Abstract
Patients with opioid addiction who receive prescription opioids for treatment of chronic non-malignant pain present a therapeutic challenge. Fifty-four patients with chronic pain and opioid addiction were randomized to receive methadone or buprenorphine/naloxone. At the 6-month follow-up, 26 (48.1%) participants who remained in the study noted a 12.75% reduction in pain (P = 0.043) and compared to 5 in the buprenorphine group, none in the methadone group reported illicit opioid use (P = 0.039). Other differences between the two conditions were not found. Long-term low-dose methadone or buprenorphine/naloxone treatment produced analgesia in patients with chronic pain and opioid addiction.
Keywords: Opioid addiction, chronic pain, buprenorphine, methadone, opioids
Chronic non-malignant pain (i.e., pain unrelated to cancer that persists beyond the usual course of disease or injury) is often treated with prescription opioids. However, .5% to 31% of patients with chronic back pain who are prescribed opioids over an extended period exhibit aberrant medication-taking behaviors.1–5 Patients who receive long-term opioid treatment might develop physical dependence, indicated by pharmacological tolerance and withdrawal symptoms, and opioid-induced hyperalgesia.6, 7 In addition, up to 43% of patients with chronic pain receiving prescription opioids present with a co-existent addiction (i.e., compulsive drug-taking behavior).3, 5
Patients who have coexistent chronic pain and addiction to short-acting opioids present a significant clinical challenge. The expert recommendation is discontinuation of short-acting opioids followed by the use of methadone, buprenorphine, naltrexone, or a non-opioid analgesic combined with behavioral addiction treatment to reduce pain and cravings and improve functioning.8 When non-opioid analgesics are used, the basic rationale is to provide a “strategic treatment interruption” or “drug holiday,” which is thought to address analgesic failure associated with long-term opioid use.9–12 However, this approach might provide inadequate pain control13 and make it difficult for patients to abstain from short-acting opioid analgesics.
Long-acting opioids may offer pain control with a decreased risk for medication misuse.14 Methadone is a long-acting μ-opioid receptor agonist and an NMDA-receptor antagonist with a half-life of 27 ± 12 hours that is effective in treating pain and opioid addiction.15–17 However, it has bothersome side effects (e.g., sedation, constipation, hyperalgesia, dizziness, nausea, vomiting, and sweating) and is associated with serious adverse events (e.g., drug overdose, respiratory depression, cardiac rhythm disturbances, QT interval prolongation and serious arrhythmia [torsades de pointes], and death) that limit its use.8,18–20 Buprenorphine, a partial μ-opioid receptor agonist and κ-opioid receptor antagonist with a half-life of 2.33 ± 0.24 hours might be an alternative to methadone because it is effective in treating opioid addiction or pain.21–26 Although methadone is superior to buprenorphine in improving addiction outcomes in opioid-dependent patients, buprenorphine has a better safety profile.21–23, 25 Buprenorphine’s ceiling on its agonist activity limits its abuse liability and reduces the probability of respiratory depression and overdose death, resulting in a safety profile superior to methadone.27 Buprenorphine might also have a ceiling on its analgesic effects.
Clinicians need evidence-based guidelines to more effectively manage patients who have both chronic pain and evidence of opioid misuse or addiction, which few studies have investigated. According to Blondell and his associates, treatment with steady doses of buprenorphine/naloxone for 6 months results in treatment retention superior to detoxification followed by abstinence in chronic pain patients with a co-existent opioid addiction.13 Similarly, Tennant and Rawson showed that methadone or propoxyphene treatment produced better treatment retention than detoxification followed by psychotherapy in patients with chronic pain and co-existent addiction.28 A case study of 4 chronic pain patients with co-existent opioid addiction who received methadone treatment found that 3 stayed in treatment for 19–21 months and stopped intravenous use.29 Pain as measured by the visual analogue scale did not significantly decrease.
Randomized clinical trials have not compared methadone and buprenorphine in patients with chronic pain and co-existent opioid addiction. The objective of this preliminary study was to compare the influence of 6-months of methadone and buprenorphine/naloxone treatment on analgesia, illicit drug use, treatment retention, and functioning in a group of patients with chronic non-cancer pain and co-existent opioid addiction in a primary care setting.
Methods
The study protocol was approved by the Institutional Review Board of the university and by the Medical Director for Research at the host hospital that was the location of the ambulatory primary care office where the study was conducted. It was registered with the Food and Drug Administration (www.ClinicalTrials.gov) and was given the identifier number NCT00879996.
Patients eligible for randomization were men and women aged 18 years or older with well-documented chronic non-malignant pain related to the spine or a large joint (e.g., hip, knee, shoulder) and an addiction to prescription opioids. Potential participants were screened during a telephone interview for chronic pain and self-identified prescription “drug addiction” and then assessed during a face-to-face interview. A diagnosis of opioid dependence was confirmed using the DAST score (> 4) and a 7-item checklist based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) for “opioid dependence.”30 The diagnosis of a chronic pain condition originating from the spine or large joints was confirmed by clinical examination and the use of diagnostic imaging (e.g., X-rays, computed tomography [CT] scan, magnetic resonance imaging [MRI]). Individuals were excluded from the study, if (1) they were homeless or placed on parole; (2) were unable to give consent (e.g., due to neurological disorders, including dementia or cognitive dysfunction, psychosis) or lacked consent from the attending physician; (3) had a co-occurring psychiatric disorder (e.g., schizophrenia); (4) had an EKG showing prolonged QT and/or previous cardiac/pulmonary issues; (5) were taking a medication that is contraindicated with methadone or buprenorphine; (6) they had a prior history of methadone or buprenorphine maintenance treatment; or (7) were pregnant. Patients were screened for liver disease because methadone cannot be prescribed in patients with hepatic impairment.
After informed consent was obtained, participants were randomized into one of two groups that were pre-determined by drawing lots using a 3:3 ratio, block randomization procedure.
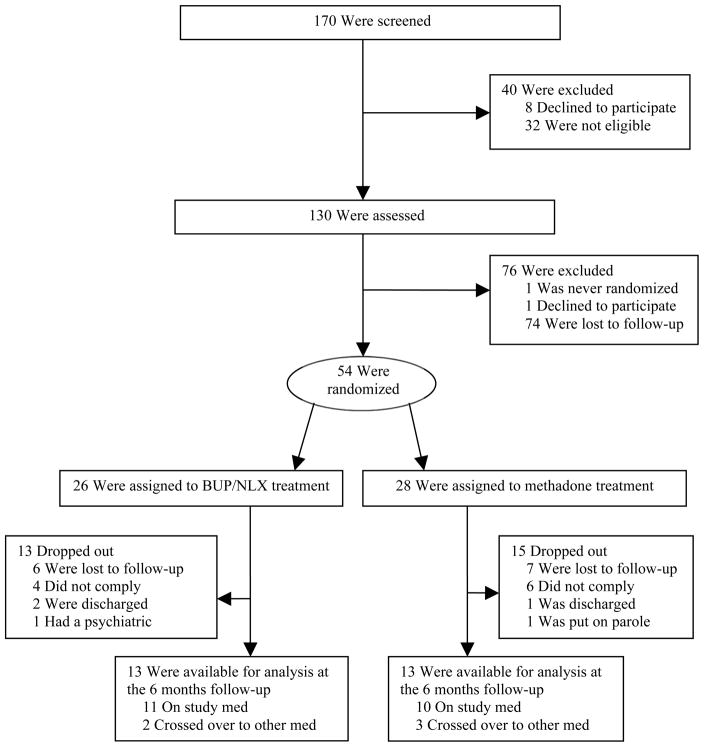
Of the 170 individuals screened, 8 declined to participate, 32 were not eligible, and 130 individuals were eligible and were assessed. After the assessment, 1 declined to participate and 75 were lost to follow-up. In this open-label trial, the remaining 54 participants with chronic pain and opioid addiction were randomly assigned to one of two 6-month treatment protocols: 1) sublingual (SL) buprenorphine/naloxone 4–16 mg/1–4 mg/day (experimental group) (n = 26) or 2) oral (PO) methadone tablets 10–60 mg/day (active comparator group) (n = 28); doses were divided 1–4 times daily. The dose range of buprenorphine originated from a study by Malinoff and associates showing that these doses of buprenorphine were effective in relieving chronic pain.31 Based on this information we used this dose range of buprenorphine in our previous study, which showed that patients with chronic pain and opioid addiction receiving buprenorphine treatment for 6 months remained in treatment longer than patients receiving detoxification followed by behavioral therapy.13 In the United States, SL buprenorphine is combined with naloxone to reduce the potential for intravenous use and is FDA-approved for the office-based treatment of opioid dependence, a diagnosis that all patients in this study had.32
The dose range of methadone was chosen according to the recommendation by Hardman and associates stating that the typical initial oral dose of methadone for pain is 2.5 to 15 mg, depending on the severity of pain.16 Higher doses of methadone were avoided due to increased risk of adverse events. Oral methadone tablets are FDA-approved for the office-based treatment of pain.33 All patients in this study had a well-documented diagnosis of chronic pain.
Participants were asked to cease self-administering opioid medications after the midnight before the morning that they were to be started on the study medication. Upon arrival for the initiation of the study medication, they were examined by a physician who confirmed that the participant was eligible for the study and documented the symptoms and signs of opioid withdrawal based on the Clinical Opiate Withdrawal Score (COWS).34
Those assigned to the methadone active comparator group were given an initial dose of 10 mg methadone orally, and were observed. If needed, an additional dose of 5 mg methadone was administered after 2–4 hours to control the symptoms and signs of opioid withdrawal. The participants received a prescription to take 5 mg methadone 4 times per day. A follow-up appointment was scheduled within 5–10 days. The dosage of methadone was then titrated to 20–60 mg per day (divided 3–4 times daily) over the following few weeks based on clinical response.
Those assigned to the buprenorphine/naloxone group were given an initial dose of 4 mg buprenorphine and were observed. If needed, an additional dose of 2 mg buprenorphine was administered after 2–4 hours to control the symptoms and signs of opioid withdrawal. The participants received a prescription to take buprenorphine/naloxone at gradually increasing doses (8/2 mg on Day 1, 12/3 mg on Day 2, and 16/4 mg on Day 3 and thereafter). A follow-up appointment was scheduled within 5–10 days.
Participants were permitted to change to the other study medication, if they requested to do so because of their perception of an inadequate response to the study medication (i.e., inadequate pain relief and control of cravings, presence of side effects, etc.).
Participants were advised not to drink any alcoholic beverages, not to obtain prescriptions from other physicians for any controlled substances, not to return to taking any of their previously prescribed opioids, and not to use any illicit drugs; however, they were permitted to take non-opioids (e.g., acetaminophen or non-steroidal anti-inflammatory drugs) for breakthrough pain. Participants were requested to engage in chemical dependency treatment for 12–16 weeks and were encouraged to attend meetings of self-help programs (e.g., Narcotics Anonymous). Participants were permitted to opt out of this protocol at any time during follow-up.
Patients were seen for monthly follow-up appointments and were required to provide urine samples at every visit. Possible diversion was monitored by counting left-over pills, requiring patients to have a lockbox for their medication, and completing a daily treatment diary. After the 6-month follow-up period, participants were permitted to choose one of the following final treatment plans: 1) begin an abstinence-oriented approach (i.e., non-opioid analgesics only), 2) initiate a tapering schedule leading to opioid discontinuation, 3) continue methadone or buprenorphine treatment, or 4) return to using their previous opioid medications.
Baseline data included demographic characteristics, medical history, and substance use history. Although participants returned for follow-up data collection every month, the primary outcome was self-reported analgesia at 6 months compared to the initial visit. Participants were asked to rate their pain using a 0–10 point numerical rating scale (NRS). Other outcomes included treatment retention, retention on study medication, self-reported functioning (0–10 point NRS), self-reported drug use (e.g., benzodiazepines, cocaine, marijuana, opiates), and self-reported alcohol use. Urine samples were collected under monitored circumstances at least once a month and were tested for buprenorphine, methadone, opiates, benzodiazepines, cannabis, and cocaine. Urine toxicology screens were performed using an “enzyme linked immunoassay” procedure.
Data were analyzed using SPSS version 16 (SPSS Inc., Chicago, IL) and GraphPad Prism 5 (GraphPad Software, La Jolla, CA). An alpha level of 0.05 was selected for all statistical tests. Fisher’s exact test was used to compare dichotomous variables. A two-tailed, unequal or equal (where appropriate), variance t-test or an ANOVA was calculated to compare continuous variables.
Because the baseline characteristics did not differ between completers and non-completers of the study only the completers were included in the analysis of analgesia, functioning, and illicit drug use (Table 1).
Table 1.
Participant characteristics at baseline by completion of study and changing study medication
| Characteristic | Completion of study | Changing the study med | ||||
|---|---|---|---|---|---|---|
| Completers (n = 26) | Non-comp. (n = 28) | p-value | Switchers (n = 14) | Non-switch. (n = 40) | p-value | |
| Retention on study med or in study, No. (% yes) | 5 (19.2) | 9 (32.1) | 0.358 | 5 (35.7) | 21 (52.5) | 0.358 |
| Demographics | ||||||
| Age, mean (SD) | 36.0 (8.2) | 40.4 (10.6) | 0.097 | 37.5 (8.3) | 38.6 (10.2) | 0.719 |
| Gender, No. (% male) | 14 (53.8) | 15 (53.6) | 1.000 | 4 (28.6) | 25 (62.5) | 0.035* |
| Race, No. (% white) | 22 (84.6) | 24 (85.7) | 1.000 | 14 (100) | 32 (80) | 0.095 |
| Marital Status, No. (% married) | 9 (34.6) | 5 (17.9) | 0.218 | 5 (35.7) | 9 (22.5) | 0.479 |
| Education, No. (% attended college) | 10 (38.5) | 11 (39.3) | 1.000 | 5 (35.7) | 16 (40) | 1.000 |
| Employment, No. (% unemployed) | 17 (65.4) | 20 (71.4) | 0.771 | 4 (28.6) | 13 (32.5) | 1.000 |
| Health Insurance, No. (% Medicaid) | 11 (42.3) | 18 (64.3) | 0.172 | 8 (57.1) | 21 (52.5) | 1.000 |
| Drug and Alcohol History | ||||||
| Age of onset of opioid use, mean (SD) | 27.4 (5.8) | 31.6 (11.1) | 0.079 | 31.1 (6.3) | 29.1 (9.9) | 0.481 |
| Age of onset of opioid problem, mean (SD) | 30.7 (6.0) | 34.1 (11.0) | 0.164 | 33.2 (6.7) | 32.2 (9.7) | 0.727 |
| Family History, No. (% pos alcohol or drug hx) | 12 (46.2) | 19 (67.9) | 0.168 | 10 (71.4) | 21 (52.5) | 0.347 |
| Treatment History | ||||||
| Prior outpatient treatment, No. (% yes) | 12 (46.2) | 13 (46.4) | 1.000 | 7 (50) | 18 (45) | 0.766 |
| Prior inpatient treatment, No. (% yes) | 11 (42.3) | 14 (50) | 0.597 | 3 (21.4) | 22 (55) | 0.060 |
| Prior treatment for mental illness, No. (% yes) | 15 (57.7) | 13 (46.4) | 0.430 | 7 (50) | 21 (52.5) | 1.000 |
| Criminal History | ||||||
| Number of arrests, mean (SD) | 2.7 (5.3) | 5.0 (7.0) | 0.187 | 3.5 (5.9) | 4.0 (6.5) | 0.801 |
| Ever convicted of a crime, No. (%yes) | 9 (34.6) | 16 (57.1) | 0.111 | 6 (42.9) | 19 (47.5) | 1.000 |
| Number of convictions, mean (SD) | 1.9 (4.3) | 2.5 (4.1) | 0.544 | 1.9 (3.3) | 2.3 (4.4) | 0.718 |
| Outcomes at baseline | ||||||
| Pain score, mean (SD) | 6.3 (1.2) | 6.5 (2.2) | 0.671 | 6.8 (1.0) | 6.3 (2.0) | 0.294 |
| Functioning score, mean (SD) | 5.0 (1.7) | 5.1 (2.1) | 0.801 | 6.0 (1.5) | 4.6 (2.0) | 0.022* |
| Positive urine for opiates, No. (% yes) | 11 (42.3) | 9 (32.1) | 0.409 | 5 (35.7) | 15 (37.5) | 1.000 |
| Positive urine for cocaine, No. (% yes) | 1 (3.9) | 6 (21.4) | 0.104 | 3 (21.4) | 4 (10) | 0.343 |
| Positive urine for any other drugs, No. (% yes) | 12 (46.2) | 8 (28.6) | 0.168 | 5 (35.7) | 15 (37.5) | 1.000 |
| Self-reported use of opiates, No. (%yes) | 11 (42.3) | 10 (35.7) | 0.573 | 5 (35.7) | 16 (40) | 0.758 |
| Self-reported use of alcohol, No. (%yes) | 7 (26.9) | 5 (17.9) | 0.510 | 2 (14.3) | 10 (25) | 0.475 |
| Self-reported use of any other drugs, No. (%yes) | 9 (34.6) | 9 (32.1) | 0.774 | 4 (28.6) | 14 (35) | 0.746 |
p-value: α < 0.05
Results
Participant Characteristics
The participant characteristics are summarized in Table 2. The average daily dose of methadone was 29.09 mg and the average daily dose of buprenorphine/naloxone was 14.93 mg/3.73 mg. The overall mean baseline pain score was 6.4 (SD = 1.8) and the overall mean baseline functioning score was 5.0 (SD = 1.9). At baseline, 20 (37%) participants had a positive urine screen for opiates, 7 (13%) for cocaine, and 20 (37%) for other drugs. Twenty-one (38.9%) participants reported use of opiates in the past 30 days, 12 (22.2%) reported alcohol use, and 18 (33.3%) reported the use of other drugs.
Table 2.
Overall participant cha racteristics
| Participant characteristics | |
|---|---|
| Age, mean (SD) | 38.3 (9.7) |
| Gender, male, No. (%) | 29 (53.7) |
| Ethnicity, White, No. (%) | 46 (85.2) |
| Marital status, | |
| never married, No. (%) | 24 (44.4) |
| married, No. (%) | 14 (25.9) |
| divorced, No. (%) | 14 (25.9) |
| widowed, No. (%) | 2 (3.7) |
| Education, attended college, No. (%) | 21 (38.9) |
| Employment, unemployed, No. (%) | 37 (68.5) |
| Health insurance, Medicaid, No. (%) | 29 (53.7) |
| Criminal history, | |
| History of arrests, No. (%) | 31 (57.4) |
| No. of arrests, mean (SD) | 6.74 (7.1) |
| Previous conviction for a crime, No. (%) | 25 (46.3) |
| No. of convictions, mean (SD) | 4.76 (5.0) |
| Pain diagnoses, | |
| Failed back syndrome (other than postlaminectomy), No. (%) | 20 (37) |
| Degeneration of lumbar intervertebral disc with myelopathy, No. (%) | 10 (18.5) |
| Lumbago, No. (%) | 10 (18.5) |
| Degeneration of lumbar or lumbosacral intervertebral disc, No. (%) | 4 (7.4) |
| Displacement of lumbar disc without myelopathy, No. (%) | 3 (5.6) |
| Lumbar spinal stenosis, No. (%) | 2 (3.7) |
| Chronic pain syndrome with significant psychosocial dysfunction, No. (%) | 2 (3.7) |
| Degenerative spondylolysis, No. (%) | 1 (1.9) |
| Degeneration of cervical intervertebral disc with myelopathy, No. (%) | 1 (1.9) |
| Postlaminectomy, No. (%) | 1 (1.9) |
| Age of onset of opioid use, mean (SD) | 29.6 (9.1) |
| Age of onset of opioid problem, mean (SD) | 32.5 (9.0) |
| Prior outpatient and inpatient treatment for addiction, No. (%) | 25 (46.3) |
| Prior treatment for another mental illness, No. (%) | 28 (51.9) |
| Most frequently abused opioid, | |
| Hydrocodone, No. (%) | 32 (59.3) |
| Oxycodone, No. (%) | 8 (14.8) |
| Fentanyl, No. (%) | 5 (9.3) |
| Heroin, No. (%) | 2 (3.7) |
| Morphine, No. (%) | 1 (1.9) |
| Codeine, No. (%) | 1 (1.9) |
| Other, No. (%) | 4 (7.4) |
| Unknown, No. (%) | 1 (1.9) |
| Family history of alcohol or drug use, No. (%) | 31 (57.4) |
Table 3 summarizes the characteristics of those who completed treatment and all randomized participants (whether or not they completed the treatment) by comparing them among the two treatment conditions at baseline. The treatment arms did not statistically differ in participants’ demographics, drug-taking history, treatment history, criminal history, self-reported alcohol, opiate, and other drug use, positive urine for cocaine, opiates, and other drugs, and pain. Participants randomized to methadone reported a significantly greater mean baseline functioning score than participants randomized to buprenorphine/naloxone (P = 0.035). However, participants who completed the study did not significantly differ by treatment condition in percent change of functioning from baseline at 6 months (Table 4), suggesting that the baseline difference in functioning did not affect the treatment results at the 6 months follow-up. Among completers of the study, a greater proportion of participants randomized to buprenorphine/naloxone were convicted of a crime (P = 0.011) and had a greater number of convictions (P = 0.04) than participants randomized to methadone (Table 3).
Table 3.
Participant characteristics at baseline by treatment condition: buprenorphine/naloxone (BUP/NLX) or methadone (MET)
| Characteristic | All randomized participants | Completers only | ||||
|---|---|---|---|---|---|---|
| BUP/NLX (n = 26) | MET (n = 28) | p-value | BUP/NLX (n = 13) | MET (n = 13) | p-value | |
| Demographics | ||||||
| Age, mean (SD) | 39.0 (10.9) | 37.7 (8.6) | 0.621 | 34.4 (7.1) | 37.7 (9.1) | 0.311 |
| Gender, No. (% male) | 17 (65.4) | 12 (42.9) | 0.111 | 8 (61.5) | 6 (46.2) | 0.695 |
| Race, No. (% white) | 20 (76.9) | 26 (92.9) | 0.135 | 11 (84.6) | 11 (84.6) | 1.000 |
| Marital Status, No. (% married) | 5 (19.2) | 9 (32.1) | 0.358 | 4 (30.8) | 5 (38.5) | 1.000 |
| Education, No. (% attended college) | 9 (34.6) | 12 (42.9) | 0.586 | 4 (30.8) | 6 (46.2) | 0.688 |
| Employment, No. (% unemployed) | 18 (69.2) | 19 (67.9) | 1.000 | 10 (76.9) | 7 (53.9) | 0.411 |
| Health Insurance, No. (% Medicaid) | 17 (65.4) | 12 (42.9) | 0.111 | 8 (61.5) | 3 (23.1) | 0.111 |
| Drug and Alcohol History | ||||||
| Age of onset of opioid use, mean (SD) | 31.2 (11.2) | 28.0 (6.5) | 0.211 | 26.7 (6.1) | 28.0 (5.6) | 0.575 |
| Age of onset of opioid problem, mean (SD) | 33.7 (10.7) | 31.3 (7.1) | 0.324 | 29.5 (6.3) | 31.9 (5.6) | 0.320 |
| Family History, No. (% pos. alcohol or drug history) | 13 (50.0) | 18 (64.3) | 0.409 | 6 (46.2) | 6 (46.2) | 1.000 |
| Treatment History | ||||||
| Prior outpatient treatment, No. (% yes) | 13 (50.0) | 12 (42.9) | 0.785 | 7 (53.8) | 5 (38.5) | 0.695 |
| Prior inpatient treatment, No. (% yes) | 15 (57.7) | 10 (35.7) | 0.172 | 7 (53.8) | 4 (30.8) | 0.428 |
| Prior treatment for mental illness, No. (% yes) | 13 (50.0) | 15 (53.6) | 1.000 | 7 (53.8) | 8 (61.5) | 1.000 |
| Criminal History | ||||||
| Number of arrests, mean (SD) | 4.6 (7.1) | 3.2 (5.5) | 0.406 | 4.4 (6.9) | 1.0 (2.0) | 0.102 |
| Ever convicted of a crime, No. (%yes) | 14 (53.8) | 11 (39.3) | 0.413 | 8 (61.5) | 1 (7.7) | 0.011* |
| Number of convictions, mean (SD) | 3.0 (4.7) | 1.5 (3.5) | 0.196 | 3.6 (5.5) | 0.1 (0.3) | 0.040* |
| Outcomes at baseline | ||||||
| Pain score, mean (SD) | 5.9 (2.1) | 6.9 (1.4) | 0.064 | 6.3 (0.8) | 6.3 (1.5) | 0.902 |
| Functioning score, mean (SD) | 4.4 (2.0) | 5.6 (1.7) | 0.035* | 4.6 (1.7) | 5.2 (1.7) | 0.430 |
| Positive urine for opiates, No. (% yes) | 10 (38.5) | 10 (35.7) | 0.783 | 5 (38.5) | 6 (46.2) | 1.000 |
| Positive urine for cocaine, No. (% yes) | 1 (3.9) | 6 (21.4) | 0.104 | 0 (0.0) | 1 (7.7) | 1.000 |
| Positive urine for any other drugs, No. (% yes) | 10 (38.5) | 10 (35.7) | 0.783 | 6 (46.2) | 6 (46.2) | 1.000 |
| Self-reported use of opiates, No. (%yes) | 8 (30.8) | 13 (46.4) | 0.403 | 3 (23.1) | 8 (61.5) | 0.123 |
| Self-reported use of alcohol, No. (%yes) | 6 (23.1) | 6 (21.4) | 1.000 | 5 (38.5) | 2 (15.4) | 0.182 |
| Self-reported use of any other drugs, No. (%yes) | 7 (26.9) | 11 (39.3) | 0.562 | 4 (30.8) | 5 (38.5) | 1.000 |
p-value: α < 0.05
Table 4.
Clinical outcome at 24 weeks for completers
| Buprenorphine n = 13 | Methadone n = 13 | OR | 95% CI | p | |
|---|---|---|---|---|---|
| Retention in study, No. (%) | 13/26 (50) | 13/28 (46.4) | 0.933 | 0.324–2.691 | 1.000 |
| Percent change of pain from baseline, mean (SD) | 87.4 (33.4) | 88.6 (24.5) | - | - | 0.918 |
| Percent change of functioning from baseline, mean (SD) | 121.9 (63.9) | 113.8 (62.5) | - | - | 0.787 |
| Positive urine for opioids, No. (%) | 5 (38.5) | 2 (15.4) | 0.280 | 0.042–1.878 | 0.371 |
| Positive urine for cocaine, No. (%) | 2 (15.4) | 0 (0) | - | - | 0.478 |
| Positive urine for other drugs, No. (%) | 5 (38.5) | 5 (38.5) | 1.000 | 0.197–5.068 | 1.000 |
| Self-reported opioid use, No. (%) | 5 (38.5) | 0 (0) | - | - | 0.039* |
| Self-reported alcohol use, No. (%) | 4 (30.8) | 2 (15.4) | 0.409 | 0.060–2.769 | 0.645 |
| Self-reported use of other drugs, No. (%) | 5 (38.5) | 3 (23.1) | 0.480 | 0.087–2.645 | 0.673 |
| Self-reported side effects, No. (%) | 8 (61.5) | 9 (69.2) | 1.125 | 0.209–6.046 | 1.000 |
p-value: α < 0.05
Completion of Study and Treatment
Twenty-six (48.1%) participants completed the study. Thirteen participants were lost to follow-up, 10 did not comply with the treatment (e.g., abuse of illicit substances, misuse of prescriptions, aberrant medication-taking behaviors), 3 were discharged, 1 had a psychiatric problem, and 1 was placed on parole (Figure 1). Twenty-one (38.9%) participants completed the study on their randomized study medication. Fourteen (25.9%) participants changed study medications during the study because they perceived the effect of the medication that they were randomized to as inadequate in relieving pain or controlling cravings or due to side effects. Five of these participants completed the study: two participants started on buprenorphine/naloxone and finished on methadone and 3 participants started on methadone and finished on buprenorphine/naloxone. There were differences between switchers and non-switchers at baseline (Table 1). Male gender was associated with staying on the study medication (P = 0.035). Participants who did not switch the study medication reported lower functioning than participants who switched the study medication (P = 0.022).
Figure 1.
Participant flow chart
Completion of the study on the study medication was associated with younger age (P = 0.038), not being covered by Medicaid (P = 0.025), younger age of onset of opioid use (P = 0.016), and younger age of onset of an opioid problem (P = 0.032). Of the 7 participants who tested positive for cocaine at baseline, none (0%) completed the study on the study medication, while of the 46 participants who tested negative for cocaine, 21 (45.7%) completed the study on the study medication (P = 0.034).
Outcome Variables
A 2 × 2 design [Treatment (buprenorphine/naloxone, methadone) x Follow-up (initial visit, at 6 months)] was used to analyze the pain scores to determine analgesia (Figure 2). The results of this repeated-measures ANOVA revealed a main effect of Follow-up (F = 4.65, df = 1, P = 0.043). Across both treatment condition, participants reported significantly less pain at 6 months (M = 5.5, SD = 1.9) than at the initial visit (M = 6.3, SD = 1.2) with a 12.75% reduction in pain at medium effect size (Cohen’s d = 0.52). This suggests that both continuous treatments resulted in analgesia after 6 months following the start of treatment. To exclude the possibility that differences at baseline masked the results at 6 months, the data were transformed to percent change in pain from baseline. However, there were no significant differences between the treatment groups (Table 3).
Figure 2.
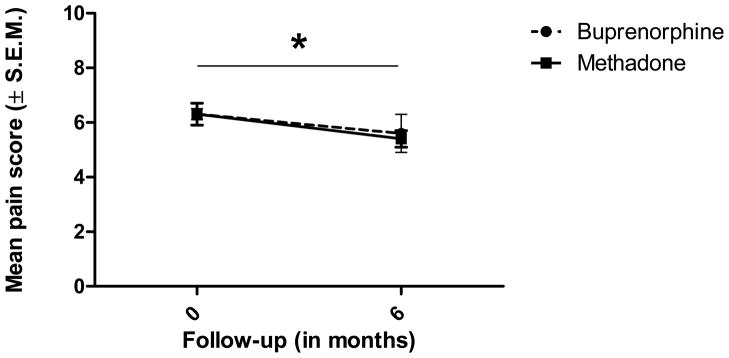
Pain as a function of Treatment and Follow-up. No significant interaction (Treatment x Follow-up) was found, but a main effect of Follow-up: Both 6-months buprenorphine/naloxone and methadone treatments resulted in a significant analgesia (p = 0.043).
Twenty-six (48.2%) participants completed the treatment at 6 months, 13 in each treatment condition. Therefore, treatment retention did not significantly differ between the two treatments.
At 6 months, 5 participants in the buprenorphine/naloxone group reported the use of opioids compared to none of the participants in the methadone group (Table 3); this difference was statistically significant (P = 0.039).
A 2 × 2 design [Treatment (buprenorphine/naloxone, methadone) x Follow-up (initial visit, at 6 months)] was used to analyze functioning. There were no significant differences. No significant differences were found in percent change in functioning from baseline (Table 3).
Discussion
To our knowledge, this is the first randomized prospective clinical trial that compared buprenorphine/naloxone and methadone among patients with chronic pain and co-existent opioid dependence. Both 6-month continuous buprenorphine/naloxone and methadone treatment resulted in analgesia. These results suggest that both long-term treatments can be used to treat chronic pain in patients who are dependent on prescription opioids. The total analgesic effect produced by methadone and buprenorphine/naloxone was small (12.75%); however, this is not surprising considering the severity of chronic pain.
Although clinically relevant improvement was reported by patients and their families in terms of personality, mood, energy, motivation, coping with pain, and functioning, no statistical improvement in functioning was found. The measures for pain and functioning used in this study (i.e., NRS) might not have been sensitive enough to assess the nuances of improvement in functioning and pain. However, the lack of dose escalations and worsening of pain further suggests the effectiveness of the studied treatments.
Treatment retention and analgesia did not differ between the two treatment conditions after 6 months of continuous treatment. This is consistent with a study by Mattick and associates, which showed that treatment retention did not differ between methadone and buprenorphine treatment in opioid-dependent patients.25
We found that participants reported more drug use than we observed with urine toxicology. For example, although none of the participants randomized to methadone reported illicit opioid use at 6 months, 5 participants receiving buprenorphine did. This is consistent with previous literature in patients with only opioid addiction, which showed that methadone is superior to buprenorphine in reducing illicit opioid use.21–23, 25 The discrepancy between self-report and urine toxicology can be explained by the timing of drug use. Urine toxicology detects the presence of opioids up to 3–5 days after the last use. However, the participants in our study were asked to report their opioid use of the past 30 days. Therefore, patients in this study were surprisingly honest about the self-report of drug use because they reported to have used more drugs than was found in the urine. In the present study, alcohol use, cocaine use, and other illicit drug use did not differ between the two treatment conditions. However, neither treatment has been found effective for cocaine and alcohol dependence in the past.
In line with previous literature, positive urine toxicology for cocaine in the beginning of the study was associated with non-completion of treatment on the study medication.35, 36
This is a preliminary study with a small convenience sample of treatment-seeking individuals and high drop-out rate that was not a double-blind and double-dummy study. The participants of this study represented a heterogeneous group regarding their pain condition and the imaging evidence for a pain condition. The small sample size limited our ability to identify sub-groups of patients (e.g., those with no prior treatment for substance abuse or with no prior arrests) who might do well with non-opioid pain management. This was an open-label trial without a placebo and a control group. The outcomes might have been different, if a placebo had been used and the treatment conditions were masked to the participants, to the clinicians who provided care, and to the investigators who collected the follow-up data. Therefore, the observed analgesia could have been the result of a placebo effect, the treatment of pain by other non-opioid medications, physical therapy, alternative treatments (e.g., acupuncture), or the use of opioid analgesic medications that was not approved by the study protocol. Future research is needed to exclude alternative explanations and confirm these results using more refined measures for pain and functioning (e.g., visual analogue scale). Although all patients in this study had a diagnosis of opioid dependence, only 21 reported the use of opiates at the initial visit. This might be due to way the question was asked: “Have you taken prescription drugs such as opiates (e.g., Lortab, OxyCotin) in the last 30 days?” Patients might have not considered other opioids such as fentanyl or opioids obtained from the street when answering the question.
We conclude that both buprenorphine/naloxone and methadone treatment for 6 months reduce chronic non-malignant pain in patients with co-existent opioid addiction. Patients receiving methadone treatment reported less use of other opioids at 6 months than patients receiving buprenorphine/naloxone treatment, but buprenorphine has a better safety profile. These medications might not only target the physiological component of pain, but might also improve the psychological component of chronic pain. Future research needs to investigate the influence of both treatments on patients’ functioning and on the physiological versus psychological component of pain as well as the neurobiology of both chronic pain and opioid addiction and their treatment.
Acknowledgments
Sources of support: National Institute on Drug Abuse (R03 DA 029768) (R.D.B. and A.M.N.).
This study was supported, in part, by a grant (R03 DA 029768) from the National Institute on Drug Abuse (R.D.B. and A.M.N.). The authors are grateful for the help of Shaun S. Bath, M.D., Alisa Li, Tenzing Namgyal Lama, and Gaurang Joshi for their assistance in data collection and Andy Danzo for his editing assistance in preparation of the manuscript.
Footnotes
Trial Registration: clinicaltrials.gov Identifier: NCT00879996
References
- 1.Brown RL, Papasouliotis O, Patterson JJ, Rounds LA. Substance abuse among patients with chronic back pain. J Fam Pract. 1996;43:152–160. [PubMed] [Google Scholar]
- 2.Reid MC, Engles-Horton LL, Weber MB, Kerns RD, Rogers EL, O’Connor PG. Use of opioid medications for chronic noncancer pain syndromes in primary care. J Gen Intern Med. 2002;17:173–179. doi: 10.1046/j.1525-1497.2002.10435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breckenridge J, Clark JD. Patient characteristics associated with opioid versus nonsteroidal anti-inflammatory drug management of chronic low back pain. J Pain. 2003;4:344–350. doi: 10.1016/s1526-5900(03)00638-2. [DOI] [PubMed] [Google Scholar]
- 4.Mahowald ML, Singh JA, Majeski P. Opioid use by patients in an orthopedics spine clinic. Arthritis Rheum. 2005;52:312–321. doi: 10.1002/art.20784. [DOI] [PubMed] [Google Scholar]
- 5.Martell BA, O’Connor PG, Kerns RD, et al. Systematic review: opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146(2):116–127. doi: 10.7326/0003-4819-146-2-200701160-00006. [DOI] [PubMed] [Google Scholar]
- 6.Cowan DT, Wilson-Barnett J, Griffiths P, Allan LG. A survey of chronic noncancer pain patients prescribed opioid analgesics. Pain Med. 2003;4(4):340–351. doi: 10.1111/j.1526-4637.2003.03038.x. [DOI] [PubMed] [Google Scholar]
- 7.Chu LF, Clark DJ, Angst MS. Opioid tolerance and hyperalgesia in chronic pain patients after one month of oral morphine therapy: a preliminary prospective study. J Pain. 2006;7:43–48. doi: 10.1016/j.jpain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Kotz M, Clark M, Compton P, et al. Dept. HHS, editor. Managing chronic pain in adults with or in recovery from substance use disorders: A treatment improvement protocol TIP 54. Rockville, MD: SAMHSA; 2012. [Google Scholar]
- 9.Veterans Health Administration, Department of Defense. VA/DoD clinical practice guideline for the management of opioid therapy for chronic pain. Washington, DC: 2003. [Accessed: September 18, 2012]. Retrieved from http://www.guideline.gov/summary/summary.aspx?doc_id=4812&nbr=003474&string=chronic+AND+paln. [Google Scholar]
- 10.Blondell RD, Ashrafioun L. Treating opioid dependency and coexistent chronic nonmalignant pain. Am Fam Physician. 2008;78:1132–1133. [PubMed] [Google Scholar]
- 11.Jackman R, Purvis JM, Mallett BS. Chronic nonmalignant pain in primary care. Am Fam Physician. 2008;78:1155–1162. [PubMed] [Google Scholar]
- 12.Last AR, Hulbert K. Chronic low back pain: evaluation and management. Am Fam Physician. 2009;79(12):1067–1074. [PubMed] [Google Scholar]
- 13.Blondell RD, Ashrafioun L, Dambra CM, Foschio EM, Zielinski AL, Salcedo DM. A clinical trial comparing tapering doses of buprenorphine with steady doses for chronic pain and coexistent opioid addiction. J Addict Med. 2010;4(3):140–146. doi: 10.1097/ADM.0b013e3181ba895d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savage SR. Opioid medications in the management of pain. In: Graham AW, Schultz TK, Mayo-Smith MF, Ries RK, Wilford BB, editors. Principles of Addiction Medicine. Chevy Chase, MD: American Society of Addiction Medicine; 2003. pp. 1451–1463. [Google Scholar]
- 15.Carpenter KJ, Chapman V, Dickenson AH. Neuronal inhibitory effects of methadone are predominantly opioid receptor mediated in the rat spinal cord in vivo. Eur J Pain. 2000;4:19–26. doi: 10.1053/eujp.1999.0147. [DOI] [PubMed] [Google Scholar]
- 16.Hardman JG, Limbird LE, Gilman AG, editors. Goodman & Gilman’s The pharmacological basis of therapeutics. 10. New York: McGraw-Hill; 2001. [Google Scholar]
- 17.Altier N, Dion D, Boulanger A, Choinière M. Management of neuropathic pain with methadone: a review of 13 cases. Clin J Pain. 2005;21(4):364–369. doi: 10.1097/01.ajp.0000125247.95213.53. [DOI] [PubMed] [Google Scholar]
- 18.Doverty M, White JM, Somogyi AA, Bochner F, Ali R, Ling W. Hyperalgesic responses in methadone maintenance patients. Pain. 2001;90:91–96. doi: 10.1016/s0304-3959(00)00391-2. [DOI] [PubMed] [Google Scholar]
- 19.Batki SL, Kauffman JF, Marlon I, Parrino MW, Woody GE. Dept. HHS, editor. Medication-assisted treatment for opioid addiction in opioid treatment programs: A treatment improvement protocol TIP 43. Rockville, MD: SAMHSA; 2005. [Google Scholar]
- 20.Rhodin A, Grönbladh L, Nilsson LH, Gordh T. Methadone treatment of chronic non-malignant pain and opioid dependence - a long-term follow-up. Eur J Pain. 2006;10(3):271–278. doi: 10.1016/j.ejpain.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Kosten TR, Schottenfeld R, Ziedonis D, Falcioni J. Buprenorphine versus methadone maintenance for opioid dependence. J Nerv Ment Dis. 1993;181(6):358–364. doi: 10.1097/00005053-199306000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Barnett PG, Rodgers JH, Bloch DA. A meta-analysis comparing buprenorphine to methadone for treatment of opiate dependence. Addict. 2001;96:683–690. doi: 10.1046/j.1360-0443.2001.9656834.x. [DOI] [PubMed] [Google Scholar]
- 23.Bridge TP, Fudala PJ, Herbert S, Leiderman DB. Safety and health policy considerations related to the use of buprenorphine/naloxone as an office-based treatment for opiate dependence. Drug Alcohol Dependence. 2003;70(2 Suppl):S79–85. doi: 10.1016/s0376-8716(03)00061-9. [DOI] [PubMed] [Google Scholar]
- 24.Kakko J, Svanborg KD, Kreek MJ, Heilig M. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo-controlled trial. Lancet. 2003;361(9358):662–668. doi: 10.1016/S0140-6736(03)12600-1. [DOI] [PubMed] [Google Scholar]
- 25.Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database of Systematic Rev. 2008;1:CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- 26.Woody GE, Poole SA, Subramaniam G, et al. Extended vs Short-term Buprenorphine Naloxone for Treatment of Opioid-Addicted Youth: A Randomized Trial. JAMA. 2008;300(17):2003. doi: 10.1001/jama.2008.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh SL, Preston KL, Bigelow GE, Stitzer ML. Acute administration of buprenorphine in humans: Partial agonist and blockade effects. J Pharmacol Expl Ther. 1995;274:361–372. [PubMed] [Google Scholar]
- 28.Tennant FS, Jr, Rawson RA. Outpatient treatment of prescription opioid dependence: Comparison of two methods. Arch Int Med. 1982;142:1845–1847. [PubMed] [Google Scholar]
- 29.Kennedy JA, Crowley TJ. Chronic pain and substance abuse: a pilot study of opioid maintenance. JSAT. 1990;7:233–238. doi: 10.1016/0740-5472(90)90046-s. [DOI] [PubMed] [Google Scholar]
- 30.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington DC: American Psychiatric Press; 2000. [Google Scholar]
- 31.Malinoff HL, Barkin RL, Wilson G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther. 2005;12:379–384. doi: 10.1097/01.mjt.0000160935.62883.ff. [DOI] [PubMed] [Google Scholar]
- 32.Suboxone [package insert] Richmond, VA: Reckitt Benckiser Pharmaceuticals, Inc; 2010. [Google Scholar]
- 33.Dolophine [package insert] Columbus, OH: Roxane Laboratories, Inc; 2006. [Google Scholar]
- 34.Wessen DR, Ling W. The clinical opiate withdrawal score (COWS) J Psychoactive Drugs. 2003;35:253–259. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- 35.Schottenfeld RS, Pakes JR, Oliveto A, Ziedonis D, Kosten TR. Buprenorphine vs methadone maintenance treatment for concurrent opioid dependence and cocaine abuse. Arch Gen Psychiatry. 1997;54(8):713–720. doi: 10.1001/archpsyc.1997.01830200041006. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan LE, Moore BA, O’Connor PG, et al. The association between cocaine use and treatment outcomes in patients receiving office-based buprenorphine/naloxone for the treatment of opioid dependence. Am J Addict. 2009;19:53–8. doi: 10.1111/j.1521-0391.2009.00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]