Abstract
During the past two decades, the exploration of function of two incretin hormones, namely glucagon-like peptide-1 (GLP-1) and gastric inhibitory peptide (GIP), has led to the development of two categories of novel therapeutic agents for diabetes and its complications, known as GLP-1 receptor (GLP-1R) agonists and DPP-IV inhibitors. Mechanisms underlying the function of GLP-1, however, still need to be further explored. GLP-1 not only functions as an incretin hormone in stimulating insulin secretion in response to nutritional, hormonal and neuronal stimulations, but also acts as an “insulin-like” factor in β-cell and extra-pancreatic organs. In addition to these insulinotropic and insulinomimetic effects, GLP-1 was shown to exert its protective effect in β-cell by repressing the expression of TxNIP, a mediator of glucolipotoxicity. A number of recent studies have shown that the Wnt signaling pathway effector, the bipartite transcription factor β-catenin/TCF, controls not only the production of GLP-1, but also the function of GLP-1. Furthermore, previously assumed “degradation” products of GLP-1(7–36)amide, including GLP-1(9–36)amide and GLP-1(28–36)amide, have been shown to exert beneficial effect in pancreas and extra-pancreatic tissues or cell lineages. Here we summarized our current knowledge on the metabolic, proliferative and protective effects of GLP-1(7–36)amide and its cleavage fragments, mainly focusing on pancreatic β-cells and the involvement of the Wnt signaling pathway effector β-catenin.
Keywords: GLP-1, TCF7L2, TxNIP, Wnt signaling pathway, β-catenin
Introduction
The proglucagon gene (gcg) is expressed in pancreatic α-cells, L-type endocrine cells in the gut, and certain neuronal cells in the brainstem.1,2 The three organs express the same gcg cDNA, which encodes the identical pro-hormone known as proglucagon. Tissue specific posttranslational processes lead to the generation of different profiles of proglucagon derived peptides, including the three major peptide hormones known as glucagon, glucagon-like peptide-1 (GLP-1) and glucagon-like peptide-2 (GLP-2). Glucagon is mainly produced in the pancreas, while the gut produces GLP-1 and GLP-2, but not glucagon. The pancreatic glucagon is a major counter-regulatory hormone of insulin in regulating glucose homeostasis. The gut produced GLP-1, however, is an incretin, exerting an opposite effect with glucagon via stimulating insulin secretion in a glucose concentration dependent manner. GLP-2 is known as a growth factor for small intestinal epithelium.1,2
During the past two decades, the exploration of mechanisms underlying the function of GLP-1 and another incretin, gastric inhibitory polypeptide (GIP), has led to the development of two categories of novel therapeutic agents for diabetes and potentially its complications, namely GLP-1 receptor (GLP-1R) agonists and DPP-IV inhibitors.3,4 Extensive investigations have shown that in addition to functioning as an incretin, GLP-1 possesses “insulin-like” or insulinomimetic effect on pancreatic β-cells and extra-pancreatic tissues or cell lineages. Furthermore, previously assumed “degradation” fragments of GLP-1, namely GLP-1(9–36)amide and GLP-1(28–36)amide, were shown to possess certain beneficial effects in both in vitro and in vivo settings.5-9 In addition to its insulinotropic and insulinomimetic effects, GLP-1 was also shown to exert protective effects in pancreatic β-cells, exemplified by reducing the levels of TxNIP, a major mediator of glucotoxicity.10 In exploring mechanisms underlying GLP-1 function, a few recent studies have demonstrated that the Wnt signaling pathway effector cat/TCF, a complex formed by free β-catenin (β-cat) and a member of the T-cell factor (TCF) family, regulates not only gut gcg mRNA transcription and GLP-1 production, but also GLP-1 function in pancreatic β-cells and brain neuronal cells.11-13 Here we present our current knowledge on GLP-1 and its “degradation” products, and summarize our current understanding on the metabolic, proliferative and protective effects of GLP-1. We mainly focus on GLP-1 and its “degradation” fragments in pancreatic β-cells, as well as the involvement of the Wnt signaling pathway effector β-cat. The involvement of another Wnt pathway effector, the diabetes risk gene TCF7L2, has been summarized elsewhere by our group and by others.14-17 For the function of GLP-1 in extra-pancreatic organs and tissues, please refer to a large body of excellent recent review articles elsewhere.7,18
GLP-1 and Its “Degradation” Fragments
GLP-1 is encoded by gcg and produced by intestinal endocrine L cells throughout the entire small intestine and colon, with the highest levels within the distal ileum.19 Gcg also encodes glucagon, produced in and secreted by pancreatic α-cells. In addition, it encodes GLP-2 in the gut, which functions as a growth factor for the small intestinal epithelium.20Figure 1A shows the overall structure of the prohormone that is encoded by gcg and the cleavage sites of the two prohormone convertases, PC2 and PC1/3. In pancreatic α-cells, the main products of the cleavage include glucagon, glicentin-related pancreatic polypeptide (GRPP), intervening peptide-1 (IP1) and major proglucagon fragment (MPGF) (Fig. 1A). During embryonic developmental stages or after pancreatic islets encounter certain stress, small amounts of GLP-1 can be detected in pancreatic α-cells.21 In both the intestine and the brainstem, the post-translational products include glicentin, GLP-1, GLP-2, GRPP, oxyntomodulin and the intervening peptide-2 (IP2) (Fig. 1A). GLP-1(7–37) and GLP-1(7–36)amide are the two biologically active forms of the incretin. For convenience, the term GLP-1(7–36) will be utilized to represent both of them hereafter. The half life of GLP-1(7–36) is very short in circulation. This is due to the presence of the degrading enzymes dipeptidyl peptidase IV (DPP-IV) and neutral endopeptidase NEP24.11 (neprilysin or CD10).22 The cleavage of GLP-1(7–36) by DPP-IV leads to the generation of GLP-1(9–36), while the cleavage of GLP-1(7–36) or GLP-1(9–36) by NEP24.11 leads to the generation of GLP-1(28–36), the C-terminal nonapeptide FIAWLVKGR (Fig. 1B). Although inhibition of either DPP-IV or NEP24.11 has been demonstrated to enhance the incretin effect of GLP-1;23,24 GLP-1(9–36) and GLP-1(28–36) have been shown to exert beneficial effects on glucose and metabolic homeostasis,5,7-9,25 indicating that they have the potential to be developed as therapeutic agents for the treatment of diabetes or other metabolic disorders.
Figure 1. Proglucagon and its cleavage products. (A) The gcg gene encode proglucagon, a pro-hormone with 160 amino acid residues (top panel). This pro-hormone contains three PC2 and four PC1/3 cleavage sites. A schematic presentation of the cleavage products of proglucagon in the pancreas (middle panel) and in the intestine and brain (bottom panel). (B) Amino acid sequences of GLP-1(7–36)amide, GLP-1(9–36)amide and GLP-1(28–36)amide. The cleavage sites for DPP-IV and NEP24.11 are indicated with arrows. GRPP, glycentin related polypeptide; IP1 and IP2, intervening peptide 1 and 2; MPGF, major proglucagon fragment; DPP-IV, dipeptidyl peptidase-4; NEP 24.11, neutral endopeptidase 24.11.
Insulinotropic Effect of GLP-1
Postprandial GLP-1 secretion or GLP-1(7–36)/exendin-4 administration leads to a rapid stimulation of insulin secretion, which is mediated through the G-protein coupled receptor GLP-1R in the presence of high levels of glucose. This involves the closure of ATP-sensitive K+ channels (KATP), followed by cell membrane depolarization, elevation of the intracellular Ca2+ level and Ca2+-induced insulin secretion.2,26,27 Glucose is required for GLP-1-mediated activation of insulin secretion. Twenty years ago, Holz and colleagues have demonstrated that GLP-1 confers glucose sensitivity to glucose-resistant β-cells, a phenomenon they termed as glucose competence.28 The α-subunit of the G protein that is coupled by GLP-1R is a Gs, which mediates the activation of adenylyl cyclase (AC), the elevation of cytoplasmic cAMP level and the stimulation of Protein Kinase A (PKA) signaling pathway. In addition, phosphatidylinositol 3-kinase (PI3K)/Protein Kinase B (PKB/Akt), mitogen-activated protein kinase (MEK)/extracellular regulated kinase (ERK), as well as epidermal growth factor receptor (EGFR) signaling can also be activated by GLP-1(7–36).29 Studies by several laboratories have shown that both PKA and exchange protein activated by cAMP (Epac) signaling pathways are involved in GLP-1 mediated insulin secretion.26,30-33 Furthermore, GLP-1(7–36) may also stimulate insulin secretion via the inhibition of voltage-dependent K+ channels.34
Insulinomimetic and Protective Effects of GLP-1
The “insulin-like” effect of GLP-1(7–36) was first demonstrated in the stimulation of hepatic glucose uptake in obese human subjects.35 An ex vivo study with rat hepatocytes indicated that GLP-1(7–36) activates glycogen synthase, possibly via PI3K and Akt signaling cascades.36 Although an early study with the infusion of GLP-1(9–36) into non-obese individual generated no improvement on insulin release or glucose disposal,37 a later study in obese and insulin resistance subjects showed a profound inhibitory effect of GLP-1(9–36) on hepatic glucose production.38 There is still an ongoing debate about the existence of GLP-1R in hepatocytes, and it is not clear whether GLP-1(7–36) and GLP-(9–36) exert their insulinomimetic effect in hepatocytes via a yet to be identified novel receptor, or through a receptor-independent mechanism, or through their further cleaved fragment [such as GLP-1(28–36)].7
Both GLP-1(7–36) and GLP-1(9–36) were found to exert insulin-like action in the heart and vasculatures. They improve cardiac function and increase glucose uptake in isolated mouse heart with ischemic myocardial damage.39 At least some of the beneficial effects by GLP-1(7–36) and GLP-1(9–36) are not mediated by the canonical GLP-1R.5 A recent study shows that GLP-1(7–36) is able to recruit microvasculature and increases glucose usage in muscle via a nitric oxide-dependent mechanism.40
GLP-1(7–36) and the GLP-1R agonist exendin-4 are known to stimulate pancreatic β-cell proliferation, enhance β-cell survival and neogenesis. More recently, a study by Liu and Habeber indicated that the nonapeptide GLP-1(28–36) possesses the cytoprotective effect in the rat pancreatic clonal cell line INS-1. This nonapeptide may target mitochondria and improve mitochondrial membrane potential, elevate cellular ATP level, inhibit cytochrome c mediated cell apoptosis, and enhance the viability and survival of this β-cell line.25
Another important protective effect of GLP-1(7–36) was demonstrated in studying a major mediator of glucotoxicity. The thiol oxidoreductase, thioredoxin (TRX) is ubiquitously expressed and participates in maintaining cell redox balance, protecting cells against oxidative stress via scavenging reactive oxygen species. TRX binding protein-2 (TBP-2), also known as thioredoxin-interacting protein (TxNIP), negatively regulates the function of TRX.41 The intracellular TxNIP level is highly correlated with the blood glucose level.42 Several investigations have revealed the causative role of TxNIP in β-cell apoptosis, providing a mechanistic link between glucotoxicity, β-cell death, and thus, the progression of type 2 diabetes.43-46 A few recent studies have shown that exendin-4 is able to reduce TxNIP level in pancreatic β-cells.42,46 We found that this is at least partially due to cAMP/PKA and cAMP/Epac mediated degradation of TxNIP protein.10
Wnt Signaling Pathway and Its Effector β-Cat in GLP-1 Production and Function
Introduction of the Wnt signaling pathway and its effector β-cat
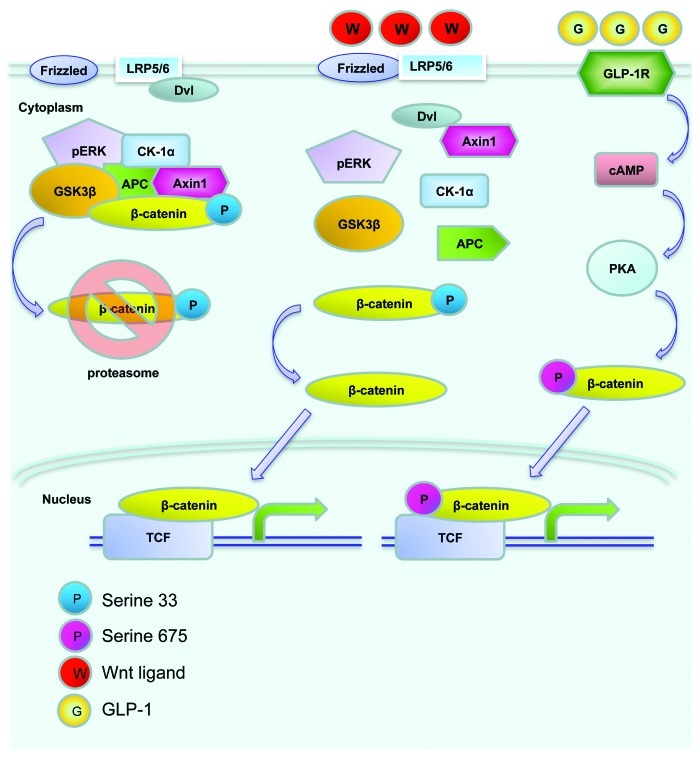
The Wnt signaling pathway was initially recognized in cancer research and in the study of embryonic development in Xenopus and Drosophila. The involvement of this signaling cascade in organogenesis in mammals has also been extensively investigated. Wnt ligands, through their cell membrane bound Frizzled receptors and the LRP5/6 co-receptor, exert various fundamental physiological and pathophysiological functions in mammals and other species. As shown in Figure 2, the major downstream effector of the canonical Wnt signaling pathway (defined as Wnt pathway hereafter) is β-cat/TCF or cat/TCF. This bipartite transcription factor consists of free β-cat and a member of the TCF family (TCF7, LEF-1, TCF7L1 and TCF7L2). The level of cytosolic free β-cat is tightly controlled by the proteasome-mediated degradation process, involving the tumor suppressors adenomatous polyposis coli (APC) and axin, as well as the serine/threonine kinases glycogen synthase kinase-3 (GSK-3) and casein kinase 1α (CK1α). β-cat is phosphorylated by GSK-3 and CK1α at Ser33 and three other adjacent serine positions to facilitate its proteasome degradation. Wnt ligands exert their regulatory effects via a Frizzled receptor and the LRP5/6 co-receptor. Upon Wnt ligand binding, the Wnt receptor associates with the protein known as dishevelled (Dvl). This event triggers the disruption of the complex containing APC, axin, GSK-3 and β-cat, preventing it from phosphorylation-mediated degradation. β-cat will be able to enter the nucleus and form the β-cat/TCF bipartite transcription factor, leading to elevated expression of target genes that are downstream of Wnt (or cat/TCF) signaling cascade.
Figure 2. An illustration of the Wnt signaling pathway. Without Wnt ligand stimulation, β-cat is trapped within the “destruction complex,” phosphorylated by the protein kinase GSK-3 and CK-1α at Ser33 and adjacent Ser positions, and subsequently degraded by proteasome (left). Following Wnt ligand stimulation and Dishvelled (Dvl) activation, β-cat escapes the trapping, enters the nucleus and forms the bipartite transcription factor cat/TCF, which leads to the stimulation of Wnt target gene expression (middle). GLP-1 was shown to activate cAMP-dependent protein kinase A (PKA), and stimulate β-cat Ser675 phosphorylation, which is positively associated with its nuclear translocation and Wnt target gene expression (right).
Extensive recent studies have revealed that cat/TCF also mediates the effect of signaling molecules other than the Wnt ligands.47 A battery of peptide hormones, including GLP-1, that utilize cAMP as their second messenger, have been shown to exert their functions through the Wnt effector β-cat/TCF.47 This has been attributed to PKA mediated activation of β-cat phosphorylation at a C-terminal Ser675 or Ser552 position (Fig. 2).
β-cat and GLP-1 production
We demonstrated previously that both lithium (which mimics the function of the Wnt ligands)48 and a constitutively active β-cat molecule (carrying S33Y mutation) can stimulate the activity of the rat gcg promoter.49 Lithium was also shown to stimulate endogenous gcg mRNA expression and GLP-1 production in mouse intestinal gcg-expressing cell lines and in primary fetal rat intestinal cell cultures.49 More recently, we found that gcg promoter and mRNA expression can be stimulated by Wnt-3A.50 Activation of gcg promoter by lithium treatment is dependent upon a TCF binding motif located within the G2 element of the gcg promoter.51 As this G2 enhancer element is known to mediate the stimulatory effects of cAMP and calcium,52 this observation suggested that cAMP activates gcg expression via cross-talking with the Wnt signaling pathway effector cat/TCF. Using chromatin immunoprecipitation (ChIP), we have then demonstrated an in vivo physical interaction between TCF7L2 and the G2 element.51 Interestingly, GIP expression was also found to be stimulated by the Wnt signaling cascade by García-Martínez and colleagues.53
β-cat and GLP-1 function
cat/TCF can mediate the function of many hormonal and other regulatory peptides during the adulthood.54 This can be achieved by β-cat Ser675 phosphorylation by cAMP/PKA or insulin/PAK-1 signaling cascade.50,55-57 Liu and Habener have assessed the involvement of TCF7L2, β-cat and Wnt signaling pathway in the function of GLP-1 and the GLP-1R agonist exendin-4.12 They demonstrated the expression of TCF7L2 in the rat INS-1 cell line and determined the presence of Wnt activity in mouse pancreatic islets using the TOPGAL transgenic mouse model. In this mouse, the expression of β-galactosidase reporter is controlled by a regulatory DNA element consisting of consensus LEF/TCF binding site upstream of a minimal c-fos promoter.58 Liu and Habener found that islets from TOPGAL mice show increased LacZ expression in response to exendin-4 treatment.12 This, along with a battery of other observations obtained by Liu and Habener, suggests that β-cat/TCF, the effector of the Wnt signaling, mediates the function of GLP-1 in stimulating β-cell proliferation. They have also demonstrated the activation of β-cat Ser675 phosphorylation by exendin-4.12 We found very recently that GLP-1(28–36) can also stimulate β-cat Ser675 phosphorylation, along with the stimulation of cAMP/PKA signaling cascade (unpublished data of Shao et al.).
Summary and Perspective
Although the studies on the two incretin hormones during the past two decades has led to the development of GLP-1R agonists and DPP-IV inhibitors as therapeutic agents for diabetes and possibly other metabolic disorders, further exploration of mechanisms underlying the function of GLP-1 will be helpful not only for the identification of novel drug targets, but also for the improvement of the usage of existing drugs.
GLP-1 is different from another incretin hormone GIP, as it possesses the insulinomimetic effect in pancreatic β-cells and extra-pancreatic tissues. The pancreatic insulinomimetic effect of GLP-1 makes its analogs or GLP-1R agonists more attractive agents in the treatment of not only type 2 diabetes mellitus, but also type 1 diabetes mellitus. The exploration of mechanisms underlying the extra-pancreatic insulinomimetic effect of GLP-1, including that in heart, liver, muscle, brain and elsewhere, may lead to the identification of novel therapeutic targets for type 2 diabetes mellitus, obesity, and other metabolic disorder.
It becomes clear to us that previously assumed “degradation” products of GLP-1(7–36) possess, at least, potential pharmacological functions. Although the degradation process may serve as a negative feedback for attenuating its acute insulinotropic effect, the products generated may exert their insulinomimetic effects on various organs. In the next few years, we anticipate to see the studies in elucidating whether these effects are mediated by a novel receptor or through a receptor-independent mechanism.
Basic research on the Wnt signaling pathway in different disciplines have revealed that the bipartite transcription factor cat/TCF mediates not only the effect of the Wnt ligands, but also the cell signaling of certain peptide hormones, especially those that utilize cAMP as a second messenger.47 Evidently, β-cat posttranslational modification, especially its C-terminal phosphorylation will be the focus for further functional study of GLP-1. As shown in Figure 3, GLP-1(7–36)amide is capable of stimulating β-cat Ser 675 phosphorylation, leading to Wnt signaling pathway activation. Since the GLP-1R agonist exendin-4 can also stimulate β-cat Ser-675 phosphorylation, we anticipate that this event can be mediated by GLP-1R (12). GLP-1(9–36)amide generated by DPP-IV cleavage exerts certain beneficial effects in a GLP-1R independent manner.5 The further cleavage by NEP 24.11 leads to the generation of GLP-1(28–36)amide. This peptide is known to possess the cytoprotective effect for pancreatic β-cells in vitro, and inhibits weight gain and attenuates diabetes and hepatic steatosis in high fat diet-induced obese mouse model.8,9,25 Whether this nonapeptide activates β-cat and functions as a powerful insulinomimetic hormone for both pancreatic β-cells and extra-pancreatic organs are worth to be examined.
Figure 3. Summary of insulinotropic and insulinomimetic effects of GLP-1 and its cleavage products in pancreatic β-cells. The cleavage of GLP-1(7–36)amide (defined as 7–36amide) by DPP-IV leads to the production of GLP-1(9–36)amide (defined as 9–36amide). The cleavage by NEP 24.11 leads to the production of GLP-1 (28–36)amide (defined as 28–36amide). GLP-1R mediates the insulinotropic effect of 7–36amide and GLP-1R agonists, such as exendin-4, involving both cAMP/PKA and cAMP/Epac. PKA can activate β-cat via increasing its Ser675 phosphorylation, which is at least partially responsible for the insulinomimetic effect of GLP-1. Whether 28–36amide exerts its insulinomimetic effect in pancreatic β-cells via a yet to be identified receptor, or a receptor independent mechanism remain to be further investigated. Whether or not 28–36amide exerts its insulinomimetic effect via stimulating β-cat Ser675 phosphorylation is also worth to be further examined.
Acknowledgments
Studies on the production and function of GLP-1 in T.J.’s laboratory are supported by Canadian Institutes of Health Research (MOP-89987, MOP-97790) and Canadian Diabetes Association (OG-3–10–3040). We appreciate Banting and Best Diabetes Centre (BBDC) for providing studentship/fellowship for our graduate students and research fellows. We regret not being able to cite all of the excellent contributions in the field due to space limitations.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/islets/article/23345
References
- 1.Jin T. Mechanisms underlying proglucagon gene expression. J Endocrinol. 2008;198:17–28. doi: 10.1677/JOE-08-0085. [DOI] [PubMed] [Google Scholar]
- 2.Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 1999;20:876–913. doi: 10.1210/er.20.6.876. [DOI] [PubMed] [Google Scholar]
- 3.Holst JJ, Orskov C. The incretin approach for diabetes treatment: modulation of islet hormone release by GLP-1 agonism. Diabetes. 2004;53(Suppl 3):S197–204. doi: 10.2337/diabetes.53.suppl_3.S197. [DOI] [PubMed] [Google Scholar]
- 4.Holst JJ. Glucagon-like peptide-1: from extract to agent. The Claude Bernard Lecture, 2005. Diabetologia. 2006;49:253–60. doi: 10.1007/s00125-005-0107-1. [DOI] [PubMed] [Google Scholar]
- 5.Ban K, Kim KH, Cho CK, Sauvé M, Diamandis EP, Backx PH, et al. Glucagon-like peptide (GLP)-1(9-36)amide-mediated cytoprotection is blocked by exendin(9-39) yet does not require the known GLP-1 receptor. Endocrinology. 2010;151:1520–31. doi: 10.1210/en.2009-1197. [DOI] [PubMed] [Google Scholar]
- 6.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–50. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 7.Tomas E, Habener JF. Insulin-like actions of glucagon-like peptide-1: a dual receptor hypothesis. Trends Endocrinol Metab. 2010;21:59–67. doi: 10.1016/j.tem.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomas E, Stanojevic V, Habener JF. GLP-1-derived nonapeptide GLP-1(28-36)amide targets to mitochondria and suppresses glucose production and oxidative stress in isolated mouse hepatocytes. Regul Pept. 2011;167:177–84. doi: 10.1016/j.regpep.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Tomas E, Wood JA, Stanojevic V, Habener JF. GLP-1-derived nonapeptide GLP-1(28-36)amide inhibits weight gain and attenuates diabetes and hepatic steatosis in diet-induced obese mice. Regul Pept. 2011;169:43–8. doi: 10.1016/j.regpep.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Shao W, Yu Z, Fantus IG, Jin T. Cyclic AMP signaling stimulates proteasome degradation of thioredoxin interacting protein (TxNIP) in pancreatic beta-cells. Cell Signal. 2010;22:1240–6. doi: 10.1016/j.cellsig.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Ip W, Chiang YT, Jin T. The involvement of the wnt signaling pathway and TCF7L2 in diabetes mellitus: The current understanding, dispute, and perspective. Cell Biosci. 2012;2:28. doi: 10.1186/2045-3701-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z, Habener JF. Glucagon-like peptide-1 activation of TCF7L2-dependent Wnt signaling enhances pancreatic beta cell proliferation. J Biol Chem. 2008;283:8723–35. doi: 10.1074/jbc.M706105200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao W, Wang D, Chiang YT, Ip W, Zhu L, Xu F, et al. The Wnt Signaling Pathway Effector TCF7L2 Controls Gut and Brain Proglucagon Gene Expression and Glucose Homeostasis. Diabetes. 2012 doi: 10.2337/db12-0365. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin T, Liu L. The Wnt signaling pathway effector TCF7L2 and type 2 diabetes mellitus. Mol Endocrinol. 2008;22:2383–92. doi: 10.1210/me.2008-0135. [DOI] [PubMed] [Google Scholar]
- 15.Jin T. The WNT signalling pathway and diabetes mellitus. Diabetologia. 2008;51:1771–80. doi: 10.1007/s00125-008-1084-y. [DOI] [PubMed] [Google Scholar]
- 16.Grant SF. Understanding the elusive mechanism of action of TCF7L2 in metabolism. Diabetes. 2012;61:2657–8. doi: 10.2337/db12-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyssenko V, Jonsson A, Almgren P, Pulizzi N, Isomaa B, Tuomi T, et al. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med. 2008;359:2220–32. doi: 10.1056/NEJMoa0801869. [DOI] [PubMed] [Google Scholar]
- 18.Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev. 2012;33:187–215. doi: 10.1210/er.2011-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansotia T, Drucker DJ. GIP and GLP-1 as incretin hormones: lessons from single and double incretin receptor knockout mice. Regul Pept. 2005;128:125–34. doi: 10.1016/j.regpep.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 20.Drucker DJ, Erlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci U S A. 1996;93:7911–6. doi: 10.1073/pnas.93.15.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thyssen S, Arany E, Hill DJ. Ontogeny of regeneration of beta-cells in the neonatal rat after treatment with streptozotocin. Endocrinology. 2006;147:2346–56. doi: 10.1210/en.2005-0396. [DOI] [PubMed] [Google Scholar]
- 22.Hupe-Sodmann K, McGregor GP, Bridenbaugh R, Göke R, Göke B, Thole H, et al. Characterisation of the processing by human neutral endopeptidase 24.11 of GLP-1(7-36) amide and comparison of the substrate specificity of the enzyme for other glucagon-like peptides. Regul Pept. 1995;58:149–56. doi: 10.1016/0167-0115(95)00063-H. [DOI] [PubMed] [Google Scholar]
- 23.Plamboeck A, Holst JJ, Carr RD, Deacon CF. Neutral endopeptidase 24.11 and dipeptidyl peptidase IV are both mediators of the degradation of glucagon-like peptide 1 in the anaesthetised pig. Diabetologia. 2005;48:1882–90. doi: 10.1007/s00125-005-1847-7. [DOI] [PubMed] [Google Scholar]
- 24.Davidson EP, Coppey LJ, Dake B, Yorek MA. Effect of Treatment of Sprague Dawley Rats with AVE7688, Enalapril, or Candoxatril on Diet-Induced Obesity. J Obes. 2011;2011:9 pages. doi: 10.1155/2011/686952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z, Stanojevic V, Brindamour LJ, Habener JF. GLP1-derived nonapeptide GLP1(28-36)amide protects pancreatic β-cells from glucolipotoxicity. J Endocrinol. 2012;213:143–54. doi: 10.1530/JOE-11-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacDonald PE, El-Kholy W, Riedel MJ, Salapatek AM, Light PE, Wheeler MB. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes. 2002;51(Suppl 3):S434–42. doi: 10.2337/diabetes.51.2007.S434. [DOI] [PubMed] [Google Scholar]
- 27.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–65. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Holz GG, 4th, Kühtreiber WM, Habener JF. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1(7-37) Nature. 1993;361:362–5. doi: 10.1038/361362a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brubaker PL, Drucker DJ. Minireview: Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004;145:2653–9. doi: 10.1210/en.2004-0015. [DOI] [PubMed] [Google Scholar]
- 30.Kwan EP, Gao X, Leung YM, Gaisano HY. Activation of exchange protein directly activated by cyclic adenosine monophosphate and protein kinase A regulate common and distinct steps in promoting plasma membrane exocytic and granule-to-granule fusions in rat islet beta cells. Pancreas. 2007;35:e45–54. doi: 10.1097/mpa.0b013e318073d1c9. [DOI] [PubMed] [Google Scholar]
- 31.Takeda Y, Amano A, Noma A, Nakamura Y, Fujimoto S, Inagaki N. Systems analysis of GLP-1 receptor signaling in pancreatic β-cells. Am J Physiol Cell Physiol. 2011;301:C792–803. doi: 10.1152/ajpcell.00057.2011. [DOI] [PubMed] [Google Scholar]
- 32.Seino S, Takahashi H, Fujimoto W, Shibasaki T. Roles of cAMP signalling in insulin granule exocytosis. Diabetes Obes Metab. 2009;11(Suppl 4):180–8. doi: 10.1111/j.1463-1326.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- 33.Holz GG. New insights concerning the glucose-dependent insulin secretagogue action of glucagon-like peptide-1 in pancreatic beta-cells. Horm Metab Res. 2004;36:787–94. doi: 10.1055/s-2004-826165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacDonald PE, Salapatek AM, Wheeler MB. Glucagon-like peptide-1 receptor activation antagonizes voltage-dependent repolarizing K(+) currents in beta-cells: a possible glucose-dependent insulinotropic mechanism. Diabetes. 2002;51(Suppl 3):S443–7. doi: 10.2337/diabetes.51.2007.S443. [DOI] [PubMed] [Google Scholar]
- 35.Egan JM, Meneilly GS, Habener JF, Elahi D. Glucagon-like peptide-1 augments insulin-mediated glucose uptake in the obese state. J Clin Endocrinol Metab. 2002;87:3768–73. doi: 10.1210/jc.87.8.3768. [DOI] [PubMed] [Google Scholar]
- 36.Redondo A, Trigo MV, Acitores A, Valverde I, Villanueva-Peñacarrillo ML. Cell signalling of the GLP-1 action in rat liver. Mol Cell Endocrinol. 2003;204:43–50. doi: 10.1016/S0303-7207(03)00146-1. [DOI] [PubMed] [Google Scholar]
- 37.Vahl TP, Paty BW, Fuller BD, Prigeon RL, D’Alessio DA. Effects of GLP-1-(7-36)NH2, GLP-1-(7-37), and GLP-1- (9-36)NH2 on intravenous glucose tolerance and glucose-induced insulin secretion in healthy humans. J Clin Endocrinol Metab. 2003;88:1772–9. doi: 10.1210/jc.2002-021479. [DOI] [PubMed] [Google Scholar]
- 38.Elahi D, Egan JM, Shannon RP, Meneilly GS, Khatri A, Habener JF, et al. GLP-1 (9-36) amide, cleavage product of GLP-1 (7-36) amide, is a glucoregulatory peptide. Obesity (Silver Spring) 2008;16:1501–9. doi: 10.1038/oby.2008.229. [DOI] [PubMed] [Google Scholar]
- 39.Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, et al. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110:955–61. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- 40.Chai W, Dong Z, Wang N, Wang W, Tao L, Cao W, et al. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes. 2012;61:888–96. doi: 10.2337/db11-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spindel ON, World C, Berk BC. Thioredoxin interacting protein: redox dependent and independent regulatory mechanisms. Antioxid Redox Signal. 2012;16:587–96. doi: 10.1089/ars.2011.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J, Couto FM, Minn AH, Shalev A. Exenatide inhibits beta-cell apoptosis by decreasing thioredoxin-interacting protein. Biochem Biophys Res Commun. 2006;346:1067–74. doi: 10.1016/j.bbrc.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 43.Shalev A. Lack of TXNIP protects beta-cells against glucotoxicity. Biochem Soc Trans. 2008;36:963–5. doi: 10.1042/BST0360963. [DOI] [PubMed] [Google Scholar]
- 44.Masson E, Koren S, Razik F, Goldberg H, Kwan EP, Sheu L, et al. High beta-cell mass prevents streptozotocin-induced diabetes in thioredoxin-interacting protein-deficient mice. Am J Physiol Endocrinol Metab. 2009;296:E1251–61. doi: 10.1152/ajpendo.90619.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corbett JA. Thioredoxin-interacting protein is killing my beta-cells! Diabetes. 2008;57:797–8. doi: 10.2337/db08-0055. [DOI] [PubMed] [Google Scholar]
- 46.Chen J, Hui ST, Couto FM, Mungrue IN, Davis DB, Attie AD, et al. Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic beta-cell mass and protects against diabetes. FASEB J. 2008;22:3581–94. doi: 10.1096/fj.08-111690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin T, George Fantus I, Sun J. Wnt and beyond Wnt: multiple mechanisms control the transcriptional property of beta-catenin. Cell Signal. 2008;20:1697–704. doi: 10.1016/j.cellsig.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 48.Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6:1664–8. doi: 10.1016/S0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 49.Ni Z, Anini Y, Fang X, Mills G, Brubaker PL, Jin T. Transcriptional activation of the proglucagon gene by lithium and beta-catenin in intestinal endocrine L cells. J Biol Chem. 2003;278:1380–7. doi: 10.1074/jbc.M206006200. [DOI] [PubMed] [Google Scholar]
- 50.Chiang YA, Shao W, Xu XX, Chernoff J, Jin T. P21-Activated Protein Kinase 1 (Pak1) Mediates the Cross Talk between Insulin and beta-Catenin on Proglucagon Gene Expression and Its Ablation Affects Glucose Homeostasis in Male C57BL/6 Mice. Endocrinology. 2013;154:77–88. doi: 10.1210/en.2012-1781. [DOI] [PubMed] [Google Scholar]
- 51.Yi F, Brubaker PL, Jin T. TCF-4 mediates cell type-specific regulation of proglucagon gene expression by beta-catenin and glycogen synthase kinase-3beta. J Biol Chem. 2005;280:1457–64. doi: 10.1074/jbc.M411487200. [DOI] [PubMed] [Google Scholar]
- 52.Fürstenau U, Schwaninger M, Blume R, Jendrusch EM, Knepel W. Characterization of a novel calcium response element in the glucagon gene. J Biol Chem. 1999;274:5851–60. doi: 10.1074/jbc.274.9.5851. [DOI] [PubMed] [Google Scholar]
- 53.García-Martínez JM, Chocarro-Calvo A, Moya CM, García-Jiménez C. WNT/beta-catenin increases the production of incretins by entero-endocrine cells. Diabetologia. 2009;52:1913–24. doi: 10.1007/s00125-009-1429-1. [DOI] [PubMed] [Google Scholar]
- 54.Yi F, Sun J, Lim GE, Fantus IG, Brubaker PL, Jin T. Cross talk between the insulin and Wnt signaling pathways: evidence from intestinal endocrine L cells. Endocrinology. 2008;149:2341–51. doi: 10.1210/en.2007-1142. [DOI] [PubMed] [Google Scholar]
- 55.Hino S, Tanji C, Nakayama KI, Kikuchi A. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase stabilizes beta-catenin through inhibition of its ubiquitination. Mol Cell Biol. 2005;25:9063–72. doi: 10.1128/MCB.25.20.9063-9072.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu G, Wang Y, Huang B, Liang J, Ding Y, Xu A, et al. A Rac1/PAK1 cascade controls β-catenin activation in colon cancer cells. Oncogene. 2012;31:1001–12. doi: 10.1038/onc.2011.294. [DOI] [PubMed] [Google Scholar]
- 57.Ip W, Shao W, Chiang YT, Jin T. The Wnt signaling pathway effector TCF7L2 is upregulated by insulin and represses hepatic gluconeogenesis. Am J Physiol Endocrinol Metab. 2012;303:E1166–76. doi: 10.1152/ajpendo.00249.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–68. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]