Abstract
Vγ4+ cells, a subpopulation of peripheral γδ T cells, are involved in West Nile virus (WNV) pathogenesis, but the underlying mechanism is unclear. In this study, we found that WNV-infected Vγ4+ cell-depleted mice had lower viremia and a reduced inflammatory response in the brain. Vγ4+ cells produced interleukin (IL)-17 during WNV infection, but blocking IL-17 signaling did not affect host susceptibility to WNV encephalitis. We also noted that there was an enhanced magnitude of protective splenic Vγ1+ cell expansion in Vγ4+ cell-depleted mice compared to that in controls during WNV infection. Additionally, Vγ4+ cells of WNV-infected mice had a higher potential for producing transforming growth factor (TGF)-β. γδ T cells of WNV-infected Vγ4+ cell-depleted mice had a higher proliferation rate than those of WNV-infected controls upon ex vivo stimulation with anti-CD3, and this difference was diminished in the presence of TGF-β inhibitor. Finally, Vγ4+ cells of infected mice contributed directly and indirectly to the higher level of IL-10, which is known to play a negative role in immunity against WNV infection. In summary, Vγ4+ cells suppress Vγ1+ cell expansion via TGF-β and increase IL-10 level during WNV infection, which together may lead to higher viremia and enhanced brain inflammation.
Keywords: West Nile virus, Pathogenesis, γδ T cell
Introduction
West Nile virus (WNV) belongs to the family of Flaviviridae, a group of plus-sense, single-stranded RNA viruses. Since its first appearance in 1999 in New York City, the virus has spread across most of the United States, parts of Canada, Mexico, Guatemala, the Caribbean and to several countries in South America (Petersen & Hayes, 2008, Murray, et al., 2011). Although most WNV infections in humans are asymptomatic, a small percentage of them result in encephalitis and death, mainly in the elderly and immunocompromised. At present, there is no specific therapeutic agent for treatment of the infection or an approved vaccine for its prevention (Petersen & Hayes, 2008). WNV can gain access to the central nervous system (CNS) after a brief viremia in the periphery, a process called neuroinvasion that may turn a mild viral infection into severe lethal encephalitis or death within 7–10 days (Ben-Nathan, et al., 1996, Diamond, et al., 2003). The mechanisms by which WNV enters the CNS are not yet clearly understood. It was suggested that the virus infects the CNS in part via hematogenous spread, as increased viremia correlates with earlier viral entry into the brain (Diamond, et al., 2003). Therefore, it is critical to control virus dissemination in the periphery. Once inside the brain, WNV-induced CNS disease might be caused by neuronal cell death directly by viral infection, and/or by bystander damage from the immune response to the pathogen, including lymphocyte and macrophage/microglia responses (Sampson & Armbrustmacher, 2001, Xiao, et al., 2001, Shrestha, et al., 2003, Wang, et al., 2003).
γδ T cells are a minority of the CD3+ T cells in lymphoid tissue and blood, but are well represented at epithelial and mucosal sites (Hayday, 2000). They can rapidly produce cytokines in response to microbial antigens (Ferrick, et al., 1995, Wang, et al., 2001, Wang, et al., 2001)– γδ T cells are divisible into functionally distinct subsets which have direct and indirect effects on host immunity to infectious pathogens (Bank, et al., 1986). For example, Vγ1+ and Vγ4+ T cells are two major subpopulations of peripheral γδ T cells in mice. Splenic Vγ1-bearing T cells are important in the elimination of Listeria by their interferon (IFN)-γ– producing activity (Matsuzaki, et al., 2002). In Coxsackievirus-infected mice, Vγ4+ T cells enhance CD4+ Th1 cell activation through IFN-γ and CD1-dependent mechanisms and promote the activation of autoimmune CD8+ T cells, which could ultimately lead to myocarditis (Huber, et al., 2000).
Our earlier work shows that γδ T cells respond rapidly following WNV infection, limiting viremia and invasion of the CNS partially via IFN-γ- production, thereby protecting the host from lethal encephalitis (Wang, et al., 2003). Following this study, we have found that γδ T cell subsets may play distinct roles in protection and pathogenesis during WNV infection (Welte, et al., 2008). Vγ1+ T cells were the major source of IFN-γ which helps to protect the host from lethal encephalitis. Mice depleted of these cells had enhanced viremia and higher mortality to WN encephalitis. In contrast, the depletion of Vγ4+ T cells resulted in a decreased viral load in the brain and a lower mortality due to WN encephalitis. In this study, we further investigated the role of Vγ4+ T cells in WNV-induced encephalitis.
Materials and methods
Infection in mice
6- to 10-week-old C57BL/6 (B6) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Groups were age- and sex-matched for each experiment and housed under identical conditions. All animal experiments were approved by the Animal Care and Use Committee at Colorado State University or the University of Texas Medical Branch. Mice were injected intraperitoneal (i.p.) with 800 plaque-forming units (PFU) of WNV 385-99 ((Tesh, et al., 2005), a dose close to LD100). Infected mice were monitored twice daily for morbidity, lethargy, anorexia and ataxia.
In vivo depletion of γδ subpopulations or blocking IL-17 signaling
T-cell depletion was achieved by two consecutive injections of 100 µg of hamster anti-Vγ4 (mAb UC3, purified from hybridoma culture supernatants (Hahn, et al., 2004) or purchased from BD Biosciences (San Diego, CA).) i.p. at 2 days and 24 h before WNV challenge (Welte, et al., 2008). Sham Ab treatments were performed with the same amount of hamster IgG isotype (Innovative Research, Southfield, MI). To block IL-17 signaling, B6 mice were administered with anti-mouse IL-17A (100 μg/day; eBioscience, San Diego, CA) or mouse IgG1 (100 μg/day; Sigma-Aldrich, St. Louis, MO) at days 0 and 5 post-infection, as described in an earlier study (Hou, et al., 2009).
Purification of spleen T cells and brain leukocytes
Splenic total T cells and γδ T cells were purified by anti-CD90 magnetic beads or a TCRγ/δ+ T Cell Isolation Kit according to the manufacturer’s instructions (Miltenyi Biotec, Auburn, CA) with a purity of 92% and 90%, respectively. Brain leukocytes were isolated based on a previously described method (Glass, et al., 2005). Prior to harvest, extensive cardiac perfusion was done by using PBS to deplete intravascular leukocytes. Brains were collected and homogenized. Cell homogenates were centrifuged and re-suspended in 7 ml PBS with 2% fetal bovine serum mixed with 3 ml of 90% Percoll (Sigma-Aldrich) in PBS. The suspension was next underlayed with 1 ml of 70% Percoll in RPMI and centrifuged at 800×g for 20 min at 22°C. Leukocytes at the interface were harvested and counted.
Quantitative PCR (Q-PCR) or PCR for determining viral load, T cell levels and cytokine production
RNA was extracted from blood and brain of non-infected and WNV-infected mice by using an RNAeasy extraction kit (Qiagen, Valencia, CA) and was employed to synthesize cDNA by using the ProSTAR First-strand RT-PCR kit (Stratagene, Cedar Creek, TX). The sequences of the primer-probe sets for WNVE, CD11b, CD8, IL-10, IL-17 and TGF-β genes (Phares, et al., 2006, Kang, et al., 2009, Xu, et al., 2009) and PCR reaction conditions were described previously (Lanciotti, et al., 2000, Wang, et al., 2004). The primers for the Gr1 gene were purchased from SABiosciences (Frederick, MD). Q-PCR analysis was performed with iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) on a CFX96 real-time PCR system (Bio-Rad). To normalize the samples, we used the same amount of cDNA in a Q-PCR for β-actin. The ratio of the amount of amplified gene compared with the amount of β-actin cDNA represented the relative levels in each sample. For Il-10 and tgf-β genes, it was calculated based on Ct values using the formula 2^ −[Ct(cytokine gene)−Ct(β-actin)] as described in RT2 Profiler™ PCR Array User Manual (SA Biosciences). For Vγ4 expression in the brain, the cDNA of infected mice brain homogenates or spleen samples was amplified by using primers described in an early study (Andrew, et al., 2005).
Flow cytometry
Brain leukocytes were stained with antibodies for cell surface markers, including Gr-1, CD3, TCRγδ, CD45 (BD Biosciences) and Vγ4. Fixed cells were examined by using a C6 Flow Cytometer (Accuri cytometers, Ann Arbor, MI) or a FACSCantu (BD Biosciences). To measure intracellular cytokine production, CD90+ splenocytes or brain leukocytes were stimulated with 50 ng/ml PMA (Sigma-Aldrich) and 500 ng/ml ionomycin (Sigma-Aldrich) for 4 h or 24h at 37°C in a Golgi-plug (BD Biosciences) containing medium. Cells were harvested, stained with Abs for TCRαβ (BD Biosciences) and Vγ1, or Vγ4, fixed in 2% paraformaldehyde, and permeabilized with 0.5% saponin before adding PE-conjugated anti-TGF-β, anti-IL-10, anti-IL-17A, or control PE-conjugated rat IgG1 (BD Biosciences). FoxP3 expression was analyzed by using a kit from eBiosciences (San Diego, CA) according to the manufacturer’s instructions. Data were analyzed by using CFlow Plus (Accuri cytometers) or Summit 4 software (Dako Cytomation).
Cytokine measurement
CD90+ splenocytes were stimulated with 50 ng/ml PMA and 500 ng/ml ionomycin (Sigma-Aldrich) for 24 h at 37°C. Supernatant was collected for cytokine measurement by using a Bio-Plex Pro Mouse Cytokine Assay (Biorad).
In vitro T cell proliferation assay
CD90+ splenocytes or γδ T cells were labeled with 2.5 µM CFSE according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA) and cultured at 1×105 cells/well for 72 h in anti-CD3-coated (10 µg/ml, eBioscience) plates with medium containing hIL-2 (5 ng/ml, eBioscience). In some experiments, cells were treated with TGFβ inhibitor SB-505124 (1 µM, Sigma-Aldrich), IL-10R neutralization antibody (10 µg/ml, Biolegend), or isotype control rIgG (10 µg/ml, Jackson ImmunoResearch). Cells were harvested and examined by a C6 Flow Cytometer (Accuri cytometers). αβ or γδ T cell proliferation was assessed by flow cytometric analysis of CFSE dilution.
Histologic examination of tissues
Anesthetized mice were perfused with 30 ml of ice cold PBS. Brains were removed and fixed in 4% paraformaldehyde. Subsequently, specimens were transferred to 70% ethanol and processed. Then, 10-micron paraffin sections were prepared for staining with hematoxylin & eosin. Stained sections were examined and scored by a pathologist, who was blinded to the origin of the samples. A para-saggital section of each mouse brain (including olfactory bulb, cerebellum, cerebrum, and brainstem) was submitted for histology, and from each block, one to four sections were examined.
Statistical analysis
Data analysis was performed by using Prism software (Graph-Pad) statistical software. Values for phenotype analysis, viral burden, and cytokine production were presented as means ± SEM. The P values of these experiments were calculated with a non-paired Student’s t test. Statistical significance was accepted at P < 0.05.
Results
V γ4+ cell-depleted mice had less viremia, accompanied by a reduced inflammation in the brain at the later stage of infection
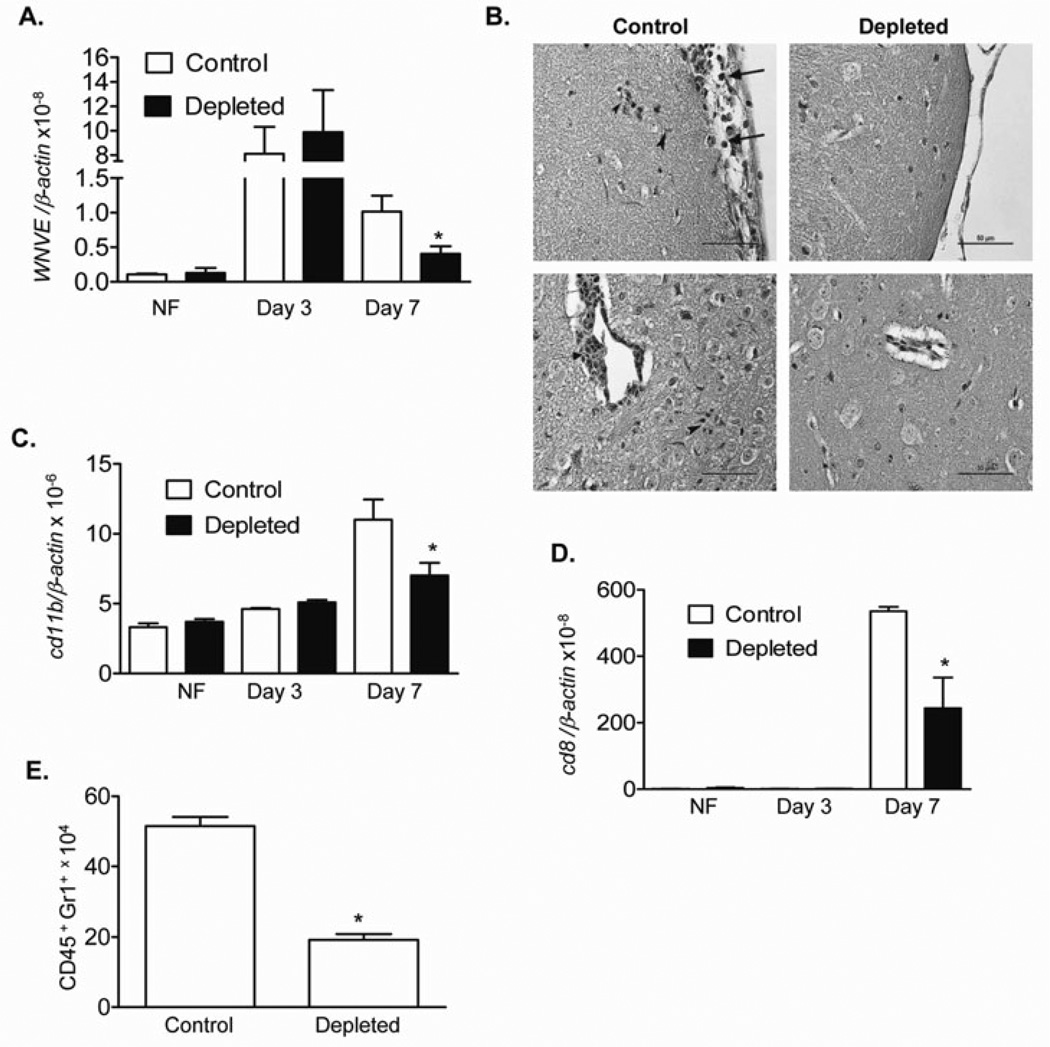
In previous work (Welte, et al., 2008), we showed that depletion of Vγ4+ T cells in mice resulted in a decreased viral load in the brain and a lower mortality due to WN encephalitis, partially because of the cells’ higher potential for producing tumor necrosis factor (TNF)-α, a cytokine known to be involved in blood brain barrier compromise and WNV entry into the brain (Wang, et al., 2004). Here, to further investigate its role in WNV pathogenesis, we performed Vγ4+ cell-depletion in mice as described before (Welte, et al., 2008) followed by i.p. infection with 800 PFU of WNV. We found that there was no difference in viremia between the Vγ4+ T cell-depleted animals and controls at an early stages of infection (day 3). Vγ4+ T cell-depleted animals had reduced levels of viremia at the later stage of infection (day 7) than did the controls (Fig. 1A, P < 0.05). These animals also exhibited reduced histologic evidence of inflammation and neuronal damage at day 10 post-infection when compared to equivalent findings in controls (Fig. 1B). In further phenotype analysis of brain leukocytes by Q-PCR, we noted that the levels of macrophages/monocytes (CD11b+) and CD8+ T cells were significantly reduced in Vγ4+ T cell-depleted mouse brains at day 7 post-infection (Figs. 1C & D, P < 0.05). The total number of infiltrating neutrophils detected in Vγ4+ T cell-depleted mouse brains was also reduced by 63% (Fig. 1E, P < 0.05) at the later stages of infection. Overall, Vγ4+ cell-depleted mice displayed less viremia, accompanied by a reduced inflammation and viral load in the brain at the later stages of infection.
Figure 1. Vγ4+-cell-depleted mice had less viremia and a reduced inflammatory response and pathology in the brain following WNV infection.
A. Viral load was determined in blood by Q-PCR in non-infected (NF) and WNV-infected mice. n = 3–7. B. Hematoxylin and eosin stained mouse brain sections at day 10 post-infection. Top left panel. Control showing leptomeningitis (arrows) and subpial microglial hypertrophy (arrowheads). Top right panel. Vγ4+ T cell-depleted animal showing absence of leptomeningitis. Bottom left panel. Control showing perivascular cuffing (arrow), microglial proliferation, and an inflammatory nodule (arrowhead). Scale bar = 50 microns. Bottom right panel. Vγ4+ T cell-depleted animal showing absence of perivascular cuffing or parenchymal hypercellularity due to inflammation. C- D. CD11b (C) and CD8 levels (D) of non-infected (NF) and WNV-infected mouse brains were measured by Q-PCR. E. Number of neutrophils in mouse brains at day 10 post-infection. n = 4–7. Data are presented as means ± SEM. *P < 0.05 for control vs. Vγ4+-T-cell-depleted mice.
Vγ4+ cells produced interleukin (IL)-17 during WNV infection. Blocking IL-17 signaling did not affect host susceptibility to WNV encephalitis
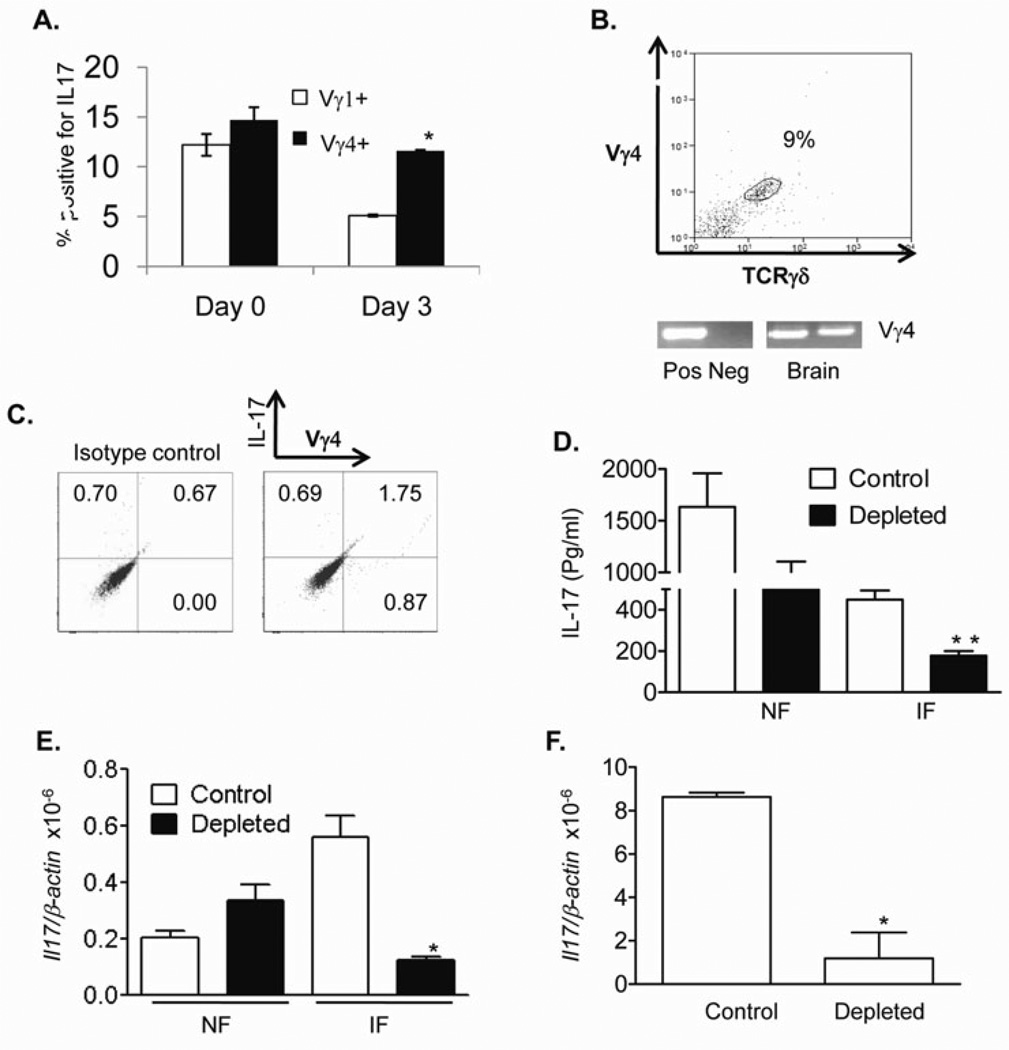
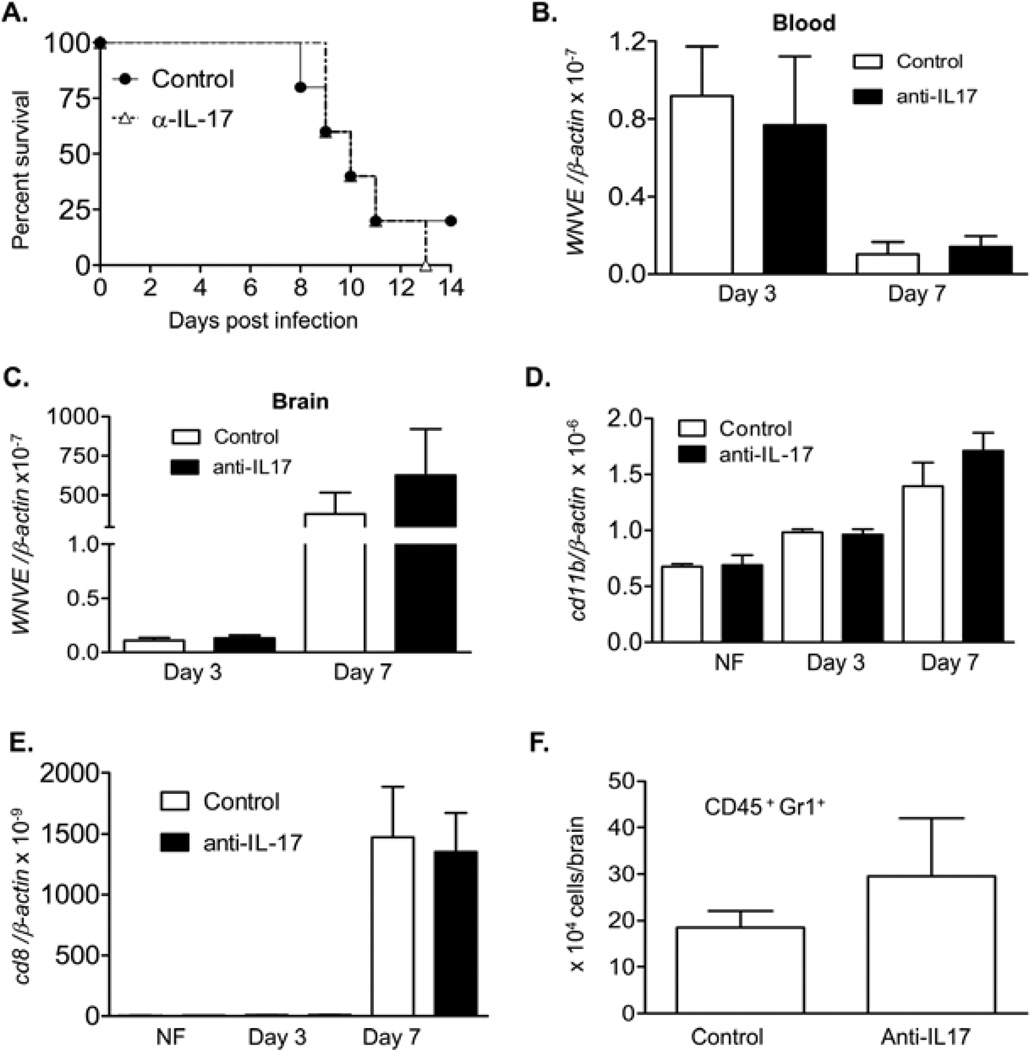
Recent reports have demonstrated that IL-17-producing γδ T cells play a key role in the pathogenesis of several disease models (Roark, et al., 2007, Flierl, et al., 2008). Therefore, we next determined the IL-17-producing activity of Vγ4+ cells during WNV infection. As shown in Fig. 2A, both splenic Vγ1+ and Vγ4+ populations of naïve mice produced IL-17, whereas Vγ4+ cells had a higher potential at day 3 post-infection (P < 0.05). At day 7 post-infection, by flow cytometry analysis, we detected 9% of Vγ4+ cells among the overall CD3+ brain leukocytes of WNV-infected mice (Fig.2B top panel). The infiltration of Vγ4+ cells in the brain was further confirmed by PCR analysis of WNV-infected brain samples (Fig. 2B bottom panel). Among the gated brain leukocytes, almost all IL-17-producing cells were stained positive for Vγ4 (Fig. 2C). To verify these results, we measured IL-17 levels in Vγ4+ cell-depleted mice. Splenic T cells of Vγ4+ cell-depleted mice produced 60% less IL-17 upon ex vivo stimulation with PMA and ionomycin, (P < 0.01, Fig. 2D). IL-17 expression in blood of these mice was also reduced by over 80% (Fig. 2E, P < 0.05). This difference was not observed in non-infected mice (Figs. 2D & 2E, P > 0.05). Moreover, there was an about 85% decrease in IL-17 expression in Vγ4+ T cell-depleted mouse brains compared to those of controls (Fig. 2F, P < 0.05). To further test if the IL-17-producing activity of Vγ4+ T cells contributes to viral pathogenesis, we treated mice with neutralizing antibody to IL-17 or isotype control antibody followed by WNV infection. Surprisingly, we found no statistical differences in the survival rates (0% vs. 20%, anti-IL-17-treated vs. controls, P > 0.05, Fig. 3A). There was no difference in viral load in the blood and brain between the two groups (Figs. 3B & 3C, P > 0.05). Further, we did not observe any difference in either levels or number of the infiltrating monocytes, CD8+ T cells or neutrophils between the two groups as measured by Q-PCR analysis and flow cytometry (Figs. 3D-F, P > 0.05). Combined together, these data indicate that the Vγ4+ T cells produce IL-17 during WNV infection. However, this activity seems to be dispensable in promoting WNV-induced encephalitis or lethality in mice.
Figure 2. Vγ4+ T cells produced IL-17 during WNV infection.
A. Splenic T cells of naïve (day 0) or day 3-infected mice were cultured ex vivo with PMA plus ionomycin and stained for Vγ1 or Vγ4, and IL-17 and gated on each γδ T cell subset for analysis of the percentage of Vγ1+ IL-17+ or Vγ4+ IL-17+. n = 3–4. B. Vγ4+ T-cells were detected in WNV-infected mouse brains (day 7) by flow cytometry (top panel) and PCR analysis (bottom panel). Top panel: Cells were gated on CD3+CD45+ leukocytes. Bottom panel: Brain cDNA samples from two WNV-infected mice were used. Spleen cDNA was used as a positive control (Pos). Negative control (Neg): non-infected mouse brain. C. Brain leukocytes of day 7-infected mice were cultured ex vivo with PMA plus ionomycin and stained for Vγ4 and IL-17 and gated on total leukocytes for analysis of the percentage of Vγ4+ IL-17+. D. Supernatant of splenic T cells of non-infected (NF) or WNV-infected mice (IF) were treated with PMA plus ionomycin for 24 h and measured for IL-17 production, n = 7. E-F. Vγ4+-cell-depleted mice had a reduced IL17 production in blood (E) at day 3 and in brain (F) at day 7 post-infection, as determined by Q-PCR. n = 3–4. *P < 0.05 or **P < 0.01 for control vs. Vγ4+-T-cell-depleted mice.
Figure 3. Viral load and inflammatory responses in anti-IL-17-treated mice.
Mice were treated with neutralizing antibody to IL-17 or isotype control antibody, followed by WNV infection. A. mice were monitored daily for survival following infection. P > 0.05 for control antibody-treated mice (n = 5) vs. anti-IL-17-treated (n = 5). B- C. Viral loads in blood (B) and brain (C) of control and anti-IL-17-treated mice were measured by Q-PCR. D- E. Monocytes (D) and CD8 T cell levels (E) in non-infected (NF) and WNV infected mouse brains were measured by Q-PCR. F. Neutrophil number in mouse brains at day 7 post-infection. n = 4–7.
Vγ4+ cells suppressed the proliferation of Vγ1+ T cell subset via production of transforming growth factor (TGF)-β
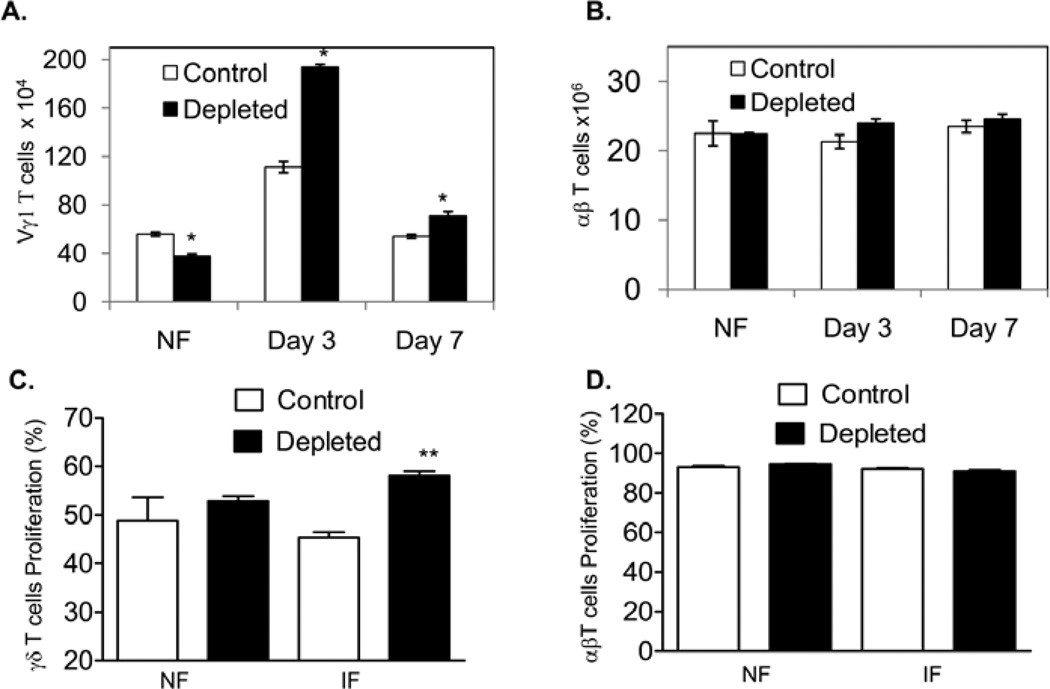
We have previously shown that splenic Vγ1+ T cells respond rapidly following WNV infection, limiting viremia and virus invasion of the CNS partially via their IFN-γ- producing activity and protect the host from lethal encephalitis (Wang, et al., 2003, Welte, et al., 2008). Here, we noted that at day 3 post-infection, the total number of splenic Vγ1+ T cells in the Vγ4 T cell-depleted mice was significantly enhanced compared to those in controls (Figs. 4A, P < 0.05). This difference was sustained even at later stages of infection (d7). The number of αβ T cells, nevertheless, was not different between the two groups (Fig. 4B, P > 0.05). To further determine if Vγ4+ cells suppress other T cell subset proliferation, splenic γδ T cells or CD90+ T cells were isolated from controls and Vγ4+ cell-depleted mice at days 0 and 2 post-infection and were CFSE labeled, treated with anti-CD3 for 3 days. T cell proliferation was measured by flow cytometry analysis. As shown in Fig. 4C, γδ T cells of day 2-infected mice had a higher proliferation rate in the absence of Vγ4+ T cells (58% ± 0.9%, P < 0.01) compared to those of controls (45% ± 1.0%). There was no difference in the proliferation rate for either naïve γδ T cells or αβ T cells isolated from naïve or infected mice (Figs. 4C & 4D, P > 0.05). Together, these results suggest that Vγ4 T cells may be involved in regulating the other splenic γδ subset (Vγ1+) T cell response during WNV infection.
Figure 4. There were more Vγ1+-cells in the spleens of Vγ4+-cell-depleted mice during WNV infection.
A- B. splenic T cells of non-infected (NF) or WNV-infected mice were stained for Vγ1 or TCRαβ. Total number of Vγ1+-cells (A) or αβ T cells (B) per spleen was shown. n = 4–6. * P < 0.05 for control vs. Vγ4+-T-cell-depleted mice. C- D. In vitro T cell proliferation assay. γδ+ (C) or CD90+ splenocytes (D) of controls or Vγ4+-cell-depleted mice were isolated from non-infected (NF) or WNV-infected mice (day 2, IF), labeled with CFSE, and cultured for 72 h with anti-CD3. Data shown are the percentage of T cell proliferation by flow cytometric analysis of CFSE. In D, CD90+ splenocytes were stained TCRαβ, αβ T cells were gated for analysis of the proliferation rate.
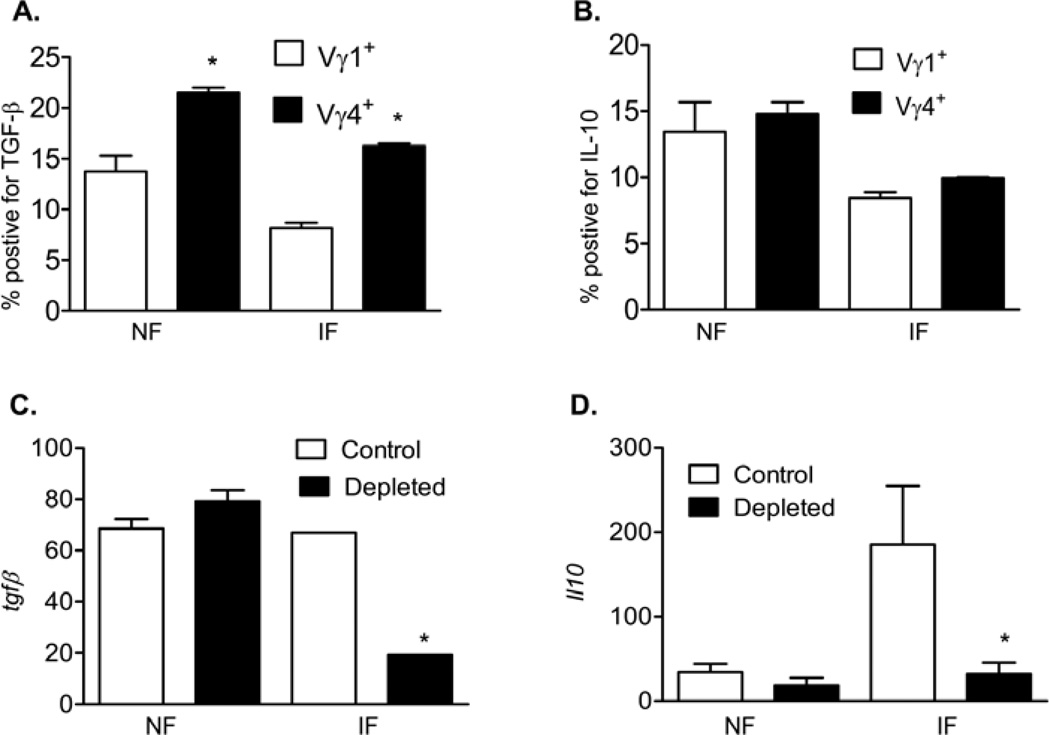
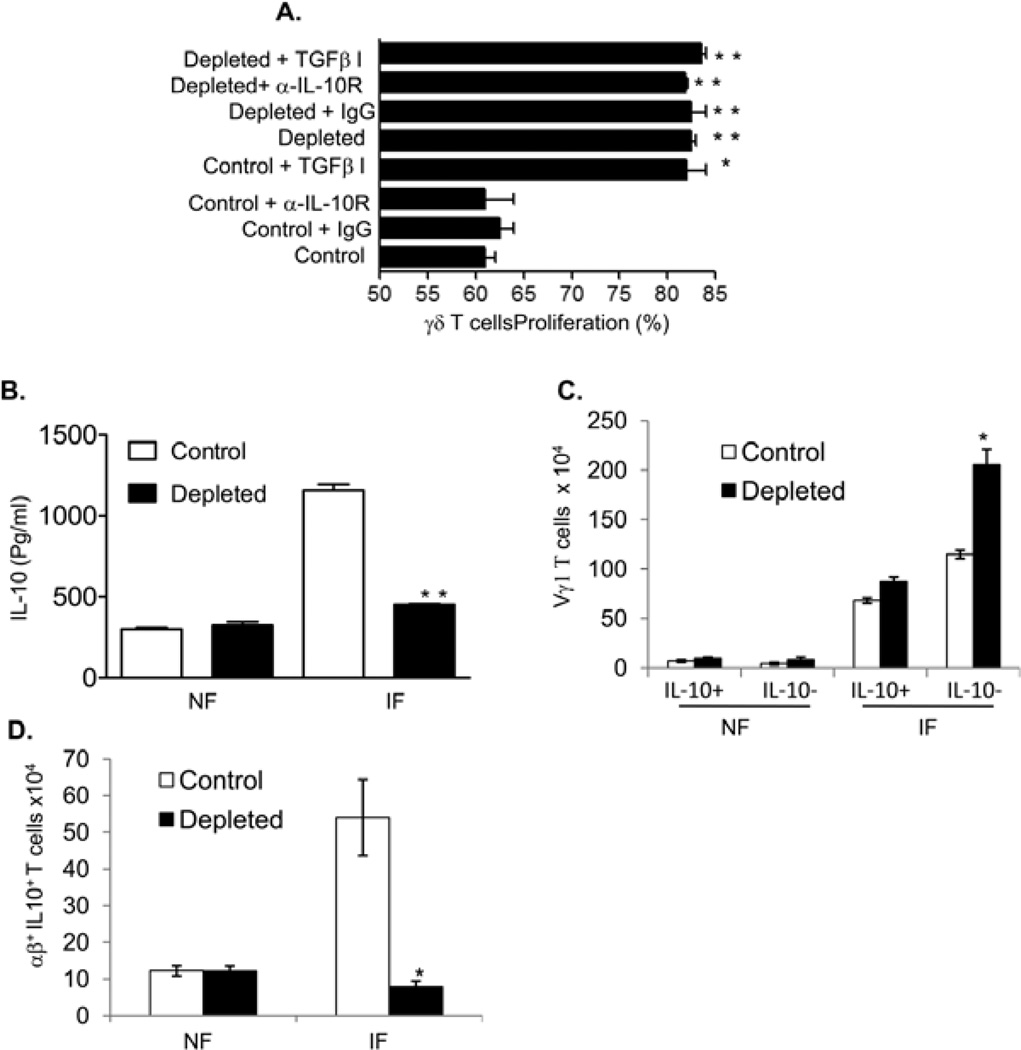
To understand the underlying mechanisms by which Vγ4+ cells suppress splenic Vγ1+ cell expansion, we measured the production of the regulatory cytokines TGF-β and IL-10 by Vγ4+ cells in WNV-infected mice. While both splenic Vγ1+ and Vγ4+ populations of naïve or WNV infected mice produced TGF-β, the latter had a greater potential (Figs. 5A, P < 0.05). Both splenic Vγ1+ and Vγ4+ populations produced IL-10 upon ex vivo stimulation with PMA and ionomycin; but there was no difference in IL-10 production between the two splenic γδ T cell subsets (Fig. 5B). In Vγ4+ T cell-depleted mice, there was a 70% and 82% reduction in TGF-β (Fig. 5C) and IL-10 expressions (Fig. 5D) in blood at day 3 post-infection. We next asked whether these regulatory cytokines played a role in anti-CD3-stimulated γδ T cell proliferation in vitro. Our results showed that the difference in the proliferation rate of γδ T cells between WNV-infected controls and Vγ4+ cell-depleted mice was diminished, if treated with a TGF-β inhibitor, but not with the neutralization antibody for the IL-10 receptor (Fig. 6A). These data further suggest that Vγ4+ T cells may suppress the proliferation of other γδ T cell subsets during WNV infection by production of TGF-β.
Figure 5. Vγ4+ T cells produced TGF-β and IL-10 during WNV infection.
A- B. Splenic T cells of non-infected (NF) and day 3 infected mice (IF) were cultured ex vivo with PMA plus ionomycin and stained for Vγ1 or Vγ4, and TGF-β (A) or IL-10 (B). Cells were gated on each γδ T cell subset for analysis of the percentage of Vγ1+ TGF-β+ or Vγ4+ TGF-β+. C- D. TGF-β (C) or IL-10 (D) expression in blood in non-infected mice or at day 3 post-infection as determined by Q-PCR. *P < 0.05 or **P < 0.01 compared to control alone.
Figure 6. Vγ4+ cells suppressed Vγ1+-T-cell expansion via TGF-β and contributed to higher level of IL-10 during WNV infection.
A. In vitro γδ T cell proliferation assay. γδ T cells of controls or Vγ4+-T-cell-depleted mice at day 2-post infection were labeled with CFSE and stimulated with anti-CD3 for 72h in the presence of anti-IL-10 receptor (α-IL-10 R) or isotype control (IgG) or TGF-β inhibitor (TGFβ-I). n = 3–4 per group. B- D. Splenic T cells of non-infected (NF) or WNV-infected (day 3, IF) controls or Vγ4+-T-cell-depleted mice were cultured ex vivo with PMA plus ionomycin. B, supernatant was measured for IL-10 production by Bioplex, n = 7. C- D, cells were harvested and stained for Vγ1 or TCRαβ, and IL-10. Total splenic T cells were gated for analysis of the percentage of IL-10 producing or non-producing Vγ1 or TCRαβ populations. *P < 0.05 or **P < 0.01 for control vs. Vγ4+-T-cell-depleted mice.
Vγ4+ cells contributed to increased levels of IL-10 during WNV infection
IL-10 is known to be involved in WNV pathogenesis (Schneider, et al., 2007, Bai, et al., 2009). WNV infection was diminished in IL-10-deficient mice, and this ultimately increased the survival rate (Schneider, et al., 2007, Bai, et al., 2009). Here, we noted that there was a 61% reduction in IL-10 production by splenic T cells of WNV-infected Vγ4+ T cell-depleted mice upon stimulation with PMA and ionomycin (Fig. 6B). These findings, when combined with the results from Fig. 5D, indicate that Vγ4+ T cells might promote IL-10 levels during WNV infection. Although Vγ4+ T cells produced IL-10 during WNV infection, they also suppressed the expansion of Vγ1+ T cells, which were shown to produce IL-10 (Fig. 5B). To investigate this possible conflict, we measured IL-10-producing Vγ1+ T cells in control and Vγ4+ T cell-depleted mice. We have found that the number of Vγ1+ IL-10- T cells significantly increased in Vγ4+ cell-depleted mice at day 3 post-infection (Fig. 6C). Nevertheless, there was no difference in the number of Vγ1+ IL10+ splenic T cells between these two groups of mice (Fig. 6C). This suggests that Vγ4+ T cells suppress the proliferation of non-IL-10 producing-Vγ1+ T cells during WNV infection. Furthermore, Bai et al. reported that CD4+ T cells were the major cellular resource for IL-10 during WNV infection (Bai, et al., 2009). We also noted that IL-10-producing αβ T cells were significantly reduced in Vγ4+ cell-depleted mice (Fig. 6D), which may indicate a role of Vγ4+ cells in promoting IL-10-producing αβ T cells during WNV infection. Combined together, these results suggest to us that Vγ4+ cells directly or indirectly contribute to the increasing levels of IL-10 during WNV infection.
Discussion
WNV can gain access to the brain after a brief viremia in its periphery. Further, WNV induced-CNS disease might be caused by direct infection in neurons, and/or by bystander damage from the immune response to the pathogen (Sampson & Armbrustmacher, 2001, Xiao, et al., 2001, Shrestha, et al., 2003, Wang, et al., 2003). Hence, it is critical to control virus dissemination in the periphery and to limit virus entry and inflammation in the CNS. In this study, we found Vγ4+ T cells might contribute to a higher viremia and/or more inflammatory responses in the brain, including an increase in infiltrating monocytes, CD8+ T cells and neutrophils at the late stage of infection.
IL-17 producing γδ T cells were involved in the exacerbation of the disease in a collagen-induced arthritis model (Roark, et al., 2007) or autoimmune encephalomyelitis (Sutton, et al., 2009). IL-17 is known to increase inflammation by recruiting cells, such as neutrophils or macrophages to the sites of infection (DiTirro, et al., 1998, Weaver, et al., 2007). Here, we noted that Vγ4+ T cells were one of the major sources of IL-17 production during WNV infection, although the percentage of IL-17-producing γδ T cells was much lower than those reported in our earlier study for IFN-γ and TNF-α production (Welte, et al., 2008). Surprisingly, in vivo blocking of IL-17 signaling led to no differences in host susceptibility to WNV infection. There were also no differences in the viral load of blood and brain and levels of brain leukocytes between the two groups. These results may indicate that IL-17 production by Vγ4+ cells is not needed for the promotion of the viral pathogenesis that we noted previously (Welte, et al., 2008).
γδ T cell subsets can be immunoregulatory, either in autoimmune and allergic diseases, or in limiting tissue pathology in response to infection (Hayday & Tigelaar, 2003). Here, we also reported that following WNV infection, Vγ4+ cells suppressed the protective Vγ1+ T cell expansion, but not of αβ T cells in a TGF-β–dependent manner. This differential suppression effect could be due to differences in the expansion patterns of the two subsets of T cells during WNV infection. Earlier studies suggested that γδ T cell expansion was more dramatic than that of αβ T cells in response to WNV infection (Wang, et al., 2003). γδ T cells induced by TGF-β were reported to mediate a potent immunosuppressive effect on anti-CD3-stimulated T cell activation and proliferation partially by upregulation of FoxP3 expression (Kang, et al., 2009). In line with this finding, we also noted a numeric decrease in FoxP3 expression by γδ T cells in Vγ4+ T cell-depleted vs. sham-depleted mice at day 3 post-infection, as assessed by the percentage and mean fluorescence intensity, whereas no differences were detected in αβ T cells between these two groups of mice (data not shown). The underlying mechanism of TGF-β–mediated suppression on γδ T cell-expansion during WNV infection is still under investigation.
The suppressive effect of Vγ4+ cells on Vγ1+ cell-expansion may contribute to viral pathogenesis in two ways. First, Vγ1+ T cells are the major splenic γδ T cell subpopulation to expand during WNV infection, and they produce IFN-γ, limit virus replication, and contribute to ultimate protection of the host from lethal encephalitis. Inhibition of Vγ1+ T cell-expansion thereby enhances viremia, which could lead to more virus dissemination into the CNS and induce encephalitis, as shown in our earlier work (Welte, et al., 2008). Second, the suppressive effect of Vγ4+ cells on Vγ1+ cell-expansion may indirectly contribute to higher IL-10 levels during WNV infection. IL-10 is known to be involved in WNV pathogenesis (Schneider, et al., 2007, Bai, et al., 2009). In this study, both Vγ1+ and Vγ4+ T cells were shown to produce IL-10 following WNV infection. In addition, Vγ4+ T cells suppressed the expansion of the non-IL-10-producing Vγ1+ cells and promote IL-10 production by αβ T cells. The mechanisms by which Vγ4+ cells promote the IL-10 production by CD4+ T cells are not clear yet. An early study reported that Vγ1+ cells reduced IL-10-producing CD4+CD25+ αβ T cells in the lungs of ovalbumin-sensitized and challenged mice (Hahn, et al., 2008). We speculate that Vγ4+ cells may increase IL-10 producing CD4+ αβ T cells by suppressing the non-IL-10-producing Vγ1+ cells. Vγ1+ cells are known to expand and be activated at the early stage of WNV infection. In comparison, induction of IL-10 expression by CD4+αβ T cells reaches the peak at the late stage of infection (day 5, Bai, et al., 2009). Here, we observed an apparent effect of Vγ4+ cell- depletion on reduction of viremia only at day 7 post-infection. Although viremia may extend by 1–2 days due to a high dose of viral challenge, it is likely that Vγ4+ T cells enhance viremia predominantly by promoting IL-10- producing αβ T cells indirectly via the production of TGF-β. This effect may ultimately lead to more WNV entry into the brain, which are associated with increased neuronal damage, and a greater inflammatory response in the CNS. Our previous findings suggest that Vγ4+ T cells have a higher potential for producing TNF-α, a cytokine known to be involved in blood brain barrier compromise, which also leads to more WNV entry and inflammatory cell infiltration into the CNS (Welte, et al., 2008). Taken together, Vγ4+ T cells might contribute to a higher viremia and/or more inflammatory responses in the brain via production of both proinflammatory cytokine and regulatory cytokines during WNV infection.
Little is known about the role of T cell-mediated pathology in WNV-related brain damage. Our data now provide the first evidence that Vγ4+ cell-mediated immune responses play an important role in WNV pathogenesis.
Acknowledgements
This work was supported by an NIH grant to T.W. (AI072060) and start-up funds to T.W. from The University of Texas Medical Branch. We thank Allison Poussard and Jennifer Smith for assistance in setting up WNV infection studies at UTMB, Dr. Y. Cong for helpful discussion, and Mardelle Susman for assisting in manuscript preparation.
References
- Andrew EM, Newton DJ, Dalton JE, et al. Delineation of the function of a major gamma delta T cell subset during infection. J Immunol. 2005;175:1741–1750. doi: 10.4049/jimmunol.175.3.1741. [DOI] [PubMed] [Google Scholar]
- Bai F, Town T, Qian F, et al. IL-10 signaling blockade controls murine West Nile virus infection. PLoS Pathog. 2009;5:e1000610. doi: 10.1371/journal.ppat.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank I, DePinho RA, Brenner MB, Cassimeris J, Alt FW, Chess L. A functional T3 molecule associated with a novel heterodimer on the surface of immature human thymocytes. Nature. 1986;322:179–181. doi: 10.1038/322179a0. [DOI] [PubMed] [Google Scholar]
- Ben-Nathan D, Huitinga I, Lustig S, van Rooijen N, Kobiler D. West Nile virus neuroinvasion and encephalitis induced by macrophage depletion in mice. Arch Virol. 1996;141:459–469. doi: 10.1007/BF01718310. [DOI] [PubMed] [Google Scholar]
- Diamond MS, Shrestha B, Mehlhop E, Sitati E, Engle M. Innate and adaptive immune responses determine protection against disseminated infection by West Nile encephalitis virus. Viral Immunol. 2003;16:259–278. doi: 10.1089/088282403322396082. [DOI] [PubMed] [Google Scholar]
- Diamond MS, Sitati EM, Friend LD, Higgs S, Shrestha B, Engle M. A critical role for induced IgM in the protection against West Nile virus infection. J Exp Med. 2003;198:1853–1862. doi: 10.1084/jem.20031223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiTirro J, Rhoades ER, Roberts AD, et al. Disruption of the cellular inflammatory response to Listeria monocytogenes infection in mice with disruptions in targeted genes. Infect Immun. 1998;66:2284–2289. doi: 10.1128/iai.66.5.2284-2289.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrick DA, Schrenzel MD, Mulvania T, Hsieh B, Ferlin WG, Lepper H. Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gammadelta T cells in vivo . Nature. 1995;373:255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- Flierl MA, Rittirsch D, Gao H, et al. Adverse functions of IL-17A in experimental sepsis. FASEB J. 2008;22:2198–2205. doi: 10.1096/fj.07-105221. [DOI] [PubMed] [Google Scholar]
- Glass WG, Lim JK, Cholera R, Pletnev AG, Gao JL, Murphy PM. Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J Exp Med. 2005;202:1087–1098. doi: 10.1084/jem.20042530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn YS, Ji XY, Woo SI, et al. Vgamma1+ gammadelta T cells reduce IL-10-producing CD4+CD25+ T cells in the lung of ovalbumin-sensitized and challenged mice. Immunol Lett. 2008;121:87–92. doi: 10.1016/j.imlet.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn YS, Taube C, Jin N, et al. Different potentials of gamma delta T cell subsets in regulating airway responsiveness: V gamma 1+ cells, but not V gamma 4+ cells, promote airway hyperreactivity, Th2 cytokines, and airway inflammation. J Immunol. 2004;172:2894–2902. doi: 10.4049/jimmunol.172.5.2894. [DOI] [PubMed] [Google Scholar]
- Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat Rev Immunol. 2003;3:233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- Hayday AC. Gammadelta cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- Hou W, Kang HS, Kim BS. Th17 cells enhance viral persistence and inhibit T cell cytotoxicity in a model of chronic virus infection. J Exp Med. 2009;206:313–328. doi: 10.1084/jem.20082030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SA, Graveline D, Newell MK, Born WK, O'Brien RL. V gamma 1+ T cells suppress and V gamma 4+ T cells promote susceptibility to coxsackievirus B3-induced myocarditis in mice. J Immunol. 2000;165:4174–4181. doi: 10.4049/jimmunol.165.8.4174. [DOI] [PubMed] [Google Scholar]
- Kang N, Tang L, Li X, et al. Identification and characterization of Foxp3(+) gammadelta T cells in mouse and human. Immunol Lett. 2009;125:105–113. doi: 10.1016/j.imlet.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Lanciotti RS, Kerst AJ, Nasci RS, et al. Rapid detection of West Nile virus from human clinical specimens, field- collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki G, Yamada H, Kishihara K, Yoshikai Y, Nomoto K. Mechanism of murine Vgamma1+ gamma delta T cell-mediated innate immune response against Listeria monocytogenes infection. Eur J Immunol. 2002;32:928–935. doi: 10.1002/1521-4141(200204)32:4<928::AID-IMMU928>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Murray KO, Walker C, Gould E. The virology, epidemiology, and clinical impact of West Nile virus: a decade of advancements in research since its introduction into the Western Hemisphere. Epidemiol Infect. 2011:1–11. doi: 10.1017/S0950268811000185. [DOI] [PubMed] [Google Scholar]
- Petersen LR, Hayes EB. West Nile virus in the Americas. Med Clin North Am. 2008;92:1307–1322. doi: 10.1016/j.mcna.2008.07.004. ix. [DOI] [PubMed] [Google Scholar]
- Phares TW, Kean RB, Mikheeva T, Hooper DC. Regional differences in blood-brain barrier permeability changes and inflammation in the apathogenic clearance of virus from the central nervous system. J Immunol. 2006;176:7666–7675. doi: 10.4049/jimmunol.176.12.7666. [DOI] [PubMed] [Google Scholar]
- Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O'Brien RL. Exacerbation of Collagen-Induced Arthritis by Oligoclonal, IL-17-Producing {gamma}{delta} T Cells. J Immunol. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson BA, Armbrustmacher V. West Nile encephalitis: the neuropathology of four fatalities. Ann N Y Acad Sci. 2001;951:172–178. [PubMed] [Google Scholar]
- Schneider BS, McGee CE, Jordan JM, Stevenson HL, Soong L, Higgs S. Prior exposure to uninfected mosquitoes enhances mortality in naturally-transmitted West Nile virus infection. PLoS One. 2007;2:e1171. doi: 10.1371/journal.pone.0001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha B, Gottlieb D, Diamond MS. Infection and injury of neurons by West Nile encephalitis virus. J Virol. 2003;77:13203–13213. doi: 10.1128/JVI.77.24.13203-13213.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Tesh RB, Siirin M, Guzman H, et al. Persistent West Nile virus infection in the golden hamster: studies on its mechanism and possible implications for other flavivirus infections. J Infect Dis. 2005;192:287–295. doi: 10.1086/431153. [DOI] [PubMed] [Google Scholar]
- Wang L, Das H, Kamath A, Bukowski JF. Human V gamma 2V delta 2 T cells produce IFN-gamma and TNF-alpha with an on/off/on cycling pattern in response to live bacterial products. J Immunol. 2001;167:6195–6201. doi: 10.4049/jimmunol.167.11.6195. [DOI] [PubMed] [Google Scholar]
- Wang L, Kamath A, Das H, Li L, Bukowski JF. Antibacterial effect of human V gamma 2V delta 2 T cells in vivo. J Clin Invest. 2001;108:1349–1357. doi: 10.1172/JCI13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- Wang T, Scully E, Yin Z, et al. IFN-gamma-producing gammadelta T cells help control murine West Nile virus infection. J Immunol. 2003;171:2524–2531. doi: 10.4049/jimmunol.171.5.2524. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lobigs M, Lee E, Mullbacher A. CD8+ T cells mediate recovery and immunopathology in West Nile virus encephalitis. J Virol. 2003;77:13323–13334. doi: 10.1128/JVI.77.24.13323-13334.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- Welte T, Lamb J, Anderson JF, Born WK, O'Brien RL, Wang T. Role of two distinct gammadelta T cell subsets during West Nile virus infection. FEMS Immunol Med Microbiol. 2008;53:275–283. doi: 10.1111/j.1574-695X.2008.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao SY, Guzman H, Zhang H, Travassos da Rosa AP, Tesh RB. West Nile virus infection in the golden hamster (Mesocricetus auratus): a model for West Nile encephalitis. Emerg Infect Dis. 2001;7:714–721. doi: 10.3201/eid0704.010420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Yang Y, Qiu G, et al. c-Maf regulates IL-10 expression during Th17 polarization. J Immunol. 2009;182:6226–6236. doi: 10.4049/jimmunol.0900123. [DOI] [PMC free article] [PubMed] [Google Scholar]