Abstract
Islet transplantation offers a promising treatment for type 1 diabetes (T1D). However, a major hurdle in this treatment is the rapid loss of functional islets during culture and after transplantation. The liver site, currently utilized for transplantation, is suboptimal for achieving long-term insulin independence due to a rapid islet loss followed by a chronic decline in islet function after transplantation. Herein, we report a synthetic saccharide-peptide (SP) hydrogel that allows suspending islets in liquid and injecting for in situ polymerization without forming islet clumps, indicating its potential in extrahepatic islet transplantation. In vitro, rat islets in SP hydrogel maintained a 3D structure and high glucose-stimulated insulin release similar to that observed in freshly isolated islets for 4 weeks, while control islets cultured in suspension lost their 3D structure and insulin release responses by 2 weeks. Biocompatibility of SP hydrogel was shown by the absence of cytokine mRNA activation in peripheral blood mononuclear cells (PBMC) exposed to hydrogel in vitro and by the absence of cellular infiltrates in and around the hydrogel implanted subcutaneously. Syngeneic Lewis rat islets transplanted in SP hydrogel in various extrahepatic sites stained strongly for insulin, and more effectively reversed diabetes than unencapsulated islets when transplanted in an omental pocket. In conclusion, the SP hydrogel is non-cytotoxic and supports normal islet structure and function both in vitro and in vivo. Specifically, the ability of the hydrogel to separate individual islets after transplantation is important for maintaining their function in vivo. This important property, combined with the versatility and biocompatibility, makes our SP hydrogel a promising synthetic scaffold that can facilitate transplantation of organized heterogeneous cells to preserve their micro-structure and function.
Keywords: Hydrogel, Extrahepatic Islet Transplantation, Tissue Engineering, 3D Islet Culture, Diabetes
1. INTRODUCTION
Diabetes is one of the most prevailing, costly, and debilitating diseases in the world [1]. Beta cell replacement therapy in the form of islet transplantation is a promising treatment for type 1 diabetes (T1D) without major surgery. After islet transplantation, 80% of patients were reported to be free of insulin injections at one year. However, the islet function gradually declines and at 5 years, only 15% of those remain insulin independent [2]. The current procedure is suboptimal because islets placed in the liver must survive in a non-native environment that is significantly different from that in the pancreas. In the liver, islets are exposed to lower oxygen tension, higher glucose concentration, and higher levels of toxins including bacterial products and immunosuppression [3]. The islets injected into the portal vein also suffer an acute cell loss. As many as 60% of the donor islets are destroyed shortly after infusion due to the instant blood mediated inflammation reaction (BMIR) caused by complement activation and non-specific innate immune response [4], as well as by the unique immune system of the liver consisting of liver natural killer cells [5] and higher number of natural killer T cells [6]. Islets are expected to function better and survive longer if they are transplanted to a site outside of the liver where the environment is similar to the pancreas and from where insulin released from the transplanted islets drains directly to the portal vein to achieve the maximum function [7]. The search for alternative extrahepatic sites for islet transplantation is critical to better preserve the number, viability and function of donor islets.
Encapsulation of islets with biomaterials presents tremendous potential benefitting islet transplantation to an extrahepatic site [8]. In pancreas, islets occupy only 1–2% of the total tissue mass and each islet is surrounded by native extracellular matrix (ECM) [9]. In addition to maintaining islet structure, ECM provides important signaling interactions to regulate multiple aspects of islet physiology, including survival, proliferation, and insulin secretion [10–11]. During the process of islet isolation, however, a majority of the ECM components are destroyed due to the enzymatic digestion. The disruption of ECMs leads to islet damage and fragmentation, accelerates cell clumping, and contributes to a significant reduction of islet viability and function during and after transplantation [12]. Therefore, it was hypothesized that encapsulation of islets in a biomimetic scaffold should prevent islet disintegration and aggregation, facilitate islet distribution, and enhance islets survival and function in an extrahepatic site [11]. In addition, semi-permeable polymer scaffolds can protect the encapsulated islets from direct contact with immune cells, while still allowing small molecules and proteins to diffuse through [13]. To explore this approach, we and others have evaluated several types of biomaterials for islet encapsulation [14]. Biopolymers extracted from natural sources, such as collagen, fibronectin, heparin, and polysaccharides, often suffer from the lack of tunability, batch-to-batch variations and potential pathogen contamination and immunogenicity [15]. A number of synthetic polymers have been used as scaffolds for islet encapsulation. Among them, poly (lactic acid) (PLA) and poly (lactic-co-glycolic acid) (PLGA) are rigid hydrophobic polymers that require pre-fabricated scaffolds, coating with native ECM proteins, and relatively large surgical procedure for transplantation [8, 16]. Use of poly(vinyl alcohol) (PVA) for islet encapsulation requires freezing to form scaffold, a process that results in islet loss and dysfunction [17]. Compared to other synthetic biomaterials, hydrogels are particularly attractive for islet encapsulation because, in principle, hydrogels can closely mimic the hydrophilic content of native ECM, and can deliver cells in a minimally invasive manner [18–19]. Among hydrogel scaffolds, polyethylene glycol (PEG)-based hydrogels have been investigated extensively for in vitro islet encapsulation [20–22]. Despite many positive attributes, PEG hydrogels have several limitations including non-degradable polymer backbone, the lack of functionality, and poor cell viability if without attaching cell adhesion ligands. Indeed, previous studies have shown that PEG gels alone are not optimal for maintaining long-term islet function in vivo [23]. Consequently, the design of new scaffolds for clinically relevant islet transplantation is of utmost importance to support extrahepatic transplantation.
The Guan laboratory has recently developed a saccharide-peptide (SP) hydrogel that allows both two- and three-dimensional cell cultures [24–25]. The SP hydrogel is composed of natural building blocks (amino acids and saccharides), fully biodegradable and nontoxic, inexpensive to produce, versatile and highly functional. The SP hydrogel also exhibits several unique properties, such as biocompatibility, ease of handling, crosslinkable at mild physiological conditions, and injectable for in situ polymerization for transplantation. Our previous study with chondrocytes has shown that SP hydrogel can be modified to provide instructional cues to control cell behavior [26]. Utilization of SP hydrogel in extrahepatic islet transplantation would bridge the gap between disease treatment and biomaterial development. Given these positive attributes of the SP hydrogel, we envisioned that the SP hydrogel should be suited for islet encapsulation and transplantation. To test our hypothesis, in this study we sought to specifically investigate (i) the biocompatibility of SP hydrogel in vivo; (ii) the ability of the SP hydrogel in maintaining viability in rat islets post-isolation; and (iii) the potential of SP hydrogel in maintaining islet insulin secretion function in response to high glucose in vitro and in vivo, which is easily lost in isolated islets (Figure 1).
Figure 1.
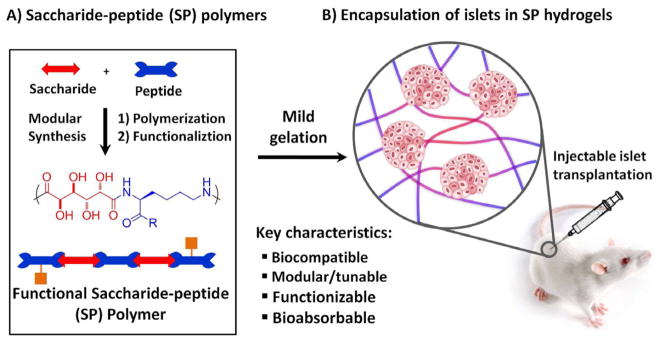
Schematic of islet encapsulation in the SP-hydrogel. (A) Saccharide-peptide polymers were generated through interfacial polymerization, then functionalized with either vinyl sulfone (VS) or cysteine (Cys). (B) Hydrogelation occurred by Michael addition in the presence of islets, when VS and Cys polymers were combined at equimolar ratio [25–26].
2. MATERIALS AND METHODS
2.1. Experimental animals
Male Lewis (LEW) rats were purchased from Charles River Laboratories, Inc. (Wilmington, MA). Rats weighing 300–350 g were used as pancreas donors and those weighing 200–250 g were used as recipients of hydrogel encapsulated islets. Luciferase transgenic (Firefly) LEW rats developed by Dr. Eiji Kobayashi [27] were obtained from the colony from University of Missouri, maintained at the City of Hope Animal Resource Center and used as pancreas donors. Skin grafts exchanged between Wild-type and Firefly LEW rats survived without rejection. All animals were maintained in specific pathogen-free conditions at the Animal Resource Center of the Beckman Research Institute/ City of Hope.
2.2. Preparation of SP hydrogels
SP hydrogels were prepared by following our previously reported procedure [25]. Briefly, polymers were prepared by dissolving VS and Cys copolymers at a concentration of 65 mg/mL in Dulbecco’s Modified Eagle’s Medium (Gibco Life Technologies, Carlsbad, CA). Addition of VS-polymer to cells, followed by mixing of Cys-polymer afforded hydrogel through mild Michael type addition reaction within 10 min as previously established.
2.3. In vitro biocompatibility assay of SP hydrogel by stimulation assay of peripheral blood mononuclear cells
PBMC from a normal LEW rat were isolated using Histopaque (Sigma-Aldrich, St. Louis, MO) and 1x106 cells were incubated with either 0.5 μL phosphate buffered saline (PBS), 0.5 mg vicryl suture, 0.5 mg hydrogel, 0.5 mg VS polymer, 0.5 mg Cys polymer, or 5 μg of LPS for 20 h at 37°C. To measure cytokine mRNA levels of PBMCs, total messenger RNA (mRNA) was extracted from each sample using a RNeasy Mini Kit (Qiagen, Valencia, CA) following the manufacturer’s protocol and cDNA was synthesized using 0.5 μg RNA from each sample by Fermentas Maxima First Strand cDNA Synthesis Kit (Thermo Scientific, Hampton, NH). TaqMan Probes (Applied Biosystems, Foster City, CA) were used to analyze gene expression by RT-PCR for tumor necrosis factor alpha (TNF-α), granulocyte-macrophage colony stimulation factor (GMCSF), interferon gamma (IFN-γ), interleukin 6 IL6), chemokine (C-C-C motif) ligand 1 (CXCL1 ), using beta actin (Actb) as the internal control. The cycle thresholds (Ct) were determined using analytical software (Viia7, Applied Biosystems, Foster City, CA). Differences in Ct between the target and control (Actb) mRNA (ΔCt) were used to quantify the relative level of each mRNA expression.
2.4. Islet isolation, SP hydrogel encapsulation and culture
Islets were isolated from LEW pancreata using our standard procedure described previously [28]. Immediately after the isolation, hand-picked islets were encapsulated in SP hydrogel by first suspending in VS-polymer by gentle pipetting followed by an immediate addition of Cys polymer. For culture 40 μL islet suspension was then transferred into a Transwell® (Fisher Scientific, Pittsburgh, PA) insert to allow for complete hydrogelation. Hydrogel-encapsulated islets or control islets on the Transwell were cultured in a 24-well plate with 300 μL RPMI-1640 culture medium supplemented with 10% FBS at 37°C in air plus 5% CO2, changing media every two days.
2.5. Islet viability assessment
Viability of islets from “Firefly” rats was quantitatively monitored by the luminescence intensity as described by Negishi K et al.[29] Hydrogel encapsulated-islets or control-islets isolated from Firefly rats (100 islets/well) were cultured for up to 28 days and luminescence intensity was measured using the Xenogen image system (Caliper Life Sciences, Hopkinton, MA) by adding 0.75 mg/mL d-luciferin to the islet culture medium. Islet viability was also qualitatively assessed using Live/Dead staining at desired time points. Live/Dead staining was done by incubating cell-laden hydrogels in PBS containing 0.24 μM fluorecein diacetate and 7.5 μM propidium iodide for 10 min. Hydrogels were then rinsed in PBS and imaged by fluorescence microscopy (Olympus IX50, Olympus America, Central Valley, PA).
2.6. In vitro islet function assessment in SP hydrogel
2.6.1. Glucose consumption during islet culture
Culture media were collected every 48 h during medium exchange, and monitored for glucose level with One Touch glucose meter (LifeScan, Milpitas, CA) in duplicate and averaged. Control medium (Ct) without islets was used as control for each time point. Differences (C0-I0) in the medium glucose level with (I0) and without (C0) islets in the first 48 h are set as baseline cell glucose consumption for each sample. Subsequent changes in glucose levels in samples with islets (It) are calculated as: Percent glucose uptake = (Ct−It)*100/(C0−I0).
2.6.2. Long-term response to glucose stimulated insulin release
Islets cultured with or without hydrogel as described above were hand-picked after 14 days in culture and collected in micro-tubes (single islet/ tube, sexplicate), washed with RPMI 1640 medium containing 3.3 mM glucose (low-glucose media) supplemented with 5% FBS 3 times. Islets were cultured in a non-tissue culture coated 96 well plate with either low-glucose or high-glucose (17 mM glucose) media for 16 h (100 μL/ well) at which time supernatants were collected to measure insulin release.
2.6.3. Short-term response to glucose stimulated insulin release by perifusion assay
Islets were cultured with or without hydrogel as previously described (200 islets/sample on day 0). On day 28, islets were collected and sandwiched-trapped between a bi-layer of microbeads (Biorad, Hercules, CA) in a minicolumn and perifused at 37°C with Krebs-Ringer’s buffer for 45 min, followed by a low-glucose buffer (3.3 mM glucose) for 10 min, a high-glucose (17 mM glucose) buffer for 15 min, and finally a low-glucose buffer [30]. Effluents were collected every 2 min starting from the first 3.3 mM buffer. The medium samples or effluents collected in the experiment were analyzed for insulin concentration using an Enzyme-Linked ImmunoSorbent Assay (ELISA) kit (Mercodia, Winston Salem, NC) for rat insulin following the manufacturer’s protocol.
2.7. In vivo biocompatibility assessment of SP hydrogel
VS-polymer and Cys-polymer were mixed to a total volume of 20 μL, aspirated in a 1mL syringe, and injected into the pinna of non-diabetic LEW rats prior to gelation. In the opposite pinna, the same amount PBS was injected. The pinnas were excised on day 0 and 13 for preparation of histological slides. Samples were fixed in 10% formalin overnight, then embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E) stains. Results were documented by bright field microscopy using an Olympus BX51.
2.8. In vivo survival of hydrogel encapsulated islets in syngeneic rats
Islets were encapsulated in SP hydrogel and transplanted into various extrahepatic sites of euglycemic syngeneic LEW recipients. Reversal of diabetes was tested by transplanting encapsulated islets in two different sites, under the renal capsule and a pocket made by the omentum. Recipient rats were treated with streptozotocin (65mg/kg, Sigma-Aldrich) via tail vein injection 7 days before transplantation. Diabetes was confirmed by blood glucose levels higher than 350 mg/dL for two consecutive measurements. Diabetic rats were transplanted with 500 islets with or without hydrogel. After transplantation, blood glucose was measured twice a week to monitor islet graft function.
2.9. Statistical analysis
Data are reported as mean ± standard error (SEM). Effects of time and the presence or absence of SP hydrogel were assessed by student t-test. P< 0.05 denotes statistical significance.
3. RESULTS
3.1. Ex vivo biocompatibility of soluble polymers and SP hydrogel
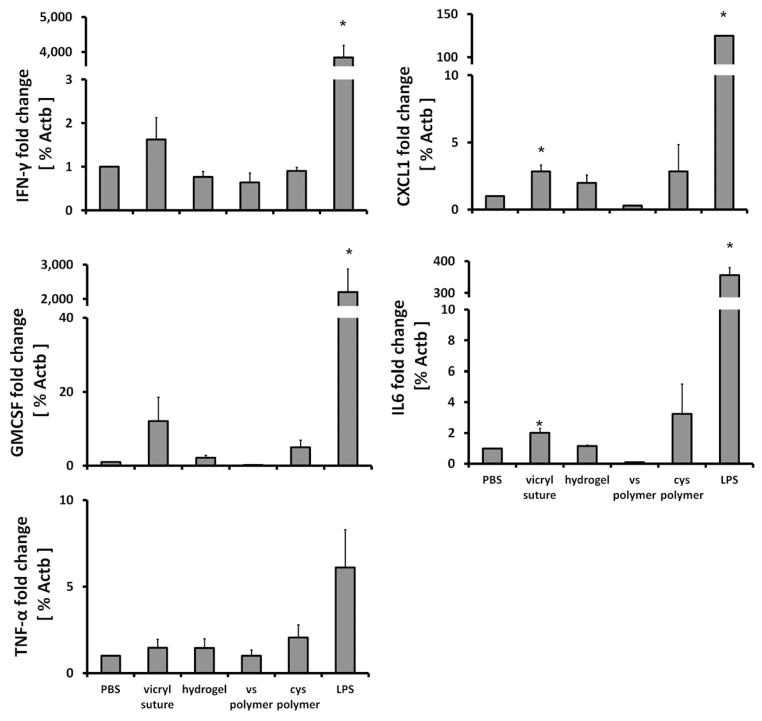
The SP hydrogel was formed by in situ crosslinking by mixing a cysteine-functionalized (Cys-polymer) and a vinyl sulfone-functionalized (VS-polymer) saccharide-peptide copolymer under mild condition [25–26]. To evaluate possible cytokine activation caused by the hydrogel and its individual polymer components, rat PBMCs were incubated in vitro with Cys-polymer, VS-polymer, SP hydrogel, negative controls, PBS or vicryl suture, or a positive control, lipopolysaccharide (LPS), for 18h in a tissue culture incubator. The mRNA expression of inflammatory cytokines released by PBMC, including TNF-α (killer cell), IFN-γ (T-cell activation), CXCL1 (IL-8 analog, recruiter), IL6 (killer cell), and GMCSF (antigen presentation) were assessed to detect any immune stimulatory effect. LPS showed the highest stimulatory effect. Compared to LPS (positive control), PBMC exposed to other materials showed minimal mRNA response as measured by IFN-γ, CXCL1, IL6, and GMCSF mRNA levels (p<0.05) (Figure 2). Responses to SP hydrogel or the individual components were not significantly different than PBS (negative control) (p = 0.06–0.49).
Figure 2.
Biocompatibility of hydrogel assessed in vitro. Peripheral blood mononuclear cells were incubated with PBS (negative control), vicryl suture, SP hydrogel, VS polymer, Cys polymer or LPS (positive control) and evaluated for cytokine gene activation after 18 hours. Gene activation in the presence of hydrogel and its constituents were not significantly different from PBS control. *(p<0.05). Mean ± SEM, n=3.
3.2. Evaluation of viability in hydrogel cultured islets
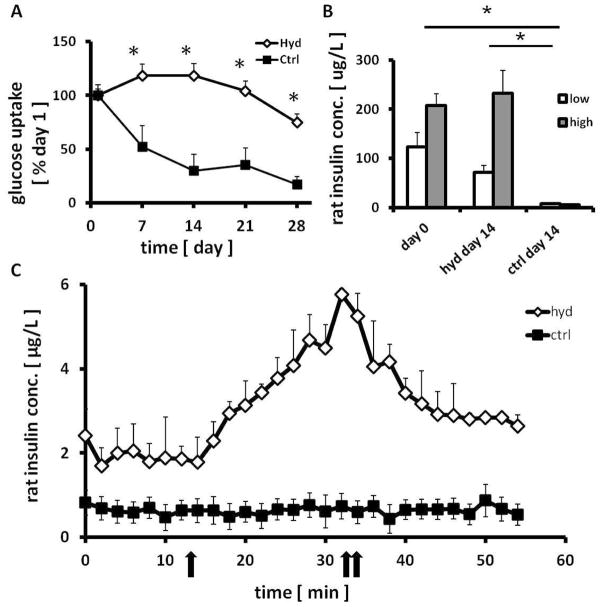
Islets encapsulated in SP hydrogel and cultured in a transwell culture insert maintained normal 3D structure, whereas the 3D structure gradually degraded in non-encapsulated control islets. The viability of islets isolated from “Firefly” LEW rats (Rosa/luc LEW Tg) was evaluated through luminescence measurements taken over the 28 day period (Figure 3A). Luminescent activity of the islets dramatically decreased over 28 days in cultures without hydrogel encapsulation (Figure 3B) and the islets rapidly disintegrated (p<0.01). In contrast, the viability of hydrogel-encapsulated islets was maintained through day 7 and then decreased, but non-significantly (p=0.07) (Figure 3B). Throughout the period, hydrogel-encapsulated islet viability was significantly higher than control unencapsulated islets (p<0.05). This data was further confirmed with qualitative images of islet viability using Live/Dead staining. Islets encapsulated in hydrogel were highly viable with intact morphology even after 28 days in culture. In contrast, unencapsulated islets aggregated and showed peripheral damage, and the culture contained many dissociated single cells most of which were dead cells (Figure 4).
Figure 3.
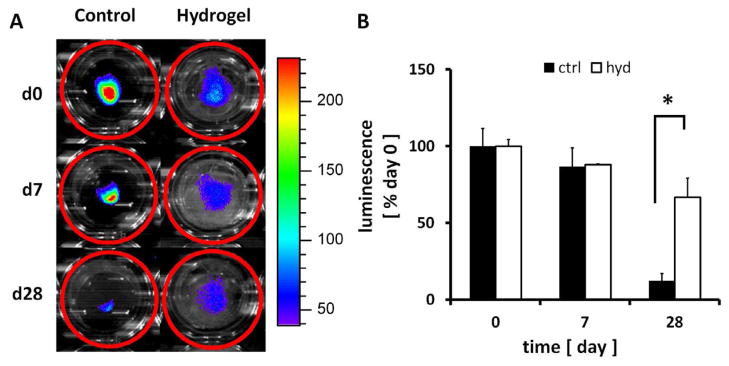
Effects of encapsulation on “Firefly” rat islet viability as evaluated by luminescence. (A) Luminescence signals from islets were monitored and (B) quantified. Data are normalized by the luminescence measured on day 0. * indicates significantly different islet viability as compared to controls without hydrogel (p<0.05). Mean ± SEM, n=3.
Figure 4.
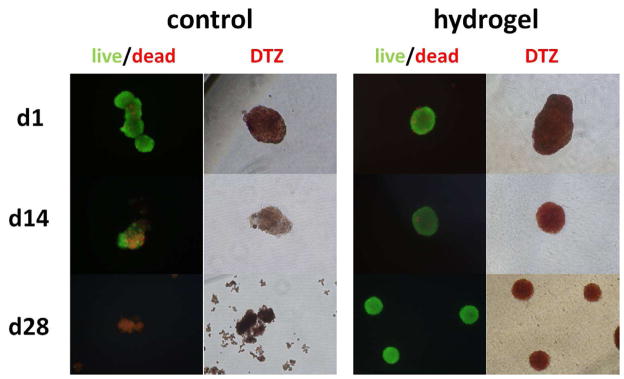
Effects of hydrogel encapsulation on islet viability (Live/Dead) and function (DTZ). Islets were stained to detect live (green) and dead (red) cells, and also DTZ stained (red) to detect insulin granules on day 1, 14, and 28. Encapsulated islets were mostly alive and stained strongly for insulin granules by DTZ throughout, as compared to control islets.
3.3. Function of in vitro cultured islets encapsulated in hydrogel
3.3.1. Islet glucose consumption
Glucose consumption in culture media was used to measure the metabolic activity of hydrogel encapsulated islets maintained in culture. Glucose metabolism is measured primarily by the glucose utilization in medium by the cultured islets and shown as an indicator not only for islet viability, but also cellular activity [31]. The uptake of glucose in medium was measured every 48h over the 28 day period to assess islet quality. Glucose uptake in encapsulated islets was maintained near 100% of day 1 level until day 21 (Figure 5A). The level then decreased slightly to 88% on day 28, which coincided with hydrogel degradation and the gradual loss of the 3D islet structure. In control islets, glucose consumption decreased to 56% by day 7, and further declined as time progressed. By day 21 and 28, and 34% and 25% of the glucose was utilized, respectively. Overall, encapsulated islets maintained much higher metabolic activity as compared to control islets (p<0.01).
Figure 5.
Maintenance of islet metabolism and function with SP hydrogel. Effects of encapsulation on (A) rat islet glucose consumption (n=3) and (B) insulin secretion in static incubation on day 14 (n=4). (C) On day 28, dynamic insulin release of islets were assessed by sequential stimulating by low- (3 mM), high- (17 mM, one arrow), and low- (3 mM, double arrow) glucose media using a perifusion system (n=3). * indicates significantly different to control islets (without hydrogel, p<0.05). Mean ± SEM.
3.3.2. Islet insulin synthesis
To quantify the biosynthetic capacity of insulin, cultured islets were incubated in either low- (3.3 mM) or high- (17 mM) glucose medium for 16 hours on day 0 and day 14 (Figure 5B). Hydrogel encapsulated islets maintained insulin secretion levels of freshly isolated islets (day 0) in both high and low glucose exposure (p=0.21–0.40). In contrast, the glucose responsiveness was not preserved in the control cultured islets tested on day 14 (p<0.05, vs. control on day 0 and encapsulated islets on day 14). The decreased insulin storage/secretion ability of the control islets was also shown by decreased insulin staining by dithizone (DTZ) on day 14 (Figure 4).
3.3.3. Dynamic insulin release in a perifusion system
To further assess beta cell function, dynamic glucose stimulated insulin release was tested in a perifusion system using islets cultured with or without hydrogel encapsulation for 28 days. Although the same number of islets was present in both the encapsulated and control samples on day 0, islets in the control group disintegrated over time and did not show stimulated insulin secretion. In contrast, encapsulated islets responded well to glucose stimulation (Figure 5C).
3.4. In vivo biocompatibility of hydrogel
To evaluate the foreign body response elicited by the hydrogel in vivo, hydrogels were injected into the rat pinna, which was later biopsied for histological examination. Sections taken on day 1 and 13 showed minimal cell infiltration into the hydrogel and surrounding area (Figure 6A). On day 1, the implanted hydrogel was spread out between subcutaneous tissues and remained intact. By day 13, hydrogel degradation was detectable with different shades of intermixed purple on H&E-stained sections with only few mononuclear cells found in a very confined area adjacent to hydrogel. These results indicate that SP hydrogel is non-cytotoxic and biodegradable in vivo.
Figure 6.
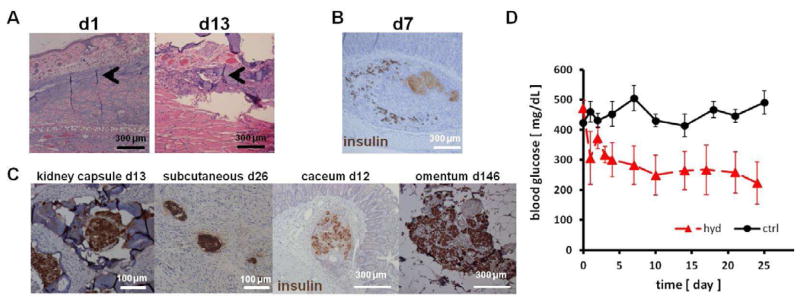
In vivo biocompatibility of hydrogel and islet function. (A) H&E stained histological sections of the rat pinna after 1 and 13 days of hydrogel injection. Minimal cell infiltration was observed in a small area adjacent to hydrogel (arrowheads). (B) Viable islets were scattered around the large mass of necrosis (light brown) observed after naked islets were injected under the submucosa of the stomach. (C) When transplanted with hydrogel, sygeneic islets stained positive for insulin (brown) were detected in various extrahepatic sites. (D) Blood glucose levels of diabetic rats after islet transplantation. 500 SP hydrogel encapsulated syngeneic islets were placed in an omentum pouch on day 0 (hyd, n=4). Controls were transplanted without the hydrogel (ctrl, n=5). Islet in hydrogel lowered blood glucose levels more effectively than control islets (p<0.05).
3.5. Survival of islets transplanted in non-hepatic sites depends on hydrogel
3.5.1. Transplantation of unencapsulated islets in non-hepatic sites
Islets were isolated and suspended in a small volume of culture medium and injected to the submucosa of the stomach of LEW rats without success in reversing diabetes. Although different methods were used with the intent to distribute islets widely without clumping, histological examination revealed that unencapsulated islets aggregated to form large clumps and developed necrosis as examined after 7 days (Figure 6B). On the same slide adjacent to the large aggregates, some single islets were found and stained insulin-positive. These results exemplify the need to develop a means to distribute islets separately and in thin layers to maintain viability and function and achieve diabetes reversal in extrahepatic sites.
3.5.2. Transplantation of hydrogel-encapsulated islets to extrahepatic sites
To examine the potential use of SP hydrogel for islet transplantation outside of the liver, islets encapsulated with SP hydrogel were placed in various extrahepatic sites, including under the kidney capsule, within the intestinal wall, in a pocket constructed in the omentum, and in subcutaneous sites of syngeneic recipients (Figure 6C). Contrary to the studies conducted without the use of hydrogel, encapsulated islets survived and stained positive for insulin at various time points in all these extraheptic sites. In addition, no tightly aggregated clumps were observed.
Finally, to evaluate the efficacy of encapsulated islets in lowering hyperglycemia, 500 syngeneic islets were transplanted with or without SP hydrogel in an omental pouch and monitored for diabetes reversal. Encapsulated islets functioned significantly better in reversing diabetes as compared to control islets (Figure 6D). From day 7 on, blood glucose levels of the hydrogel group were significantly lower than controls (p<0.05).
4. DISCUSSION
In this study, we have demonstrated the biocompatibility and suitability of the SP hydrogel for islet encapsulation both in vitro and in vivo. The hydrogel is formed by mixing two liquid components: a VS-polymer solution in which the islets are suspended and a Cys-polymer solution which is added to initiate cross-linking. Due to the mild nature of the Michael type addition reaction for cross-linking, islets maintained high viability throughout the polymerization. In vitro, both the individual polymer components (VS- and Cys-polymer) and the SP hydrogel induced minimal inflammatory cytokine activation in PBMC, implying that the degraded products from the hydrogel should cause a minimal immune response (Figure 2). In agreement with the in vitro results, degradable SP hydrogel induced a very minimal inflammatory cell infiltration in vivo (Figure 6A). The mild Michael type addition reaction also permits the suspension of isolated islets in a liquid without clumping and allows injecting islet suspension into a desired location. After gelation is initiated, islets remain separated from each other and do not clump which is a critical problem to be solved in extrahepatic islet placement to preventing massive islet necrosis. Utilization of a synthetic ECM scaffold, in our case, the SP hydrogel, immobilized islets at the site of injection, supports the 3D structure, and alleviates further islet damage. Histological examination of biopsied graft site indicated that by the time hydrogel starts to degenerate, islets have embedded in the surrounded tissue and survive.
Our results have further demonstrated the capability of our SP hydrogel in maintaining islet viability and function in vitro. The 3D islet structure is supported by the SP hydrogel surrounding the islets, resulting in maintenance of islet function and viability for over 4 weeks (67% viability) until hydrogel degradation (Figure 3B). In contrast, the viability of PEG-encapsulated single beta cells, such as MIN-6 cells and RIN-m5F cells, maintained only 20% viability on day 10 when encapsulated without the addition of adhesion ligands [32–33]. The viability of alginate-encapsulated islets demonstrated 41% viability by day 12 without the presence of supporting sertoli cells [34]. Stimulated insulin production in SP hydrogel-encapsulated islets was superior to that in suspension cultured islets in both static incubation (day 14) and dynamic insulin release assay (day 28) in vitro (Figure 5). Compared to previously reported hydrogels, SP hydrogel afford high efficiency in maintaining islet viability, structure, and function, without the need of functionalization with cell adhesion ligand or inclusion of supporting cells. Considering islets are highly sensitive cells that are more susceptible to functional impairment compared to beta cell lines, our in vitro results are very promising, which encouraged us to go forward with in vivo studies. Using the gold standard in the islet research, insulin production and responsiveness was evaluated in vivo by diabetes reversal in the rodent model. In order for rats to become normoglycemic, the transplanted islets have to survive, be connected with the vasculature, release insulin in response to glucose, and the released insulin has to enter into the blood circulation. Our hydrogel-encapsulated islets were able to lower hyperglycemia in an extraheptic site, the omentum, while control islets did not (Figure 6D). These results have demonstrated our SP hydrogel as a very promising new biomaterial for islet encapsulation and transplantation outside of the liver.
We propose that the following positive features of our SP hydrogel could contribute to its superior performance for islet encapsulation and transplantation. Firstly, the SP hydrogel is composed of natural building blocks, amino acid and saccharide (Figure 1), making both the hydrogel and its degradation products non-toxic and minimally immunogenic (Figure 2). In addition, its intrinsic biomimetic functionality affords SP hydrogel excellent in vivo biocompatibility (Figure 6A) and cytocompatibility for islets (Figure 4). Secondly, the mild in situ cross-linking condition should minimize damage to islets during cross-linking, allow for simple injection without major surgery, and capture islets in the hydrogel matrix to prevent islets from aggregation and necrosis (Figure 4). Thirdly, the semi-permeable SP hydrogel matrix may protect the encapsulated islets from direct contact with blood [13], preventing blood mediated inflammation reaction (BMIR) and direct contact with immune cells at least for the initial post-transplantation period, resulting in high viability and function for transplanted islets (Figure 6).
Given the versatility and high functionality of the SP hydrogel, we expect that further functionalization with various signaling ligands and factors will lead to significant enhancement of islet function and viability in vivo. Specifically, the functionalization of hydrogel will promote the rapid vascularization necessary to ensure adequate oxygen tension in the transplant site. In our current SP hydrogel platform, we hypothesize that vascular cells are recruited to the vicinity to provide oxygen to the transplanted graft, similar to non-degradable alginate and PEG-based hydrogels. Our previous publication indicated that vascularization around transplanted islets can occur within 6 days in the absence of hydrogel [28]. In our current platform, vascular cells may penetrate into the biodegradable hydrogel over time in response to the metabolic needs of encapsulated islets. Tailoring our biodegradable SP hydrogel to specifically facilitate the rapid recruitment of native vascular cells should increase islet survival and function post-transplantation. Modifications toward this goal as well as mechanistic investigations for islet encapsulation and transplantation are currently ongoing.
4. CONCLUSIONS
We have shown the SP hydrogel can promote islet viability and function both in vitro and in vivo. Therefore, the SP hydrogel is potentially useful for in vitro culture and maintenance of islets as well as for in vivo transplantation applications. For treating T1D patients, there is an urgent need in the diabetes community for improvements in islet transplantation outcome. SP hydrogel has the potential to fill that gap with many highly desirable properties, such as the ability to preserve stimulated insulin production in vitro for islets one month after isolation, as well as the ability to facilitate islet transplantation to an extrahepatic site to lower blood glucose level. Since rat islets undergo a similar isolation procedure as human islets, SP hydrogel has potential applications in clinical islet transplantation. Specifically, further optimization of the SP hydrogel may prevent post-transplant, acute donor islet destruction and increase percentage of T1D patients achieving long-term insulin-independence by allowing islet transplantation outside of the liver. This unique biomaterial opens up nonconventional (extrahepatic) sites for islet transplantation and may be developed as a new generation of islet transplantation method for treatment of T1D.
Acknowledgments
The authors would like to acknowledge the Nora Eccles Treadwell Foundation and the National Institute of Health (R01 EB006797) for the grant support, Dr. Fouad Kandeel, Director of Department of Diabetes, Endocrinology and Metabolism, for supporting the project, Dr. Masato Mitsuhashi, Hitachi Chemical Research Center, Irvine, for valuable comments, and Dr. Ivan Todorov for assessment of histology slides. Authors would like to especially acknowledge City of Hope pathology core lab for timely processing histology samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Economic costs of diabetes in the U.S. In 2007. Diabetes Care. 2008;31:596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 2.McCall M, James Shapiro AM. Update on islet transplantation. Cold Spring Harb Perspect Med. 2012;2:a007823. doi: 10.1101/cshperspect.a007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlsson PO, Palm F, Andersson A, Liss P. Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes. 2001;50:489–95. doi: 10.2337/diabetes.50.3.489. [DOI] [PubMed] [Google Scholar]
- 4.Bennet W, Sundberg B, Groth CG, Brendel MD, Brandhorst D, Brandhorst H, et al. Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes. 1999;48:1907–14. doi: 10.2337/diabetes.48.10.1907. [DOI] [PubMed] [Google Scholar]
- 5.Ishiyama K, Rawson J, Omori K, Mullen Y. Liver natural killer cells play a role in the destruction of islets after intraportal transplantation. Transplantation. 2011;91:952–60. doi: 10.1097/TP.0b013e3182139dc1. [DOI] [PubMed] [Google Scholar]
- 6.Toyofuku A, Yasunami Y, Nabeyama K, Nakano M, Satoh M, Matsuoka N, et al. Natural killer T-cells participate in rejection of islet allografts in the liver of mice. Diabetes. 2006;55:34–9. [PubMed] [Google Scholar]
- 7.Brown J, Mullen Y, Clark WR, Molnar IG, Heininger D. Importance of hepatic portal circulation for insulin action in streptozotocin-diabetic rats transplanted with fetal pancreases. J Clin Invest. 1979;64:1688–94. doi: 10.1172/JCI109631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blomeier H, Zhang X, Rives C, Brissova M, Hughes E, Baker M, et al. Polymer scaffolds as synthetic microenvironments for extrahepatic islet transplantation. Transplantation. 2006;82:452–9. doi: 10.1097/01.tp.0000231708.19937.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, et al. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell. 2006;10:397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Stendahl JC, Kaufman DB, Stupp SI. Extracellular matrix in pancreatic islets: relevance to scaffold design and transplantation. Cell Transplant. 2009;18:1–12. doi: 10.3727/096368909788237195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng JY, Raghunath M, Whitelock J, Poole-Warren L. Matrix components and scaffolds for sustained islet function. Tissue Eng Part B Rev. 2011;17:235–47. doi: 10.1089/ten.TEB.2011.0004. [DOI] [PubMed] [Google Scholar]
- 12.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–62. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 13.Nafea EH, Marson A, Poole-Warren LA, Martens PJ. Immunoisolating semi-permeable membranes for cell encapsulation: focus on hydrogels. J Control Release. 2011;154:110–22. doi: 10.1016/j.jconrel.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 14.Borg DJ, Bonifacio E. The use of biomaterials in islet transplantation. Curr Diab Rep. 2011;11:434–44. doi: 10.1007/s11892-011-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228–32. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 16.Salvay DM, Rives CB, Zhang X, Chen F, Kaufman DB, Lowe WL, Jr, et al. Extracellular matrix protein-coated scaffolds promote the reversal of diabetes after extrahepatic islet transplantation. Transplantation. 2008;85:1456–64. doi: 10.1097/TP.0b013e31816fc0ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saudek F, Cihalova E, Karasova L, Kobylka P, Lomsky R. Increased glucagon-stimulated insulin secretion of cryopreserved rat islets transplanted into nude mice. J Mol Med (Berl) 1999;77:107–10. doi: 10.1007/s001090050313. [DOI] [PubMed] [Google Scholar]
- 18.Seliktar D. Designing cell-compatible hydrogels for biomedical applications. Science. 2012;336:1124–8. doi: 10.1126/science.1214804. [DOI] [PubMed] [Google Scholar]
- 19.Cushing MC, Anseth KS. Materials science. Hydrogel cell cultures. Science. 2007;316:1133–4. doi: 10.1126/science.1140171. [DOI] [PubMed] [Google Scholar]
- 20.Weber LM, He J, Bradley B, Haskins K, Anseth KS. PEG-based hydrogels as an in vitro encapsulation platform for testing controlled beta-cell microenvironments. Acta Biomater. 2006;2:1–8. doi: 10.1016/j.actbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Su J, Hu BH, Lowe WL, Jr, Kaufman DB, Messersmith PB. Anti-inflammatory peptide-functionalized hydrogels for insulin-secreting cell encapsulation. Biomaterials. 2010;31:308–14. doi: 10.1016/j.biomaterials.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2011;8:153–70. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin CC, Anseth KS. Glucagon-like peptide-1 functionalized PEG hydrogels promote survival and function of encapsulated pancreatic beta-cells. Biomacromolecules. 2009;10:2460–7. doi: 10.1021/bm900420f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao SW, Yu TB, Guan Z. De novo design of saccharide-peptide hydrogels as synthetic scaffolds for tailored cell responses. J Am Chem Soc. 2009;131:17638–46. doi: 10.1021/ja907097t. [DOI] [PubMed] [Google Scholar]
- 25.Chawla K, Yu TB, Liao SW, Guan Z. Biodegradable and biocompatible synthetic saccharide-Peptide hydrogels for three-dimensional stem cell culture. Biomacromolecules. 2011;12:560–7. doi: 10.1021/bm100980w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chawla K, Yu TB, Stutts L, Yen M, Guan Z. Modulation of chondrocyte behavior through tailoring functional synthetic saccharide-peptide hydrogels. Biomaterials. 2012;33:6052–60. doi: 10.1016/j.biomaterials.2012.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hakamata Y, Murakami T, Kobayashi E. “Firefly rats” as an organ/cellular source for long-term in vivo bioluminescent imaging. Transplantation. 2006;81:1179–84. doi: 10.1097/01.tp.0000203137.06587.4a. [DOI] [PubMed] [Google Scholar]
- 28.Ito T, Itakura S, Todorov I, Rawson J, Asari S, Shintaku J, et al. Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function. Transplantation. 2010;89:1438–45. doi: 10.1097/tp.0b013e3181db09c4. [DOI] [PubMed] [Google Scholar]
- 29.Negishi K, Teratani T, Iwasaki J, Kanazawa H, Kasahara N, Lefor AT, et al. Luminescence technology in preservation and transplantation for rat islet. Islets. 2011;3:111–7. doi: 10.4161/isl.3.3.15626. [DOI] [PubMed] [Google Scholar]
- 30.Omori K, Mitsuhashi M, Todorov I, Rawson J, Shiang KD, Kandeel F, et al. Microassay for glucose-induced preproinsulin mRNA expression to assess islet functional potency for islet transplantation. Transplantation. 2010;89:146–54. doi: 10.1097/TP.0b013e3181c4218d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashcroft SJ, Hedeskov CJ, Randle PJ. Glucose metabolism in mouse pancreatic islets. Biochem J. 1970;118:143–54. doi: 10.1042/bj1180143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weber LM, Hayda KN, Haskins K, Anseth KS. The effects of cell-matrix interactions on encapsulated beta-cell function within hydrogels functionalized with matrix-derived adhesive peptides. Biomaterials. 2007;28:3004–11. doi: 10.1016/j.biomaterials.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Weber LM, Hayda KN, Anseth KS. Cell-matrix interactions improve beta-cell survival and insulin secretion in three-dimensional culture. Tissue Eng Part A. 2008;14:1959–68. doi: 10.1089/ten.tea.2007.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jourdan G, Dusseault J, Benhamou PY, Rosenberg L, Halle JP. Co-encapsulation of bioengineered IGF-II-producing cells and pancreatic islets: effect on beta-cell survival. Gene Ther. 2011;18:539–345. doi: 10.1038/gt.2010.166. [DOI] [PubMed] [Google Scholar]