Abstract
Curcumin (diferuloylmethane), the active ingredient in turmeric (Curcuma longa), is a highly pleiotropic molecule with anti-inflammatory, anti-oxidant, chemopreventive, chemosensitization, and radiosensitization activities. The pleiotropic activities attributed to curcumin come from its complex molecular structure and chemistry, as well as its ability to influence multiple signaling molecules. Curcumin has been shown to bind by multiple forces directly to numerous signaling molecules, such as inflammatory molecules, cell survival proteins, protein kinases, protein reductases, histone acetyltransferase, histone deacetylase, glyoxalase I, xanthine oxidase, proteasome, HIV1 integrase, HIV1 protease, sarco (endo) plasmic reticulum Ca2+ ATPase, DNA methyltransferases 1, FtsZ protofilaments, carrier proteins, and metal ions. Curcumin can also bind directly to DNA and RNA. Owing to its β-diketone moiety, curcumin undergoes keto–enol tautomerism that has been reported as a favorable state for direct binding. The functional groups on curcumin found suitable for interaction with other macromolecules include the α, β-unsaturated β-diketone moiety, carbonyl and enolic groups of the β-diketone moiety, methoxy and phenolic hydroxyl groups, and the phenyl rings. Various biophysical tools have been used to monitor direct interaction of curcumin with other proteins, including absorption, fluorescence, Fourier transform infrared (FTIR) and circular dichroism (CD) spectroscopy, surface plasmon resonance, competitive ligand binding, Forster type fluorescence resonance energy transfer (FRET), radiolabeling, site-directed mutagenesis, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS), immunoprecipitation, phage display biopanning, electron microscopy, 1-anilino-8-naphthalene-sulfonate (ANS) displacement, and co-localization. Molecular docking, the most commonly employed computational tool for calculating binding affinities and predicting binding sites, has also been used to further characterize curcumin’s binding sites. Furthermore, the ability of curcumin to bind directly to carrier proteins improves its solubility and bioavailability. In this review, we focus on how curcumin directly targets signaling molecules, as well as the different forces that bind the curcumin–protein complex and how this interaction affects the biological properties of proteins. We will also discuss various analogues of curcumin designed to bind selective targets with increased affinity.
1 Introduction
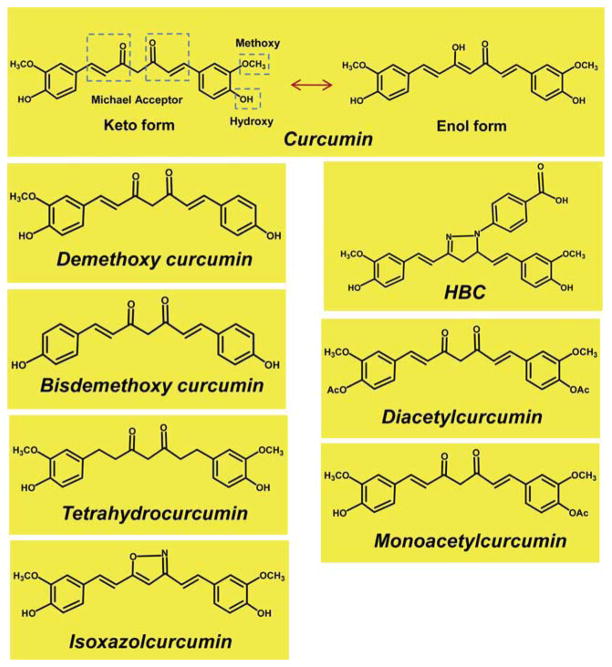
Curcumin (Fig. 1) is the major active component of turmeric, a yellow compound originally isolated from the plant Curcuma longa. It is a member of the curcuminoid family and has been used for centuries in traditional medicines. Curcumin has also long been part of the daily diet in Asian countries and has not been shown to cause any toxicity.1 Extensive research over the past 30 years has indicated that this molecule has therapeutic potential against a wide range of diseases, such as cancer, lung diseases, neurological diseases, liver diseases, metabolic diseases, autoimmune diseases, cardiovascular diseases, and various other inflammatory diseases.2,3 How a single agent can possess these diverse effects has been an enigma over the years. However, numerous lines of evidence indicate that curcumin is highly pleiotropic with anti-inflammatory,4–6 hypoglycemic,7,8 anti-oxidant,9 wound healing,10 and anti-microbial activities.11 It has been shown to possess chemosensitization, chemotherapeutic, and radiosensitization activities as well.4,12,13 Many clinical trials using curcumin as a therapeutic agent are under way.14
Fig. 1.
The molecular structures of curcumin and curcumin analogues known to interact directly with various proteins.
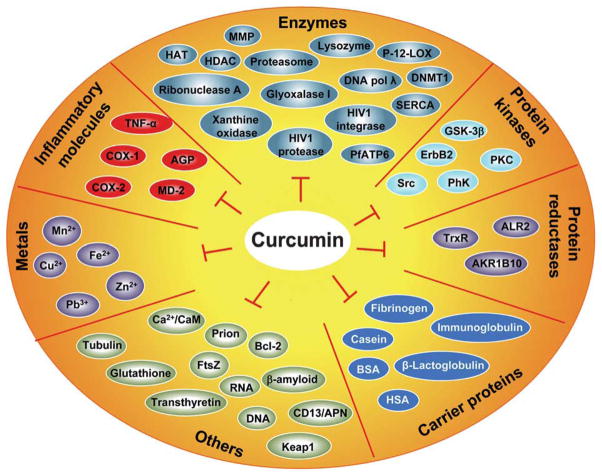
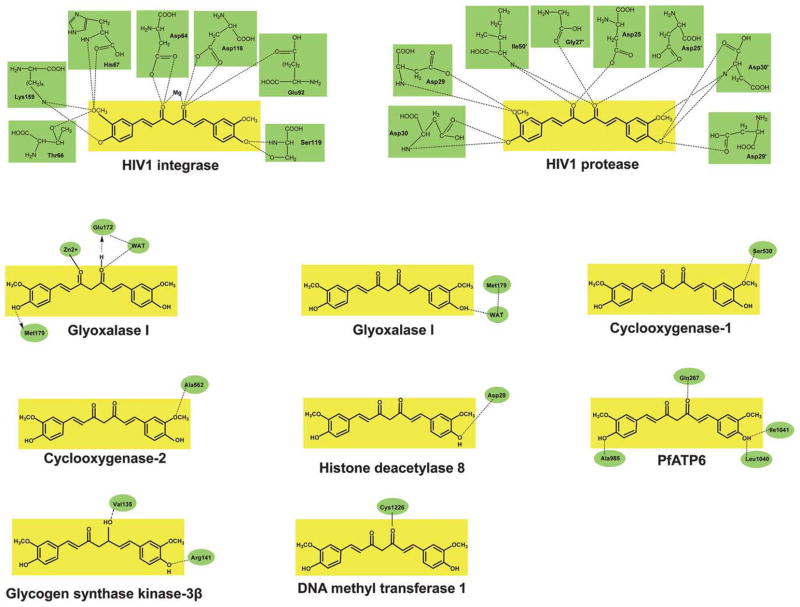
The molecular basis of a disease is related to dysregulation of an array of signaling molecules. With the advent of advanced molecular tools, we now know that over 500 different genes of the signaling pathways control any given disease.2 However, most currently available treatments are based on the modulation of a specific single target. Curcumin is a functionally labile molecule with the potential to modulate the biological activity of a number of signaling molecules either indirectly or directly by binding through covalent, non-covalent hydrophobic, and hydrogen bonding interactions. Curcumin has been shown to directly interact with a number of signaling molecules, including inflammatory molecules, cell survival proteins, histone acetyltransferases (HATs), histone deacetylases (HDAC), protein kinases, protein reductases, glyoxalase I (GLOI), proteasome, sarco (endo) plasmic reticulum Ca2+ ATPase (SERCA), Human immunodeficiency virus type 1 (HIV1) integrase, HIV1 protease, DNA methyltransferases 1 (DNMT1), FtsZ protofilaments, carrier proteins, DNA, RNA, and metal ions (Fig. 2). Curcumin interacts with these signaling molecules through numerous amino acids; some of these interactions are shown in Fig. 3. Various biophysical tools, including spectrophotometry, Fourier transform infrared (FTIR), circular dichroism (CD) spectroscopy, fluorescence quenching, Forster type fluorescence resonance energy transfer (FRET), surface plasmon resonance, competitive ligand binding, radiolabeling, site directed mutagenesis, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS), immunoprecipitation, phage display biopanning, electron microscopy, 1-anilino-8-naphthalene-sulfonate (ANS) displacement, and co-localization techniques, have been employed to show direct interaction of curcumin with other proteins. Most of these studies have utilized molecular docking as a computational tool to study the mode and site of binding. Because molecular docking is not as accurate as an experimentally determined structure, there remains a need to characterize protein–curcumin interactions through X-ray crystallography or nuclear magnetic resonance (NMR) spectroscopy studies. For most of the proteins, the binding of curcumin to the protein has been detected with a binding constant typically in the nanomolar to micromolar range (Table 1, Table 2).
Fig. 2.
Direct molecular targets of curcumin and curcumin analogues.
Fig. 3.
Curcumin interacts with signaling molecules through amino acids. Images are adapted from the references as described under their respective sections. A dotted line indicates a hydrogen bond interaction, whereas a solid line indicates covalent interaction. WAT, water.
Table 1.
A list of targets that interact directly with curcumin and its analoguesa
| Target | Technique | Functional group | Constant (μM M−1) | Reference |
|---|---|---|---|---|
| Inflammatory molecules | ||||
| Tumor necrosis factor-α | Docking | ND | ND | 31 |
| Cyclooxygenase-1 | Docking | Methoxy | ND | 34 |
| Cyclooxygenase -2 | Docking | Methoxy | 35b | 35 |
| Cyclooxygenase -2i | Docking | Methoxy | 0.05b | 35 |
| α1-acid glycoprotein | CD | Phenyl, carbonyl | 4c | 40 |
| MD-2 | SDM, docking | ND | 0.37e | 41 |
| Enzymes | ||||
| Histone acetylase (p300/CBP) | IP, radiolabeling | Carbonyl | ND | 21 |
| Histone deacetylase | Docking | Hydroxyl | 0.539d | 42 |
| Glyoxalase I | Docking | Carbonyl | 2.6–4.6d | 44,45 |
| Xanthine oxidase | Docking | ND | 141–186e | 49 |
| Proteasome | Docking | Carbonyl | 1.85b | 50 |
| Proteasomei | Docking | Carbonyl | ND | 51 |
| SERCA | ATP binding, FITC labeling | ND | 7–15b | 52 |
| SERCA2 | Docking, co-localization | ND | ND | 53 |
| PfATP6 | Docking | Hydroxyl | 1.2–2.5f | 55 |
| HIV type 1 integrase | SDM, radiolabeling | Hydroxyl | 40b | 56,57 |
| HIV type 1 protease | Docking | Hydroxyl | 100b | 56,57,156 |
| DNA methyltransferase 1 | Docking | Carbonyl | 0.03b | 59 |
| DNA polymerase λi | MALDI-TOF MS | ND | 3.9b | 60 |
| Ribonuclease Ai | CD, FTIR, Docking | Carbonyl | 1g | 61 |
| Platelet 12-lipoxygenase | Docking | ND | 66b | 65 |
| Matrix metalloproteinasesi | Docking | Phenyl | ND | 70 |
| Lysozyme | Absorption spectroscopy | ND | ND | 73 |
| Hen egg-white lysozyme | Fluorescence quenching | Methoxy | 7.7b | 74 |
| Protein kinases | ||||
| Protein kinase C | Docking, fluorescence quenching | Carbonyl | 4–11h | 78 |
| Src | IP | ND | ND | 80 |
| Glycogen synthase kinase-3β | Docking | Hydroxyl | 0.066b | 89 |
| ErbB2 | SDM, IP, radiolabeling | ND | ND | 91 |
| Phosphorylase kinase | Protein kinase assay | ND | 75d | 93 |
| Protein reductases | ||||
| Thioredoxin reductase | Docking | Methoxy | 3.6b | 20,95 |
| Aldose reductase 2 | Docking | ND | 10b | 97 |
| AKR1B10i | SDM, docking | Hydroxyl | 0.022d | 100 |
| Carrier proteins | ||||
| Casein micelles | Fluorescence | ND | 1.5g | 110 |
| αS1-casein | CD, fluorescence quenching | ND | 6.3–201c | 111 |
| Human serum albumin | Fluorescence quenching | ND | 1, 10g | 118 |
| Human serum albumini | Fluorescence quenching, FRET, FTIR, CD, docking | ND | ND | 119 |
| Bovine serum albumin | FTIR, CD, fluorescence quenching | ND | 3.33g | 120 |
| Bovine serum albumini | CD, FTIR, docking | ND | 10g | 121 |
| Fibrinogen | Fluorescence quenching | ND | 10g | 118 |
| β-Lactoglobulin | Forster energy transfer, competitive ligand binding, fluorescence quenching, docking, FRET, CD | Methoxy, hydroxyl | 10c | 123,124 |
| Immunoglobulin | FTIR, CD, fluorescence quenching | Carbonyl | ND | 126 |
| Others | ||||
| Bcl-2 | CD | ND | 0.0022d | 129,157 |
| FtsZ | Docking, microscopy | Methoxy | 7.3e | 135,136 |
| Prion protein | CD, fluorescence quenching | ND | 0.07e | 138 |
| DNA | FTIR, UV-visible spectrophotometry | ND | 4.3g | 16 |
| DNAi | UV-visible spectrophotometry, CD, fluorescence quenching, docking | ND | 1g | 15 |
| RNA | FTIR, UV-visible spectrophotometry | ND | 1.3g | 16 |
| Transthyretin | Fluorescence quenching, ANS displacement | Methoxy | 2.3e | 140 |
| Ca2+/Calmodulini | Phage display biopanning, surface plasmon resonance, docking | Hydroxyl | 8.11e | 141 |
| Tubulin | Microscopy, UV-visible spectrophotometry | ND | 2.4e | 142 |
| CD13/Aminopeptidase N | Surface plasmon resonance, antibody competition assay | Carbonyl | 10b | 143 |
| β-Amyloid | Fibril aggregation assay, electron microscopy | Carbonyl | 0.8b | 144,145 |
| Glutathione | FAB-MS and MALDI-MS | Carbonyl | 8d | 148 |
| Metals | ||||
| Cu2+ | Spectrophotometry | ND | 3–12e | 19 |
| Fe2+ | Spectrophotometry | ND | 2.5–5e | 19 |
ANS, 8-anilino 1-naphthalene sulfonic acid; Bcl-2, B-cell lymphoma-2; CBP, cAMP-response element-binding protein; CD, circular dichroism; FAB-MS, fast atom bombardment mass spectrometry; FITC, fluorescein isothiocyanate; FRET, Forster-type fluorescence resonance energy transfer; FTIR, Fourier transform infrared spectroscopy; HIV, human immunodeficiency virus; IP, immunoprecipitation; MALDI-MS, matrix-assisted laser desorption ionization mass spectrometry; MALDI-TOF MS, matrix-assisted laser desorption ionization time of flight mass spectrometry; MD-2, myeloid differentiation protein-2; SDM, site directed mutagenesis; SERCA, sarco (endo) plasmic reticulum Ca2+-ATPase; Src, sarcoma.
IC50.
association constant.
inhibition constant (Ki).
dissociation constant (Kd).
binding affinity.
binding constant.
half maximal effective concentration (EC50).
ND, not determined.
These targets interact directly with curcumin analogues.
The functional groups shown are of curcumin. Association constant and binding constant are in × 104 M−1.
Table 2.
Mode of direct interaction of curcumin and curcumin analogues with various biomoleculesa
| Molecule | Finding |
|---|---|
| Inflammatory molecules | |
| TNF-α | Interacted with TNF-α by hydrophobic, van der Waals forces, and H-bond. Cys129 in TNF-α was found as the binding site for curcumin.31 |
| COX-1 | Inhibited the enzyme activity by direct binding through Ser530.34 |
| COX-2 | Inhibited the enzyme activity by direct binding through Val523, Val116, Ala516 and Tyr355;34 interacted by hydrogen bonds with Ala562 and inhibited PGE-2 production.35 |
| b Interacted by forming hydrogen bonds with Glu346, Phe580, Asn101, and Gln350 and inhibited PGE-2 production.35 | |
| AGP | Binds at two sites on the outer region of AGP, the open end of the central hydrophobic cavity and on a surface cleft.40 |
| MD-2 | Inhibited LPS signaling by binding to the Cys133 residue inside a hydrophobic pocket of MD-2 through Michael addition reaction.41 |
| Enzymes | |
| HAT (p300/CBP) | Formed a covalent association with p300, promoted proteasome-dependent degradation of p300, and inhibited acetyltransferase activity.21 |
| HDAC | Made several close contacts with the active site residues of enzyme and exhibited potent HDAC inhibitory activity.42 |
| GLOI | Inhibited enzyme activity (Ki, 2.6–4.6 μM), coordinated with Zn2+ in the active site of GLOI through oxygen atoms of carbonyl group;44 the keto and enol forms interacted through hydrophobic interactions with binding free energies of −24.16 and −30.38 kcal mol−1, respectively.45 |
| XO | Degradation product of curcumin exhibited effective inhibitory activity in comparison to curcumin; the binding pocket for interaction consisted of Phe914, Phe1009, and Thr1010 on XO.49 |
| Proteasome | Inhibited proteasome activity by direct binding to the amino-terminal threonine (Thr1) of the β5 CT-like subunit of the proteasome.50 |
| *Exhibited potent anti-proliferative and proteasome inhibition activity by direct binding to the β5 subunit.51 | |
| SERCA | Stabilized the E1 conformation of SERCA, bind to a site that induced conformational changes and precluded ATP from binding to SERCA-Ca2+ pump.52 |
| SERCA2 | Induced ER stress and inhibited survival of human liposarcoma cells, co-localized with SERCA2 in ER and inhibited the enzyme activity by direct interaction with Asp254, Arg264, and Gln56 residues.53 |
| PfATP6 | Exhibited anti-malarial effects by binding directly to the PfATP6 through hydrophobic interactions and hydrogen bonds.55 |
| HIV-1 IN | Bind directly to the active site of enzymes, o-hydroxyl and/or keto–enol structures were important for IN inhibitory actions;56 interacted with integrase catalytic core.57 |
| HIV-1 PR | Bind directly to the active site of enzymes, o-hydroxyl and/or keto–enol structures were important for PR inhibitory actions.56 |
| DNMT1 | Exerted inhibitory effect by covalently blocking catalytic Cys1226 of DNMT1.59 |
| DNA Pol λb | Bind selectively to the N-terminal domain, binding site consisted of β-sheet (Thr51 of sheet-1), the α-helix (residues 57–69) and the two loops (residues 51–56 and 70–75).60 |
| RNase Ab | Bind to the RNase with binding constant of 104 M−1. The oxygen atoms at positions 3 and 5 of DAC formed a hydrogen bond with Tyr97, Gln11 and Lys7 of RNase.61 |
| Lipoxygenase | Inhibit soybean lipoxygenase L3 activity by blocking the active site,63 binds in a non-competitive manner and undergoes photodegradation in the crystallographic X-rays.64 |
| P-12-LOX | Binds to the enzyme, inhibited enzyme activity, and reduced sprout formation in an in vitro model of angiogenesis.65 |
| MMPsb | Interaction was formed by three hydrogen bonds and was associated with a docking energy of −11.46 kcal mol−1.70 |
| Lysozyme | Binds to lysozyme with a binding constant of 1.2 × 103 M−1.73 |
| HEWL | Exhibited inhibitory activity against the fibrillation of hen lysozyme, showed interaction predominantly by van der Waals force or by hydrogen bonding.74 |
| Protein kinases | |
| PKC | Binds to the C1B subdomains of PKC by forming hydrogen bonds with the residues at the activator binding site of enzyme (EC50, 4–11 μM).78 |
| Src | Inhibited v-Src kinase activity, decreased tyrosyl substrate phosphorylation of Shc, cortactin, FAK, and reduced proliferation of v-Src transformed cells.80 |
| GSK-3β | Inhibited the activity by direct binding to the Val135, Ile62, Arg141, and Lys85 concomitant with an increase in liver glycogen reserves in fasting BALB/c mice.89 |
| ErbB2 | Increased association of CHIP with ErbB2, induced ubiquitination and depletion of ErbB2 by binding to the kinase domain.91 |
| PhK | Inhibited enzyme activity selectively in a non-competitive manner (Ki, 75 μM).93 |
| Protein reductases | |
| TrxR | Inhibited activity by inducing alkylation of Cys496/Sec497 in the catalytically active site of the enzyme;20 at least one methoxy group in curcumin is necessary for interaction with TrxR.95 |
| ALR2 | Inhibited activity in a non-competitive manner (IC50, 10 μM), interacted with Tyr48, Lys21, Thr19, Gln183, Leu300 and Trp111 of ALR2.97 |
| AKR1B10b | Exhibited selectivity and potency by interacting with Trp21, Gln114, Trp220, Val301 and Ser304 residues.100 |
| Carrier proteins | |
| CMs | Formed a complex with CMs (CM-curcumin) through hydrophobic interactions, the IC50 of CM-curcumin complex was reduced from 14.85 to 12.69 μM.110 |
| αS1-casein | Binds to the αS1-casein at two binding sites, one with high affinity (2.01 × 106 M−1) and the other with low affinity (6.3 × 104 M−1) predominantly by hydrophobic interactions.111 |
| HSA | Exhibited strong association with the hydrophobic domains of HSA, interaction suppressed curcumin degradation due to hydrolysis.118 |
| b Interacted through hydrophobic forces with Arg218, Asn295, and Tyr452 of HSA.119 | |
| BSA | Binds via hydrophilic and hydrophobic interactions with a binding constant of 3.33 ± 0.8 × 104 M−1.120 |
| b Enol form docked to hydrophobic subdomain preferentially near Trp213 of BSA.121 | |
| Fibrinogen | Exhibited strong association with the hydrophobic domains of fibrinogen, interaction suppressed curcumin degradation due to hydrolysis.118 |
| βLG | Interacted through hydrophobic contacts, encapsulation of curcumin in βLG nanoparticles enhanced the solubility and stability of curcumin;123 interacted through phenolic hydroxyl group, two tryptophan residues (Trp19 and Trp61) in β-LG were critical for interaction.124 |
| Ig | Interacted with an average affinity constant of 1.170 × 104 predominantly through hydrogen bonds and hydrophobic forces.126 |
| Others | |
| Bcl-2 | Interacted directly with cavity 2 through multiple amino acids, abrogated Bcl-2 activity and enhanced apoptosis.129 |
| FtsZ | Inhibited the assembly of FtsZ protofilaments and bacterial cytokinesis and increased the GTPase activity of FtsZ;135 interacted with the active site ‘pocket 1’ of FtsZ by hydrogen bonds.136 |
| PrP | Binds selectively to the non-native β-forms and α-helical intermediate of PrP.138 |
| DNA | Binds to the major and minor grooves of DNA duplex with overall binding constants of 4.255 × 104 M−1.16 |
| b Interacted with the minor groove of ct-DNA, the binding site was 3 base pairs long and involved AT residues.15 | |
| RNA | Binds to the RNA bases and also to the backbone phosphate group with overall binding constants of 1.262 × 104 M−1.16 |
| TTR | Binds to the active site of TTR with a molar ratio of 1.2 : 1 and with a Kd of 2.3 × 10−6 M, stabilizes the TTR by preventing denaturant induced tertiary and quaternary structural changes.140 |
| Ca2+/CaMb | Antagonizes Ca2+/CaM functions by binding directly to the C-terminal hydrophobic pocket of enzyme and inhibited the cell cycle progression of colon cancer cells.141 |
| Tubulin | Inhibited proliferation of HeLa and MCF-7 cells, inhibited tubulin assembly into microtubules by direct binding, reduced GTPase activity and induced aggregation of tubulin dimers.142 |
| CD13/APN | Inhibited APN activity irreversibly by direct binding that was concomitant with its ability to inhibit invasion of APN-positive tumor cells.143 |
| Abeta | Enol form of curcumin exhibited strong binding.144,145 |
| Glutathione | Formed mono- and di-glutathionyl-adducts of curcumin; presence of GSTP1-1 significantly accelerated the initial rate of GSH- mediated consumption of curcumin.148 |
| Keap1 | Disrupts the Nrf2-Keap1 complex by interaction with the thiol group of Keap1 through a Michael addition reaction.151 |
| Metals | |
| Cu2+, Fe2+, Zn2+ | Exerted anti-AD effects by preventing amyloid aggregation and inducing Cu2+, Fe2+, and Zn2+ chelation.19 |
Abeta, β-amyloid; AD, Alzheimer’s disease; AGP, α1-acid glycoprotein; Ala, alanine; ALR, aldose reductase; APN, aminopeptidase N; Arg, arginine; Asn, asparagines; Asp, aspartic acid; Bcl-2, B-cell lymphoma-2; BSA, bovine serum albumin; Ca2+/CaM, Ca2+/calmodulin; CBP, CREB-binding protein; CHIP, carboxyl terminus of Hsc70-interacting protein; CMs, casein micelles; COX, cyclooxygenase; ct, calf thymus; CT, chymotrypsin; Cys, cysteine; DNMT, DNA methyltransferase; ER, endoplasmic reticulum; FAK, focal adhesion kinase; Gln, glutamine; GLOI, glyoxalase I; Glu, glutamic acid; GSK-3β, glycogen synthase kinase-3β; GST, glutathione S-transferase; GTPase, guanosine triphosphatase; HAT, histone acetylase; HDAC, histone deacetylase; HEWL, hen egg-white lysozyme; HIV-1 IN, human immunodeficiency virus type 1 integrase; HIV-1 PR, human immunodeficiency virus type 1 protease; HSA, human serum albumin; Ile, isoleucine; Ig, immunoglobulin; Keap1, Kelch-like ECH-associated protein 1; Kd, dissociation constant; Ki, inhibition constant; Leu, leucine; LPS, lipopolysaccharide; Lys, lysine; MD-2, myeloid differentiation protein-2; MMPs, matrix metalloproteinases; P-12-LOX, platelet 12-lipoxygenase; PGE, prostaglandin; Phe, phenylalanine; PhK, phosphorylase kinase; PKC, protein kinase C; Pol λ, polymerase λ; PrP, prion protein; RNase, ribonuclease; Sec, selenocysteine; Ser, serine; SERCA, sarco (endo) plasmic reticulum Ca2+-ATPase; Shc, src homology/collagen protein; Thr, threonine; TNF-α, tumor necrosis factor-α; Trp, tryptophan; TrxR, thioredoxin reductase; TTR, transthyretin; Tyr, tyrosine; Val, valine; Src, sarcoma; XO, xanthine oxidase; βLG, β-lactoglobulin.
These targets interact directly with the curcumin analogues.
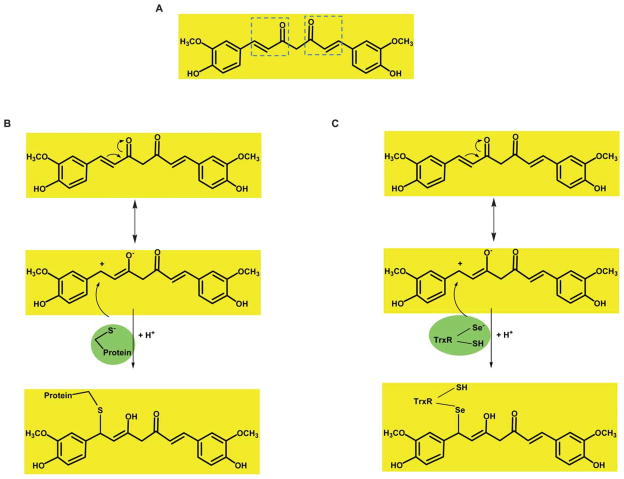
Curcumin’s ability to bind directly to diverse proteins with high affinity stems from its molecular structure and functionality. Chemically, curcumin is a diferuloyl methane molecule (1,7-bis (4-hydroxy-3-methoxyphenol)-1,6-heptadiene-3,5-dione) containing two ferulic acid residues joined by a methylene bridge. Curcumin has two hydrophobic phenyl domains that are connected by a flexible linker (Fig. 1), and molecular docking studies have found that curcumin can adopt many different conformations suitable for maximizing hydrophobic contacts with the protein to which it is bound. For example, the phenyl rings of curcumin can participate in π–π van der Waals interactions with aromatic amino acid side chains. Within curcumin’s generally hydrophobic structure, the phenolic and carbonyl functional groups, which are located on the ends and in the center of the molecule, can participate in hydrogen bonding with a target macromolecule. This structure provides a strong and directed electrostatic interaction to increase favorable free energies of association. Indeed, curcumin binds to DNA not through intercalation of the phenyl rings but by hydrogen bonding interactions with the minor groove in AT-rich regions.15,16 Owing to its β-diketone moiety, curcumin undergoes keto–enol tautomerism and exists entirely in the enol form both in solution and in solid phase.17,18 This keto–enol tautomerization provides curcumin with additional chemical functionality. The predominant enol form allows the midsection of the molecule to both donate and accept hydrogen bonds. The enol form also makes an ideal chelator of positively charged metals, which are often found in the active sites of target proteins.19 Finally, the keto–enol tautomerization allows curcumin to act as a Michael acceptor to nucleophilic attack, and curcumin has been found to bind covalently to nucleophilic cysteine sulfhydryls and the selenocysteine Se− moiety (Fig. 4).20,21 The combination of hydrophobic interactions, including π–π interactions, extensive hydrogen bonding, metal chelation, and covalent bonding, covering such a large surface area gives curcumin many possible mechanisms to interact with target proteins.
Fig. 4.
A. Curcumin structure showing functional groups that can serve as Michael reaction acceptors (in dotted boxes). B, C. The proposed mechanism of Michael addition of a reduced sulfhydryl and selenocysteine (Se−) moiety to the keto form of curcumin. Figure adapted from Fang et al.20 and Jung et al.91
Although curcumin has been shown to modulate several targets, one of the major limitations is its poor bioavailability. The problems with stability and bioavailability have been partly overcome by using curcumin’s ability to bind to numerous carrier proteins. During the past two decades, researchers have made a number of modifications in the curcumin structure to improve its activity and bioavailability. Like curcumin, these analogues have been shown to interact directly with numerous macromolecules. These curcumin analogues reported to interact with various targets are 4-[3,5-bis-[2-(4-hydroxy-3-methoxy-phenyl)-ethyl]-4,5-dihydro-pyrazol-1-yl]-benzoic acid (HBC), monoacetylcurcumin (MAC), diacetylcurcumin (DAC), tetra-hydrocurcumin (THC), isoxazolcurcumin (IOC), difluorinated curcumin (CDF), demethoxy curcumin (DMC), and bis demethoxy curcumin (BDMC) (Fig. 1). The major direct targets of curcumin analogues reported to date are Ca2+/calmodulin (Ca2+/CaM), DNA polymerase λ, ribonuclease A (RNase A), matrix metalloproteinases (MMPs), AKR1B10, XO, platelet 12-lip-oxygenase (P-12-LOX), DNMT1, Bcl-2, thioredoxin reductase (TrxR), COX-2, calf thymus-DNA (ct-DNA), and some carrier proteins (Table 2).
Apart from binding directly to numerous molecules, accumulating evidence suggests that curcumin has a diverse range of indirect molecular targets. These targets modulated by curcumin can be upregulated or downregulated depending upon the target. Included among these molecular targets are transcription factors, enzymes, inflammatory mediators, protein kinases, drug resistance proteins, cell-cycle regulatory proteins, adhesion molecules, growth factors, receptors, cell-survival proteins, chemokines, and chemokine receptors.22–29 Given these diverse targets, it can be envisioned how curcumin could mediate pleiotropic activity.
In this review, we will describe direct molecular targets of curcumin, different forces that bind the curcumin–protein complex, and the biological consequences of this interaction. The estimated physical parameters for the binding of ligands such as curcumin or its derivatives to different proteins are 1) IC50 values, expressed in concentration units (M), defined as the concentration of ligand necessary to inhibit the activity by 50%; 2) the inhibition constant (Ki), also expressed in concentration units (M), defined as the equilibrium constant for the dissociation of the protein–inhibitor complex; 3) the dissociation constant (Kd), expressed in concentration units (M), defined as the equilibrium constant for the dissociation of protein–ligand complex; and 4) the binding constant or association constant (Ka), expressed in inverse concentration units (M−1), and defined as the equilibrium constant for the formation of protein–ligand complexes. Studies of different types have addressed the binding of curcumin or its analogues to different proteins, in accordance with the above-mentioned binding parameters. The binding parameters are reported here as in the original articles with no attempt made to convert them to a single form. The results of these studies and any outstanding questions raised by our current understanding of these interactions are summarized in this review. Our criterion for literature review was to select only conclusive studies. If a study was inconclusive, we have discussed it only in terms of the analysis still needed. Where reported, the pharmacological relevance of these interactions are also discussed.
2 Direct molecular targets of curcumin
2.1 Curcumin binds directly to inflammatory molecules
Curcumin has been shown to exert anti-inflammatory activity by binding directly to pro-inflammatory molecules. Various inflammatory molecules known to be direct targets of curcumin are tumor necrosis factor (TNF)-α, cyclooxygenase (COX)-1, COX-2, human α1-acid glycoprotein (AGP), and myeloid differentiation protein 2 (MD-2).
2.1.1 Tumor necrosis factor-α
Tumor necrosis factor (TNF)-α is an essential component of the immune system and is produced by several type of cells, especially macrophages. It possesses pro-inflammatory activities and can contribute to a variety of autoimmune diseases, including psoriasis, inflammatory bowel disease, rheumatoid arthritis, systemic sclerosis, systemic lupus erythematosus, multiple sclerosis, and diabetes.30 In one study, curcumin was found to dock at the receptor-binding sites of TNF-α by molecular docking. Several residues, including Leu89, Asn90, Asp105, Asn106, and Cys129, on TNF-α were required for binding to curcumin. Curcumin exhibited direct interaction with TNF-α by non-covalent interactions (such as hydrophobic and H-bonds) and also by covalent interactions (such as π–π aromatic interactions at Tyr201 and π-cation interactions at Lys126). These studies suggest that curcumin may influence or even interrupt the signal transduction between TNF-α and its receptor by direct binding and thereby may suppress inflammation induced by this cytokine.31 Structural confirmation of this mechanism through X-ray crystallography or NMR spectroscopy studies would provide important information on a mechanism by which the pharmacological properties of curcumin could be enhanced.
2.1.2 Cyclooxygenase
The enzyme cyclooxygenase (COX), specifically the isozymes COX-1 and COX-2, is involved in the conversion of arachidonic acid into inflammatory prostaglandins. Whereas COX-1 is constitutively expressed in all tissues, COX-2 is increasingly expressed under inflammatory conditions.32,33 Curcumin has been shown to inhibit COX-1 and COX-2 activity by direct binding. Molecular docking studies observed various interactions between the enzymes and curcumin. In particular, the methoxy group of curcumin formed a hydrogen bond with Ser530 of COX-1 (Fig. 3). The phenyl rings of curcumin were surrounded by the amino acid residues Tyr385, Leu384, Phe518, Met522, Ser530, Tyr355, His90, Leu357, Arg120, and Glu524 of COX-1. The energy for the binding of curcumin to COX-1 was calculated to be −36.45 kcal mol−1. Curcumin also showed interactions with Val523, Val116, Ala516, and Tyr355 of COX-2. In agreement with these observations, curcumin was found to possess anti-oxidant activity.34 Padhye et al. also found that curcumin interacts directly with COX-2 by forming H-bonds with Ala562, with an estimated binding energy of −5.71 kcal mol−1 (Fig. 3).35
The isooxazole and pyrazole analogues of curcumin also docked to COX-1 but not COX-2. The pyrazole analogue exhibited tighter binding than did curcumin, through the methoxy group. By molecular docking studies, Padhye et al. have shown that fluoro curcumin analogues, due to their inherent biostability because of strong C–F bonds, bind to the active site of COX-2. These authors concluded that CDF is more effective in inhibiting COX-2 than curcumin and other fluorocurcumins.35 The study indicated that CDF forms H-bonds with Glu346, Phe580, Asn101, and Gln350 of COX-2. Consistent with these observations, CDF caused a significant decrease in the level of prostaglandin E2, a COX-2-dependent protein, and significantly downregulated constitutive NF-κB in pancreatic cancer cells.
2.1.3 α1-Acid glycoprotein
The α1-acid glycoprotein (AGP), also known as orosomucoid, has been suggested to have anti-inflammatory effects and a role in immunomodulation.36,37 This glycoprotein has been shown to bind and transport a number of endogenous and exogenous compounds including various drugs.38,39 The serum level of AGP has been shown to greatly increase during inflammatory and immunological processes. Using CD, UV-visible absorption, and fluorescence spectroscopy, curcumin was shown to bind to the AGP in a left-handed chiral conformation. As revealed by CD displacement experiments, curcumin also interacted with two genetic variants (F1–S and A) of AGP. The association constant for the curcumin–AGP complex was estimated to be 4 × 104 M−1 and was stable only below room temperature, indicating that the binding was at the surface of the protein. Molecular docking calculations performed on the curcumin–AGP complex suggested the existence of two possible binding sites for curcumin (both of which are located on the outer region of AGP: the open end of the central hydrophobic cavity and a surface cleft) through intermolecular hydrogen bonding with the phenol and enol moieties and π–π interactions with the phenol moiety of curcumin.40 If experimental conditions that select between these two configurations could be determined, X-ray crystal structures would clarify the various mechanisms by which curcumin is capable of interacting with AGP.
2.1.4 Myeloid differentiation protein 2
Myeloid differentiation protein 2 (MD-2) is the LPS-binding component of the endotoxin surface receptor complex MD-2/TLR4 and is involved in LPS signaling. In one study employing fluorescence spectroscopy, molecular docking, and the LPS signaling inhibition assay, curcumin was shown to inhibit LPS signaling by binding directly to MD-2 with submicromolar affinity. Molecular docking indicated that curcumin can bind the hydrophobic pocket of MD-2, which also binds bacterial LPS. Although curcumin did not form a covalent bond with the Cys133 residue inside a hydrophobic pocket of MD-2, this residue was proposed as a potential target for curcumin to bind through the Michael addition reactions. The authors of this study concluded that interaction of curcumin with MD-2 may have an important physiological relevance, particularly for some types of chronic inflammation that are caused by bacterial infection.41
2.2 Curcumin binds directly to enzymes
2.2.1 Histone acetyltransferases
Histone acetylation plays an essential role in the epigenetic regulation of gene expression and is carried out by a group of enzymes called histone acetyl-transferases (HATs), such as p300/CBP. Marcu et al. found that curcumin can inhibit activity of the p300/CBP family of HAT proteins specifically by direct binding with no activity towards the PCAF/GCN5 HATs. Because tetrahydrocurcumin did not show p300 inhibitory activity, this group proposed that the α, β unsaturated carbonyl groups in the curcumin side chain function as the Michael reaction sites that are required for its HAT-inhibitory activity. Concomitant with these observations, curcumin promoted proteasome-dependent degradation of p300. In addition to inducing p300 degradation, curcumin inhibited the acetyltransferase activity of purified p300. Further studies employing immunoprecipitation and radiolabeling indicated that curcumin formed a covalent association with p300 and abolished histone hyperacetylation in both PC3-M prostate cancer cells and peripheral blood lymphocytes. On the basis of these observations, the authors of this study proposed that curcumin acts as a novel HAT inhibitor.21 However, structural confirmation of this effect through X-ray crystallography would demonstrate a novel mechanism of curcumin activity.
2.2.2 Histone deacetylases
The acetyltransferase activity of HAT is counterbalanced by a group of enzymes called histone deacetylases (HDAC), which deacetylate histone proteins. HDAC, in association with HAT, plays a crucial role in epigenetic regulation of gene expression. One study tested the HDAC inhibitory activity of 33 compounds using HeLa nuclear extract and a fluorimetric assay. Among all the compounds, curcumin was one of the most potent HDAC inhibitors (IC50, 115 μM). The estimated free energy of binding was −8.55 kcal mol−1, and the Ki value was 539 nM. Molecular docking revealed that curcumin complexed with HDAC8 and adopted a stable binding pose extended toward the entrance cavity of the enzyme. Curcumin was shown to make various close hydrophobic contacts with the active site residues (including Arg37, Pro35, Ile34, and Phe152) of the enzyme but did not react with the zinc ion located in the cavity. In addition, two hydrogen bonds, one between the Asp29 carbonyl group and the hydroxy group of the curcumin (2.46 Å) and the second between the Tyr100 carbonyl group and the phenolic oxygen of the curcumin (1.80 Å), contributed to the low binding energy (Fig. 3).42
2.2.3 Glyoxalase I
Glyoxalase I (GLOI) is a key metal-loenzyme in the glycolytic pathway and is involved in detoxification of reactive α-ketoaldehydes, such as methylglyoxal. It is one of the main detoxification enzymes in both cancerous and normal cells.43 One study investigated the potential of curcumin and its 26 derivatives to inhibit GLOI activity by direct binding. In vitro studies using recombinant protein indicated that curcumin and curcumin derivatives inhibited human GLOI, with a Ki value in the range 2.6–4.6 μM. Molecular docking showed that the enol form of curcumin coordinated with Zn2+ in the active site of GLOI through oxygen atoms of the carbonyl group and formed a strong hydrogen bond with the Lys156, Arg122, and Arg37 residues of GLOI.44 Liu et al. also showed that the keto and enol forms of curcumin bind to the active site of GLOI and that the enol form interacted with sixteen residues, including Glu172 and Met179. Curcumin interacted chiefly through hydrophobic interactions. In addition, the carbonyl oxygen of curcumin formed a coordination bond with the zinc ion, which also contributed to curcumin’s binding within the active site pocket of GLOI. Furthermore, a water molecule (WAT) entered the active site and contributed to the binding. A hydrogen bond was observed between the oxygen atom of the water molecule and the O11 of curcumin. Another hydrogen bond was formed between the water molecule and Glu172 (Fig. 3). Furthermore, the water molecule contributed to the binding of the keto form of curcumin with GLOI by binding to the phenolic hydroxyl of curcumin and Met179 of GLOI (Fig. 3). The calculated binding free energies for the keto and enol complexes were − 24.16 and − 30.38 kcal mol−1, respectively, indicating that the enol form of curcumin is more potent for binding to the GLOI, possibly because of metal chelation. The authors also reported that BDMC binds to GLOI less effectively than does curcumin.45
2.2.4 Xanthine oxidase
Xanthine oxidase (XO) is an enzyme that catalyzes the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid. It is capable of generating reactive oxygen species.46 This enzyme plays an important role in many pathological conditions and is also involved in the pathogenesis of many diseases.47 In an experimental study, Pauff and Hille reported that curcumin neither inhibited XO activity nor affected the in vitro superoxide production,48 but many other in vivo studies have described curcumin as an inhibitor of XO and scavenger of superoxide. Molecular docking studies have shown that XO is a direct target of curcumin. Interestingly, degradation products of curcumin, such as trans-6-(4′-hydroxy-3′-methoxyphenyl)-2, 4-dioxo-5-hexenal, ferulic aldehyde, ferulic acid, feruloyl methane, and vanillin, have been shown to bind XO more efficiently than parent curcumin. The study also revealed that the binding pocket for interaction on XO consisted of Phe914, Phe1009, and Thr1010.49 On the basis of their observations, these authors suggested that the degradation products of curcumin exhibit better biological activities than curcumin itself under physiological conditions.49 X-ray crystallography or NMR spectroscopy would provide further structural information on these small molecules and their docking with XO, which could elucidate important information on the different mechanisms of interactions of curcumin and its degradation products.
2.2.5 Proteasomes
Proteasomes are also important targets of curcumin. Employing nucleophilic susceptibility and in silico docking studies, Milacic et al. showed that both carbonyl carbons of the β-diketone moiety of curcumin are highly susceptible to a nucleophilic attack by the hydroxyl group of the amino-terminal threonine (Thr1) of the β5 chymotrypsin-like (CT-like) subunit of the proteasome. Curcumin was also shown to form a hydrogen bond with Ser96 with a distance of 2.18 Å in the β5-subunit. The direct binding of curcumin to β5-subunit was concomitant with inhibition of CT-like activity of a purified rabbit 20S proteasome (IC50, 1.85 μM) and 26S proteasome in human colon cancer HCT-116 and SW480 cells. The inhibition of proteasome activity by curcumin in human colon cancer cells led to an accumulation of ubiquitinated proteins and several proteasome target proteins and a subsequent induction of apoptosis. Furthermore, treatment of SCID mice bearing HCT-116 colon tumors with curcumin resulted in decreased tumor growth. These authors concluded that proteasome inhibition could be one mechanism for the chemopreventive and/or therapeutic roles of curcumin in human colon cancer.50
Curcumin analogues have also been shown to bind to the proteasome directly. In one study, curcumin acetates and amino acid conjugates of curcumin were investigated in terms of their proteasome inhibitory and anti-proliferative effects against several human cancer cell lines. The water-soluble amino acid conjugates of curcumin showed a potent anti-proliferative and proteasome inhibitory activity. Further molecular docking studies indicated that, like curcumin, the amino acid conjugates of curcumin inhibited proteasome activity by direct binding to the β5 subunit of the proteasome.51 Structures of these systems, as determined by crystallography or NMR spectroscopy, would provide further evidence for different mechanisms of interactions displayed by curcumin and its analogs.
2.2.6 Sarco (endo) plasmic reticulum Ca2+ ATPase
The sarco (endo) plasmic reticulum Ca2+ ATPase (SERCA) is the major Ca2+ transport protein and plays an important role in regulating intracellular calcium during the muscle contraction-relaxation cycle. In one study, curcumin was shown to inhibit SERCA activity by direct binding. Curcumin was found to stabilize the E1 conformation of SERCA. Employing a fluorescence labeling technique and ATP binding, curcumin was shown to bind to a site within the ATPase that induced a conformational change to prevent ATP from binding. This eventually led to an inhibition of ATP-dependent SERCA activity.52 In another study, curcumin was shown to induce endoplasmic reticulum stress and inhibit the survival of human liposarcoma cells. These effects of curcumin were attributed to its inhibitory effect on SERCA2 activity by direct binding and induction of apoptosis in a CHOP-DR5-caspase dependent manner. Curcumin was found to co-localize with SERCA2 in the endoplasmic reticulum. Further docking studies revealed that curcumin interacts directly through Asp254, Arg264, and Gln56 residues of enzyme.53 These authors suggested that curcumin may serve as a potent agent for curing human liposarcoma by targeting SERCA2 directly.53
PfATP6 is a parasite orthologue of mammalian SERCA and has been shown to be the molecular target of anti-malarials.54 In one study, curcumin was shown to possess anti-malarial effects by binding directly to PfATP6. Molecular docking studies indicated that curcumin interacts with PfATP6 mainly through hydrophobic interactions and hydrogen bonds involving both its phenolic hydroxyl and keto–enol moiety and that it can efficiently inhibit PfATP6. In the complex of PfATP6 with the keto form of curcumin, four residues were involved in the hydrogen bond formation: Gln267 with the keto oxygen, Leu1040 and Ile1041 with one of the phenolic oxygens, and Ala985 with the other phenolic oxygen (Fig. 3). The estimated binding affinities for the keto and enol forms of curcumin with PfATP6 were 1.2 μM and 2.5 μM, respectively. Furthermore, methylcurcumin, which lacks the phenolic hydroxyl groups, showed lesser binding affinity.55
2.2.7 Human immunodeficiency virus type 1 (HIV1) integrase and HIV1 protease
Human immunodeficiency virus type 1 (HIV-1) is the etiological agent of acquired immunodeficiency syndrome (AIDS). Following infection, the retrovirus uses an integrase (IN) and a protease (PR) to propagate its life cycle, thus making these enzymes potential targets for therapeutic intervention. As indicated by molecular docking studies, curcumin was shown to have a potential inhibitory effect on these enzymes by direct binding to their active sites. For IN, the binding site was formed by residues Asp64, His67, Thr66, Glu92, Thr93, Asp116, Ser119, Asn120, and Lys159. Docked curcumin was found to contact the catalytic residues adjacent to Asp116 and Asp64, and near the divalent metal Mg2+ (Fig. 3). In the PR docking, the curcumin structure fit well to the active site, interacting with residues Asp25, Asp29, Asp30, Gly27′, Asp29′, and Asp30′ (Fig. 3). These results suggested that extensive hydrogen bonding promoted by the o-hydroxyl and/or keto–enol structures are important for both IN and PR inhibitory actions.56 In another study, Mazumder et al. investigated the inhibitory affect of curcumin on purified HIV-1 IN and observed that curcumin interacted with the catalytic core of the IN enzyme. They further observed that the anti-IN activity of curcumin was due to an intramolecular stacking of two phenyl rings that brought the hydroxyl groups into close proximity.57
2.2.8 DNA methyltransferase 1
DNA methyltransferases (DNMTs) are involved in the methylation of promoter CpG of tumor-suppressor genes (TSGs), resulting in transcriptional silencing of these genes in a variety of solid and blood cancers.58 Therefore, approaches aiming to modulate DNMT activity have therapeutic potential. Curcumin was recently shown to exert its inhibitory effect on M.SssI (a DNMT1 analogue) by covalently blocking the catalytic Cys1226 of DNMT1 (Fig. 3). Curcumin could inhibit the activity of M.SssI with an IC50 value of 30 nM. Tetrahydrocurcumin also showed similar activity, whereas hex-ahydrocurcumin did not show any inhibitory activity. In agreement with these observations, curcumin induced global DNA hypomethylation in a leukemia cell line.59 The calculated binding affinities for different curcumin derivatives from docking studies correlated with the experimentally observed IC50 values. The same studies also suggested that the α, β-unsaturated group of curcumin covalently binds to the catalytic cysteine of DNMT. However, the limited results from docking studies cannot substantiate such covalent binding, and further studies are necessary to confirm these claims.
2.2.9 DNA polymerase λ
DNA polymerase (pol) λ is a eukaryotic polymerase that is involved in DNA repair processes. Takeuchi et al. recently investigated the molecular structural relationship of curcumin and 13 chemically synthesized curcumin derivatives in terms of their ability to inhibit DNA pol λ. Curcumin was found to be an effective inhibitor of pol λ. Among all the derivatives investigated, MAC was one of the strongest pol λ inhibitors, even stronger than curcumin. The compound did not influence the activities of replicative pols, such as α, β, δ, and ε. As indicated by matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF MS) analysis, MAC bound selectively to the N-terminal domain of pol λ but did not bind to the C-terminal region. Molecular docking studies revealed that the binding site of MAC on pol λ consisted of a β-sheet (Thr51 of sheet-1), the α-helix (residues 57–69), and the two loops (residues 51–56 and 70–75). Further studies indicated that curcumin derivatives lacking the unsaturated β-diketone moiety did not show any inhibitory effect.60
2.2.10 Ribonuclease A
Ribonucleases (RNases) act at the junction of transcription and translation processes and may be cytotoxic because of their undesired cleavage of RNA. Specifically, they adsorb to certain cells via the cytosol, degrade RNA, and in turn inhibit protein synthesis and cause cell death. The curcumin analogue DAC has been found to interact with RNase, as evaluated by multiple biophysical methods, including fluorescence quenching, CD, FTIR, and molecular docking. The binding constant was 104 M−1. By FRET analysis, the distance between the tyrosine residue of RNase A and DAC was estimated to be 2.6 nm. No substantial conformational variation in the protein after binding was observed. Docking studies indicated that the oxygen atoms of the keto–enol group of DAC were hydrogen bonded with Tyr97, Gln11, and Lys7 of RNase, which was in addition to extensive nonspecific hydrophobic interactions.61
2.2.11 Lipoxygenase
Lipoxygenases (LOXs) are a family of iron-containing enzymes that catalyze the dioxygenation of arachidonic acid into hydroxyperoxyeicosatetraenoic acids, which is followed by conversion to their corresponding eicosa-noids. Numerous lines of evidence from preclinical and clinical studies have revealed various roles of LOX isoforms in carcinogenesis. Different LOXs have been shown to exhibit pro-tumorigenic or anti-tumorigenic activities and to modulate the tumor response in a tissue-specific manner. The LOX pathways have been shown to play a role in the spread and metastasis of several cancers.62 Therefore, many LOX inhibitors are currently being studied for their anti-carcinogenic properties. One study using soybean LOX L3 demonstrated that curcumin can inhibit the enzyme’s activity by blocking the active site. Further X-ray diffraction and mass spectrometry studies revealed an electron mass near the soybean L3 catalytic site. However, the mechanism of curcumin’s interaction with soybean L3 was not demonstrated, and the electron mass was found to be an unusual degradation product of curcumin located near the soybean L3 catalytic site.63 In a subsequent study, the group demonstrated that curcumin binds to LOX in a non-competitive manner. In a complex with LOX, curcumin was found to undergo photo-degradation in the X-ray beam and utilizes the enzyme’s catalytic ability to form the peroxy complex 4-hydroperoxy-2-methoxy-phenol, which later transformed into 2-methoxycyclohexa-2,5-diene-1,4-dione.64
P-12-LOX is another enzyme that is involved in cancer cell angiogenesis and metastasis and represents a potential therapeutic target. Using a homology model of the three-dimensional structure of human P-12-LOX, computational docking of synthetic curcumin derivatives (mainly substituted on the aromatic rings) was performed in one study. Over 75% of the compounds were successfully docked into the active site of P-12-LOX, and many of them shared similar binding modes. Curcuminoids that did not dock into the active site did not inhibit P-12-LOX. The hydrophobic groups consisting of aromatic rings of curcumin attached to a flexible linker were necessary for binding and LOX inhibition. The amino acid residues of P-12-LOX found to be critical for hydrophobic interactions were Glu355, Ile592, Phe351, Phe413, His364, Leu360, Ile398, Leu406, Ala402, Trp143, Leu407, Leu360, and Leu365. The curcuminoids inhibiting P-12-LOX were tested for their ability to reduce sprout formation of endothelial cells (an in vitro model of angiogenesis). Only curcuminoids inhibiting human P-12-LOX and the known inhibitor nordihydroguaiaretic acid (NDGA) reduced sprout formation.65
2.2.12 Matrix metalloproteinases
Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases capable of degrading various extracellular matrix proteins. These are thought to play a major role in cell proliferation, migration, differentiation, angiogenesis, apoptosis, and host defense. They have also been implicated in a variety of pathological conditions, such as inflammatory, vascular, and autoimmune disorders.66–69 Docking analysis of curcumin derivatives THC and BDMC with MMPs was performed in one study. Although both THC and BDMC showed affinity to MMPs, BDMC had higher affinity than THC. Further docking analysis revealed that the interaction of BDMC with MMPs was associated with a docking energy of −11.46 kcal mol−1 and the formation of three hydrogen bonds. One hydrogen bond was formed between the hydroxyl group of the phenyl ring and the carboxyl group of the hydrophobic amino acid Pro421. Another hydrogen bond formed a bridge between the oxygen of the curcumin heptane branch and the amine group of Arg424.70
2.2.13 Lysozyme
Lysozyme is an enzyme that destroys bacterial cell walls by hydrolyzing the polysaccharide component of the cell wall. It is abundantly present in a number of secretions, including tears. This enzyme is also present in high concentration in egg white. It has been reported to be responsible for the formation of systemic amyloidosis in the human body.71,72 In one study that used absorption spectroscopy and fluorescence techniques, curcumin was found to bind to lysozyme. The binding constant between curcumin and lysozyme was estimated to be 1.2 × 103 M−1.73 The hen egg-white lysozyme (HEWL) has a structure highly homologous to human lysozyme. In another study, curcumin was shown to exhibit inhibitory activity against the fibrillation of hen lysozyme. In a study that used quenching and Van’t Hoff analysis, an interaction between curcumin and lysozyme was observed that was governed predominantly by van der Waals force or hydrogen bonding. Curcumin was also found to acquire disaggregating ability on preformed lysozyme fibrils. Interestingly, curcumin that was pre-incubated at 25 °C for 1 week exhibited better inhibitory activity towards lysozyme aggregation than did untreated curcumin. This superior inhibitory potency of pre-incubated curcumin was highly associated with curcumin dimeric species formed during the course of its preincubation.74 Given the propensity for lysozyme to form high-quality crystals for X-ray structure determination, this system could serve as an excellent structural model for examining the mechanism of curcumin–protein interactions.
2.3 Curcumin binds directly to protein kinases
Protein kinases that are the integral part of the signal transduction pathway have been reported as direct targets of curcumin. Among these protein kinases are protein kinase C (PKC), v-Src, GSK-3β, and ErbB2 (HER2/neu), as detailed below.
2.3.1 Protein kinase C
The protein kinase C (PKC) family is a group of serine/threonine kinases that play a central role in cellular signal transduction pathways. This family has been reported to govern a wide range of physiological processes, including differentiation, proliferation, membrane transport, and the organization of cytoskeletal and extracellular matrix proteins.75–77 In an effort to develop curcumin derivatives as effective PKC activators, Majhi et al. synthesized several long-chain derivatives of curcumin, characterized their absorption and fluorescence properties, and studied their interaction with the activator-binding second cysteine-rich C1B subdomain of PKCδ, PKCε, and PKCθ. As shown from fluorescence spectroscopic studies, curcumin and its analogue, in which the phenolic OH was alkoxylated with a C16 alkyl chain, quenched the intrinsic fluorescence of PKCδC1B, PKCεC1B, and PKCθC1B in a manner similar to that of the PKC activator 12-O-tetradeca-noylphorbol 13-acetate (TPA). The binding was confirmed by blue-shifted fluorescence spectrum and increased fluorescence anisotropy of curcumin and its analogues in the presence of PKC activators. Molecular docking of curcumin and its C16 analogue with PKC C1B revealed that both the molecules form hydrogen bonds with the tyrosine and tryptophan residues of the protein, and these authors proposed probable conformational changes in the protein and ligands.78
2.3.2 Viral sarcoma
Cellular sarcoma (c-Src), a protein that is encoded by the cellular homologue of viral sarcoma (v-src), is a ubiquitously expressed cytoplasmic tyrosine kinase whose overexpression has been implicated in human tumors.79 Curcumin has been shown to inhibit the kinase activity of v-Src, which led to a decrease in tyrosyl substrate phosphorylation of Shc, cortactin, and FAK. Immunoprecipitation and in vitro kinase assays revealed that the inhibitory effect of curcumin on Src could be direct. This effect was associated with a reduction in the Src-mediated Shc-Tyr317 phosphorylation, FAK phosphorylation, decreased ERK activation, and reduced proliferation of v-Src-transformed cells.80
2.3.3 Glycogen synthase kinase-3β
Glycogen synthase kinase (GSK)-3β is a multi-tasking serine/threonine kinase that in humans is encoded by the GSK3B gene.81 Although originally isolated from skeletal muscle,82,83 the enzyme is widely expressed in all tissues, with an abundance in the brain.84 It is involved in the pathogenesis of several diseases including type II diabetes, cancer, Alzheimer’s disease, mood disorders, bipolar disorders, and stroke.85–88
In one study, curcumin was found to optimally fit within the binding pocket of GSK-3β via several attractive interactions with key amino acids.89 Further docking studies indicated that the conjugated keto–enol system of curcumin forms hydrogen bonds in such a way that the enolic hydroxyl group of curcumin interacts with the amidic carbonyl of Val135, whereas the conjugated ketone of curcumin was hydrogen bonded to the NH of the same amino acid residue. Other amino acids of GSK-3β involved in the interaction were Ile62, Arg141, and Lys85 through multiple hydrogen bonds (Fig. 3). Concomitant with this direct binding, curcumin inhibited GSK-3β (IC50, 66.3 nM). Curcumin also significantly increased liver glycogen reserves in food-deprived BALB/c mice, an effect that could be attributed to its inhibitory effect on GSK-3β.89
2.3.4 ErbB2
ErbB2 (HER2/neu) is a transmembrane tyro-sine kinase whose overexpression has been shown to increase a cell’s metastatic potential and resistance to anti-cancer agents.90 Therefore, therapeutic strategies that downregulate the level of ErbB2 protein and/or its activity could be potential treatments for ErbB2-overexpressing cancers. Recently, curcumin was shown to increase the association of carboxyl terminus of Hsc70-interacting protein (CHIP), a chaperone-dependent ubiquitin ligase with ErbB2, and eventually induced ubiquitination and depletion of ErbB2. As indicated by site-directed mutagenesis and molecular docking, curcumin exerted its effect by binding to the kinase domain of ErbB2. Further studies indicated that curcumin’s Michael acceptor functionality was required for both covalent association of curcumin with ErbB2 and curcumin-mediated ErbB2 depletion.91
2.3.5 Phosphorylase kinase
Phosphorylase kinase is a serine/threonine-specific protein kinase that phosphorylates glycogen phosphorylase. Phosphorylation activates glycogen phosphorylase, allowing it to metabolize glycogen to supply energy for muscle contraction.92 In one of our early studies, we examined the effects of curcumin on the activity of six different protein kinases (PKA, PKC, protamine kinase, phosphorylase kinase, autophosphorylation-activated protein kinase, and a tyrosine kinase) using highly purified protein. As shown by results of the protein kinase assay, curcumin inhibited all the kinases; however, only phosphorylase kinase was completely inhibited at relatively lower curcumin concentrations. Although the exact amino acid involved in binding was not identified, Lineweaver–Burk plot analysis indicated that curcumin is a non-competitive inhibitor of phosphorylase kinase, with a Ki of 75 μM.93
2.4 Curcumin binds directly to protein reductases
Protein reductases are enzymes that catalyze the reduction of other proteins. Curcumin has been reported to inhibit protein reductase activity by direct binding as well. Among these reductases are thioredoxin reductase (TrxR) and aldose reductase (ALR2), as discussed below.
2.4.1 Thioredoxin reductase
Thioredoxin reductase catalyzes NADPH-dependent reduction of the redox-active disulfide (S–S) in thioredoxin (Trx), which serves a wide range of functions in cellular proliferation, defense against oxidative stress, apoptosis, and redox control.94 Fang et al. found that curcumin has potential to inhibit TrxR1, one of the isoenzymes of TrxR. The inhibition occurred only in the presence of NADPH and persisted after removal of curcumin. By using mass spectrometry and blotting analysis, the authors showed that irreversible inhibition by curcumin was caused by covalent binding of Cys496/Sec497 in the catalytically active site of the enzyme through Michael addition. Further analysis indicated that modification of TrxR by curcumin shifted the enzyme from an anti-oxidant to a pro-oxidant.20 In another study, Singh and Misra compared the potency of curcumin, DMC, and BDMC for inhibiting TrxR by molecular docking. The residues His108, Arg351, Lys29, and Leu112 of the F chain of TrxR were found to interact with the curcumin molecule. Further docking analysis indicated that Se atom of the SeCys498 residue formed a hydrophobic contact with the sp2 carbon of the β-diketone moiety of curcumin at a distance of 3.23 Å. It was concluded that at least one methoxy group in curcuminoids is necessary for interaction with catalytic residues of thioredoxin.95 Structural data confirming this observation would further demonstrate the mechanism of this interaction.
2.4.2 Aldose reductase
Aldose reductase (ALR) 2, a member of the aldo–keto reductase (AKR) super family, is the first and rate-limiting enzyme in the polyol pathway and reduces glucose to sorbitol utilizing NADPH as a cofactor. Sorbitol is subsequently metabolized to fructose by sorbitol dehydrogenase.96 Accumulation of intracellular sorbitol due to increased ALR2 activity has been implicated in the development of various complications of diabetes. The potential of curcumin as an agent to prevent or treat diabetic complications was investigated in one study. Curcumin inhibited ALR2 activity in a non-competitive manner (IC50, 10 μM). The inhibitory effect of curcumin on ALR2 was specific because curcumin failed to inhibit ALR1 under similar experimental conditions. Molecular docking revealed that curcumin interacts with ALR2 at active site residues Tyr48, Lys21, Thr19, Gln183, Leu300, and Trp111 in a closed type of conformation. Consistent with these observations, curcumin was found to suppress sorbitol accumulation in human erythrocytes under high glucose conditions, demonstrating an in vivo potential of curcumin to prevent sorbitol accumulation.97 X-ray crystallography determination of the structure of curcumin when docked to ALR2 could provide important information about novel mechanisms of curcumin binding.98
The human small intestine reductase (HSIR or AKR1B10) is another member of the AKR family that has been reported to play a role in tumor cell survival and proliferation.99,100 It is overexpressed in a number of cancer cells. Muthena et al. found that curcumin has the potential to inhibit AKR1B10 as well, although the potency was far less than for ALR2 (IC50, 30.0 ± 3.0 μM).97 In another study, the curcumin analogue BDMC was found to be a highly potent and selective inhibitor of AKR1B10. On the other hand, curcumin and DMC exhibited >3-fold less potency and selectivity than BDMC. Molecular docking studies of the curcuminoids in the AKR1B10-NADP+ complex and site-directed mutagenesis of the putative binding residues suggested that Trp21, Gln114, Trp220, Val301, and Ser304 formed a hydrogen bond with the hydroxyl group on the phenyl ring of BDMC.100
2.5 Curcumin binds directly to carrier proteins
The major limitation of the clinical efficacy of curcumin is its low solubility in aqueous solution (2.99 × 10−8 M) and poor bioavailability.101,102 To overcome this problem, attempts have been made through encapsulation in polymeric micelles, liposomes, polymeric nanoparticles, lipid-based nanoparticles, and hydrogels.103–107 Various proteins have been shown to act as carriers by binding directly to the curcumin. These proteins include milk casein, human serum albumin (HSA), bovine serum albumin (BSA), β-lactoglobulin (βLG), and immunoglobulin (Ig).
2.5.1 Casein
Caseins, which are the major milk proteins, have excellent emulsification, gelation, and water-binding properties. Microspheres of casein that are prepared by glutaraldehyde cross-linking have been used for the oral delivery of anticancer drugs, such as doxorubicin and mitoxantrone.108,109 Sahu et al. reported the complexation of curcumin with the natural nanostructure of casein micelles (CMs) and its application in drug delivery to cancer cells. As shown by fluorescence spectroscopy, curcumin was shown to form a complex with CMs through hydrophobic interactions. The binding constant for the CM-curcumin interaction was 1.48 × 104 M−1. It was suggested that curcumin could interact through Trp164 and Trp199 of αS1-casein and through Trp143 of β-casein. Determination of cytotoxicity in HeLa cells indicated that the IC50 of free curcumin and the CM-curcumin complex were 14.85 and 12.69 μM, respectively.110 In another study, CD and spectroscopic measurements indicated that curcumin has the ability to bind to αS1 casein with two binding sites, one with high affinity (2.01 × 106 M−1) and the other with low affinity (6.3 × 104 M−1), predominantly by hydrophobic interactions. The conformation of αS1-casein was not changed because of this interaction. The biostability of curcumin was, however, enhanced significantly because of this interaction. The chaperone-like activity of αS1-casein was also slightly enhanced on binding to curcumin.111
2.5.2 Albumin
Human serum albumin (HSA) and bovine serum albumin (BSA), the two major albumins, are the most widely studied carrier proteins, owing to their structural homology. Both HSA and BSA have been shown to serve as carriers of curcumin by direct binding. Absorption, fluorescence, and CD spectroscopy have been extensively used to study the binding of curcumin to HSA. The estimated binding constants have been shown to vary from 105 to 104 M−1. Two binding sites have been identified for curcumin in HSA. FRET analysis has suggested that the high affinity binding site of curcumin from Trp214 is at 2.74 nm, in the IIA domain of HSA. The estimated enthalpy change for curcumin–HSA formation has been found to be −13.6 kcal mol−1. Furthermore, a number of studies have shown that the binding is governed by hydrophobic and hydrogen bonding interactions.112–117 In one study, curcumin was shown by UV-visible spectrophotometry to exhibit strong association with the hydrophobic domains of HSA. The binding inhibited interaction with the surrounding water, leading to a suppression of curcumin degradation due to hydrolysis. These results suggested that the stabilization effect of HSA may enable curcumin to maintain its medicinal properties at a wound site to promote healing.118
Apart from binding directly to curcumin, HSA has also been shown to serve as a carrier of curcumin analogues by direct binding. For example, one study investigated the interaction of IOC with HSA employing various biophysical tools. Thermodynamic analysis indicated that the interaction, as in curcumin, was entropy driven, with hydrophobic forces as the major binding force. CD and FTIR studies revealed an absence of significant conformational changes upon binding. From FRET analysis, the curcumin-binding site from Trp214 in HSA was calculated to be 3.2 nm. Molecular docking studies confirmed that IOC is located within the binding pocket of the hydrophobic subdomains of HSA. Furthermore, Arg218, Asn295, and Tyr452 residues of HSA were involved in the interaction. The authors of this study suggested that this interaction might be useful for delivery of IOC.119
Bourassa et al. investigated complexation of curcumin with BSA using multiple biophysical methods, such as FTIR, CD, and fluorescence quenching. Structural analysis revealed that curcumin binds BSA via hydrophilic and hydrophobic interactions, with a binding constant of 3.33 ± 0.8 × 104 M−1. Curcumin binding altered BSA conformation with a major reduction of α-helix and an increase in β-sheet and turn structures, indicating a partial protein unfolding. Further analysis indicated that the curcumin-binding site was mainly in the vicinity of Trp212 and Trp134 located in protein domains I and II.120 Curcumin analogues have also shown potential to interact with BSA. For example, one study investigated the interaction of IOC and DAC with BSA. The average binding distances between the donor (BSA) and acceptors (IOC and DAC) were found to be 3.79 and 4.27 nm, respectively. CD and FTIR indicated minor conformational changes of BSA on binding. A docking study indicated that both IOC and DAC (enol form) preferentially docked to the hydrophobic subdomain near Trp213 of BSA.121
2.5.3 Fibrinogen
Fibrinogen, a soluble protein present in blood plasma, is converted to fibrin by the action of thrombin. Fibrinogen comprises around 4% of total plasma proteins and plays a major role in the formation of blood clots. As revealed by UV-visible spectrophotometry, curcumin was shown to exhibit strong association (with binding constant of 105 M−1) to the hydrophobic domains of fibrinogen in one study. The binding inhibited interaction with surrounding water, leading to suppression of curcumin degradation due to hydrolysis. These authors concluded that the stabilization effects of this protein may enable curcumin to maintain its medicinal properties at a wound site to promote healing.118
2.5.4 β-Lactoglobulin
β-LG is a low-molecular-weight whey protein capable of binding and transporting small hydrophobic molecules.122 The potential of βLG as a carrier molecule for curcumin was investigated by following the interaction between curcumin and βLG using spectroscopic techniques; the binding site was visualized by molecular modeling. This protein was found to interact with curcumin at pH 7.0 with an association constant of 1.0 × 105 M−1. The interaction was hydrophobic in nature and did not affect either the conformation or the state of association of βLG. As revealed by molecular docking, curcumin binds to the central calyx of βLG. Further inspection of the binding site suggested the closer contact of the methoxy phenyl moiety of curcumin with the aromatic amino acid residues of βLG. The authors of this study concluded that nanoparticles of βLG, by virtue of their ability to enhance the solubility and stability of curcumin, may serve as carrier molecules.123 In another study, the ability of curcumin and DAC to bind to bovine β-LG was investigated using various biophysical tools, including fluorescence quenching, CD, and FRET. Curcumin was found to possess greater affinity to bind to β-LG compared with DAC. By FRET analysis, the average distances between Trp19 and Trp61 and the ligands curcumin and DAC were estimated to be 3.383 and 3.509 nm, respectively. The stronger interaction of curcumin with β-LG suggested the critical role of the hydroxyl phenolic group in the para position in the binding process. Further studies indicated that two tryptophan residues (Trp19 and Trp61) in β-LG are critical for interaction.124
2.5.5 Immunoglobulin
Intravenous immunoglobulin (IVIG), the human serum immunoglobulin (Ig) fraction, is an important transport protein for drugs.125 Recently, the interaction of curcumin with IVIG was studied by fluorescence quenching and FTIR spectroscopy. The binding parameters for the interaction suggested that the binding of IVIG to curcumin was characterized by two binding sites with the average affinity constant at 1.17 × 104 M−1. FRET analysis gave the average binding distance between curcumin and the chromophore of IVIG as 5.57 nm. The observed spectral changes indicated a partial unfolding of the protein structure, but the typical β-structural conformation of IVIG was still retained. The molecules of curcumin were mainly located in the complement-determining Fab region of IgG. Molecular docking revealed the existence of hydrogen bonds between curcumin and His35, Arg96, Tyr99, Tyr91, Ala92, Tyr94, and Tyr98 residues of IgG. These authors suggested that IVIG can serve as a carrier for curcumin.126
2.6 Curcumin binds directly to cell survival proteins
A major reason for the failure of current cancer therapy is that, over time, cancer cells develop resistance to currently available treatments, partly owing to the expression of cell survival proteins such as Bcl-2.127,128 Luthra et al. examined the interaction of curcuminoids (curcumin, DMC, and BDMC) with Bcl-2 by molecular docking studies. The curcuminoids were found to interact directly with Bcl-2. Seven cavities on Bcl-2 were apparently available for binding. Multiple analyses run on selected cavities demonstrated that cavity-2 had more promising binding affinity (ΔG) and Ki with curcuminoids. Of the three curcuminoids, DMC showed stronger binding (Ki, 0.56 nM; ΔG, −6.97 kcal mol−1) than curcumin (Ki, 2.21 nM; ΔG, −4.53 kcal mol−1) or BDMC (Ki, 4.68 nM; ΔG, −6.4 kcal mol−1). The binding site of cavity-2 consisted of several residues including Tyr108, Glu136, Gly141, Asn143, Trp144, Gly145, Arg146, His184, Trp188, and Tyr202. Consistent with these observations, DMC possessed a significant anti-proliferative effect and affectedBcl-2-regulated apoptotic pathways more efficiently on glioma U87 cells.129
2.7 Curcumin binds directly to FtsZ protofilaments
FtsZ is a cytoskeletal protein that plays a pivotal role in bacterial cytokinesis.130–134 Therefore, FtsZ may be considered as an important anti-bacterial drug target. In one study, curcumin was found to induce filamentation in Bacillus subtilis 168, suggesting that it inhibits bacterial cytokinesis. Curcumin strongly inhibited the formation of the cytokinetic Z-ring in B. subtilis 168 without affecting the segregation and organization of the nucleoids. Curcumin inhibited the assembly of FtsZ protofilaments and also increased the GTPase activity of FtsZ. It was also found to bind to FtsZ in vitro with a dissociation constant of 7.3 μM. The number of binding sites of curcumin on FtsZ was 0.7 per FtsZ molecule. The authors of this study concluded that curcumin has the potential to inhibit bacterial cell division by perturbing the cytokinetic Z-ring through a direct interaction with FtsZ.135 Employing molecular docking, Kaur et al. also showed that curcumin interacts with the active site of FtsZ through amino acid residues Val19, Gly104, Gly22, Gly107, Gly108, Thr109, Asn166, Ala186, and Asp187 both by nonspecific hydrophobic interactions and by forming hydrogen bonds.136
2.8 Curcumin binds directly to prion protein
Curcumin has been shown to inhibit plaque formation, which is a hallmark of a number of neurodegenerative diseases, such as transmissible spongiform encephalopathies.137 These diseases are characterized by conversion of the native, predominantly α-helical conformation of prion protein (PrP) into the β-stranded conformation. In one study, curcumin showed an affinity to bind selectively to the non-native β-forms and α-helical intermediate of PrP. As revealed by CD and fluorescence quenching, curcumin recognized the converted β-form of PrP both as oligomers and fibrils but not the native form. Although the exact amino acids involved in the interaction were not identified, curcumin was found to bind to the prion fibrils in the left-handed chiral arrangement.138
2.9 Curcumin binds directly to DNA and RNA
Curcumin has also been shown to bind to DNA and RNA directly and to affect their physiological functions. One study investigated the interactions of curcumin with calf thymus (ct)-DNA and yeast RNA in aqueous solution at physiological conditions using constant DNA and RNA concentrations. FTIR and UV-visible spectroscopic methods were used to determine the ligand binding modes, the binding constants, and the stability of curcumin–DNA and curcumin–RNA complexes in aqueous solution. Spectroscopic evidence showed that curcumin binds DNA through thymine O2 (minor groove) and guanine and adenine N7 (major groove), as well as to the backbone PO2 group with overall binding constants of 4.3 × 104 M−1. Curcumin–RNA binding was mediated by hydrogen bonding with uracil O2 and guanine and adenine N7 atoms as well as the backbone phosphate group, with overall binding constants of 1.3 × 104 M−1. No conformational changes were observed upon curcumin interaction with these biopolymers.16
The curcumin analogue IOC has also been shown to interact with ct-DNA. As investigated by UV-visible, fluorescence, and CD spectroscopies, viscosity measurements and docking studies, IOC was found to be a minor groove binder, mediated by hydrogen bonding interactions. The binding constant of IOC to DNA calculated from both UV-visible and CD spectra was 104 M−1. Further analyses indicated that the binding site of IOC on ct-DNA consisted of three base pairs involving AT residues within the minor groove.15 Like IOC, DMC has also been found to interact with ct-DNA, with a binding constant of 4.4 × 104 M−1.139 The physiological consequence of binding of curcumin and its derivatives to DNA is not yet understood and needs to be explored further, especially in view of its observed nuclear localization in tumor cells.
2.10 Curcumin binds directly to transthyretin
Transthyretin (TTR) is a homotetrameric protein involved in the transport of thyroxin (T4) and retinol in human plasma. Mis-folding and aggregation of TTR is implicated in the pathogenesis of familial amyloid polyneuropathy (FAP) and senile systemic amyloidosis (SSA). As shown by fluorescence quenching and ANS displacement studies, curcumin bound to the active site of TTR and stabilized it by preventing denaturant-induced tertiary and quaternary structural changes. Fluorescence quenching analysis indicated that curcumin binds to TTR with a molar ratio of 1.2 : 1 and Kd of 2.3 × 10−6 M. Curcumin was unable to bind to TTR at acidic pH. Protonation and isomerization of the phenolic and enolic hydroxyl groups of curcumin at low pH hampered the binding. These results suggested that curcumin binds to and stabilizes TTR, thereby highlighting the importance of the side chain conformations of the ligand in binding to TTR.140
2.11 Curcumin binds directly to Ca2+/CaM
The curcumin derivative HBC has been shown to exhibit potent inhibitory activities against the proliferation of several tumor cell lines. Using phage display biopanning, HBC was found to bind directly to the Ca2+/calmodulin (CaM). Molecular docking indicated that HBC was compatible with the binding cavity for a known inhibitor, W7, in the C-terminal hydrophobic pocket of Ca2+/CaM. Consistent with these observations, HBC induced prolonged phosphorylation of ERK1/2 and activated p21 (WAF1) expression, resulting in the induction of G0/G1 cell cycle arrest in HCT15 colon cancer cells. The authors of this study suggested that HBC has the potential to inhibit the cell cycle progression of colon cancer cells by antagonizing Ca2+/CaM functions.141 To our knowledge, however, curcumin has not been reported to bind to Ca2+/CaM directly.
2.12 Curcumin binds directly to tubulin, APN, and β-amyloid aggregates
Curcumin has also been shown to bind to tubulin,142 CD13/aminopeptidase N (APN),143 and β-amyloid (Aβ) aggregates.144,145 Curcumin was shown to bind to tubulin at a single binding site, with a Kd of 2.4 μM, and the binding also induced conformational changes in tubulins.129 APN is a membrane-bound, zinc-dependent metalloproteinase that plays a key role in tumor invasion and angiogenesis. The in vitro binding of curcumin to APN was confirmed by fluorescence and surface plasmon resonance analysis, and its in vivo binding was confirmed by APN-specific antibody competition assay. Curcumin binds strongly to APN and irreversibly inhibits its activity, with a Ki of 11.2 μM. This inhibition of APN by curcumin also resulted in inhibition of angiogenesis.130 In one study, binding of curcumin and its derivatives to Aβ-aggregates was studied by UV-visible spectroscopy. The results indicated that curcumin binds to Aβ-aggregates through the enol form and that enolization is crucial for such binding.131
2.13 Curcumin binds directly to glutathione
Glutathione (GSH) is a tripeptide and antioxidant with potential to prevent oxidative damage to important cellular components caused by reactive oxygen species.146 Spectrophotometric analyses of curcumin and GSH has indicated complex interactions.147 The reactions of curcumin with GSH and the effect of recombinant human glutathione S-transferase (GST) P1-1 on reaction kinetics were investigated in one study. As identified by FAB-MS and MALDI-MS, curcumin was found to form glutathionylated products that included mono- and diglutathionyl adducts of curcumin. The presence of GSTP1-1 significantly accelerated the initial rate of GSH-mediated consumption of curcumin. GSTP1-1 kinetics (as determined using HPLC) indicated substrate inhibition (Km, 25 ± 11 μM; Ki, 8 ± 3 μM).148
2.14 Curcumin binds directly to keap1
Kelch-like ECH-associated protein 1 (Keap1) is a cysteine-containing chaperone that is encoded by the Keap1 gene in humans. It helps maintain the transcription factor Nrf2 in an inactive state in the cytosol.149 When stimulated by agents that can modify thiol groups, Keap1 no longer represses Nrf2 activity, and Nrf2 translocates to the nucleus. There, it binds to the antioxidant-responsive element and initiates the transcription of genes involved in detoxification and cytoprotection.150 Curcumin has been shown to alter the Nrf2–Keap1 interaction in rat kidney epithelial cells and to enhance the expression of Nrf2 dependent heme oxygenase-1. Although the mechanism by which curcumin interrupts Nrf2-Keap1 interactions was not investigated, it was postulated that curcumin’s Michael addition to the thiols in Keap1 may promote a conformational change in the Nrf2–Keap1 complex that helps release and translocate Nrf2 into the nucleus.151
2.15 Curcumin binds directly to metal ions
The α,β unsaturated β-diketone moiety of curcumin can form chelates with transition metals. Several reports have considered the activity of metal chelates of curcumin. Metal chelates of curcumin of the type 1 : 1 and 1 : 2 have been reported for the ions Cu2+, Fe2+, Mn2+, Pb3+, and others. Metal chelates of curcumin are mostly non-fluorescent, although absorption spectra show significant changes. Cu2+ and Mn2+ chelates of curcumin and its derivatives have been found to act as antioxidants and superoxide dismutase mimics.112,152–154 Manganese complexes of curcumin and curcumin derivatives have been shown to exhibit capacity to protect brain lipids against peroxidation.155 One study used spectrophotometry to quantify the affinity of curcumin for Cu2+, Fe2+, and Zn2+. All three metal ions showed affinity towards curcumin, although Zn2+ showed little binding. Given that amyloid aggregation induced by these metals plays a pivotal role in the pathogenesis of Alzheimer’s disease, the authors concluded that curcumin could act as an agent against Alzheimer’s disease by inducing metal chelation. However, how curcumin binds to these metals was not investigated.19
3 Summary, conclusions, and future perspective
Curcumin has a novel chemical structure that allows it to participate in a number of physical, chemical, and biological processes. It has three important functionalities: an aromatic o-methoxy phenolic group; α,β-unsaturated β-diketo linker; and keto–enol tautomerism. The aromatic groups provide hydrophobicity, and the linker gives flexibility. The tautomeric structures also influence the hydrophobicity and polarity of the curcumin molecule. All these unique features of curcumin make it able to bind to a variety of biomacromolecules.
A number of experimental and theoretical methods have been used to study the interactions of curcumin with many target proteins, enzymes, DNA, RNA, and carrier molecules. The common factor in almost all curcumin interactions is that curcumin is located in the hydrophilic pockets near cysteine residues, and its α, β-unsaturated-β-diketone is the main group participating in the interactions. The distinctive changes in the spectroscopic properties of curcumin during binding have provided an excellent experimental tool to monitor these interactions. Because of their relative ease of operation and high sensitivity, fluorescence-based methods have been most commonly employed for understanding curcumin–protein binding, and a wealth of qualitative and semi-quantitative information has been provided to understand the in vitro binding between curcumin and its target proteins. Other sophisticated techniques, such as X-ray crystallography and NMR spectroscopy, would no doubt provide more authentic and conclusive information on such binding interactions. However, they are not commonly employed because of the difficulties inherent in growing single crystals of protein–curcumin systems.
Molecular docking has been the most commonly employed tool for computations. Using this method, several researchers have calculated the binding affinities and also indicated the probable site and mode of binding between curcumin and other biomolecules. However, the level of explanation is not the same in all the studies, and this review does not make any attempt to compare the results with the strength of calculations. It is, of course, very important to note that docking studies alone cannot provide complete information, and more advanced calculations based on ab initio molecular orbital methods are necessary to correlate the experimentally observed binding phenomena.
In this review, we have thus made an attempt to compile all the experimental and theoretical studies on the interactions of curcumin with various targets. As is clear from this compilation, curcumin is effective against a variety of inflammatory ailments and modulates multiple cell signaling pathways. Curcumin’s ability to bind to carrier proteins improves its solubility and bioavailability. Most of the biomolecules that curcumin binds to are integral components of cell signaling pathways and therefore may be pharmacologically relevant. However, most of the direct interaction data obtained to date are based on in vitro studies. Only limited studies have shown functional consequences of curcumin interaction. In many instances, what may occur in vivo is still not clear. In spite of numerous reports showing pleiotropic activity, curcumin has yet not been approved for treatment of any human disease, even though it has been reported to be safe for humans at gram dosages. Therefore, future studies should be focused more toward understanding the real functional meaning of these direct interactions and bringing this fascinating molecule to the forefront of therapeutic agents for the treatment of human diseases.
Acknowledgments
We thank Michael Worley, Zachary S. Bohannan and the Department of Scientific Publications for carefully editing the manuscript and providing valuable comments. Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research. Dr. Webb holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund. This work was supported by a core grant from the National Institutes of Health (CA-16672), a program project grant from the National Institutes of Health (NIH CA-124787-01A2), and a grant from the Center for Targeted Therapy of MD Anderson Cancer Center.
Biography

Dr. Bharat B. Aggarwal is a Ransom Horne, Jr. Distinguished Professor of Experimental Therapeutics at the University of Texas MD Anderson Cancer Center, Houston, Texas. Dr. Aggarwal has been investigating the “Role of Inflammatory Pathways Mediated through TNF, NF-kappaB and STAT3, for the Prevention and Therapy of Cancer and other Chronic Diseases”. While searching for novel and safe anti-inflammatory agents, his group has identified more than 50 novel compounds from natural sources that interrupt these cell-signaling pathways. He has published more than 600 papers, and is one of the most highly cited scientists in the world by ISI since 2001.
References
- 1.Ammon HP, Wahl MA. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal BB, Harikumar KB. Int J Biochem Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kannappan R, Gupta SC, Kim JH, Reuter S, Aggarwal BB. Mol Neurobiol. 2011;44:142–159. doi: 10.1007/s12035-011-8168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta SC, Kim JH, Prasad S, Aggarwal BB. Cancer Metastasis Rev. 2010;29:405–434. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu Y, Zheng S, Lin J, Ryerse J, Chen A. Mol Pharmacol. 2008;73:399–409. doi: 10.1124/mol.107.039818. [DOI] [PubMed] [Google Scholar]
- 6.Kohli K, Ali J, Ansari MJ, Raheman Z. Indian J Pharmacol. 2005;37:141–147. [Google Scholar]
- 7.Sharma S, Kulkarni SK, Chopra K. Clin Exp Pharmacol Physiol. 2006;33:940–945. doi: 10.1111/j.1440-1681.2006.04468.x. [DOI] [PubMed] [Google Scholar]
- 8.Nishiyama T, Mae T, Kishida H, Tsukagawa M, Mimaki Y, Kuroda M, Sashida Y, Takahashi K, Kawada T, Nakagawa K, Kitahara M. J Agric Food Chem. 2005;53:959–963. doi: 10.1021/jf0483873. [DOI] [PubMed] [Google Scholar]
- 9.Sharma OP. Biochem Pharmacol. 1976;25:1811–1812. doi: 10.1016/0006-2952(76)90421-4. [DOI] [PubMed] [Google Scholar]
- 10.Sidhu GS, Singh AK, Thaloor D, Banaudha KK, Patnaik GK, Srimal RC, Maheshwari RK. Wound Repair Regener. 1998;6:167–177. doi: 10.1046/j.1524-475x.1998.60211.x. [DOI] [PubMed] [Google Scholar]
- 11.Negi PS, Jayaprakasha GK, Jagan Mohan Rao L, Sakariah KK. J Agric Food Chem. 1999;47:4297–4300. doi: 10.1021/jf990308d. [DOI] [PubMed] [Google Scholar]
- 12.Goel A, Aggarwal BB. Nutr Cancer. 2010;62:919–930. doi: 10.1080/01635581.2010.509835. [DOI] [PubMed] [Google Scholar]
- 13.Gupta SC, Patchva S, Koh W, Aggarwal BB. Clin Exp Pharmacol Physiol. 2011 in press. [Google Scholar]
- 14.Reuter S, Gupta SC, Park B, Goel A, Aggarwal BB. Genes Nutr. 2011;6:93–108. doi: 10.1007/s12263-011-0222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bera R, Sahoo BK, Ghosh KS, Dasgupta S. Int J Biol Macromol. 2008;42:14–21. doi: 10.1016/j.ijbiomac.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Nafisi S, Adelzadeh M, Norouzi Z, Sarbolouki MN. DNA Cell Biol. 2009;28:201–208. doi: 10.1089/dna.2008.0840. [DOI] [PubMed] [Google Scholar]
- 17.Pederson U, Rasmussen PB, Lawesson SO. Liebigs Ann Chem. 1985;8:1557–1569. [Google Scholar]
- 18.Tonnesen HH, Karlsen J, Mostad A. Acta Chem Scand, Ser B. 1982;B36:475–479. [Google Scholar]
- 19.Baum L, Ng A. J Alzheimer’s Dis. 2004;6:367–377. doi: 10.3233/jad-2004-6403. discussion 443–369. [DOI] [PubMed] [Google Scholar]
- 20.Fang J, Lu J, Holmgren A. J Biol Chem. 2005;280:25284–25290. doi: 10.1074/jbc.M414645200. [DOI] [PubMed] [Google Scholar]
- 21.Marcu MG, Jung YJ, Lee S, Chung EJ, Lee MJ, Trepel J, Neckers L. Med Chem. 2006;2:169–174. doi: 10.2174/157340606776056133. [DOI] [PubMed] [Google Scholar]
- 22.Kunnumakkara AB, Anand P, Aggarwal BB. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Goel A, Kunnumakkara AB, Aggarwal BB. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Aggarwal BB, Sung B. Trends Pharmacol Sci. 2009;30:85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed T, Gilani AH. Pharmacol, Biochem Behav. 2009;91:554–559. doi: 10.1016/j.pbb.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Dairaku I, Han Y, Yanaka N, Kato N. Biosci, Biotechnol, Biochem. 2010;74:185–187. doi: 10.1271/bbb.90568. [DOI] [PubMed] [Google Scholar]
- 27.Hu GX, Liang G, Chu Y, Li X, Lian QQ, Lin H, He Y, Huang Y, Hardy DO, Ge RS. Bioorg Med Chem Lett. 2010;20:2549–2551. doi: 10.1016/j.bmcl.2010.02.089. [DOI] [PubMed] [Google Scholar]
- 28.Chen YC, Tsai SH, Shen SC, Lin JK, Lee WR. Eur J Cell Biol. 2001;80:213–221. doi: 10.1078/0171-9335-00158. [DOI] [PubMed] [Google Scholar]
- 29.Lin JK. Adv Exp Med Biol. 2007;595:227–243. doi: 10.1007/978-0-387-46401-5_10. [DOI] [PubMed] [Google Scholar]
- 30.Sethi G, Sung B, Kunnumakkara AB, Aggarwal BB. Adv Exp Med Biol. 2009;647:37–51. doi: 10.1007/978-0-387-89520-8_3. [DOI] [PubMed] [Google Scholar]
- 31.Wua ST, Suna JC, Leeb KJ, Sunc YM. Int J Eng Sci Technol. 2010;2:4263–4277. [Google Scholar]
- 32.Vane JR, Botting RM. Inflammation Res. 1998;47(Suppl 2):S78–87. doi: 10.1007/s000110050284. [DOI] [PubMed] [Google Scholar]
- 33.van Ryn J, Trummlitz G, Pairet M. Curr Med Chem. 2000;7:1145–1161. doi: 10.2174/0929867003374255. [DOI] [PubMed] [Google Scholar]
- 34.Selvam C, Jachak SM, Thilagavathi R, Chakraborti AK. Bioorg Med Chem Lett. 2005;15:1793–1797. doi: 10.1016/j.bmcl.2005.02.039. [DOI] [PubMed] [Google Scholar]
- 35.Padhye S, Banerjee S, Chavan D, Pandye S, Swamy KV, Ali S, Li J, Dou QP, Sarkar FH. Pharm Res. 2009;26:2438–2445. doi: 10.1007/s11095-009-9955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fournier T, Medjoubi NN, Porquet D. Biochim Biophys Acta, Protein Struct Mol Enzymol. 2000;1482:157–171. doi: 10.1016/s0167-4838(00)00153-9. [DOI] [PubMed] [Google Scholar]
- 37.Hochepied T, Berger FG, Baumann H, Libert C. Cytokine Growth Factor Rev. 2003;14:25–34. doi: 10.1016/s1359-6101(02)00054-0. [DOI] [PubMed] [Google Scholar]
- 38.Israili ZH, Dayton PG. Drug Metab Rev. 2001;33:161–235. doi: 10.1081/dmr-100104402. [DOI] [PubMed] [Google Scholar]
- 39.Kremer JM, Wilting J, Janssen LH. Pharmacol Rev. 1988;40:1–47. [PubMed] [Google Scholar]
- 40.Zsila F, Bikadi Z, Simonyi M. Bioorg Med Chem. 2004;12:3239–3245. doi: 10.1016/j.bmc.2004.03.074. [DOI] [PubMed] [Google Scholar]
- 41.Gradisar H, Keber MM, Pristovsek P, Jerala R. J Leukocyte Biol. 2007;82:968–974. doi: 10.1189/jlb.1206727. [DOI] [PubMed] [Google Scholar]
- 42.Bora-Tatar G, Dayangac-Erden D, Demir AS, Dalkara S, Yelekci K, Erdem-Yurter H. Bioorg Med Chem. 2009;17:5219–5228. doi: 10.1016/j.bmc.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 43.Padmanabhan PK, Mukherjee A, Madhubala R. Biochem J. 2006;393:227–234. doi: 10.1042/BJ20050948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan M, Luo M, Song Y, Xu Q, Wang X, Cao Y, Bu X, Ren Y, Hu X. Bioorg Med Chem. 2011;19:1189–1196. doi: 10.1016/j.bmc.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 45.Liu M, Yuan M, Luo M, Bu X, Luo HB, Hu X. Biophys Chem. 2010;147:28–34. doi: 10.1016/j.bpc.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Griot C, Vandevelde M, Richard A, Peterhans E, Stocker R. Free Radical Res. 1990;11:181–193. doi: 10.3109/10715769009088915. [DOI] [PubMed] [Google Scholar]
- 47.Harrison R. Free Radical Biol Med. 2002;33:774–797. doi: 10.1016/s0891-5849(02)00956-5. [DOI] [PubMed] [Google Scholar]
- 48.Pauff JM, Hille R. J Nat Prod. 2009;72:725–731. doi: 10.1021/np8007123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen L, Ji HF. Bioorg Med Chem Lett. 2009;19:5990–5993. doi: 10.1016/j.bmcl.2009.09.076. [DOI] [PubMed] [Google Scholar]
- 50.Milacic V, Banerjee S, Landis-Piwowar KR, Sarkar FH, Majumdar AP, Dou QP. Cancer Res. 2008;68:7283–7292. doi: 10.1158/0008-5472.CAN-07-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wan SB, Yang H, Zhou Z, Cui QC, Chen D, Kanwar J, Mohammad I, Dou QP, Chan TH. Int J Mol Med. 2010;26:447–455. doi: 10.3892/ijmm_00000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bilmen JG, Khan SZ, Javed MH, Michelangeli F. Eur J Biochem. 2001;268:6318–6327. doi: 10.1046/j.0014-2956.2001.02589.x. [DOI] [PubMed] [Google Scholar]
- 53.Wang L, Song R, Shen Y, Sun Y, Gu Y, Shu Y, Xu Q. Mol Cancer Ther. 2011;10:461–471. doi: 10.1158/1535-7163.MCT-10-0812. [DOI] [PubMed] [Google Scholar]
- 54.Eckstein-Ludwig U, Webb RJ, Van Goethem ID, East JM, Lee AG, Kimura M, O’Neill PM, Bray PG, Ward SA, Krishna S. Nature. 2003;424:957–961. doi: 10.1038/nature01813. [DOI] [PubMed] [Google Scholar]
- 55.Ji HF, Shen L. Bioorg Med Chem Lett. 2009;19:2453–2455. doi: 10.1016/j.bmcl.2009.03.060. [DOI] [PubMed] [Google Scholar]
- 56.Vajragupta O, Boonchoong P, Morris GM, Olson AJ. Bioorg Med Chem Lett. 2005;15:3364–3368. doi: 10.1016/j.bmcl.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 57.Mazumder A, Raghavan K, Weinstein J, Kohn KW, Pommier Y. Biochem Pharmacol. 1995;49:1165–1170. doi: 10.1016/0006-2952(95)98514-a. [DOI] [PubMed] [Google Scholar]
- 58.Herman JG, Baylin SB. N Engl J Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 59.Liu Z, Xie Z, Jones W, Pavlovicz RE, Liu S, Yu J, Li PK, Lin J, Fuchs JR, Marcucci G, Li C, Chan KK. Bioorg Med Chem Lett. 2009;19:706–709. doi: 10.1016/j.bmcl.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 60.Takeuchi T, Ishidoh T, Iijima H, Kuriyama I, Shimazaki N, Koiwai O, Kuramochi K, Kobayashi S, Sugawara F, Sakaguchi K, Yoshida H, Mizushina Y. Genes Cells. 2006;11:223–235. doi: 10.1111/j.1365-2443.2006.00937.x. [DOI] [PubMed] [Google Scholar]
- 61.Sahoo BK, Ghosh KS, Dasgupta S. Protein Pept Lett. 2009;16:1485–1495. doi: 10.2174/092986609789839278. [DOI] [PubMed] [Google Scholar]
- 62.Comba A, Pasqualini ME. Pancreatology. 2009;9:724–728. doi: 10.1159/000235623. [DOI] [PubMed] [Google Scholar]
- 63.Skrzypczak-Jankun E, McCabe NP, Selman SH, Jankun J. Int J Mol Med. 2000;6:521–526. doi: 10.3892/ijmm.6.5.521. [DOI] [PubMed] [Google Scholar]
- 64.Skrzypczak-Jankun E, Zhou K, McCabe NP, Selman SH, Jankun J. Int J Mol Med. 2003;12:17–24. [PubMed] [Google Scholar]
- 65.Jankun J, Aleem AM, Malgorzewicz S, Szkudlarek M, Zavodszky MI, Dewitt DL, Feig M, Selman SH, Skrzypczak-Jankun E. Mol Cancer Ther. 2006;5:1371–1382. doi: 10.1158/1535-7163.MCT-06-0021. [DOI] [PubMed] [Google Scholar]
- 66.Page-McCaw A, Ewald AJ, Werb Z. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parks WC, Wilson CL, Lopez-Boado YS. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 68.Egeblad M, Werb Z. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 69.Nagase H, Visse R, Murphy G. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 70.Girija CR, Karunakar P, Poojari CS, Begum NS, Syed AA. J Proteomics Bioinf. 2010;3:200–203. [Google Scholar]
- 71.Pepys MB, Hawkins PN, Booth DR, Vigushin DM, Tennent GA, Soutar AK, Totty N, Nguyen O, Blake CC, Terry CJ, et al. Nature. 1993;362:553–557. doi: 10.1038/362553a0. [DOI] [PubMed] [Google Scholar]
- 72.Dumoulin M, Kumita JR, Dobson CM. Acc Chem Res. 2006;39:603–610. doi: 10.1021/ar050070g. [DOI] [PubMed] [Google Scholar]
- 73.Kapoor S, Priyadarsini KI. Biophys Chem. 2001;92:119–126. doi: 10.1016/s0301-4622(01)00188-0. [DOI] [PubMed] [Google Scholar]
- 74.Wang SS, Liu KN, Lee WH. Biophys Chem. 2009;144:78–87. doi: 10.1016/j.bpc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 75.Battaini F, Mochly-Rosen D. Pharmacol Res. 2007;55:461–466. doi: 10.1016/j.phrs.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Griner EM, Kazanietz MG. Nat Rev Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 77.Alkon DL, Sun MK, Nelson TJ. Trends Pharmacol Sci. 2007;28:51–60. doi: 10.1016/j.tips.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 78.Majhi A, Rahman GM, Panchal S, Das J. Bioorg Med Chem. 2010;18:1591–1598. doi: 10.1016/j.bmc.2009.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Biscardi JS, Tice DA, Parsons SJ. Adv Cancer Res. 1999;76:61–119. doi: 10.1016/s0065-230x(08)60774-5. [DOI] [PubMed] [Google Scholar]
- 80.Leu TH, Su SL, Chuang YC, Maa MC. Biochem Pharmacol. 2003;66:2323–2331. doi: 10.1016/j.bcp.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 81.Lau KF, Miller CC, Anderton BH, Shaw PC. Genomics. 1999;60:121–128. doi: 10.1006/geno.1999.5875. [DOI] [PubMed] [Google Scholar]
- 82.Embi N, Rylatt DB, Cohen P. Eur J Biochem. 1980;107:519–527. [PubMed] [Google Scholar]
- 83.Rylatt DB, Aitken A, Bilham T, Condon GD, Embi N, Cohen P. Eur J Biochem. 1980;107:529–537. [PubMed] [Google Scholar]
- 84.Woodgett JR. EMBO J. 1990;9:2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martinez A, Castro A, Dorronsoro I, Alonso M. Med Res Rev. 2002;22:373–384. doi: 10.1002/med.10011. [DOI] [PubMed] [Google Scholar]
- 86.Alonso M, Martinez A. Curr Med Chem. 2004;11:755–763. doi: 10.2174/0929867043455738. [DOI] [PubMed] [Google Scholar]
- 87.Mohammad MK, Al-Masri IM, Taha MO, Al-Ghussein MA, Alkhatib HS, Najjar S, Bustanji Y. Eur J Pharmacol. 2008;584:185–191. doi: 10.1016/j.ejphar.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 88.Taha MO, Bustanji Y, Al-Ghussein MA, Mohammad M, Zalloum H, Al-Masri IM, Atallah N. J Med Chem. 2008;51:2062–2077. doi: 10.1021/jm7009765. [DOI] [PubMed] [Google Scholar]
- 89.Bustanji Y, Taha MO, Almasri IM, Al-Ghussein MA, Mohammad MK, Alkhatib HS. J Enzyme Inhib Med Chem. 2009;24:771–778. doi: 10.1080/14756360802364377. [DOI] [PubMed] [Google Scholar]
- 90.Anderson NG, Ahmad T. Front Biosci. 2002;7:d1926–1940. doi: 10.2741/A889. [DOI] [PubMed] [Google Scholar]
- 91.Jung Y, Xu W, Kim H, Ha N, Neckers L. Biochim Biophys Acta, Mol Cell Res. 2007;1773:383–390. doi: 10.1016/j.bbamcr.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 92.Johnson L. Biochem Soc Trans. 2007;35:7–11. doi: 10.1042/BST0350007. [DOI] [PubMed] [Google Scholar]
- 93.Reddy S, Aggarwal BB. FEBS Lett. 1994;341:19–22. doi: 10.1016/0014-5793(94)80232-7. [DOI] [PubMed] [Google Scholar]
- 94.Arner ES, Holmgren A. Eur J Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 95.Singh DV, Misra K. Bioinformation. 2009;4:187–192. doi: 10.6026/97320630004187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kinoshita JH. Exp Eye Res. 1990;50:567–573. doi: 10.1016/0014-4835(90)90096-d. [DOI] [PubMed] [Google Scholar]
- 97.Muthenna P, Suryanarayana P, Gunda SK, Petrash JM, Reddy GB. FEBS Lett. 2009;583:3637–3642. doi: 10.1016/j.febslet.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 98.Howard EI, Sanishvili R, Cachau RE, Mitschler A, Chevrier B, Barth P, Lamour V, Van Zandt M, Sibley E, Bon C, Moras D, Schneider TR, Joachimiak A, Podjarny A. Proteins: Struct, Funct, Bioinf. 2004;55:792–804. doi: 10.1002/prot.20015. [DOI] [PubMed] [Google Scholar]
- 99.Yan R, Zu X, Ma J, Liu Z, Adeyanju M, Cao D. Int J Cancer. 2007;121:2301–2306. doi: 10.1002/ijc.22933. [DOI] [PubMed] [Google Scholar]
- 100.Matsunaga T, Endo S, Soda M, Zhao HT, El-Kabbani O, Tajima K, Hara A. Biochem Biophys Res Commun. 2009;389:128–132. doi: 10.1016/j.bbrc.2009.08.107. [DOI] [PubMed] [Google Scholar]
- 101.Letchford K, Liggins R, Burt H. J Pharm Sci. 2008;97:1179–1190. doi: 10.1002/jps.21037. [DOI] [PubMed] [Google Scholar]
- 102.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Mol Pharmaceutics. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 103.Ma Z, Haddadi A, Molavi O, Lavasanifar A, Lai R, Samuel J. J Biomed Mater Res A. 2008;86:300–310. doi: 10.1002/jbm.a.31584. [DOI] [PubMed] [Google Scholar]
- 104.Li L, Ahmed B, Mehta K, Kurzrock R. Mol Cancer Ther. 2007;6:1276–1282. doi: 10.1158/1535-7163.MCT-06-0556. [DOI] [PubMed] [Google Scholar]
- 105.Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, Maitra A. J Nanobiotechnol. 2007;5:3. doi: 10.1186/1477-3155-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sou K, Inenaga S, Takeoka S, Tsuchida E. Int J Pharm. 2008;352:287–293. doi: 10.1016/j.ijpharm.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 107.Vemula PK, Li J, John G. J Am Chem Soc. 2006;128:8932–8938. doi: 10.1021/ja062650u. [DOI] [PubMed] [Google Scholar]
- 108.Willmott N, Magee GA, Cummings J, Halbert GW, Smyth JF. J Pharm Pharmacol. 1992;44:472–475. doi: 10.1111/j.2042-7158.1992.tb03649.x. [DOI] [PubMed] [Google Scholar]
- 109.Knepp WA, Jayakrishnan A, Quigg JM, Sitren HS, Bagnall JJ, Goldberg EP. J Pharm Pharmacol. 1993;45:887–891. doi: 10.1111/j.2042-7158.1993.tb05614.x. [DOI] [PubMed] [Google Scholar]
- 110.Sahu A, Kasoju N, Bora U. Biomacromolecules. 2008;9:2905–2912. doi: 10.1021/bm800683f. [DOI] [PubMed] [Google Scholar]
- 111.Sneharani AH, Singh SA, Appu Rao AG. J Agric Food Chem. 2009;57:10386–10391. doi: 10.1021/jf902464p. [DOI] [PubMed] [Google Scholar]
- 112.Barik A, Mishra B, Kunwar A, Kadam RM, Shen L, Dutta S, Padhye S, Satpati AK, Zhang HY, Indira Priyadarsini K. Eur J Med Chem. 2007;42:431–439. doi: 10.1016/j.ejmech.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 113.Priyadarsini KI. J Photochem Photobiol, C. 2009;10:81–95. [Google Scholar]
- 114.Zsila F, Bikadi Z, Simonyi M. Tetrahedron: Asymmetry. 2003;14:2433–2444. [Google Scholar]
- 115.Mandeville JS, Froehlich E, Tajmir-Riahi HA. J Pharm Biomed Anal. 2009;49:468–474. doi: 10.1016/j.jpba.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 116.Pulla Reddy AC, Sudharshan E, Appu Rao AG, Lokesh BR. Lipids. 1999;34:1025–1029. doi: 10.1007/s11745-999-0453-x. [DOI] [PubMed] [Google Scholar]
- 117.Kunwar A, Barik A, Pandey R, Priyadarsini KI. Biochim Biophys Acta, Gen Subj. 2006;1760:1513–1520. doi: 10.1016/j.bbagen.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 118.Leung MH, Kee TW. Langmuir. 2009;25:5773–5777. doi: 10.1021/la804215v. [DOI] [PubMed] [Google Scholar]
- 119.Sahoo BK, Ghosh KS, Dasgupta S. Biopolymers. 2009;91:108–119. doi: 10.1002/bip.21092. [DOI] [PubMed] [Google Scholar]
- 120.Bourassa P, Kanakis CD, Tarantilis P, Pollissiou MG, Tajmir-Riahi HA. J Phys Chem B. 2010;114:3348–3354. doi: 10.1021/jp9115996. [DOI] [PubMed] [Google Scholar]
- 121.Sahoo BK, Ghosh KS, Dasgupta S. Biophys Chem. 2008;132:81–88. doi: 10.1016/j.bpc.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 122.Kontopidis G, Holt C, Sawyer L. J Dairy Sci. 2004;87:785–796. doi: 10.3168/jds.S0022-0302(04)73222-1. [DOI] [PubMed] [Google Scholar]
- 123.Sneharani AH, Karakkat JV, Singh SA, Rao AG. J Agric Food Chem. 2010;58:11130–11139. doi: 10.1021/jf102826q. [DOI] [PubMed] [Google Scholar]
- 124.Mohammadi F, Bordbar AK, Divsalar A, Mohammadi K, Saboury AA. Protein J. 2009;28:117–123. doi: 10.1007/s10930-009-9171-6. [DOI] [PubMed] [Google Scholar]
- 125.Liu J, Tian J, Hu Z, Chen X. Biopolymers. 2004;73:443–450. doi: 10.1002/bip.20000. [DOI] [PubMed] [Google Scholar]
- 126.Liu Y, Yang Z, Du J, Yao X, Lei R, Zheng X, Liu J, Hu H, Li H. Immunobiology. 2008;213:651–661. doi: 10.1016/j.imbio.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 127.Siegel DS, Zhang X, Feinman R, Teitz T, Zelenetz A, Richon VM, Rifkind RA, Marks PA, Michaeli J. Proc Natl Acad Sci U S A. 1998;95:162–166. doi: 10.1073/pnas.95.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tu Y, Renner S, Xu F, Fleishman A, Taylor J, Weisz J, Vescio R, Rettig M, Berenson J, Krajewski S, Reed JC, Lichtenstein A. Cancer Res. 1998;58:256–262. [PubMed] [Google Scholar]
- 129.Luthra PM, Kumar R, Prakash A. Biochem Biophys Res Commun. 2009;384:420–425. doi: 10.1016/j.bbrc.2009.04.149. [DOI] [PubMed] [Google Scholar]
- 130.Dai K, Lutkenhaus J. J Bacteriol. 1991;173:3500–3506. doi: 10.1128/jb.173.11.3500-3506.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nogales E, Downing KH, Amos LA, Lowe J. Nat Struct Biol. 1998;5:451–458. doi: 10.1038/nsb0698-451. [DOI] [PubMed] [Google Scholar]
- 132.Errington J, Daniel RA, Scheffers DJ. Microbiol Mol Biol Rev. 2003;67:52–65. doi: 10.1128/MMBR.67.1.52-65.2003. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Romberg L, Levin PA. Annu Rev Microbiol. 2003;57:125–154. doi: 10.1146/annurev.micro.57.012903.074300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Margolin W. Nat Rev Mol Cell Biol. 2005;6:862–871. doi: 10.1038/nrm1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rai D, Singh JK, Roy N, Panda D. Biochem J. 2008;410:147–155. doi: 10.1042/BJ20070891. [DOI] [PubMed] [Google Scholar]
- 136.Kaur S, Modi NH, Panda D, Roy N. Eur J Med Chem. 2010;45:4209–4214. doi: 10.1016/j.ejmech.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 137.Stefani M. Biochim Biophys Acta, Mol Basis Dis. 2004;1739:5–25. doi: 10.1016/j.bbadis.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 138.Hafner-Bratkovic I, Gaspersic J, Smid LM, Bresjanac M, Jerala R. J Neurochem. 2008;104:1553–1564. doi: 10.1111/j.1471-4159.2007.05105.x. [DOI] [PubMed] [Google Scholar]
- 139.Kunwar A, Simon E, Singh U, Chittela RK, Sharma D, Sandur SK, Priyadarsini IK. Chem Biol Drug Des. 2011;77:281–287. doi: 10.1111/j.1747-0285.2011.01083.x. [DOI] [PubMed] [Google Scholar]
- 140.Pullakhandam R, Srinivas PN, Nair MK, Reddy GB. Arch Biochem Biophys. 2009;485:115–119. doi: 10.1016/j.abb.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 141.Shim JS, Lee J, Park HJ, Park SJ, Kwon HJ. Chem Biol. 2004;11:1455–1463. doi: 10.1016/j.chembiol.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 142.Gupta KK, Bharne SS, Rathinasamy K, Naik NR, Panda D. FEBS J. 2006;273:5320–5332. doi: 10.1111/j.1742-4658.2006.05525.x. [DOI] [PubMed] [Google Scholar]
- 143.Shim JS, Kim JH, Cho HY, Yum YN, Kim SH, Park HJ, Shim BS, Choi SH, Kwon HJ. Chem Biol. 2003;10:695–704. doi: 10.1016/s1074-5521(03)00169-8. [DOI] [PubMed] [Google Scholar]
- 144.Yanagisawa D, Shirai N, Amatsubo T, Taguchi H, Hirao K, Urushitani M, Morikawa S, Inubushi T, Kato M, Kato F, Morino K, Kimura H, Nakano I, Yoshida C, Okada T, Sano M, Wada Y, Wada KN, Yamamoto A, Tooyama I. Biomaterials. 2010;31:4179–4185. doi: 10.1016/j.biomaterials.2010.01.142. [DOI] [PubMed] [Google Scholar]
- 145.Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 146.Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF. Biochem Pharmacol. 2003;66:1499–1503. doi: 10.1016/s0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- 147.Mathews S, Rao MN. Int J Pharm. 1991;76:257–259. [Google Scholar]
- 148.Awasthi S, Pandya U, Singhal SS, Lin JT, Thiviyanathan V, Seifert WE, Jr, Awasthi YC, Ansari GA. Chem-Biol Interact. 2000;128:19–38. doi: 10.1016/s0009-2797(00)00185-x. [DOI] [PubMed] [Google Scholar]
- 149.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 151.Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Biochem J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Barik A, Mishra B, Shen L, Mohan H, Kadam RM, Dutta S, Zhang HY, Priyadarsini KI. Free Radical Biol Med. 2005;39:811–822. doi: 10.1016/j.freeradbiomed.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 153.Koiram PR, Veerapur VP, Kunwar A, Mishra B, Barik A, Priyadarsini IK, Mazhuvancherry UK. J Radiat Res. 2007;48:241–245. doi: 10.1269/jrr.06103. [DOI] [PubMed] [Google Scholar]
- 154.Kunwar A, Narang H, Priyadarsini KI, Krishna M, Pandey R, Sainis KB. J Cell Biochem. 2007;102:1214–1224. doi: 10.1002/jcb.21348. [DOI] [PubMed] [Google Scholar]
- 155.Vajragupta O, Boonchoong P, Watanabe H, Tohda M, Kummasud N, Sumanont Y. Free Radical Biol Med. 2003;35:1632–1644. doi: 10.1016/j.freeradbiomed.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 156.Sui Z, Salto R, Li J, Craik C, Ortiz de Montellano PR. Bioorg Med Chem. 1993;1:415–422. doi: 10.1016/s0968-0896(00)82152-5. [DOI] [PubMed] [Google Scholar]
- 157.Gurudutta GU, Verma YK, Singh VK, Gupta P, Raj HG, Sharma RK, Chandra R. FEBS Lett. 2005;579:3503–3507. doi: 10.1016/j.febslet.2005.05.015. [DOI] [PubMed] [Google Scholar]