Abstract
Background
Schistosoma (S.) haematobium infection is a common cause of genital morbidity in adult women. Ova in the genital mucosal lining may cause lesions, bleeding, pain, discharge, and the damaged surfaces may pose a risk for HIV. In a heterogeneous schistosomiasis endemic area in South Africa, we sought to investigate if young girls had genital symptoms and if this was associated with urinary S. haematobium.
Methodology
In a cross-sectional study of 18 randomly chosen primary schools, we included 1057 schoolgirls between the age of 10 and 12 years. We interviewed assenting girls, whose parents had consented to their participation and examined three urines from each of them for schistosome ova.
Principal findings
One third of the girls reported to have a history of genital symptoms. Prior schistosomal infection was reported by 22% (226/1020), this was associated with current genital symptoms (p<0.001). In regression analysis the genital symptoms were significantly associated both with urinary schistosomiasis (p<0.001) and water contact (p<0.001).
Conclusions
Even before sexually active age, a relatively large proportion of the participating girls had similar genital symptoms to those reported for adult genital schistosomiasis previously. Anti-schistosomal treatment should be considered at a young age in order to prevent chronic genital damage and secondary infections such as HIV, sexually transmitted diseases and other super-infections.
Author Summary
Urogenital schistosomiasis (Bilharzia) is a common cause of gynecological disease in adult women. Reports to date indicate that genital lesions in adults become chronic and that the damages make women susceptible to HIV. This is the first study on urogenital schistosomiasis in pre-pubertal girls. We interviewed girls aged 10 to 12 years of age for urinary and gynecological symptoms. The research assistants did not know the schistosomiasis infection status in the school or the individuals. We collected three urines that were examined for schistosome eggs. We found that a significantly increased number of girls with urinary schistosomiasis have stinking, bloody discharge, ulcers, tumors and a burning sensation in their genitals. This indicates that gynecological damages due to schistosomiasis start before sexual activity, and before menstruation. By preventing urogenital schistosomiasis in girls we may have an innovative opportunity to reduce teenage HIV transmission and gynecological disease. This study presents a new aspect of a neglected disease affecting more than 100 million females, long overdue for mass intervention.
Introduction
Urogenital schistosomiasis causes gynecological morbidity in adult women [1], [2]. Schistosoma (S.) haematobium is primarily known for its effect on the urinary tract, but in endemic areas schistosomiasis may be the most common cause of genital morbidity and mucosal lesions [3]. An estimated 390 million females are at risk of schistosomiasis infection [4], [5]. It is second only to malaria in terms of public health impact of the parasitic diseases, with more than 100 million females infected, 85% of them live in rural parts of Africa.
Previous studies on urogenital schistosomiasis have been conducted in adult women of childbearing age. S. haematobium ova when deposited in the female reproductive tract seem to be equally distributed in the different genital parts, but are most commonly identified in the cervix and the vagina [6], [7], [8], [9]. Both viable and dead ova may cause tissue reactions, morbidity and symptoms long after contact with infested waters [10], [11]. The disease may manifest itself in both the genital and urinary tract and may be found exclusively in the genitals [6], [12]. In young girls there have only been a few case reports, hypothesizing that the pre-pubertal predilection site is in the vulva [7], [8], [9], [13], [14]. This may partly be because gynecological inspections are not prioritized in rural areas, controversial in virgins, but also because the causal relationship between schistosomiasis and genital lesions in young females has not been explored on a large scale [14], [15].
It has been hypothesized that female genital schistosomiasis poses an increased risk to secondary infections such as human papillomavirus and other STDs. Most importantly, these women have been found to have significantly more HIV [16], [17], [18], [19]. In the wake of the HIV epidemic and a realistic prospect of successful anti-schistosomal mass-treatment programs this study sought to explore if girls before sexual debut had signs of genital disease [20]. In Ugu District, South Africa the study aimed to explore the association between gynecologic symptoms and urinary S. haematobium in young girls.
Methods
Study design and participants
We carried out a cross-sectional study in 18 primary schools, which were randomized for inclusion from 309 primary schools in the area. We invited all girls aged 10–12 years in the included schools. Girls who were absent on the days of the invitations were excluded, as were girls with serious illnesses, or if their guardians or they refused.
Setting
The schools were visited between September 2009 and November 2010 in the predominantly rural Ugu District, KwaZulu Natal, South Africa, an S. haematobium endemic area, which covers 5866 km2 (Figure 1). It has an estimated population of 700 000 almost exclusively isiZulu speaking people, 84% reside in the rural areas, 51% are below the age of 20 years and 55% are female [21].
Figure 1. Map of Ugu district in South Africa.
The coastal areas are inhabited by the more affluent and the schools here were excluded.
Ethical considerations
The study was approved by The Biomedical Research Ethics Administration, UKZN 2009, Ref BF029/07; by the Department of Health, Pietermaritzburg, 2009, Ref HRKM010 - 08; by the Norwegian ethics committee, Ref 469 - 07066a1.2007.535, 2007, and the Departments of Health (2008) and Education (2009) in Ugu District. The Helsinki Declaration was followed. All members of the group, including students and research assistants had passed exams in Good Clinical Practice and signed Declarations of Confidentiality. The interviewers did not know the study subjects beforehand. Prior to the study there were information meetings for the parents, principals, school governing bodies and/or teachers of each school. Informed consent was given by each girl, and the parents/guardians signed consent forms. Identifying information was stored separate from the interview information (in separate towns). All were informed about their right to withdraw and to abstain from answering questions without negative consequences. In order to protect girls from stigmatization the disease was discussed in general terms as urinary schistosomiasis. Treatment for schistosomiasis was offered to all, and all were informed about possible side effects. A private psychologist was hired by the project to take care of referred cases as felt necessary; for psychological, practical and legal issues. When other medical help was required, the girls were referred to a government clinical facility, or offered private care if government services were unavailable. For ethical and community liaison reasons the project staff was not involved in any physical or psychological examinations after referral.
The interview
The interviews (30 minutes duration) were conducted face to face in isiZulu (the local language) by trained female fieldworkers. Questions were asked about recent (the last week) or previous urogenital symptoms of itch, burn, ulcers or tumors (swelling, lumps) in the genitals, malodorous discharge, color of discharge and feeling of a burning sensation in the genitals, as well as red urine, dysuria, urge and stress incontinence [1], [2]. They were also asked questions about confounders for bloody discharge (menstruation and red urine), malodorous discharge (sexual intercourse and sexual abuse) and for burning sensations in the genitals (sexual intercourse and dysuria). The girls in a pilot study (same age) had no concept of the local anatomy and we therefore decided to not include questions on exact localization of e.g. tumors. In case the child did not seem to understand, terms were explained and if she seemed too shy/uncomfortable to answer the interviewers were instructed to move to the next question. The discharge color was defined using a custom-made color chart. The study population was not familiar with the details of the questionnaire on beforehand. A water body was defined as a river, dam, lake, stream or pond. Each child was asked if she carried out any of seven specific water-related activities known in the study area (playing/swimming, washing/bathing, laundry, washing blankets, collecting water, fishing and crossing) [22]. Furthermore, there were validated demographic, social and psychological components in the questionnaire that will not be analyzed here.
Parasitology
The researchers aimed to visit each school at least three times. We obtained urine samples from each girl on three consecutive days between 10 am and 2 pm. After gentle tilting we deposited 10 ml of urine into a container with 1 ml methylate-formalin solution and the same week we investigated the specimens by microscopy [23]. After centrifuging, we transferred all of the precipitate onto microscopy slides; the last amount was washed with water before transferred. If the mean number of eggs of the three specimens was higher than 50 per 10 ml urine the infection was classified as high-intensity [24]. One stool sample per child was collected for Kato Katz and analyzed for Ascaris lumbricoides, Ancylostoma duodenale, Taenia solium, Trichuris trichiura and S. mansoni. If there was at least one ovum in a specimen it was defined as positive.
Data management and statistical analyses
Based on data from studies in adults we estimated the prevalence of genital schistosomiasis to be 30% and urinary schistosomiasis 40% [3]. We hypothesized that the expected prevalence of genital ulcers in the schistosomiasis exposed to be 9% and in the unexposed 4%. To detect a difference with a significance level of 5% and a power of 80%, the sample size would have to be 511 unexposed and 341 schistosomiasis exposed young women, in all 852 subjects. The information was recorded on paper; the personal information sheet was separated from the other information as soon as the record number had been secured. Data was entered into EpiData (interview) or Excel (urine) and subsequently exported into IBM SPSS version 19 (Chicago, Illinois, USA). Chi-square and odds ratios (OR) with 95% confidence interval (CI) were used to compare impacts of water contact or current urinary schistosomiasis infection on genital symptoms. In order to study the impact of other variables (for example menstruation or red urine), logistic regression analysis was applied with a 5% significance level; variables were included if the P-value in the crude association was less than 0.2 and if the Spearman rank correlation coefficient was below 0.7. When there were less than 10 cases, the variable was not included in regression analysis. The statistical analysis was computed using SPSS.
Results
Characteristics of the study group
Schools that were randomized for inclusion were visited in no particular order. We invited all pupils aged 10 to 12 years. All schools were visited several times in order to collect guardian consent forms and to find as many students as possible. The parents of 1241/1948 (64%) pupils in 18 schools provided consent. On the days we were in the schools we were able to include 1057 assenting girls. In the first 13 schools, where there was adequate time before exams, the consent forms were returned and signed by 92% (1109/1201) of the parents. The pupils were recruited from grades 1 to 7, median grade 5.
Menstruation, HIV testing and sexual history
Answers were recorded as missing if the child chose not to answer or did not understand the topics. Seven percent of the girls (71/1019) had started menstruating. Only 5% (51/981) said they had been tested for HIV. Three out of 980 (0.3%) knew they had HIV, as many as 495 said they did not know and 77 girls did not reply to this question. Less than one percent (7/1017) reported to have had intra-vaginal sex. However, two percent (22/953) reported to have been sexually abused. They were referred to psychosocial follow-up. All in all 24 of 1019 young girls had experienced voluntary or involuntary vaginal sex.
Parasitology
Out of the 1057 girls who were interviewed, 970 submitted at least one urine for examination, and out of these 791 submitted three urine samples. S. haematobium eggs were found in 32% (312/970) of the girls. High-intensity urinary infection was found in 28% (88/312) of these. In those who had ova in the urine, the mean intensity of infection was 52 eggs/10 ml urine (range 1–624/10 ml). Among the 658 girls with negative urine specimens, 79% (522) submitted three negative urine samples. There was neither any difference in urinary schistosomiasis infection intensity nor presence of symptoms between those who had submitted three urine samples versus one sample.
Symptoms
Thirty five percent reported to have had genital symptoms (356/1018), and as many as 17% (172/1008) reported genital symptoms the last week. Eighteen girls reported having a genital tumor or an ulcer this last week. Table 1 shows the association between urinary schistosomiasis and symptoms in girls. Controlled for confounders in multivariate analyses the table shows that urinary schistosomiasis remained associated with bloody discharge, a burning sensation in the genitals, genital ulcers, tumors and incontinence. Having had vaginal sex was not significantly associated with any of the symptoms; however the variable ‘vaginal sex’ was forced into the multivariate analyses. It did not influence the association between the symptoms and urinary schistosomiasis or water contact. Likewise, having soil-transmitted helminths did not influence the associations (data not shown) and only one person had S. mansoni. The discharge color was white in 51% (84/164) of the cases; cream color in 35% (58/164) and yellow in 9% (14/164). The discharge had streaks/traces of red in 13% (11/87) of the cases, light red in 66% (57/87) of the cases and an even lighter shade of red (light pink) in 9% (8/87). Patients with symptoms were referred but not investigated by the project.
Table 1. Association between the urogenital symptoms in rural 10–12 year old girls and urinary schistosomiasis.
| Symptoms and frequencies | In 298 urinary schistosomiasis positivea (%) | In 628 urinary schistosomiasis negative (%) | OR (95% CI)b | P | Adj. OR (95%CI)c | P |
| Bloody discharge | ||||||
| Never | 247 (83) | 608 (97) | 1.0 | 1.0 | ||
| Sometimes | 32 (11) | 13 (2) | 6.1(3.1–11.7) | <0.001 | 4.2 (2.1–8.5) | <0.001 |
| Always | 19 (6) | 7 (1) | 6.7 (2.8–16.1) | <0.001 | 3.3 (1.3–8.5) | 0.01 |
| Red urined | ||||||
| Never | 207 (69) | 568 (90) | 1.0 | 1 | ||
| Sometimes | 37 (12) | 34 (5) | 2.9 (1.8–4.9) | <0.001 | 2.4 (1.5–4.1) | 0.001 |
| This week | 54 (18) | 26 (4) | 5.7 (3.5–9.3) | <0.001 | 4.3 (2.5–7.2) | <0.001 |
| Malodorous discharge | ||||||
| Never | 254 (85) | 565 (90) | 1.0 | 1.0 | ||
| Sometimes | 20 (7) | 39 (6) | 1.1 (0.7–2.0) | 0.64 | 1.1 (0.6–2.0) | 0.69** |
| Always | 24 (8) | 24 (4) | 2.2 (1.2–4.0) | 0.007 | 2.2 (1.2–4.0) | 0.008 |
| Genital itch | ||||||
| Never | 237 (80) | 524 (83) | 1.0 | 1.0 | ||
| Sometimes | 39 (13) | 62 (10) | 1.4 (0.9–2.1) | 0.13 | 1.4 (0.9–2.2) | 0.12** |
| This week | 22 (7) | 42 (7) | 1.2 (0.7–2.0) | 0.59 | 1.2 (0.7–2.0) | 0.57** |
| Burning sensation in the genitals | ||||||
| Never | 243 (82) | 561 (89) | 1.0 | 1.0 | ||
| Sometimes | 34 (11) | 43 (7) | 1.8 (1.1–2.9) | 0.01 | 1.6 (1.0–2.6) | 0.05 |
| This week | 21 (7) | 24 (4) | 2.0 (1.1–3.7) | 0.02 | 1.9 (1.0–3.5) | 0.05 |
| Dysuria | ||||||
| Never | 220 (74) | 516 (82) | 1.0 | 1.0 | ||
| Sometimes | 55 (18) | 75 (12) | 1.7 (1.2–2.5) | 0.005 | 1.6 (1.1–2.3) | 0.02 |
| This week | 23 (8) | 37 (6) | 1.5 (0.9–2.5) | 0.17 | 1.3 (0.7–2.2) | 0.42** |
| Genital ulcer | ||||||
| Never | 266 (89) | 598 (95) | 1.0 | 1.0 | ||
| Sometimes | 27 (9) | 25 (4) | 2.4 (1.4–4.3) | 0.002 | 2.4 (1.4–4.3) | 0.002 |
| This week | 5 (2) | 5 (1) | 2.2 (0.6–7.8) | 0.20 | 2.2 (0.6–7.7) | 0.21** |
| Genital tumor | ||||||
| Never | 283 (95) | 613 (98) | 1.0 | 1.0 | ||
| Sometimes | 12 (4) | 13 (2) | 2.0 (0.9–4.4) | 0.09 | 2.0 (0.9–4.4) | 0.09 |
| This week | 3 (1) | 2 (0) | 3.2 (0.5–19.6) | 0.19 | 3.3 (0.5–19.6) | 0.19** |
| Urge incontinence | ||||||
| Never | 184 (62) | 443 (71) | 1.0 | 1.0 | ||
| Sometimes | 77 (26) | 109 (17) | 1.7 (1.2–2.4) | 0.002 | 1.7 (1.2–2.4) | 0.002 |
| This week | 37 (12) | 76 (12) | 1.2 (0.8–1.8) | 0.47 | 1.2 (0.8–1.8) | 0.44** |
| Stress incontinence | ||||||
| Never | 212 (71) | 530 (84) | 1.0 | 1.0 | ||
| Sometimes | 44 (15) | 66 (11) | 1.7 (1.1–2.5) | 0.02 | 1.7 (1.1–2.5) | 0.02 |
| This week | 42 (14) | 32 (5) | 3.3 (2.0–5.3) | <0.001 | 3.3 (2.0–5.3) | <0.001 |
Eight separate multivariate analyses.
Age was forced into each model and did not influence the results (data not shown).
The presence of at least one schistosome ova in any of the urine examined specimens.
Odds ratio (OR) with 95% confidence interval (CI).
Adjusted odds ratio, different confounding variables were included in each multivariate analysis for the specific genital symptom.
Red urine as seen by the child.
If recalculated as ‘ever had the symptom’ it is significantly associated with urinary schistosomiasis.
Nuances in urinary schistosomiasis negative and positive groups
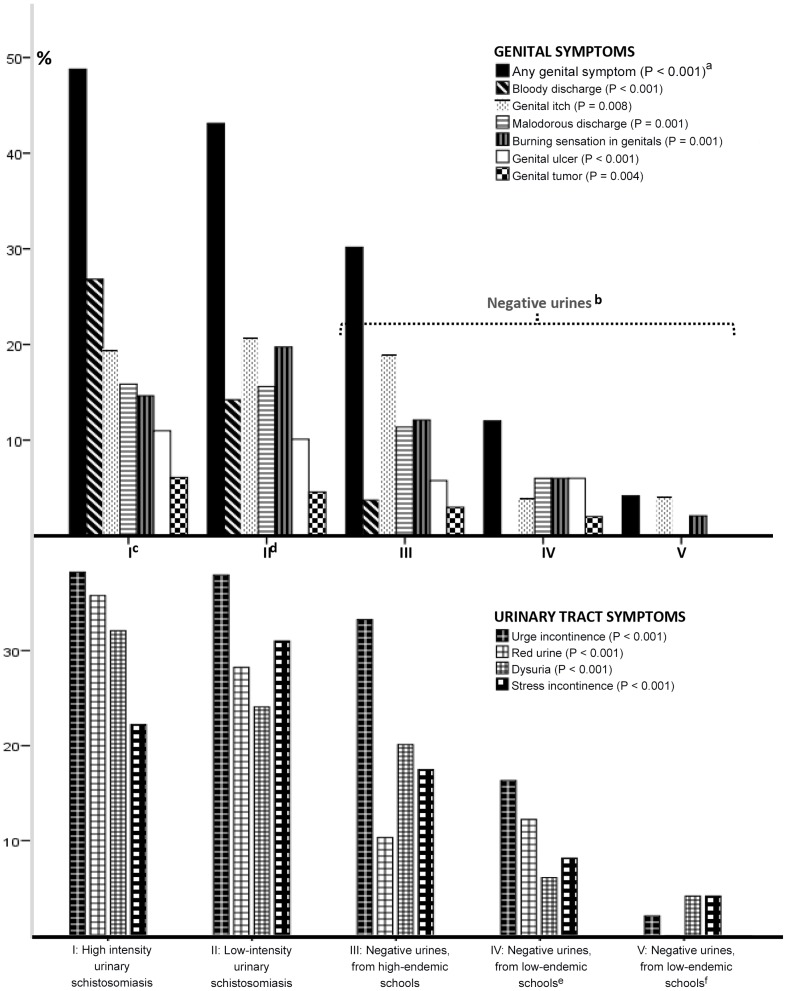
In order to explore the urinary negative girls in more detail they were first divided into two groups (Figure 2), those in high-endemic school versus those in low endemic. The pupils from the low-endemic schools were further split into two, those who admitted water body contact and those who did not. Figure 2 shows that symptoms are significantly more common in those that have a high intensity of infection. The figure also highlights that low-endemic schools (the ‘most negative’ in the district) have a low prevalence of genital symptoms. Amongst the girls with high-intensity schistosomiasis almost 50% had genital symptoms, compared to less than 5% in the negative girls who lived in non-endemic areas and had no water contact. These girls denied having genital tumors, ulcers, bloody or smelly discharge. Bloody discharge was found in the high-endemic schools only, notably also in those individuals of these schools who were negative for schistosomiasis in three urines (Figure 1, category III). Urge incontinence and genital itch were both relatively constant in the high-endemic schools, but significantly higher than in the low-endemic schools.
Figure 2. Genital and urinary symptoms in girls of two S. haematobium positive groups and three negative risk groups.
aLikelihood ratio. bThree urines investigated for S. haematobium ova, all were negative. cMore than 50 S. haematobium ova per 10 ml urine. d1–49 ova per 10 ml urine. eThese girls have water body contact (e.g. river, dam or lake). fThese girls deny water body contact.
History of water contact
We found a significant association between water contact and all the listed symptoms (data not shown). However, among the 364 who denied water contact, 21% (76) had urinary schistosomiasis. Sixty three percent of the girls reported water contact (667/1057), among these 606 submitted urines and 39% (236/606) had S. haematobium ova in urine. Among the girls with three negative urines, 54% (281/522) reported water contact. These reported significantly more genital symptoms the last week than their peers without water contact (p = 0.001).
Prior urinary infection with S. haematobium and symptoms
Twenty two percent of the young girls (226/1020) reported having had urinary schistosomiasis previously. This was significantly associated with current genital symptoms such as bloody discharge (Chi square, p<0.001), malodorous discharge (p<0.001), genital itch (p<0.017) and genital ulcer (p = 0.001). Furthermore 29% (251/853) knew of a family member who had had urinary schistosomiasis or red urine and these factors too were associated with all the queried symptoms (p≤0.002), except genital tumor (p = 0.08).
Twelve percent (129/1057) reported that they had been treated for schistosomiasis previously. Girls who said that they had not been treated had significantly more urinary schistosomiasis than those who had been treated (p<0.001), but not more symptoms (sample size small and p-values range from p = 0.43 to p = 1.0).
Discussion
In an S. haematobium endemic area girls aged 10 to 12 years with schistosomiasis had significantly more often unpleasant symptoms such as genital ulcers, bloody discharge, malodorous white to yellow cultured discharge, genital itch or tumors than those without this infection. Even before sexual debut and independent of menstruation more than 40% of girls with S. haematobium ova in urine reported having had gynecological symptoms previously, one third reported having it the last week or ‘always’. Girls living in endemic areas without urinary schistosomiasis also had significantly more genital symptoms than their peers in low-endemic schools. This study shows that urinary schistosomiasis, water contact, history of red urine and family history of schistosomiasis (‘Isichenene’ in Zulu) are also associated with the full range of symptoms. As shown in adults previously, the history of water contact was an excellent predictor for genital symptoms also in girls [3], [7], [8], [9], [25]. The girls who had been treated for schistosomiasis previously had the same symptoms as those who denied having received treatment.
In adults the grainy sandy patches have been found to be diagnostic of S. haematobium infection and are significantly associated with discharge [1], [2], [3]. However, the findings in this young population could not be corroborated by a clinical examination. These results are therefore circumstantial, since the gynecological symptoms are not specific for genital schistosomiasis. Without the physical examination and intravaginal tests we cannot confirm schistosomiasis as the etiological factor. Furthermore, vaginal discharge, ulcers and genital itch may have other causes that were not controlled for in this study, such as the sexually transmitted diseases, atopic, irrigative dermatitis or other dermatoses like psoriasis or lichen sclerosis, lice, scabies, or non-specific etiology [26], [27]. Furthermore, one cannot preclude that the current symptoms, although caused by infection with S. haematobium in the lower genital tract may make the genital mucosa more susceptible to super-infections by other agents such as bacterial infections, which in turn may cause the reported symptoms. Likewise, the association between water contact and symptoms may be influenced by social and other practices. Poor perinea hygiene may be more common in a group that has limited access to water; and cultural cleansing rituals may also be hypothetical reasons for the association between water contact and symptoms [28].
In this study three urines were collected and the presence of schistosome ova defined the urinary schistosomiasis positive group. However, this study confirms that even urinary negative cases in endemic areas have gynecological morbidity. Hence the prevalence is most likely higher in this population. A more sensitive diagnostic method, such as antigen detection or PCR, would likely have made the reported finding more apparent, though this was not possible in our study [29].
Some girls denied having had water contact, but were found to still have S. haematobium ova in urine. Girls may be shy, worried about repercussions or not be able to differentiate between urinary and genital symptoms. Some girls were ignorant of some phenomena in the questionnaire such as menstruation or discharge. The interviewers – all female – were trained to explain the differences, however information sessions using dolls followed by more thorough questioning of genital symptoms could perhaps have produced more reliable answers and less under-reporting. This was not done in the present study. Further, the most reliable method to determine water contact is by direct observation, although many schistosomiasis studies have used self-reported water body data [30], [31].
It is well documented that many adult women may have genital schistosomiasis even without having detectable schistosome ova in the urine [3], [12], [32]. Urine investigations may therefore be of limited use in the diagnosis of genital schistosomiasis. Studies have shown that female genital schistosomiasis may cause pathologic blood vessel morphology and fragile blood vessels that may lead to mucosal bleeding [3], [33], [34]. Bloody discharge may be a result of this. Inter-menstrual bleeding, post-coital bleeding, malodorous and abnormally colored discharge and genital itch have been found to be associated with S. haematobium ova in the genitals of adults, even after correcting for sexually transmitted diseases [1], [19], [25], [34].
The girls in this study report the same symptoms as adult women in previous studies [1], [2]. One may fear that young children's genital mucosa are already imbued with calcified S. haematobium ova [16], [25], [35]. Childhood water contact may start very early and these girls may have had S. haematobium infection for several years [36]. One study found that such lesions were refractory to treatment in adults, whereas treatment received before the age of 20 years seemed to offer some protection against genital mucosal pathology [25]. Even so, the morbidity prevalence levels were unacceptably high even in those who had received treatment once in childhood and treatment may have to be given in infanthood in order to prevent genital damage [36]. Furthermore, siblings and people sharing the same water bodies should be given simultaneous treatment in order to reduce re-infection rate and intensity; treatment should be given in low-transmission seasons, and the effect should be secured by several rounds [37].
At the present time there are no suitable tools for the diagnosis of genital schistosomiasis in girls. Abnormal malodorous or bloody genital discharge are mucosal symptoms [2]. In our young study population gynecological investigations were not possible for cultural and technical reasons [3]. Further studies are needed to triangulate the analyses of (1) symptoms and (2) water contact/family history with (3) the objective mucosal findings in genital schistosomiasis.
For the rural clinician history taking and urine analyses are simpler sources of information than gynecological examinations, and especially so in virgins. The findings in this study suggest that young girls in S. haematobium endemic areas have gynecological symptoms as a result of schistosoma infection. Mucosal damages may be present as these young girls enter into their first sexual relationships, making them particularly susceptible to HIV or human papillomavirus infection [16], [17], [36]. Further studies are needed to explore the effects of treatment on the prolific symptomatic manifestations and on decreasing the susceptibility to super-infections before sexual debut.
Supporting Information
Enclosed is a Strobe checklist for cross-sectional studies.
(DOC)
Acknowledgments
We thank the children who participated in the study and their carers. We are grateful for help from Professor in psychology I. Petersen, University of KwaZulu-Natal, Clinical social workers and family therapists G. Ottesen and U. Fauske, Family Therapy Unit Ullevaal, Child and Adolescent Psychiatric Clinic, Oslo University Hospital, Norway. We are thankful for much good advice and data assistance from R.F. Manyaira. We thank F.O. Pettersen and L. Sandvik for reviewing the manuscript. We also wish to thank V.P. Mkhiva, T.P. Ziqubu, M.P. Majiya, T.C. Madwe, N.H. Lubanyana, N.P. Lubanyana, and Z.H. Chiliza for adapting the interview to the local decorum, interviews and laboratory work. This study could not have been done without their effort and hard work.
Funding Statement
The study was funded by Centre for Imported and Tropical Diseases, Oslo University Hospital, Oslo University Hospital Ullevaal (VIRUUS), Ship owner Tom Wilhelmsen Foundation (http://legatsiden.no), S.G. Sønneland Foundation (http://legatsiden.no) and General Travel Scholarships (http://legatsiden.no). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Leutscher PD, Ravaoalimalala VE, Raharisolo C, Ramarokoto CE, Rasendramino M, et al. (1998) Clinical findings in female genital schistosomiasis in Madagascar. Trop Med Int Health 3: 327–332. [DOI] [PubMed] [Google Scholar]
- 2. Kjetland EF, Kurewa EN, Ndhlovu PD, Gwanzura L, Mduluza T, et al. (2008) Female genital schistosomiasis - a differential diagnosis to sexually transmitted disease: Genital itch and vaginal discharge as indicators of genital S. haematobium morbidity in a cross-sectional study in endemic rural Zimbabwe. Tropical medicine and international health 13: 1509–1517. [DOI] [PubMed] [Google Scholar]
- 3. Kjetland EF, Ndhlovu PD, Mduluza T, Gomo E, Gwanzura L, et al. (2005) Simple clinical manifestations of genital Schistosoma haematobium infection in rural Zimbabwean women. American Journal of Tropical Medicine and Hygiene 72: 311–319. [PubMed] [Google Scholar]
- 4. Hotez PJ, Fenwick A, Kjetland EF (2009) Africa's 32 Cents Solution for HIV/AIDS. PLoS Negl Trop Dis 3: e430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J (2006) Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infectious Diseases 6: 411–425. [DOI] [PubMed] [Google Scholar]
- 6. Kjetland EF, Leutscher PD, Ndhlovu PD (2012) A review of female genital schistosomiasis. Trends in Parasitology 28: 58–65. [DOI] [PubMed] [Google Scholar]
- 7. Berry A (1966) A cytopathological and histopathological study of bilharziasis of the female genital tract. Path Bact 91: 325–337. [DOI] [PubMed] [Google Scholar]
- 8. Badawy AH (1962) Schistosomiasis of the cervix. Br Med J 1: 369–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Charlewood GP, Shippel S, Renton H (1949) Schistosomiasis in gynaecology. J Obstet Gynaecol Br Emp 56: 367–385. [DOI] [PubMed] [Google Scholar]
- 10. Jourdan PM, Holmen SD, Gundersen SG, Roald B, Kjetland EF (2011) HIV target cells in Schistosoma haematobium-infected female genital mucosa. American journal of tropical medicine and hygiene 85: 1060–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jourdan PM, Roald B, Poggensee G, Gundersen SG, Kjetland EF (2011) Increased vascularity in cervicovaginal mucosa with Schistosoma haematobium infection. PLoS Negl Trop Dis 5: e1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Poggensee G, Kiwelu I, Saria M, Richter J, Krantz I, et al. (1998) Schistosomiasis of the lower reproductive tract without egg excretion in urine. American Journal of Tropical Medicine and Hygiene 59: 782–783. [DOI] [PubMed] [Google Scholar]
- 13. Feldmeier H, Poggensee G, Krantz I (1998) Puberty and age intensity profiles in schistosome infections: another hypothesis. Parasitology Today 14: 435. [DOI] [PubMed] [Google Scholar]
- 14. Savioli L, Gabrieli A, Neve H (1990) Vulvar Schistosomiasis haematobium lesion treated with Praziquantel. Trop Doc 20: 45–46. [DOI] [PubMed] [Google Scholar]
- 15. Laven JS, Vleugels MP, Dofferhoff AS, Bloembergen P (1998) Schistosomiasis haematobium as a cause of vulvar hypertrophy. Eur J Obstet Gynecol Reprod Biol 79: 213–216. [DOI] [PubMed] [Google Scholar]
- 16. Downs JA, Mguta C, Kaatano GM, Mitchell KB, Bang H, et al. (2011) Urogenital schistosomiasis in women of reproductive age in Tanzania's Lake Victoria region. American Journal of Tropical Medicine and Hygiene 84: 364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kjetland EF, Ndhlovu PD, Gomo E, Mduluza T, Midzi N, et al. (2006) Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS 20: 593–600. [DOI] [PubMed] [Google Scholar]
- 18. Mosunjac MB, Tadros T, Beach R, Majmudar B (2003) Cervical schistosomiasis, human papilloma virus (HPV), and human immunodeficiency virus (HIV): a dangerous coexistence or coincidence? Gynecol Oncol 90: 211–214. [DOI] [PubMed] [Google Scholar]
- 19. Siddappa NP, Hemashettar G, Shanmuganathan V, Semenya AA, Sweeney ED, et al. (2011) Schistosoma mansoni enhances host susceptibility to mucosal but not intravenous challenge by R5 Clade C SHIV. PLoS Negl Trop Dis 5: e1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fenwick A, Webster JP, Bosque-Oliva E, Blair L, Fleming FM, et al. (2009) The Schistosomiasis Control Initiative (SCI): rationale, development and implementation from 2002–2008. Parasitology 1–12. [DOI] [PubMed] [Google Scholar]
- 21.Statistics South Africa (2001) Census: Primary tables KwaZulu-Natal. Report No: 03-02-08.
- 22. Kvalsvig JD (1986) The effects of Schistosomiasis haematobium on the activity of school children. J Trop Med Hyg 89: 85–90. [PubMed] [Google Scholar]
- 23. Thomassen Morgas DE, Kvalsvig JD, Gundersen SG, Taylor M, Kjetland EF (2010) Schistosomiasis and water-related practices in school girls in rural KwaZulu-Natal, South Africa. Southern Afr J Epidemiol Infect 25: 30–33. [Google Scholar]
- 24.WHO editor (2011) Helminth control in school-age children. A guide for managers of control programmes. Second edition ed: Preventive Chemotherapy and Transmission Control (PCT), Department of Control of Neglected Tropical Diseases (NTD), World Health Organization, 20, Avenue Appia, 1211 Geneva 27, Switzerland.
- 25. Kjetland EF, Ndhlovu PD, Kurewa EN, Midzi N, Gomo E, et al. (2008) Prevention of gynecologic contact bleeding and genital sandy patches by childhood anti-schistosomal treatment. American Journal of Tropical Medicine and Hygiene 79: 79–83. [PubMed] [Google Scholar]
- 26. Fischer GO (2001) Vulval disease in pre-pubertal girls. Australas J Dermatol 42: 225–234; quiz, 235–226. [DOI] [PubMed] [Google Scholar]
- 27. Jaquiery A, Stylianopoulos A, Hogg G, Grover S (1999) Vulvovaginitis: clinical features, aetiology, and microbiology of the genital tract. Arch Dis Child 81: 64–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hull T, Hilber AM, Chersich MF, Bagnol B, Prohmmo A, et al. (2011) Prevalence, motivations, and adverse effects of vaginal practices in Africa and Asia: findings from a multicountry household survey. J Womens Health (Larchmt) 20: 1097–1109. [DOI] [PubMed] [Google Scholar]
- 29. Stothard JR, Sousa-Figueiredo JC, Standley C, Van Dam GJ, Knopp S, et al. (2009) An evaluation of urine-CCA strip test and fingerprick blood SEA-ELISA for detection of urinary schistosomiasis in schoolchildren in Zanzibar. Acta Tropica 111: 64–70. [DOI] [PubMed] [Google Scholar]
- 30. Utzinger J, N'Goran EK, Tanner M, Lengeler C (2000) Simple anamnestic questions and recalled water-contact patterns for self-diagnosis of Schistosoma mansoni infection among schoolchildren in western Cote d'Ivoire. American Journal of Tropical Medicine and Hygiene 62: 649–655. [DOI] [PubMed] [Google Scholar]
- 31. Lengeler C, Utzinger J, Tanner M (2002) Screening for schistosomiasis with questionnaires. Trends Parasitol 18: 375–377. [DOI] [PubMed] [Google Scholar]
- 32. Bland KG, Gelfand M (1970) The effects of schistosomiasis on the cervix uteri in the African female. The Journal of obstetrics and gynaecology of the British Commonwealth 77: 1127–1131. [DOI] [PubMed] [Google Scholar]
- 33. Jourdan PM, Poggensee G, Gundersen SG, Roald B, Kjetland EF (2011) Increased cervicovaginal vascularity in association with Schistosoma haematobium ova. PLoS Negl Trop Dis 5: e1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Poggensee G, Kiwelu I, Weger V, Goppner D, Diedrich T, et al. (2000) Female genital schistosomiasis of the lower genital tract: prevalence and disease-associated morbidity in Northern Tanzania. J Infect Dis 181: 1210–1213. [DOI] [PubMed] [Google Scholar]
- 35. Stothard JR (2012) Female genital schistosomiasis - icebergs of morbidity ahead? Trends in Parasitology 28: 174–175. [DOI] [PubMed] [Google Scholar]
- 36. Stothard JR, Gabrielli AF (2007) Schistosomiasis in African infants and preschool children: to treat or not to treat? Trends Parasitol 23: 83–86. [DOI] [PubMed] [Google Scholar]
- 37.WHO (1998) Report of the WHO informal consultation on schistosomiasis control. 1–45.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Enclosed is a Strobe checklist for cross-sectional studies.
(DOC)