Abstract
Objective
To determine whether men with rheumatoid arthritis (RA) are more likely to achieve remission compared to women.
Methods
RA patients enrolled in the CORRONA cohort between October 2001 and January 2010 were selected for the present analyses. Detailed clinical, demographic and drug utilization data were available at enrollment (baseline) and at subsequent follow up visits. We examined the influence of sex on CDAI remission (CDAI≤2.8) using sustained remission or point remission as the primary outcome measure in multivariate stepwise logistic regression models. We stratified the data by RA duration at baseline (≤2 years or >2 years) to investigate whether RA duration had differential effects on remission in men and women.
Results
A total of 10,299 RA patients (2,406 men and 7,893 women) were available for this study. In both early and established RA, women had more severe disease at baseline with worse disease acitivity measures, modified Health Assessment Questionnaire Disability Index, pain visual analog scale and depression. Women were also more likely to have been treated with DMARDs and anti-TNF therapy compared to men. In the regression models, maleness was associated with sustained remission in early RA (OR: 1.38, 95% CI: 1.07, 1.78, p=0.01), but not in established RA. However, for point remission, an inverse association was observed with maleness in established RA (OR: 0.65, 95% CI: 0.48, 0.87, p=0.005) and not in early RA.
Conclusion
Within the large, real-life CORRONA cohort of RA patients, men were more likely to achieve sustained remission compared to women in early RA, although not in established RA.
Introduction
With recent advances in available therapies for rheumatoid arthritis (RA) and their effectiveness in management of the disease (1), the goal of RA treatment has evolved from a reduction in pain despite ongoing inflammation and disability, to achievement and sustainability of disease remission (2). However, the ability to respond favorably to treatment and achieve remission varies widely between patients. Over the last few years, there has been a growing interest in a posible role for sex in predicting remission in RA.
A number of studies have reported better responses (3–6) and/or increased remission (4, 6–13) among men compared to women in response to both biologic and non-biologic disease modifying anti-rheumatic drugs (DMARDs), while some did not find any association between sex and remission (14, 15). Thus, although evidence of increased remission among men is accumulating, it has not firmly been established. Further, most of these studies investigated remission at only one point in time, which may not adequately reflect clinical remission.
In the present study, our goal was to examine the role of sex as a potential predictor of remission in a large cohort of patients with RA from the Consortium of Rheumatology Researchers of North America (CORRONA). We wished to test the hypothesis that male sex is a significant predictor of remission in RA.
Patients and Methods
Patients
The study population consisted of RA patients enrolled in the prospective observational CORRONA cohort between October 1, 2001 and January 8th, 2010. The CORRONA network includes 268 participating academic and community rheumatologists at 103 sites in 35 states within the USA. Details of the CORRONA network of recruitment sites and cohort have been published elsewhere (16). All patients who satisfied the 1987 American College of Rheumatology (ACR) criteria for RA (17) and were being treated by participating rheumatologists were eligible for enrollment into the cohort. Ethical approval for the study was obtained from institutional review boards of participating academic recruitment sites and from a central institutional review board for community-based private recruitment sites. All patients provided written informed consent prior to being enrolled in the cohort.
Data Collected
Detailed clinical, demographic and drug utilization data were collected for each patient at enrollment into the cohort (study baseline), and at subsequent routine follow up visits at intervals of approximately 3 months. The data collected by the participating rheumatologists included joint counts for 28 swollen (SJC28) and tender joints (TJC28), physician global assessment (GA), presence of radiographic erosions, rheumatoid factor seropositivity, comorbidities, number of previous hospitalizations and individual components of the ACR response criteria. Drug utilization data were recorded for traditional as well as biological DMARDs. Patients also completed questionnaires which collected self-reported data on global health assessment (patient GA), pain on a Visual Analog Scale (VAS), work status (full-time, part-time, not working outside home, student, disabled or retired), ethnicity, depression prior to baseline, the modified Health Assessment Questionnaire Disability Index (mHAQ-DI), and use of prednisone and/or anti-TNF therapy.
Disease Activity Scores
Disease activity was assessed as (a) Disease Activity Scores based on 28 joint counts, using 4 variables and the ESR (DAS28) (18) and (b) Clinical Disease Activity Index (CDAI) scores (19), and computed as follows:
DAS28 = [0.56*sqrt(TJC28) + 0.28*sqrt(SJC28) + 0.70*ln(ESR) + 0.014*(global)], and CDAI = SJC28 + TJC28 + Physician Global Assessment (GA) (in cm) + Patient GA (in cm).
CDAI Remission as the Endpoint
The primary outcome of interest, i.e. RA remission, was assessed based on CDAI remission (CDAI≤2.8) (20). Patients were classified as being in “sustained remission” if they were in CDAI remission at any 2 consecutive visits after baseline that were more than 2 months, and up to 6 months, apart. For those patients who did not achieve sustained remission during the study follow up, we determined whether they achieved “point remission”. Patients were considered to be in “point remission” if they satisfied the CDAI remission criteria at any single visit after baseline, and for reasons of reliability, if the CDAI score at the remission visit was at least 2.0 units lower than at the previous and subsequent visits. Since patients can often go in and out of remission at different points in time, for each patient who satisfied the point remission criteria in the present study, we chose to include only the first instance of remission.
Statistical Analyses
Baseline sex comparisons
All patients who had a diagnosis of RA at study baseline were included in the analyses unless they had missing data on sex, ethnicity and/or CDAI scores at baseline or were in CDAI remission at baseline or had had a follow up of <1 year since enrollment into the cohort. Baseline comparisons of disease characteristics and drug use between men and women were performed using Chi-square tests for categorical variables, and t-tests, using a pooled or unpooled standard error with Satterthwaite’s approximation for degrees of freedom as appropriate, for continuous variables.
Stepwise Multivariate Logistic Regression
We examined the influence of sex as the main explanatory variable (female sex as the referent category) on CDAI remission using sustained remission as the primary outcome measure in multivariate logistic regression models. Patients who did not attain sustained remission, but satisfied our point remission criteria were excluded from these analyses. We also performed a separate analysis for the patients who achieved point but not sustained remission. For both sustained remission and point remission, we used a stepwise regression approach to assess which covariates contributed to the prediction model. Candidate covariates included age, baseline CDAI score, ethnicity (Caucasian (reference) vs. non-Caucasian), RA duration, work status (full-time (reference) vs. working part-time/not working outside home/student or being disabled/retired), self-reported depression prior to baseline, prednisone use (yes or no), use of anti-TNF therapy (yes or no), strong DMARD use (i.e. Methotrexate, Leflunomide, Sulfasalazine, Azathioprine, Cyclosporine, Tacrolimus, Gold, etc.), presence of sub-cutaneous nodules (yes or no), pain VAS and previous hospitalization for RA (yes or no). Since the length of follow up varied between patients, depending on when they were enrolled in the cohort, and the chances of remission are likely to increase with increasing length of follow up, we also adjusted for “length of follow up” in the regression models. Further, although we stratified the data according to whether the patients had a duration of RA of at most 2 years or more than 2 years, we adjusted for differences in RA duration between patients within the strata.
Results
Baseline sex comparisons
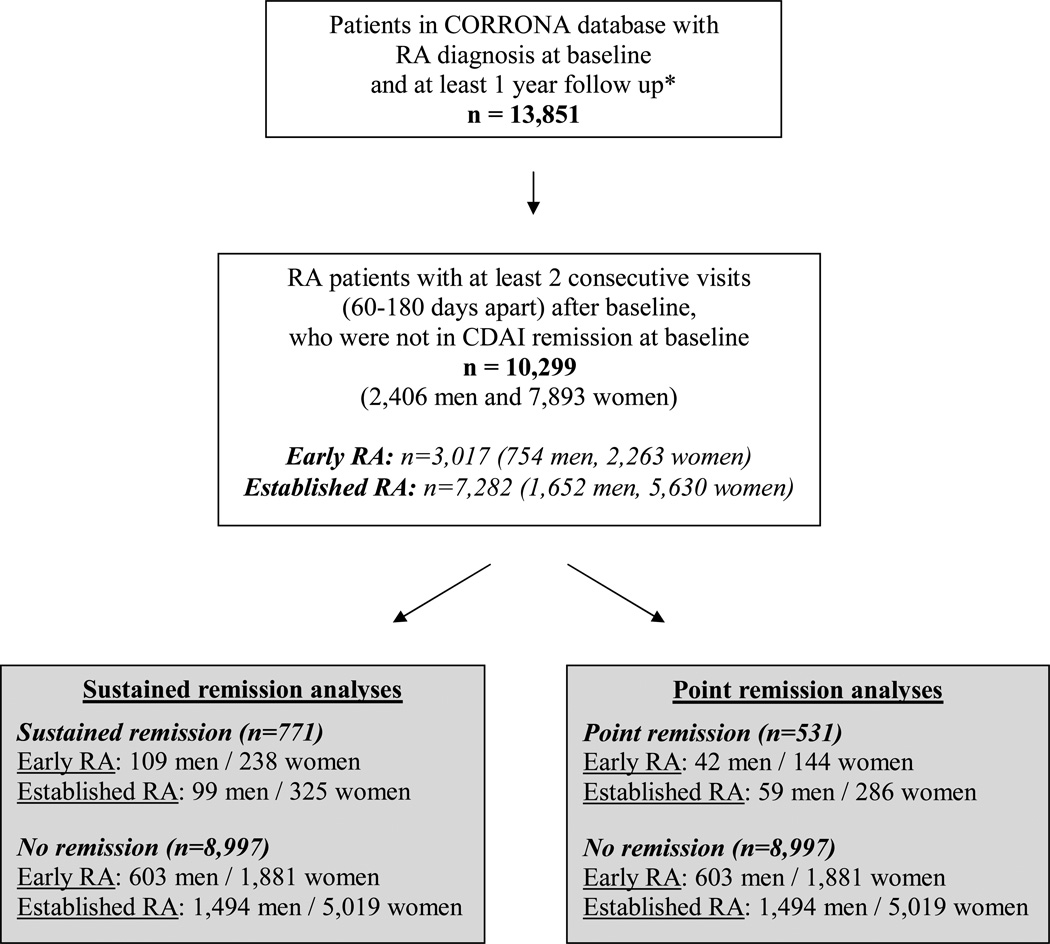
A total of 10,299 RA patients (2,406 men and 7,893 women), who were not in remission at baseline, were available for the present analyses after we excluded patients who had missing data on sex, ethnicity or CDAI scores at baseline (Figure 1). The demographic and clinical characteristics of these patients at baseline are summarized by sex and RA duration in Table 1. The patients were predominantly Caucasian (>82%). Overall, in both the early RA and established RA groups, the men were older and a significantly larger proportion of men were married (early RA: 80% vs. 62%, p<0.0001; established RA: 77% vs. 63%, p<0.0001) and/or were employed full-time (early RA: 55% vs. 44%, p<0.0001; established RA: 40% vs. 32%, p<0.0001) compared to women. Subcutaneous nodules and RF seropositivity were numerically more common among men, whereas radiographic erosions were more common among women; however, these differences were not statistically significant. Disease activity measures were worse among women; these included CDAI scores (early RA: 18.7±13.5 vs. 17.4±13.2, p=0.02; established RA: 16.6±12.3 vs. 15.8±12.0, p=0.02), tender joint counts (early RA: 6.1±6.5 vs. 4.9±6.0, p<0.0001; established RA: 5.0±6.0 vs. 4.4±5.6, p=0.0004), ESR (early RA: 25.6±21.3 vs. 23.5±23.4, p=0.12; established RA: 26.7±22.0 vs. 20.3±19.6, p<0.0001) and patient global health (early RA: 35.2±25.9 vs. 32.6±25.4, p=0.02; established RA: 35.0±25.4 vs. 32.9±24.5, p=0.003). Swollen joint counts and morning stiffness were similar between men and women. Physician global scores were higher among women (early RA: 31.0±21.0 vs. 29.5±20.9, p=0.66; established RA: 28.1±20.8 vs. 26.8±20.2, p=0.02), as were self-reported measures, including the patient pain VAS (early RA: 37.8±27.0 vs. 35.2±26.1, p=0.02; established RA: 36.8±26.0 vs. 34.1±24.9, p<0.0001), the modified HAQ disability index (early RA: 0.41±0.45 vs. 0.36±0.45, p=0.02; established RA: 0.42±0.47 vs. 0.35±0.43, p<0.0001), as well as self-reported depression prior to baseline (early RA: 27% vs. 14%, p<0.0001; established RA: 28% vs. 16%, p<0.0001). Although in early RA significantly more women had been hospitalized for RA before baseline (men: 0.8% vs. women: 2.3%, p=0.009), more men had had joint surgery (24% vs. 17%, p<0.0001); these differences were not found in established RA. Men and women did not differ in their use of strong DMARDs or prednisone, although in the established RA group, more men were using prednisone (35% vs. 32%, p=0.01). Weak DMARDs (Hydoxychloroquine, Minocycline) (early RA: 14% vs. 9%, p<0.0001; established RA: 15% vs. 11%, p=0.0002) and anti-TNF therapy (early RA: 26% vs. 21%, p=0.003; established RA: 56% vs. 50%, p<0.0001), on the other hand, were more commonly used among women. Duration of follow up did not differ between men and women (early RA: 13.7±7.8 vs. 13.5±7.9, p=0.50; established RA: 16.3±9.0 vs. 15.6±8.8, p=0.02).
Figure 1. Selection of RA Patients from the CORRONA Database.
Footnote:
* RA patients with non-missing data for sex, ethnicity and CDAI at baseline
Table 1.
Disease Characteristics at Baseline in the CORRONA Cohort of RA Patients
| Early RA | Established RA | |||||
|---|---|---|---|---|---|---|
| Characteristic | Men (N = 754) |
Women (N = 2,263) |
p | Men (N = 1,652) |
Women (N = 5,630) |
p |
| Age (years), mean ± SD | 57.9±12.6 | 53.7±14.2 | < 0.0001 | 60.0±11.8 | 57.9±12.8 | < 0.0001 |
| Disease Duration (years), mean ± SD | 0.9±0.8 | 0.9±0.8 | 0.83 | 12.9±9.1 | 13.9±9.8 | <0.0001 |
| Ethnicity, N (%) | ||||||
| White | 658 (87.3) | 1868 (82.6) | 0.002 | 1435 (86.9) | 4654 (82.7) | <0.0001 |
| Marital Status (married), N (%) | 596 (79.9) | 1380 (61.5) | <0.0001 | 1266 (77.4) | 3494 (62.8) | <0.0001 |
| Work Status, N (%) | ||||||
| Full time | 413 (55.1) | 987 (43.9) | < 0.0001 | 657 (40.3) | 1794 (32.2) | < 0.0001 |
| Part-time/Not working outside the home/Student |
97 (12.9) | 673(30.0) | 268 (16.5) | 1627 (29.2) | ||
| Disabled/Retired | 240 (32.0) | 586 (26.1) | 704 (43.2) | 2145 (38.5) | ||
| Subcutaneous nodules ever, N (%) | 66 (8.8) | 146 (6.5) | 0.03 | 455 (27.6) | 1302 (23.2) | 0.0002 |
| Rheumatoid factor seropositivity, N (%) | 351 (73.3) | 923 (69.9) | 0.16 | 745 (79.3) | 2305 (77.1) | 0.16 |
| Erosions, N (%) | 9 (1.8) | 43 (2.8) | 0.56 | 103 (9.3) | 427 (10.8) | 0.22 |
| ESR, mean ± SD | 23.5±23.4 | 25.6±21.3 | 0.12 | 20.3±19.6 | 26.7±22.0 | <0.0001 |
| Tender Joint Count, mean ± SD | 4.9±6.0 | 6.1±6.5 | <0.0001 | 4.4±5.6 | 5.0±6.0 | 0.0004 |
| Swollen Joint Count, mean ± SD | 6.3±6.5 | 6.1±6.2 | 0.48 | 5.5±5.7 | 5.3±5.6 | 0.31 |
| CDAI, mean ± SD | 17.4±13.2 | 18.7±13.5 | 0.02 | 15.8±12.0 | 16.6±12.3 | 0.02 |
| Morning Stiffness (hours), mean ± SD | 1.6±3.5 | 1.6±2.9 | 0.92 | 1.2±2.3 | 1.1±1.8 | 0.29 |
| Physician Global VAS, mean ± SD | 29.5±20.9 | 31.0±21.0 | 0.66 | 26.8±20.2 | 28.1±20.8 | 0.02 |
| Patient Global VAS, mean ± SD | 32.6±25.4 | 35.2±25.9 | 0.02 | 32.9±24.5 | 35.0±25.4 | 0.005 |
| Patient Pain VAS, mean ± SD | 35.2±26.1 | 37.8±27.0 | 0.02 | 34.1±24.9 | 36.8±26.0 | <0.0001 |
| mHAQ, mean ± SD | 0.36±0.45 | 0.41±0.45 | 0.008 | 0.35±0.43 | 0.42±0.47 | <0.0001 |
| Depression (self-reported), N (%) | 106(14.3) | 594 (27.0) | <0.0001 | 255 (15.8) | 1508 (27.6) | <0.0001 |
| MI or Stroke before BL, N (%) | 59 (7.8) | 67 (3.0) | <0.0001 | 169 (10.2) | 205 (3.6) | <0.0001 |
| Diabetes Mellitus before BL, N (%) | 59 (7.8) | 142 (6.3) | 0.14 | 160 (9.7) | 395 (7.0) | 0.0003 |
| Background Prednisone Use, N (%) | 289 (38.3) | 798 (35.3) | 0.13 | 577 (34.9) | 1782 (31.7) | 0.01 |
| Background TNFi Use, N (%) | 156 (20.7) | 589 (26.0) | 0.003 | 819 (49.6) | 3177 (56.4) | <0.0001 |
| Background "Strong" DMARD Use, N (%) | 457 (60.6) | 1418 (62.7) | 0.31 | 1064 (64.4) | 3671 (65.2) | 0.55 |
| Background "Weak" DMARD Use, N (%) | 65 (8.6) | 322 (14.2) | <0.0001 | 184 (11.1) | 834 (14.8) | 0.0002 |
| Previously hospitalized for RA, N (%) | 6 (0.8) | 52 (2.3) | 0.009 | 97 (5.9) | 294 (5.2) | 0.31 |
| Previous joint surgery | 183 (24.3) | 383 (17.0) | <0.0001 | 572 (34.8) | 1945 (34.7) | 0.96 |
| Length of follow up (quarters), mean ± SD | 13.7±7.8 | 13.5±7.9 | 0.70 | 16.3±9.0 | 15.6±8.8 | 0.009 |
Mean values and standard deviations (SD) are reported for continuous variables. Proportions are shown for categorical variables.
Stepwise Multivariate Logistic Regression
(a) Sustained remission
A total of 771 patients achieved sustained CDAI remission during the course of the study (Figure 1). In the logistic regression model with CDAI sustained remission as the outcome variable, male sex was a significant predictor of sustained remission in early RA (unadjusted OR: 1.43, 95% CI: 1.12, 1.82, p=0.004), but not in established RA (unadjusted OR: 1.02, 95% CI: 0.81, 1.29, p=0.84). As shown in Table 2, after adjusting for covariates in the multivariate model, male sex was still associated with an increased odds for sustained remission in early RA (OR: 1.38, 95% CI: 1.07, 1.78, p=0.01), whereas in established RA, no association was observed with sex (OR: 0.93, 95% CI: 0.72, 1.18, p=0.55). In early RA, covariates that were indicative of more severe disease were significantly and inversely associated with sustained remission, i.e. self-reported depression, presence of nodules, use of anti-TNF therapy and longer RA duration. Ethnicity did not influence remission status.
Table 2.
Multivariate Logistic Regression of Sex on Sustained CDAI Remission
| Outcome: CDAI sustained remission | |||
|---|---|---|---|
| RA duration<=2 years | |||
| Variable | OR | 95% CI | p value |
| Sex* | 1.38 | 1.07 – 1.78 | 0.01 |
| Ethnicity* | 0.99 | 0.71 – 1.37 | 0.96 |
| Baseline CDAI | 1.00 | 0.99 – 1.01 | 0.65 |
| RA duration | 0.75 | 0.64 – 0.89 | 0.0007 |
| Depression (self-reported) | 0.40 | 0.28 – 0.58 | <0.0001 |
| TNFi use | 0.65 | 0.47 – 0.89 | 0.008 |
| Sub-cutaneous nodules | 0.41 | 0.20 – 0.76 | 0.008 |
| Pain VAS | 0.99 | 0.98 – 0.99 | <0.0001 |
| RA duration>2 years | |||
| Variable | OR | 95% CI | p value |
| Sex* | 0.93 | 0.75 – 1.22 | 0.55 |
| Ethnicity* | 0.91 | 0.69 – 1.20 | 0.53 |
| Baseline CDAI | 0.99 | 0.97 – 0.99 | 0.03 |
| RA duration | 0.98 | 0.97 – 1.00 | 0.007 |
| Depression | 0.71 | 0.51 – 0.86 | 0.01 |
| Prednisone use | 0.66 | 0.50 – 0.81 | 0.0009 |
| Sub-cutaneous nodules | 0.53 | 0.42 – 0.76 | <0.0001 |
| Pain VAS | 0.99 | 0.98 – 0.99 | <0.0001 |
Footnote:
All covariates adjusted for in the final model are listed as independent variables.
The referent group for these variables were as follows: Sex – female; ethnicity – Caucasian; work status – full-time
(b) Point remission (among those not achieving sustained remission)
Among the patients who did not achieve sustained remission, 531 achieved point remission. As shown in Table 3, in the stepwise logistic regression model, male sex was inversely associated with point remission among established RA patients (unadjusted OR: 0.70, 95% CI: 0.52, 0.92, p=0.01), but not in the early RA group (unadjusted OR: 0.88, 95% CI: 0.61, 1.24, p=0.48). After adjusting for covariates, maleness was still inversely associated with point remission in estabished RA (OR: 0.65, 95% CI: 0.48, 0.87, p=0.005), but not early RA (OR: 0.85, 95% CI: 0.59, 1.21, p=0.39).
Table 3.
Multivariate Logistic Regression of Sex on CDAI Point Remission (among those not achieving sustained remission)
| Outcome: CDAI point remission | |||
|---|---|---|---|
| RA duration<=2 years | |||
| Variable | Odds ratio (OR) |
95% CI | p value |
| Sex* | 0.86 | 0.59 – 1.21 | 0.39 |
| Ethnicity* | 0.97 | 0.62 – 1.45 | 0.88 |
| Baseline CDAI | 0.99 | 0.98 – 1.00 | 0.06 |
| Prednisone use | 1.45 | 1.06 – 1.97 | 0.02 |
| RA duration>2 years | |||
| Variable |
Odds ratio (OR) |
95% CI | p value |
| Sex* | 0.65 | 0.48 – 0.87 | 0.005 |
| Ethnicity* | 0.82 | 0.58 – 1.13 | 0.23 |
| Baseline CDAI | 0.99 | 0.98 – 1.00 | 0.06 |
| Length of follow up | 1.01 | 1.00 – 1.03 | 0.03 |
| RA duration | 0.98 | 0.97 – 1.00 | 0.01 |
| Pain VAS | 0.99 | 0.98 – 0.99 | <0.0001 |
Footnote:
All covariates adjusted for in the final model are listed as independent variables.
The referent group for these variables were as follows: Sex – female; ethnicity – Caucasian
Discussion
In this large observational cohort of RA patients from the CORRONA database, male sex was a significant and independent predictor for sustained CDAI remission. This association was seen in early RA, and was not observed in established RA. Since there were a number of variables associated with sex at baseline in this cohort, such that women had more severe baseline disease, we identified among them those that we believed could potentially influence remission and adjusted for them in the logistic regression models. These included baseline CDAI scores, anti-TNF therapy, self-reported depression, pain VAS and the work status category (included as patients who worked part-time, did not work outside the home or were students). Adjusting for these confounders in the logistic regression models slightly reduced the strength of the association. Nonetheless, male sex remained significantly and independently associated with sustained remission in the early RA group. Thus, although the contribution of sex was relatively small, it is most likely real and not just a statistical artifact. Further, the smaller numbers of men compared to women in our analyses does not in our opinion change the results as they, in fact, represent a relatively large sample of men for RA studies. Among the patients who did not achieve sustained remission during our study follow up of this CORRONA cohort, sex did not influence point remission in early RA, although women were more likely to achieve point remission than men in the established RA group.
The present study is the first to report that male sex is a significant predictor for sustained remission in early RA in contrast to established RA. Most previous studies on this topic had examined the influence of sex on point remission in RA (7, 9, 11–13, 21). However, point remission may be limited in its clinical importance as it is based on data from a single time point and may not be as reliable or clinically relevant as sustained remission which is based on data from at least 2 time points spread over a period of time. An association with sustained remission had been reported in an early RA cohort (8), but similar investigations in established RA cohorts had not been performed. Despite variations in the definitions of remission used in the different studies based on the DAS44, DAS28, SDAI, CDAI, and ACR criteria (13), our findings are in agreement with previous reports of male sex being a significant predictor of remission in RA (4, 6–13). We also previously examined response measures other than remission in men and women with RA, including EULAR responses in two other RA cohorts (5, 22), and found that men had better EULAR responses compared to women, in early RA and not established RA (22). Thus, our findings of increased sustained remission among men in early RA and not established RA in the CORRONA cohort are in agreement with those previous findings, and support the finding that men have better responses irrespective of the treatment outcome under study, including sustained remission
In general, sex-specific investigations as presented here have not been addressed in most studies of RA remission. Among the few studies that have examined sex as a predictor for remission either as a primary predictor or as a covariate in the multivariate analyses, only two did not find an association between sex and remission (14, 15). This could be due to the very small sample sizes (195 and 105 patients) in both of these studies. In the large multi-national QUEST-RA study, although it was shown that men achieved remission more often than women irrespective of the definition of remission (13), it has also been postulated that sex differences in RA disease activity may be the result of a sex bias in reporting of disease activity measures (21). One could also speculate other possibilities to explain the data, including immunological response differences, drug dosing differences and genetic differences between men and women. Our data do not allow us to differentiate among these possibilities.
It is as yet unclear why men are more likely than women to achieve sustained remission in early RA and not established RA. As we and others have demonstrated, patients with shorter RA duration, i.e. early RA, are more likely to achieve remission (3, 23). It is therefore quite plausible that treatment responses and rates of remission may be of smaller magnitude among both men and women in established RA, thus making the sex differences less apparent especially since the difference in response observed between men and women in early RA is in itself relatively small. The increased odds of sustained remission among men in early RA is nevertheless intriguing and has not yet been explained. Presumably, such differences in treatment response between men and women could arise as a result of genetic, physiological, immunological or even psychosocial differences between the sexes. In the multivariate regression models, self-reported depression, sub-cutaneous nodules and anti-TNF therapy were significant predictors for no remission. It may be expected that more severe disease, as indicated by sub-cutaneous nodules and anti-TNF therapy, and being depressed would reduce the probability of remission as suggested from previous studies (24–26). Even after these confounders were adjusted in the logistic regression models, male sex was still a significant and independent predictor of sustained remission in the CORRONA cohort. Furthermore, there were no differences between men and women in the effect of self-reported depression, anti-TNF therapy or prednisone on sustained remission in the CORRONA cohort (data not shown). This suggests that there are additional sex-specific factors in operation in influencing the chances of sustained remission in RA, especially in the first couple of years after diagnosis. The range of possible physiological, immunological, psychological factors that may differ between men and women and that could influence remission in RA is vast, and identification of the specific culprit responsible for the increased sustained remission among men in early RA is beyond the scope of this study. There may also be “gender” differences contributing to men being more likely to undergo sustained remission. For example, CDAI scores, and hence CDAI remission, are highly dependent on pain perception. We could speculate that men may have a higher threshold for reporting joint tenderness and global health in the early stages of the disease, but as disease duration increases, adaptive mechanisms relating to pain perception may be in operation, leading to more similar reporting of symptoms between men and women. Regarding patients who did not achieve sustained remission, however, we cannot explain our findings of why women should be more likely to achieve point remission in established RA and not in early RA.
The large number of sites, relatively long follow-up, both physician derived and patent-derived outcomes and use of a “real-life” cohort represent significant strengths of the study. There are also some limitations in this study. Our results demonstrate an association between male sex and sustained remission in early RA; however, the available data and observational nature of the CORRONA cohort do not allow us to determine the cause of this association. Although our database size allows us to adjust for many potential confounders, the possibility remains that there may be additional unknown confounders that we have not recorded and so could not adjust for in the multivariate models, including the true causative agent. We used CDAI remission as our outcome measure because data on the CDAI were available on a larger number of patients in the CORRONA database and because CDAI remission is a more rigorous definition of remission. Although there is currently no gold standard for assessing clinical remission in RA, comparisons of measures of remission have shown that the CDAI remission criteria are more stringent than those for DAS28 or modified ACR remission, allowing for less residual disease activity (27). Therefore, we feel that the CDAI remission is an appropriate measure for the primary outcome in this study. Given the large sample size of the CORRONA cohort, it is possible that statistical significance may have been achieved as a result of the large sample size without reflecting clinical significance, as seen for some of the baseline comparisons in table 1. Our main findings that men are more likely to achieve sustained remission does not, however, appear to be the result of a statistical artifact. Nonetheless, we would like to point out that the number of men in our study cohort, although large for RA studies, is not large enough to make the results irrefutable. The data analyzed in the present study were collected at over 100 different sites by different rheumatologists who are part of the CORRONA network. Some subtle variation in the clinical measures recorded may have been introduced as a result of assessment by different rheumatologists or there may have been differences in routine clinical care and standard treatment practices specific to certain recruitment sites. At the same time, that same large number of sites and physicians mitigates against a systematic difference in these areas. The CORRONA cohort consists of patients in routine clinical care enrolled since 2001, and the inclusion criteria only required that the patients satisfy the 1987 ACR criteria for RA and were being treated by a rheumatologist participating in the CORRONA network. Therefore, within the cohort, patients had variable RA duration, and had been on various therapies for various lengths of time. We could not adjust for duration of therapy in the analyses presented as we did not have accurate data on drug usage before cohort enrollment. As a proxy for treatment duration, we adjusted for RA duration, under the assumption that treatment started soon after diagnosis.
In summary, within the large, real-life CORRONA cohort of RA patients, men were more likely to achieve sustained remission compared to women in early RA, although not in established RA.
Significance and Innovations.
-
-
The present study investigates the influence of sex on remission using sustained remission as the outcome of interest, in contrast to previous studies which used point remission.
-
-
This study is the first to report that male sex is a significant predictor for sustained remission in early RA, and not in established RA.
Acknowledgements
Damini Jawaheer, Ph.D. is supported by a Career Development Award (K01AR053496) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS).
The Consortium of Rheumatology Researchers of North America was supported by Abbott, Amgen, BMS, Centocor, Genentech, Lilly, and Roche through contracted subscriptions to the database.
Footnotes
Disclosures
Dr. Furst has received consultant fees, speaking fees, and/or honoraria (less than $10,000 each) from Abbott, Actelion, Amgen, BMS, Biogen Idec, Centocor, Genentech, Gilead, GSK, NI, Nitec, Novartis, Pfizer, Roche, and UCB; serves on the advisory boards for Abbott, Amgen, BMS, Centocor, Genentech, Biogen Idec, Roche, and UCB; and serves as Director of Publications for the Consortium of Rheumatology Researchers of North America (CORRONA) and receives consultant fees.
References
- 1.van Vollenhoven RF. Treatment of rheumatoid arthritis: state of the art 2009. Nat Rev Rheumatol. 2009;5(10):531–541. doi: 10.1038/nrrheum.2009.182. [DOI] [PubMed] [Google Scholar]
- 2.Lipsky PE. Are new agents needed to treat RA? Nat Rev Rheumatol. 2009;5(10):521–522. doi: 10.1038/nrrheum.2009.197. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JJ, Wells G, Verhoeven AC, Felson DT. Factors predicting response to treatment in rheumatoid arthritis: the importance of disease duration. Arthritis Rheum. 2000;43(1):22–29. doi: 10.1002/1529-0131(200001)43:1<22::AID-ANR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Kvien TK, Uhlig T, Odegard S, Heiberg MS. Epidemiological aspects of rheumatoid arthritis: the sex ratio. Ann N Y Acad Sci. 2006;1069:212–222. doi: 10.1196/annals.1351.019. [DOI] [PubMed] [Google Scholar]
- 5.Jawaheer D, Maranian P, Park G, Lahiff M, Amjadi SS, Paulus HE. Disease progression and treatment responses in a prospective DMARD-naive seropositive early rheumatoid arthritis cohort: does gender matter? J Rheumatol. 2010;37(12):2475–2485. doi: 10.3899/jrheum.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saevarsdottir S, Wallin H, Seddighzadeh M, Ernestam S, Geborek P, Petersson IF, et al. Predictors of response to methotrexate in early DMARD naive rheumatoid arthritis: results from the initial open-label phase of the SWEFOT trial. Ann Rheum Dis. 2011;70(3):469–475. doi: 10.1136/ard.2010.139212. [DOI] [PubMed] [Google Scholar]
- 7.Hyrich KL, Watson KD, Silman AJ, Symmons DP. Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford) 2006;45(12):1558–1565. doi: 10.1093/rheumatology/kel149. [DOI] [PubMed] [Google Scholar]
- 8.Forslind K, Hafstrom I, Ahlmen M, Svensson B. Sex: a major predictor of remission in early rheumatoid arthritis? Ann Rheum Dis. 2007;66(1):46–52. doi: 10.1136/ard.2006.056937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mancarella L, Bobbio-Pallavicini F, Ceccarelli F, Falappone PC, Ferrante A, Malesci D, et al. Good clinical response, remission, and predictors of remission in rheumatoid arthritis patients treated with tumor necrosis factor-alpha blockers: the GISEA study. J Rheumatol. 2007;34(8):1670–1673. [PubMed] [Google Scholar]
- 10.van der Heijde D, Klareskog L, Landewe R, Bruyn GA, Cantagrel A, Durez P, et al. Disease remission and sustained halting of radiographic progression with combination etanercept and methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 2007;56(12):3928–3939. doi: 10.1002/art.23141. [DOI] [PubMed] [Google Scholar]
- 11.Burmester GR, Ferraccioli G, Flipo RM, Monteagudo-Saez I, Unnebrink K, Kary S, et al. Clinical remission and/or minimal disease activity in patients receiving adalimumab treatment in a multinational, open-label, twelve-week study. Arthritis Rheum. 2008;59(1):32–41. doi: 10.1002/art.23247. [DOI] [PubMed] [Google Scholar]
- 12.Makinen H, Hannonen P, Sokka T. Sex: a major predictor of remission as measured by 28- joint Disease Activity Score (DAS28) in early rheumatoid arthritis? Ann Rheum Dis. 2008;67(7):1052–1053. doi: 10.1136/ard.2007.084897. [DOI] [PubMed] [Google Scholar]
- 13.Sokka T, Hetland ML, Makinen H, Kautiainen H, Horslev-Petersen K, Luukkainen RK, et al. Remission and rheumatoid arthritis: Data on patients receiving usual care in twenty-four countries. Arthritis Rheum. 2008;58(9):2642–2651. doi: 10.1002/art.23794. [DOI] [PubMed] [Google Scholar]
- 14.Mottonen T, Hannonen P, Korpela M, Nissila M, Kautiainen H, Ilonen J, et al. Delay to institution of therapy and induction of remission using single-drug or combination-disease-modifying antirheumatic drug therapy in early rheumatoid arthritis. Arthritis Rheum. 2002;46(4):894–898. doi: 10.1002/art.10135. [DOI] [PubMed] [Google Scholar]
- 15.Vazquez I, Graell E, Gratacos J, Canete JD, Vinas O, Ercilla MG, et al. Prognostic markers of clinical remission in early rheumatoid arthritis after two years of DMARDs in a clinical setting. Clin Exp Rheumatol. 2007;25(2):231–238. [PubMed] [Google Scholar]
- 16.Kremer JM. The CORRONA database. Autoimmun Rev. 2006;5(1):46–54. doi: 10.1016/j.autrev.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 18.van der Heijde DM, van 't Hof MA, van Riel PL, Theunisse LA, Lubberts EW, van Leeuwen MA, et al. Judging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity score. Ann Rheum Dis. 1990;49(11):916–920. doi: 10.1136/ard.49.11.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aletaha D, Nell VP, Stamm T, Uffmann M, Pflugbeil S, Machold K, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther. 2005;7(4):R796–R806. doi: 10.1186/ar1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S100–S108. [PubMed] [Google Scholar]
- 21.Sokka T, Toloza S, Cutolo M, Kautiainen H, Makinen H, Gogus F, et al. Women, men, and rheumatoid arthritis: analyses of disease activity, disease characteristics, and treatments in the QUEST-RA Study. Arthritis Res Ther. 2009;11(1):R7. doi: 10.1186/ar2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jawaheer D, Olsen J, Hetland ML. Gender Differences in Response to Anti-TNF Therapy in Early and Established Rheumatoid Arthritis – Results from the Longitudinal Danish DANBIO Registry. J Rheumatol. 2011 doi: 10.3899/jrheum.110548. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furst DE, Pangan AL, Harrold LR, Chang H, Reed G, Kremer JM, et al. Greater likelihood of remission in rheumatoid arthritis patients treated earlier in the disease course: Results from the Consortium of Rheumatology Researchers of North America registry. Arthritis Care Res (Hoboken) 2011;63(6):856–864. doi: 10.1002/acr.20452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kekow J, Moots R, Khandker R, Melin J, Freundlich B, Singh A. Improvements in patient-reported outcomes, symptoms of depression and anxiety, and their association with clinical remission among patients with moderate-to-severe active early rheumatoid arthritis. Rheumatology (Oxford) 2011;50(2):401–409. doi: 10.1093/rheumatology/keq327. [DOI] [PubMed] [Google Scholar]
- 25.Hider SL, Tanveer W, Brownfield A, Mattey DL, Packham JC. Depression in RA patients treated with anti-TNF is common and under-recognized in the rheumatology clinic. Rheumatology (Oxford) 2009;48(9):1152–1154. doi: 10.1093/rheumatology/kep170. [DOI] [PubMed] [Google Scholar]
- 26.Katchamart W, Johnson S, Lin HJ, Phumethum V, Salliot C, Bombardier C. Predictors for remission in rheumatoid arthritis patients: A systematic review. Arthritis Care Res (Hoboken) 2010;62(8):1128–1143. doi: 10.1002/acr.20188. [DOI] [PubMed] [Google Scholar]
- 27.Mierau M, Schoels M, Gonda G, Fuchs J, Aletaha D, Smolen JS. Assessing remission in clinical practice. Rheumatology (Oxford) 2007;46(6):975–979. doi: 10.1093/rheumatology/kem007. [DOI] [PubMed] [Google Scholar]