Abstract
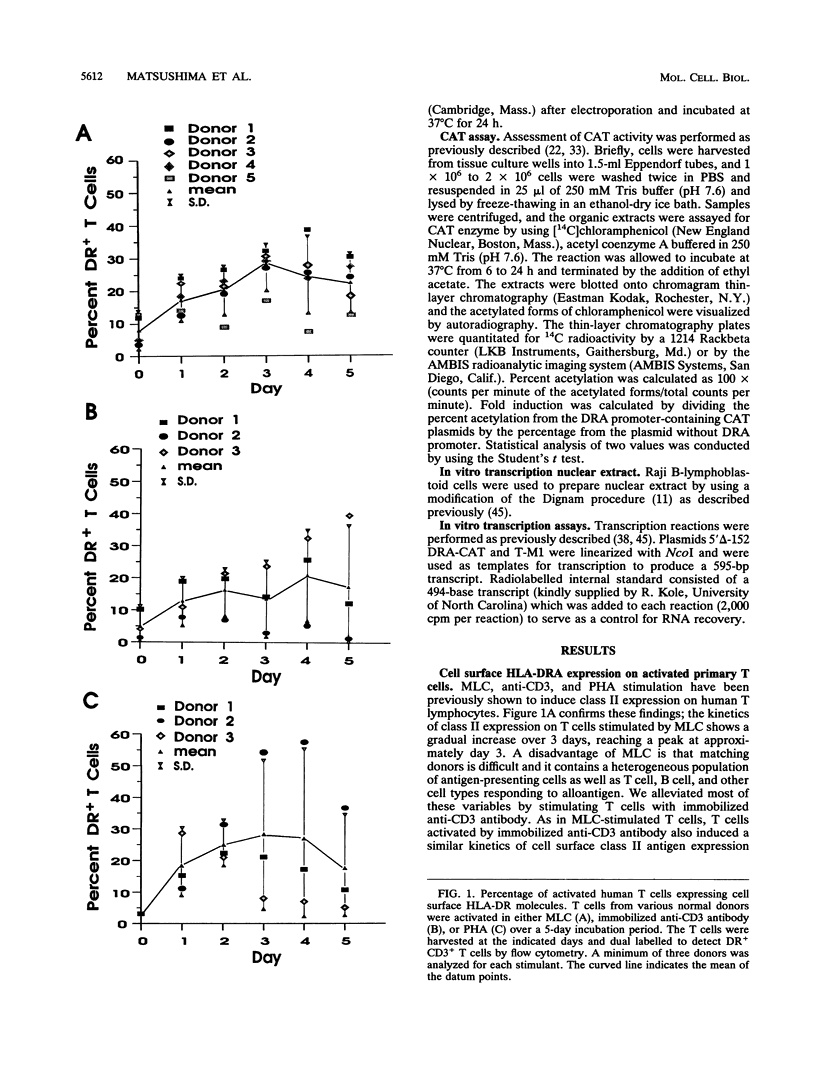
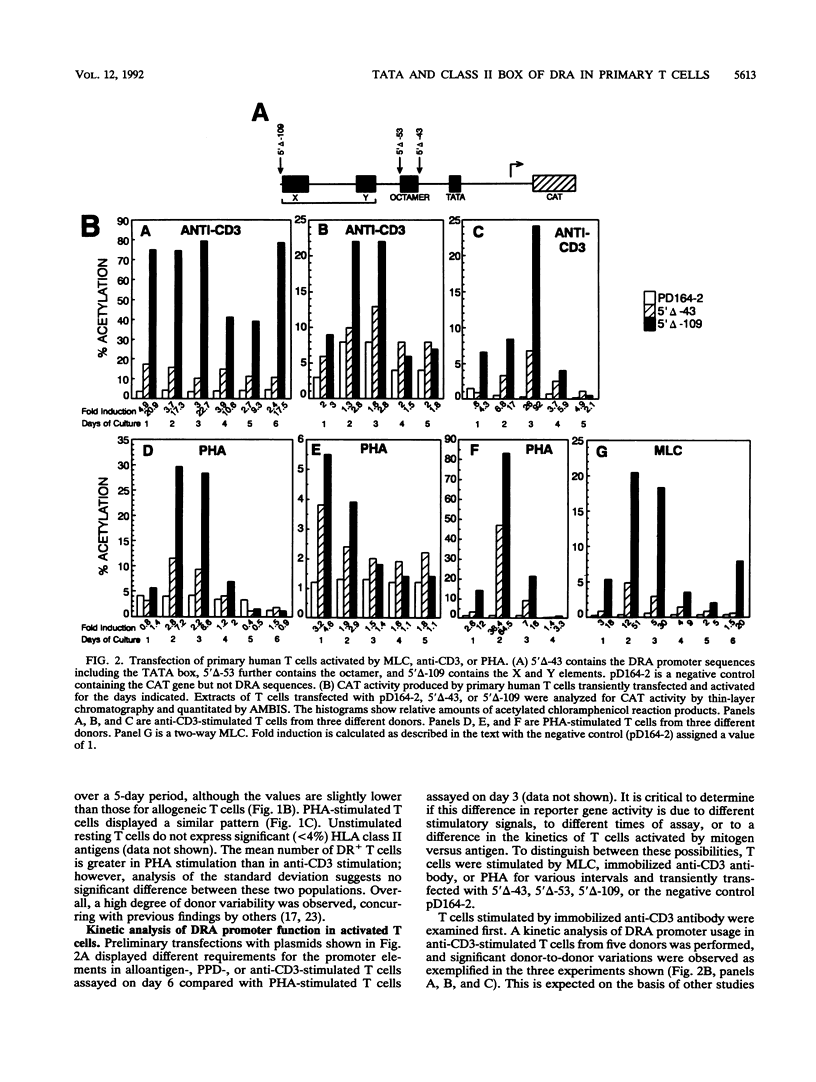
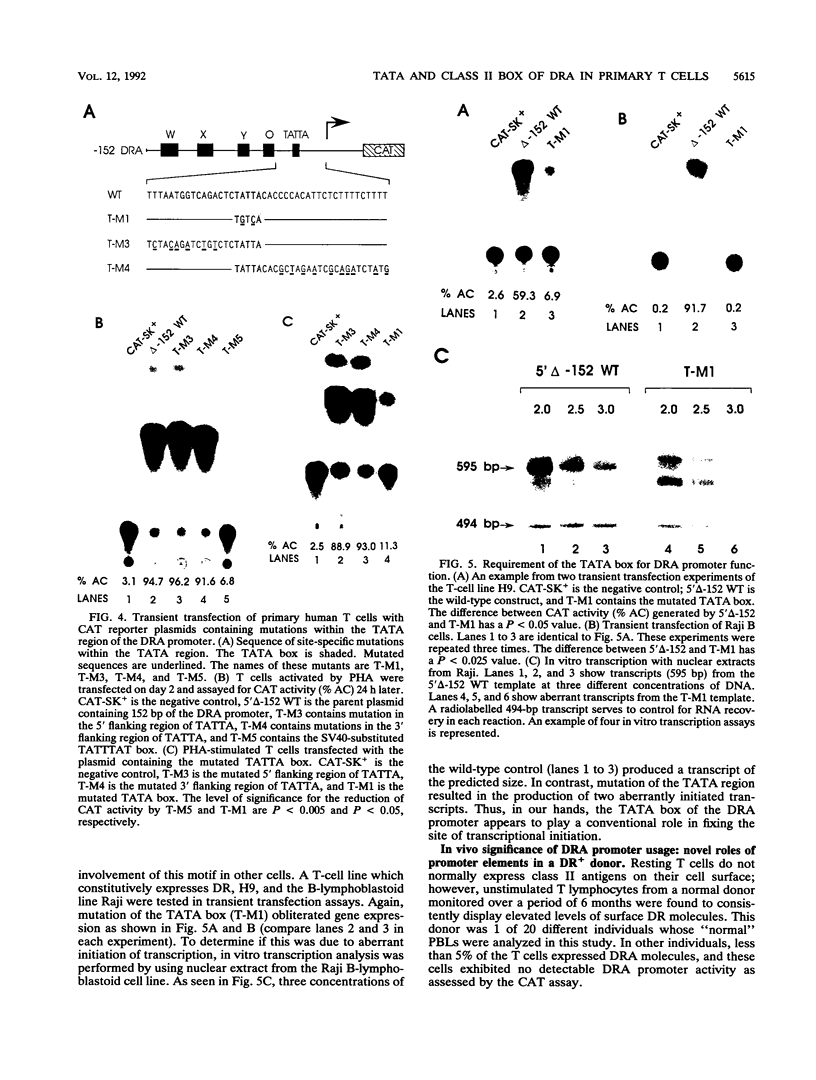
Human T lymphocytes express human leukocyte antigen (HLA)-DR-alpha (DRA) upon mitogenic or antigenic stimulation. DR+ T cells are also found in a number of inflammatory and autoimmune diseases and have a proposed role in these diseases. The molecular mechanism of DR regulation in untransformed blood T lymphocytes was studied here by transient transfection of DRA-chloramphenicol acetyltransferase reporter gene constructs. Several novel features of this regulation were observed. During the early stages of T-cell activation by mitogens or antigens, strong promoter induction was exhibited with the proximal 43 bp of the DRA promoter which contains a TATTA motif. Addition of upstream X and Y DNA elements augmented the response. This contrasts with data from transformed cell lines in which the proximal 43 bp produced no detectable promoter function, and the inclusion of X and Y elements is essential for basal level expression. Mutation of the TATTA motif or substitution with a functional but different TATA element produced errant initiation and greatly reduced gene expression. Interestingly, T lymphocytes from a normal donor were DR+ prior to in vitro stimulation, and again, strong promoter activity was observed with 43 bp of proximal sequence. Unexpectedly, the presence of the X and Y elements correlated with a suppression of class II promoter function and surface antigen expression. This study of nontransformed lymphocytes reveals several novel features of DRA gene regulation and underscores the value and necessity of such studies.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accolla R. S., Dellabona P., Scarpellino L., Carra G., Sartoris S. A family of trans-acting factors with distinct regulatory functions control expression of MHC class II genes. Immunol Res. 1990;9(1):20–33. doi: 10.1007/BF02918476. [DOI] [PubMed] [Google Scholar]
- Babiss L. E., Vales L. D. Promoter of the adenovirus polypeptide IX gene: similarity to E1B and inactivation by substitution of the simian virus 40 TATA element. J Virol. 1991 Feb;65(2):598–605. doi: 10.1128/jvi.65.2.598-605.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoist C., Mathis D. Regulation of major histocompatibility complex class-II genes: X, Y and other letters of the alphabet. Annu Rev Immunol. 1990;8:681–715. doi: 10.1146/annurev.iy.08.040190.003341. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Charron D. J., Engelman E. G., Benike C. J., McDevitt H. O. Ia antigens on alloreactive T cells in man detected by monoclonal antibodies. Evidence for synthesis of HLA-D/DR molecules of the responder type. J Exp Med. 1980 Aug 1;152(2 Pt 2):127s–136s. [PubMed] [Google Scholar]
- Cogswell J. P., Basta P. V., Ting J. P. X-box-binding proteins positively and negatively regulate transcription of the HLA-DRA gene through interaction with discrete upstream W and V elements. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7703–7707. doi: 10.1073/pnas.87.19.7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogswell J. P., Zeleznik-Le N., Ting J. P. Transcriptional regulation of the HLA-DRA gene. Crit Rev Immunol. 1991;11(2):87–112. [PubMed] [Google Scholar]
- Didier D. K., Schiffenbauer J., Woulfe S. L., Zacheis M., Schwartz B. D. Characterization of the cDNA encoding a protein binding to the major histocompatibility complex class II Y box. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7322–7326. doi: 10.1073/pnas.85.19.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didier D. K., Woulfe S. L., Schwartz B. D. Characterization of a DNA-binding protein system which is inversely correlated with class II expression. Immunol Res. 1990;9(1):69–76. doi: 10.1007/BF02918480. [DOI] [PubMed] [Google Scholar]
- Diedrichs M., Schendel D. J. Differential surface expression of class II isotypes on activated CD4 and CD8 cells correlates with levels of locus-specific mRNA. J Immunol. 1989 May 1;142(9):3275–3280. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari C., Pilli M., Penna A., Bertoletti A., Valli A., Cavalli A., Pasetti G., Fiaccadori F. Autopresentation of hepatitis B virus envelope antigens by T cells. J Virol. 1992 Apr;66(4):2536–2540. doi: 10.1128/jvi.66.4.2536-2540.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S. M., Chiorazzi N., Wang C. Y., Montrazeri G., Kunkel H. G., Ko H. S., Gottlieb A. B. Ia-bearing T lymphocytes in man. Their identification and role in the generation of allogeneic helper activity. J Exp Med. 1978 Nov 1;148(5):1423–1428. doi: 10.1084/jem.148.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher L. H., Kara C. J. Sequences and factors: a guide to MHC class-II transcription. Annu Rev Immunol. 1992;10:13–49. doi: 10.1146/annurev.iy.10.040192.000305. [DOI] [PubMed] [Google Scholar]
- Greenblatt J. Roles of TFIID in transcriptional initiation by RNA polymerase II. Cell. 1991 Sep 20;66(6):1067–1070. doi: 10.1016/0092-8674(91)90027-v. [DOI] [PubMed] [Google Scholar]
- Hooft van Huijsduijnen R. A., Bollekens J., Dorn A., Benoist C., Mathis D. Properties of a CCAAT box-binding protein. Nucleic Acids Res. 1987 Sep 25;15(18):7265–7282. doi: 10.1093/nar/15.18.7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H. S., Fu S. M., Winchester R. J., Yu D. T., Kunkel H. G. Ia determinants on stimulated human T lymphocytes. Occurrence on mitogen- and antigen-activated T cells. J Exp Med. 1979 Aug 1;150(2):246–255. doi: 10.1084/jem.150.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A., Roosnek E., Gregory T., Berman P., Abrignani S. T cells can present antigens such as HIV gp120 targeted to their own surface molecules. Nature. 1988 Aug 11;334(6182):530–532. doi: 10.1038/334530a0. [DOI] [PubMed] [Google Scholar]
- Liou H. C., Boothby M. R., Finn P. W., Davidon R., Nabavi N., Zeleznik-Le N. J., Ting J. P., Glimcher L. H. A new member of the leucine zipper class of proteins that binds to the HLA DR alpha promoter. Science. 1990 Mar 30;247(4950):1581–1584. doi: 10.1126/science.2321018. [DOI] [PubMed] [Google Scholar]
- Matsushima G. K., Stohlman S. A. Distinct subsets of accessory cells activate Thy-1+ triple negative (CD3-, CD4-, CD8-) cells and Th-1 delayed-type hypersensitivity effector T cells. J Immunol. 1991 May 15;146(10):3322–3331. [PubMed] [Google Scholar]
- Moses H., Panek R. B., Benveniste E. N., Ting J. P. Usage of primary cells to delineate IFN-gamma-responsive DNA elements in the HLA-DRA promoter and to identify a novel IFN-gamma-enhanced nuclear factor. J Immunol. 1992 Jun 1;148(11):3643–3651. [PubMed] [Google Scholar]
- Oshima S., Eckels D. D. Selective expression of class II MHC isotypes by MLC-activated human T lymphocytes. Hum Immunol. 1990 Mar;27(3):208–219. doi: 10.1016/0198-8859(90)90051-p. [DOI] [PubMed] [Google Scholar]
- Oshima S., Eckels D. D. Selective signal transduction through the CD3 or CD2 complex is required for class II MHC expression by human T cells. J Immunol. 1990 Dec 15;145(12):4018–4025. [PubMed] [Google Scholar]
- Pawelec G., Bühring H. J. Expression of MHC class II epitopes on human T lymphocyte clones. Cell Immunol. 1990 May;127(2):520–526. doi: 10.1016/0008-8749(90)90152-h. [DOI] [PubMed] [Google Scholar]
- Pellegrino M. A., Ferrone S., Dierich M. P., Reisfeld R. A. Enhancement of sheep red blood cell human lymphocyte rosette formation by the sulfhydryl compound 2-amino ethylisothiouronium bromide. Clin Immunol Immunopathol. 1975 Jan;3(3):324–333. doi: 10.1016/0090-1229(75)90019-7. [DOI] [PubMed] [Google Scholar]
- Peterlin B. M., Andersson G., Lötscher E., Tsang S. Transcriptional regulation of HLA class-II genes. Immunol Res. 1990;9(3):164–177. doi: 10.1007/BF02918176. [DOI] [PubMed] [Google Scholar]
- Popovic M., Lange-Wantzin G., Sarin P. S., Mann D., Gallo R. C. Transformation of human umbilical cord blood T cells by human T-cell leukemia/lymphoma virus. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5402–5406. doi: 10.1073/pnas.80.17.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith W., Barras E., Satola S., Kobr M., Reinhart D., Sanchez C. H., Mach B. Cloning of the major histocompatibility complex class II promoter binding protein affected in a hereditary defect in class II gene regulation. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4200–4204. doi: 10.1073/pnas.86.11.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith W., Herrero-Sanchez C., Kobr M., Silacci P., Berte C., Barras E., Fey S., Mach B. MHC class II regulatory factor RFX has a novel DNA-binding domain and a functionally independent dimerization domain. Genes Dev. 1990 Sep;4(9):1528–1540. doi: 10.1101/gad.4.9.1528. [DOI] [PubMed] [Google Scholar]
- Robbins P. A., Maino V. C., Warner N. L., Brodsky F. M. Activated T cells and monocytes have characteristic patterns of class II antigen expression. J Immunol. 1988 Aug 15;141(4):1281–1287. [PubMed] [Google Scholar]
- Servenius B., Rask L., Peterson P. A. Class II genes of the human major histocompatibility complex. The DO beta gene is a divergent member of the class II beta gene family. J Biol Chem. 1987 Jun 25;262(18):8759–8766. [PubMed] [Google Scholar]
- Sherman P. A., Basta P. V., Moore T. L., Brown A. M., Ting J. P. Class II box consensus sequences in the HLA-DR alpha gene: transcriptional function and interaction with nuclear proteins. Mol Cell Biol. 1989 Jan;9(1):50–56. doi: 10.1128/mcb.9.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P. A., Basta P. V., Ting J. P. Upstream DNA sequences required for tissue-specific expression of the HLA-DR alpha gene. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4254–4258. doi: 10.1073/pnas.84.12.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. C., Rooney R. J., Fisch T. M., Heintz N., Nevins J. R. E1A-dependent trans-activation of the c-fos promoter requires the TATAA sequence. Proc Natl Acad Sci U S A. 1990 Jan;87(2):513–517. doi: 10.1073/pnas.87.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Roberts-Thomson P. J. Lymphocyte surface marker expression in rheumatic diseases: evidence for prior activation of lymphocytes in vivo. Ann Rheum Dis. 1990 Feb;49(2):81–87. doi: 10.1136/ard.49.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick A. G., Blake M. C., Kahn J. W., Azizkhan J. C. Functional analysis of GC element binding and transcription in the hamster dihydrofolate reductase gene promoter. Nucleic Acids Res. 1989 Nov 25;17(22):9291–9304. doi: 10.1093/nar/17.22.9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang S. Y., Nakanishi M., Peterlin B. M. Mutational analysis of the DRA promoter: cis-acting sequences and trans-acting factors. Mol Cell Biol. 1990 Feb;10(2):711–719. doi: 10.1128/mcb.10.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer A. J., Flad H. D. Discontinuous density gradient separation of human mononuclear leucocytes using Percoll as gradient medium. J Immunol Methods. 1979;30(1):1–10. doi: 10.1016/0022-1759(79)90268-0. [DOI] [PubMed] [Google Scholar]
- Vilen B. J., Cogswell J. P., Ting J. P. Stereospecific alignment of the X and Y elements is required for major histocompatibility complex class II DRA promoter function. Mol Cell Biol. 1991 May;11(5):2406–2415. doi: 10.1128/mcb.11.5.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viville S., Jongeneel V., Koch W., Mantovani R., Benoist C., Mathis D. The E alpha promoter: a linker-scanning analysis. J Immunol. 1991 May 1;146(9):3211–3217. [PubMed] [Google Scholar]
- Yu D. T., McCune J. M., Fu S. M., Winchester R. J., Kunkel H. G. Two types of Ia-positive T cells. Synthesis and exchange of Ia antigens. J Exp Med. 1980 Aug 1;152(2 Pt 2):89s–98s. [PubMed] [Google Scholar]
- Zeleznik-Le N. J., Azizkhan J. C., Ting J. P. Affinity-purified CCAAT-box-binding protein (YEBP) functionally regulates expression of a human class II major histocompatibility complex gene and the herpes simplex virus thymidine kinase gene. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1873–1877. doi: 10.1073/pnas.88.5.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ewijk W., Ron Y., Monaco J., Kappler J., Marrack P., Le Meur M., Gerlinger P., Durand B., Benoist C., Mathis D. Compartmentalization of MHC class II gene expression in transgenic mice. Cell. 1988 May 6;53(3):357–370. doi: 10.1016/0092-8674(88)90156-0. [DOI] [PubMed] [Google Scholar]