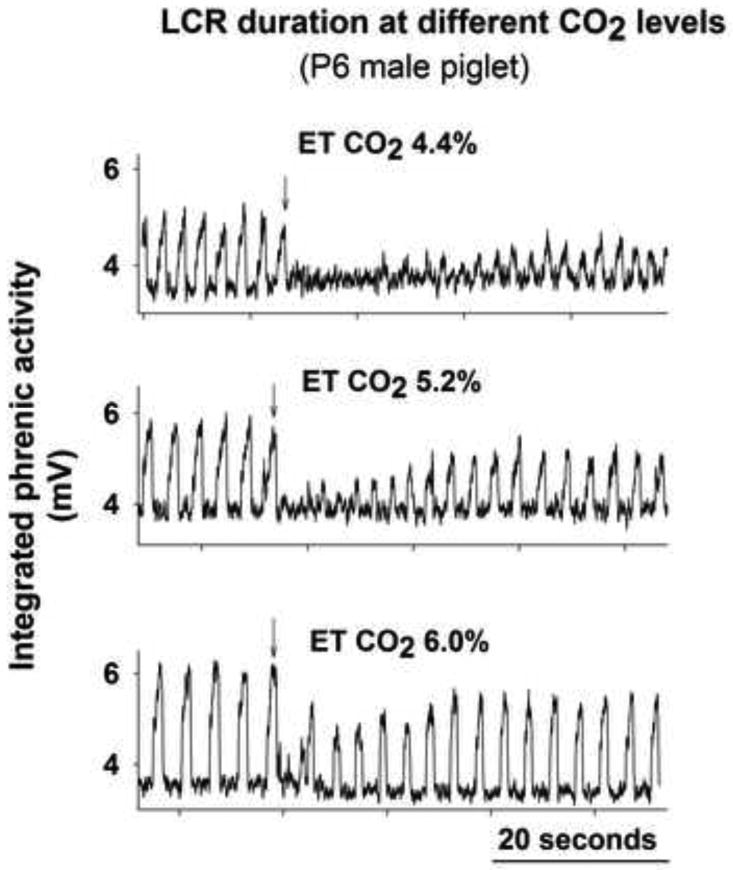
Integrated phrenic nerve activity of a P6 male piglet showing responses to intralaryngeal injection of 0.1 ml of water (at arrows) under hypocapnic, normocapnic and hypercapnic conditions.
Table 1.
Phrenic responses to 0.1 ml intralaryngeal water injections in 11 piglets during imposed hypocapnia and hypercapnia: mean values and (SE). Respiratory frequency was significantly higher during hypocapnia than during hypercapnia. The duration of the LCR was significantly longer during hypocapnia than during hypercapnia. The duration of the longest apnea showed a similar trend, which was not significant.
| Hypocapnia | Hypercapnia | P value | |
|---|---|---|---|
|
|
|||
| End-tidal CO2 (%) | 4.12 (0.07) | 6.25 (0.23) | |
| Respiratory frequency (%of normocapnic) | 131 (7.8) | 85 (1.8) | 0.01* |
| LCR duration (% of normocapnic) | 153 (17.8) | 88 (7.8) | 0.01* |
| Apnea duration (% of normocapnic) | 125 (17.5) | 92 (10.5) | 0.13 |
3.2 Experiments with hypoxia
Data suitable for analysis were obtained for only 14 of the 19 piglets subjected to hypoxia; the remaining 5 animals did not complete the protocol because of severe hypotension and/or loss of phrenic nerve activity. The responses of the animals exposed to 10% (n = 3) and 12% O2 (n = 11) were similar and were combined for analysis. The quantitative responses to hypoxia varied widely among the 14 animals, but several features were qualitatively consistent. Respiratory frequency often increased at the onset of hypoxia, but phrenic activity slowed and became more irregular toward the end of the hypoxic exposure. In the experiment illustrated in Fig 2, the duration of respiratory disruption by LCR trials was shorter than baseline near the onset of hypoxia, but became longer during the course of hypoxic exposure. This pattern was apparent in 8 of the 14 animals, but was not consistent enough to be statistically significant. Table 2 shows that for the 14 piglets, the mean durations of the LCR and of the longest apnea both increased from near the onset of hypoxia to the later part of the hypoxic exposure. Hypoxia was always accompanied by some degree of arterial hypotension, but the trends in LCR and apnea durations were not temporally correlated with the decrease in arterial pressure.
Figure 2.
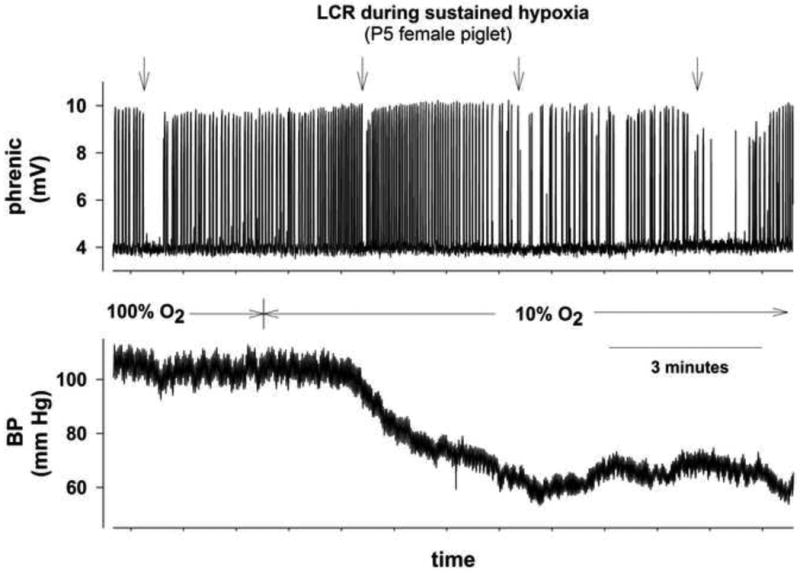
Integrated phrenic nerve activity and blood pressure record of a P5 female piglet showing responses to intralaryngeal injection of 0.1 ml of water (at arrows) under hyperoxic control conditions and at intervals during 10 min of sustained hypoxia (FIO2 = 0.10).
Table 2.
Phrenic responses to 0.1 ml intralaryngeal water injections in 14 piglets during sustained ventilation with 10% or 12% inspired O2: mean values and (SE). The data shown are for the first test, 1-3 min after the onset of hypoxia, and the last test, after 10-15 min of sustained hypoxia. Respiratory frequency did not change appreciably. The durations of the LCR and the longest associated apnea tended to increase from the first to the last test (see Fig. 2), but these trends were not statistically significant.
| First Hypoxia | Last Hypoxia | P value | |
|---|---|---|---|
|
|
|||
| Respiratory frequency (%of normoxic) | 105 (10.0) | 95 (10.0) | 0.34 |
| LCR duration (% of baseline) | 141 (28.9) | 201 (30.0) | 0.11 |
| Apnea duration (% of baseline) | 119 (27.1)) | 190 (30.9) | 0.06 |
4. Discussion
4.1 Experiments with hypercapnia and hypocapnia
The general pattern of the response of phrenic activity to changes in end-tidal CO2 is consistent with previous observations in vagotomized neonatal animals. Dreshaj et al (1999) and Curran et al. (2000) found that hypercapnia in decerebrate, vagotomized, paralyzed and ventilated piglets increased phrenic activity per breath, accompanied by a decrease in respiratory frequency. A study of decerebrate, vagotomized neonatal rats is in general agreement: the response to hypercapnia was characterized by increased phrenic activity per breath, and the youngest animals (P0-P4) slowed their frequency while ventilated with 5% CO2 (Zhou et al., 1996).
As shown in Fig. 1 and Table 1, the duration of the LCR was inversely related to the end-tidal CO2 concentration in these experiments. This finding is consistent with the idea that increasing respiratory drive acts to reduce the inhibitory effectiveness of the laryngeal protective reflex. Similarly, Lawson (1982) found that the duration of phrenic apnea following electrical stimulation of an SLN was greater under hypocapnic than hypercapnic conditions in anesthetized, vagotomized, paralyzed, ventilated piglets. Litmanovitz et al. (1994) reported similar responses of the diaphragm EMG in anesthetized, spontaneously breathing piglets. Bongianni et al. (1996) found a comparable response of phrenic activity during hypocapnia in anesthetized adult cats, but did not find a shortening of reflex apnea under hypercapnic conditions. As our vagotomized piglets slowed their respiratory frequency with increasing end-tidal CO2, the decreased LCR duration cannot be attributed to a tachypneic breathing pattern preceding laryngeal stimulation.
4.2 Experiments with hypoxia
The results of the hypoxic challenges were less consistent and more complicated. As illustrated in Fig. 2, some piglets increased respiratory frequency at the onset of hypoxia and then slowed phrenic activity as hypoxic exposure was continued, as others have reported (Darnall and Bruce, 1987; Fung et al., 1996). The responses varied widely among animals, however, and for the group as a whole, no significant trend in respiratory frequency occurred (Table 2).
As illustrated in Fig. 2, the LCR duration and the longest apnea elicited by intralaryngeal water increased during hypoxic exposure in some animals, but the data were much more variable than during hypercapnia. Both the LCR and the longest apnea showed an increasing trend with time of exposure, but neither change was statistically significant (Table 2). We speculate that adenosine may have accumulated more rapidly in the brain stems of animals that displayed this response pattern (Darnall and Bruce, 1987; Koos, 2011), as we have shown that adenosine prolongs the LCR by activation of A2A receptors in the nucleus of the solitary tract (Xia et al., 2008; Duy et al., 2010).
The variability of these results is reflected in previous studies of the LCR during hypoxia. Thus apnea elicited by intralaryngeal water in anesthetized piglets has been reported to be either shortened (Woodson and Brauel, 1992; Goding, 1998) or prolonged (Lanier et al., 1983) by 10 minutes of acute hypoxia. Depth of anesthesia may have been an important variable contributing to these contrasting results.
The problem of anesthesia has been avoided in an extensive series of studies in awake, chronically instrumented lambs. Here, too, the results with respect to hypoxia are complex: the duration of apnea with intralaryngeal water instillation has generally been shortened by acute hypoxia (Sladek et al.,1993; Milerad and Sundell, 1999; Sundell et al., 2003), although not significantly at all ages. As pointed out by Milerad and Sundell (1999), the apneic response to laryngeal stimulation during acute hypoxia may be attenuated by carotid chemoreceptor mediated ventilatory drive, but enhanced by the attendant hypocapnia. A very interesting finding is that lambs kept in 10% O2 for 12 days following birth had longer LCR apneas and slower recovery than control lambs, tested either in normoxia or hypoxia (Sladek et al., 1993). The chronic hypoxic exposure of these animals was interrupted five times each day for feeding and cleaning, so the experience of the lambs may have been what is now referred to as chronic intermittent hypoxia.
Wennergren and colleagues (1989) measured the apneic responses to intralaryngeal water in 12 human infants during normoxia and mild hypoxia (usually 15% FIo2). The average apneic duration was 2.6 sec in normoxia and 5.3 sec in hypoxia (P<0.01). Strikingly, one 4-week-old boy had 15 sec of apnea in normoxia, increasing to 30 sec in hypoxia; this child died unexpectedly six weeks later, with an ultimate diagnosis of SIDS.
4.3 Reflections
Few vertebrate physiological requirements are more fundamental and widespread than the need for an adequate supply of O2. In mammals O2 is required during both fetal and postnatal life, but the appropriate physiological strategies for dealing with reduced O2 availability are very different before and after birth (Leiter and Böhm, 2007). Thus fetal mammals, which can do little to enhance their O2 supply, respond to hypoxia by reducing non-essential activities such as fetal breathing movements and redirecting blood flow to the most essential organs, the heart and the brain (Rurak et al., 1990). These “fetal” responses are retained in the “diving” response, which is particularly prominent in young animals of many species, including humans (Pedroso et al., 2011). Once air breathing is established at birth, however, a major response to hypoxia is to increase ventilation—an appropriate strategy for increasing the O2 supply. This conversion from the fetal, O2-conserving response to ventilatory stimulation following birth must be prompt, but it is not instantaneous, as evidenced by the time course of postnatal development of the ventilatory response to hypoxia (Liu et al., 2006). Thus the variation in the airway protective responses of our 4- to 10-day-old piglets under hypoxic conditions is not surprising: they were examined at various stages of the maturation of their conversion from placental to pulmonary O2 supply, with its attendant neurological responses. There were no apparent differences in responses that could be attributed to gender. The piglets were of mixed breeds, and we cannot estimate possible genetic contributions to the varied responses.
We have avoided the problem of general anesthesia in this study, but have not dealt with the crucial variable of sleep, which prolongs the LCR in intact piglets (Van der Velde et al., 2003) and is particularly important in relation to SIDS, which usually occurs during a period of sleep (Kinney and Thach, 2009), Sleep itself undergoes extensive maturation during infancy (Scher, 2008) and may interact importantly with hypoxia in determining the response to laryngeal stimulation.
4.4 Conclusion
In decerebrate, vagotomized piglets, hypocapnia enhances the inhibitory effectiveness of the LCR, and mild hypercapnia attenuates the response, in accordance with the concept that increased respiratory drive counteracts the inhibitory action of the reflex. The influence of hypoxia is less consistent and more complex, reflecting the central and peripheral actions of hypoxia and their time-varying characteriatics. The possible impact of chronic intermittent hypoxia on the LCR was not addressed in this study but remains undefined and may be important in the pathogenesis of SIDS.
Even though the effects of hypoxia on the LCR were too variable to allow a statistically significant pattern to be defined, the progressive lengthening of LCR-induced apnea that accompanied the time-dependent reduction in ventilation during sustained hypoxia in some animals (e.g. Fig 2) may initiate some cases of SIDS. A remaining question is whether this response, when it occurs, is attributable to the central accumulation of adenosine, which occurs during sustained hypoxia in neonatal animals (Darnall and Bruce, 1987; Koos, 2011).
Highlights.
We measured the laryngeal chemoreflex (LCR) in decerebrate neonatal piglets.
LCR-induced apnea (LCR-A) was prolonged by hypocapnia and shortened by hypercapnia.
In hypoxia some piglets had biphasic responses of respiratory frequency and LCR-A.
Prolonged LCR-A in hypoxia may be a trigger for the Sudden Infant Death Syndrome.
Acknowledgments
This work was supported by grants 36379 and 42707 from the NICHD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartlett D., Jr Respiratory functions of the larynx. Physiol Rev. 1989;69:33–57. doi: 10.1152/physrev.1989.69.1.33. [DOI] [PubMed] [Google Scholar]
- Bissonnette JM. Mechanisms regulating hypoxic respiratory depression during fetal and postnatal life. Am J Physiol Regu Integr Comp Physiol. 2000;278:R1391–R1400. doi: 10.1152/ajpregu.2000.278.6.R1391. [DOI] [PubMed] [Google Scholar]
- Bongianni F, Fontana GA, Mutolo D, Pantaleo T. Effects of central chemical drive on poststimulatory respiratory depression of laryngeal origin in the adult cat. Brain Research Bull. 1996;39:267–273. doi: 10.1016/0361-9230(95)02139-6. [DOI] [PubMed] [Google Scholar]
- Cohen G, Malcolm G, Henderson-Smart D. Ventilatory response of the newborn infant to mild hypoxia. Peadiatr Pulmonol. 1997;24:163–172. doi: 10.1002/(sici)1099-0496(199709)24:3<163::aid-ppul1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Curran AK, Chen G, Darnall RA, Filiano JJ, Li A, Nattie EE. Lesion or muscimol in the rostral ventral medulla reduces ventilator output and the CO2 response in decerebrate piglets. Respir Physiol. 2000;123:23–37. doi: 10.1016/s0034-5687(00)00143-2. [DOI] [PubMed] [Google Scholar]
- Curran AK, Xia L, Leiter JC, Bartlett D., Jr Elevated body temperature enhances the laryngeal chemoreflex in decerebrate piglets. J Appl Physiol. 2005;98:780–786. doi: 10.1152/japplphysiol.00906.2004. [DOI] [PubMed] [Google Scholar]
- Darnall RA, Bruce RD. Effects of adenosine and xanthine derivatives on breathing during acute hypoxia in the anesthetized newborn piglet. Pediatr Pulmonol. 1987;3:110–116. doi: 10.1002/ppul.1950030213. [DOI] [PubMed] [Google Scholar]
- Downing SE, Lee JC. Laryngeal chemosensitivity: a possible mechanism for sudden infant death. Pediatrics. 1975;55:640–649. [PubMed] [Google Scholar]
- Dreshaj IA, Haxhiu MA, Abu-Shaweesh J, Carey RE, Martin RJ. CO2-induced prolongation of expiratory time during early development. Respir Physiol. 1999;116:125–132. doi: 10.1016/s0034-5687(99)00039-0. [DOI] [PubMed] [Google Scholar]
- Duy PM, Xia L, Bartlett D, Jr, Leiter JC. An adenosine A2A agonist injected in the nucleus of the solitary tract prolongs the laryngeal chemoreflex by a GABAergic mechanism in de cerebrate piglets. Exp Physiol. 2010;96:774–787. doi: 10.1113/expphysiol.2010.052647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger EI, Severinghaus JW. The rate of rise of PaCO2 in the apneic anesthetized patient. Anesthesiology. 1961;22:419–425. doi: 10.1097/00000542-196105000-00013. [DOI] [PubMed] [Google Scholar]
- Fung ML, Wang W, Darnall RA, St John WM. Characterization of ventilator responses to hypoxia in neonatal rats. Respir Physiol. 1996;103:57–66. doi: 10.1016/0034-5687(95)00077-1. [DOI] [PubMed] [Google Scholar]
- Goding GS. Correlation of laryngeal chemoreflex severity with laryngeal muscle response. Laryngoscope. 1998;108:863–872. doi: 10.1097/00005537-199806000-00015. [DOI] [PubMed] [Google Scholar]
- Haouzi P, Beyaert C, Gille JP, Chalon B, Marchal F. Laryngeal reflex apnea is blunted during and after hindlimb muscle contraction in sheep. Am J Physiol Regu Integr Comp Physiol. 1997;272:R586–R592. doi: 10.1152/ajpregu.1997.272.2.R586. [DOI] [PubMed] [Google Scholar]
- Johnson P, Salisbury DM, Storey AT. Apnea induced by stimulation of sensory receptors in the larynx. In: Bosma JF, Showacre J, editors. Symposium on Development of Upper Airway Respiratory Anatomy and Function Implications for Sudden Infant Death Syndrome. Washington, DC: U. S. Department of Health, Education and Welfare; 1975. pp. 160–185. [Google Scholar]
- Kinney HC, Thach BT. The sudden infant death syndrome. N Engl J Med. 2009;361:795–805. doi: 10.1056/NEJMra0803836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos BJ. Adenosine A2a receptors and O2 sensing in development. Am J Physiol Regu Integr Comp Physiol. 2011;301:R601–R622. doi: 10.1152/ajpregu.00664.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P. Systemic effects resulting from carotid body stimiulation—invited article. Adv Exp Med Biol. 2009;648:223–233. doi: 10.1007/978-90-481-2259-2_26. [DOI] [PubMed] [Google Scholar]
- Lanier B, Richardson MA, Cummings C. Effect of hypoxia on laryngeal reflex apnea—implications for sudden infant death. Otolaryngol Head Neck Surg. 1983;91:597–604. doi: 10.1177/019459988309100602. [DOI] [PubMed] [Google Scholar]
- Lawson EE. Recovery from central apnea: effect of stimulus duration and end-tidal CO2 partial pressure. J Appl Physiol. 1982;53:105–109. doi: 10.1152/jappl.1982.53.1.105. [DOI] [PubMed] [Google Scholar]
- Lee JC, Stoll BJ, Downing SE. Properties of the laryngeal chemoreflex in neonatal piglets. Am J Physiol. 1977;233:R30–R36. doi: 10.1152/ajpregu.1977.233.1.R30. [DOI] [PubMed] [Google Scholar]
- Leiter JC, Böhm I. Mechanisms of pathogenesis in the Sudden Infant Death Syndrome. Respir Physiol Neurobiol. 2007;159:127–138. doi: 10.1016/j.resp.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Litmanovitz I, Dreshaj I, Miller MJ, Haxhiu MA, Martin RJ. Central chemosensitivity affects respiratory muscle responses to laryngeal stimulation in the piglet. J Appl Physiol. 1994;76:403–408. doi: 10.1152/jappl.1994.76.1.403. [DOI] [PubMed] [Google Scholar]
- Liu Q, Lowry TE, Wong-Riley MTT. Postnatal changes in ventilation during normoxia and acute hypoxia in the rat: implication for a sensitive period. J Physiol. 2006;577:957–970. doi: 10.1113/jphysiol.2006.121970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meny RG, Carroll JL, Carbone MT, Kelly DH. Cardiorespiratory recordings from infants dying suddenly and unexpectedly at home. Pediatrics. 1994;93:44–49. [PubMed] [Google Scholar]
- Miflin SW. Laryngeal afferent inputs to the nucleus of the solitary tract. Am J Physiol. 1993;265:R269–R276. doi: 10.1152/ajpregu.1993.265.2.R269. [DOI] [PubMed] [Google Scholar]
- Milerad J, Sundell HW. Reduced inspiratory drive following laryngeal chemoreflex apnea during hypoxia. Respir Physiol. 1999;116:35–45. doi: 10.1016/s0034-5687(99)00035-3. [DOI] [PubMed] [Google Scholar]
- Pedroso FS, Riesgo RS, Gatiboni T, Rotta NT. The diving reflex in healthy infants in the first year of life. J Child Neurol. 2012;27:168–171. doi: 10.1177/0883073811415269. [DOI] [PubMed] [Google Scholar]
- Reeves SR, Gozal D. Developmental plasticity of respiratorycontrol following intermittent hypoxia. Respir Physiol Neurobiol. 2005;149:301–311. doi: 10.1016/j.resp.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Richardson MA, Adams J. Fatal apnea in piglets by way of laryngeal chemoreflex: postmortem findings as anatomic correlates of sudden infant death syndrome in the human infant. Laryngoscope. 2005;115:1163–1169. doi: 10.1097/01.MLG.0000165458.52991.1B. [DOI] [PubMed] [Google Scholar]
- Rurak DW, Richardson BS, Patrick JE, Carmichael L, Homan J. Blood flow and oxygen delivery to fetal organs and tissues during sustained hypoxemia. Am J Physiol. 1990;258(Regulatory Integrative Comp Physiol 27):R1116–R1122. doi: 10.1152/ajpregu.1990.258.5.R1116. [DOI] [PubMed] [Google Scholar]
- Sant'Ambrogio G, Mathew OP, Sant'Ambrogio FB, Fisher JT. Laryngeal receptors responding to respiratory events. Prog Clin Biol Res. 1985;176:171–182. [PubMed] [Google Scholar]
- Sant'Ambrogio G, Mathew OP. Laryngeal receptors and their reflex responses. Clin Chest Med. 1986;7:211–222. [PubMed] [Google Scholar]
- Sasaki CT, Hundal JS, Wadie M, Woo JS, Rosenblatt W. Modulating effects of hypoxia and hypercarbia on glottis closing force. Ann Otol Rhinol Laryngol. 2009;118:148–153. doi: 10.1177/000348940911800211. [DOI] [PubMed] [Google Scholar]
- Sasse SA, Berry RB, Nguyen TK, Light RW, Mahutte CK. Arterial blood gas changes during breath-holding from functional residual capacity. Chest. 1996;110:958–964. doi: 10.1378/chest.110.4.958. [DOI] [PubMed] [Google Scholar]
- Scher MS. Ontogeny of EEG-sleep from neonatal through infancy periods. Sleep Medicine. 2008;9:615–636. doi: 10.1016/j.sleep.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Sladek M, Grogaard JB, Parker RA, Sundell HW. Prolonged hypoxemia enhances and acute hypoxemia attenuates laryngeal reflex apnea in young lambs. Pediatr Res. 1993;34:813–820. doi: 10.1203/00006450-199312000-00024. [DOI] [PubMed] [Google Scholar]
- Sundell HW, Karmo H, Milerad J. Impaired cardiorespiratory recovery after laryngeal stimulation in nicotine-exposed young lambs. Pediatr Res. 2003;53:104–112. doi: 10.1203/00006450-200301000-00018. [DOI] [PubMed] [Google Scholar]
- Thach BT. Maturation and transformation of reflexes that protect the laryngeal airway from liquid aspiration from fetal to adult life. Am J Med. 2001;111(Suppl 8A):69S–77S. doi: 10.1016/s0002-9343(01)00860-9. [DOI] [PubMed] [Google Scholar]
- Thach BT. Laryngeal chemoreflexes and development. Pediatr Resp Rev. 2010;11:213–218. doi: 10.1016/j.prrv.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Van der Velde L, Curran AK, Filiano JJ, Darnall RA, Bartlett D, Jr, Leiter JC. Prolongation of the laryngeal chemoreflex after inhibition of the rostral ventral medulla in piglets: a role in SIDS? J Appl Physiol. 2003;94:1883–1895. doi: 10.1152/japplphysiol.01103.2002. [DOI] [PubMed] [Google Scholar]
- Wennergren G, Hertzberg T, Milerad J, Bjure J, Lagercrantz H. Hypoxia reinforces laryngeal reflex bradycardia in infants. Act a Paediatr Scand. 1989;78:11–17. doi: 10.1111/j.1651-2227.1989.tb10879.x. [DOI] [PubMed] [Google Scholar]
- Woodson GE, Brauel G. Arterial chemoreceptor influence on the laryngeal chemoreflex. Otolaryngol Head Neck Surg. 1992;107:775–782. doi: 10.1177/019459988910700612.1. [DOI] [PubMed] [Google Scholar]
- Xia L, Leiter JC, Bartlett D., Jr Laryngeal water receptors are insensitive to body temperature in neonatal piglets. Respir Physiol Neurobiol. 2006;150:82–86. doi: 10.1016/j.resp.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Xia L, Bartlett D, Jr, Leiter JC. An adenosine A2a antagonist injected in the NTS reverses thermal prolongation of the LCR in decerebrate piglets. Respir Physiol Neurobiol. 2008;164:358–365. doi: 10.1016/j.resp.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Huang Q, Fung ML, Li A, Darnall RA, Nattie EE, St John WM. Phrenic response to hypercapnia in the unanesthetized, decerebrate, newborn rat. Respir Physiol. 1996;104:11–22. doi: 10.1016/0034-5687(95)00098-4. [DOI] [PubMed] [Google Scholar]


